Abstract
Objective of the study was to assess effect of iron therapy on serum hepcidin levels in iron deficient pregnant women and its correlation with hemoglobin, serum iron profile and C-reactive protein (CRP). A total of 100 pregnant women were enrolled in the study; 25 were included in the “control group” having normal hematological and biochemical parameters while 75 iron deficient pregnant women were enrolled in the “patient group” with low hematological and biochemical parameters. CRP was done to rule out inflammation and to observe its association with hepcidin. Intravenous iron was administered to the patient group. Post treatment CBC, serum iron, serum ferritin and serum hepcidin were determined. Difference between pre and post treatment hemoglobin, serum iron, serum ferritin and serum hepcidin levels were determined and correlation among them was calculated. Post treatment serum hepcidin levels were significantly higher than pretreatment level (p = 0.001). However, no correlation was seen between serum hepcidin, serum iron, serum ferritin and hemoglobin. Hepcidin levels remain low during pregnancy as there is increased demand for iron in pregnancy. Iron supplementation results in increased hepcidin levels; however no mathematical correlation was found between serum hepcidin level and serum iron profile.
Keywords: Iron deficiency anemia, Pregnancy, Hepcidin, Treatment
Introduction
Insufficient amount of dietary intake of iron, excessive loss of iron or its increased demand results in iron deficiency anemia (IDA) [1]. IDA affects nearly 700–800 million people worldwide [2]. It is most prevalent in old age, lower socio-economic group and in women of child bearing age [3].
Anemia in pregnancy is a common problem. WHO has defined anemia in pregnancy as hemoglobin level below 11 g/dl. Anemia in pregnancy is multifactorial of which iron deficiency is the most common cause [4]. Iron demand increases in the second and third trimester of pregnancy. Increase in oxygen consumption by both mother and fetus tends to increase red cell mass which requires iron. Early placental development and fetal growth also requires iron [5]. Adequate nutritional supply and proper iron supplementation is required to fulfill this increased demand. Poor nutrition and lack of iron supplementation along with increased demand leads to iron deficiency anemia [6].
Treatment of IDA according to American college of Physician’s recent guidelines [7] is oral iron supplementation which is economical and effective. Ferrous sulphate is given as 200 mg twice daily [8]. Treatment of IDA with oral iron supplements is however limited by gastrointestinal absorption problems. It is also ineffective in treating IDA with coexisting acute or chronic conditions such as celiac disease, H. pylori infection and autoimmune atrophic gastritis. In pregnancy moderate to severe anemia is treated with parenteral iron sucrose infusion which has an established role in the correction of IDA in pregnancy [9, 10].
Proliferation, growth and functions of cells require sufficient amount of iron. Transferrin regulates the systemic iron homeostasis by controlling the entry of iron into the plasma. Iron bound to transferrin is obtained from various sources i.e. reticuloendothelial macrophages, senescent red cells and the absorbed dietary iron by enterocytes [11]. Iron absorbed from the enterocytes is either bound with apoferritin or ferroportin. Iron incorporated in ferritin is lost after the exfoliation of the cells. While iron bound to ferroportin is transported from the enterocytes into the plasma. Ferroportin thus plays an important role in the exit of iron from the enterocytes into the plasma [12].
Ferroportin is regulated by hepcidin; one of the most important regulators of iron metabolism with a molecular weight of approximately 28 Da [13]. It is encoded by hepcidin antimicrobial peptide (HAMP) gene located on the long arm of chromosome 19 at position 13.1(19q13.1) [14, 15]. Hepcidin binds with ferroportin, relieves it from the cell membrane which then enter into the lysosomal compartment for degradation [16]. Synthesis of hepcidin increases with increased plasma iron or stores, infections and inflammation through SMAD and STAT3 pathways. SMAD and STAT 3 pathway increases hepcidin expression which degrades ferroportin and inhibits iron absorption [17]. SMAD pathway is activated by bone morphogenic protein-6 (BMP-6) and its co-receptor hemojuvellin (HJV). BMP-6 combines with HJV and phosphorylates SMAD to form SMAD-1/-5/-8-SMAD-4 complex. This complex translocates to the nucleus and induces the transcription of HAMP gene which in turn stimulates hepcidin production [18].
Decreased plasma iron levels, anemia (hypoxia) and increased erythropoiesis suppresses the synthesis of hepcidin which in turn increases ferroportin synthesis and transports of iron from the enterocytes into the plasma [19]. Hepcidin expression is suppressed by several factors that include SMAD 7 protein, growth differentiation factor 15(GDF15), twisted grastulation factor 1(TWSG1), erythropoietin, hypoxia inducible factor (HIF) and matriptase. SMAD7 inhibits SMAD protein that mediates a negative feedback loop to both TGF-β and BMP signaling [20]. At cellular level iron is regulated by iron regulatory protein (IRP) which inhibits production of divalent metal transporter (DMT1) during high intracellular iron levels and vice versa [18].
Iron deficiency anemia is diagnosed partly on signs and symptoms but mostly by conventional laboratory investigations which include serum iron, serum ferritin and less commonly the soluble transferrin receptors estimation. These tests have certain limitation; the commonly available laboratory test such as serum iron fails to reflect accurate iron status after iron supplementation in pregnant women.
Objective of this study was to assess the effect of parenteral iron therapy on hepcidin levels in iron deficient pregnant women and to observe the correlation between serum hepcidin level with hemoglobin, serum iron and serum ferritin.
Materials and Methods
This study was conducted at Baqai institute of hematology and Fatima hospital, Baqai medical university, Karachi. Samples were collected from pregnant females. One hundred subjects were enrolled and divided into two groups. Seventy five pregnant females in the 2nd and 3rd trimesters with low hemoglobin, decreased red cell indices, increased red cell distribution width (RDW) and microcytic hypochromic red cells having no history of parenteral iron infusion or oral iron supplements in the recent pregnancy and no history of allergic reactions to parenteral iron infusion were included in the ‘patient group’. Twenty five normal healthy pregnant women in the 2nd and 3rd trimesters with normal hematological parameters and no history of recent illness or intravenous iron infusion were constituted as the ‘control group’. Written informed consent was taken from all subjects enrolled in the study which was approved by the ethics committee of Baqai Medical University, Karachi.
Design
Purposive non-probability sampling technique was used and the study was conducted in three steps.
Step I
Blood samples from pregnant females were collected from the antenatal OPD and obstetrics ward of Fatima hospital, Baqai medical university, Karachi. Before collection of the samples each subject was made aware of the entire procedure and their signatures were obtained on the consent form along with their phone number and address for follow-up. 5 ml of venous blood was collected under aseptic conditions from each individual. Each blood sample was divided into two aliquots. 2 ml of blood was added to an anticoagulant tube and was used for complete blood count (CBC). Rest of the 3 ml of blood was added to a serum separator tube (gel tube) and allowed to stand for 30 min. After 30 min, the gel tubes were centrifuged at 2000 rpm for 10 min. Serum was separated after centrifugation. Serum iron, serum ferritin, and C-reactive protein (CRP) of all blood samples were determined. Sera of both groups were stored at −20 °C for subsequent analysis of serum hepcidin.
Step II
Intravenous iron infusion was given to the pregnant iron deficient females according to the following protocol;
Two ampoules (200 mg) of iron sucrose diluted with 100 ml N/S were given as intravenous infusion over 60 min.
First 25 ml of infusion was given at 5–10 drops/min as a test dose.
In the absence of any allergic reactions the rest of the 75 ml was infused at a moderate rate of 30 drops over approximately 45 min.
Step III
Post treatment blood samples were collected from the patient group after 20 days of iron infusion. Blood samples were tested for serum iron, serum ferritin and hepcidin.
CBC (including all blood cell counts and red cell indices i.e. Hb, TWBC, platelet, PCV, MCV, MCH, MCHC, RDW) was determined on all pre and post iron blood samples collected from patients, using automated cell analyzer (Sysmex XP 100 Tokyo Japan). Peripheral smears were made, air dried and stained with Leishman’s stain. Serum iron was determined by colorimetric analysis using Spinreact iron-ferrozine kit. Serum ferritin was determined by Enzyme Linked Immuno Sorbant Assay (ELISA) using Biochek, INC kit (BC-1025). Serum hepcidin was determined by ELISA using Human Hepcidin 25 (Hepc-25) ELISA kit (SL2089Hu). CRP was determined using automated analyzer. From the control group only one random blood sample was obtained from each subject and CBC, serum iron, serum ferritin, serum hepcidin and CRP were performed. No iron treatment was given to the control group.
Statistical Analysis
Data was analyzed using SPSS Version 20.0. Descriptive statistics was used for calculating mean, range and standard deviation. Student t test was used for comparison between the two groups while Pearson correlation was used for correlation between the two groups.
Results
Complete Blood Count and Biochemical Parameters in Normal and Iron Deficient Pregnant Women Before Iron Therapy
Females of 24th to 32nd weeks with hemoglobin level greater than 11 g/dl and normal MCV, MCH and MCHC constituted the control group. Biochemical parameters including serum iron, serum ferritin and serum hepcidin were assessed. Pregnant females of 24th to 32nd weeks with decreased hemoglobin, MCV and MCHC and decreased serum iron and serum ferritin formed the patient group. Results of the control and patient groups are shown in Table 1.
Table 1.
Complete blood count and biochemical parameters of normal pregnant and iron deficient pregnant females
| Parameters | Normal (N = 25) | IDA (N = 75) | p value |
|---|---|---|---|
| Hemoglobin (g/dl) | 11.65 ± 0.77 | 8.16 ± 1.15 | 0.001 |
| PCV (%) | 37.16 ± 2.77 | 27.73 ± 2.13 | 0.001 |
| MCV (fl) | 86.3 ± 4.17 | 72.92 ± 5.32 | 0.001 |
| MCH (pg) | 29.15 ± 1.47 | 21.95 ± 2.64 | 0.001 |
| MCHC (%) | 33.08 ± 1.43 | 29.34 ± 2.62 | 0.001 |
| TLC (103/µl) | 8.92 ± 1.61 | 9.07 ± 2.09 | 0.841 |
| Platelets (103/µl) | 270.36 ± 58.23 | 296 ± 80.911 | 0.058 |
| Serum iron (µg/dl) | 94.20 ± 29.67 | 52.22 ± 32.68 | 0.001 |
| Serum ferritin (ng/dl) | 24.27 ± 11.72 | 5.68 ± 3.00 | 0.001 |
| Serum hepcidin (ng/dl) | 3.99 ± 1.74 | 2.27 ± 1.2 | 0.001 |
| CRP (mg/L) | 2.5 ± 0.45 | 2.65 ± 0.49 | 0.395 |
Data are shown as mean ± SD
Hb hemoglobin, PCV packed cell volume, MCV mean cell volume, MCH mean cell hemoglobin, MCHC mean cell hemoglobin, TLC total leukocyte count, platelets, serum iron, serum ferritin, serum hepcidin, CRP C-reactive protein
Comparison of Pre and Post Treatment Levels of Hemoglobin, Serum Iron, Ferritin and Hepcidin in Iron Deficient Pregnant Females
Table 2 shows the results of pretreatment and post treatment levels of hemoglobin, serum iron, serum ferritin and hepcidin in iron deficient pregnant females. Differences between the pre treatment and post treatment values were statistically significant.
Table 2.
Pretreatment and post treatment levels in iron deficient pregnant females
| Parameters | Pretreatment | Post treatment | p value |
|---|---|---|---|
| Hemoglobin (g/dl) | 8.16 ± 1.15 | 9.25 ± 1.13 | 0.001 |
| S. iron (µg/dl) | 52.22 ± 32.68 | 112.79 ± 52.63 | 0.001 |
| S. ferritin (ng/dl) | 5.68 ± 3.00 | 22.73 ± 16.76 | 0.001 |
| S. hepcidin (ng/dl) | 2.27 ± 1.2 | 4.89 ± 3.01 | 0.001 |
Data are shown as mean ± SD. Serum iron, serum ferritin, serum hepcidin
C-Reactive Protein (CRP) and its Correlation with Serum Hepcidin
C-reactive protein was determined to exclude any concomitant inflammatory condition in anemic and non-anemic pregnant females and to study its association with hepcidin. No correlation was found between serum hepcidin and CRP when Pearson correlation was applied (r = 0.25, p = 0.086).
Correlation Between Hemoglobin, Serum Iron, Serum Ferritin and Serum Hepcidin Levels of Iron Deficient Group
Differences of the hemoglobin, serum iron, serum ferritin and serum hepcidin of the iron deficient group were calculated by subtracting post treatment values from pretreatment baseline. Correlation between them was analyzed during Pearson correlation. A statistically significant correlation was seen between serum iron on the one hand and serum ferritin on the other with a correlation (r = 0.333) and (p < 0.001). No correlation was seen between serum iron and hemoglobin. Serum hepcidin increases when iron is given, but there is no linear correlation between it and hematological pattern as shown in Table 3.
Table 3.
Correlation of hematological parameters with hepcidin
| Parameters | Diff. hemoglobin | Diff. S. iron | Diff. S. ferritin | Diff. S. hepcidin | ||||
|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | |
| Diff hemoglobin | N/A | N/A | −0.08 | 0.487 | 0.024 | 0.836 | 0.164 | 0.154 |
| Diff. S. iron | −0.08 | 0.487 | N/A | N/A | 0.333** | 0.003 | 0.035 | 0.765 |
| Diff. S. ferritin | 0.024 | 0.836 | 0.333** | 0.003 | N/A | N/A | 0.187 | 0.104 |
| Diff. S. hepcidin | 0.164 | 0.154 | 0.035 | 0.765 | 0.187 | 0.104 | N/A | N/A |
r pearson correlation, p 0.01, S serum, Diff difference, N/A not applicable
** Correlation is significant at 0.01 level
Discussion
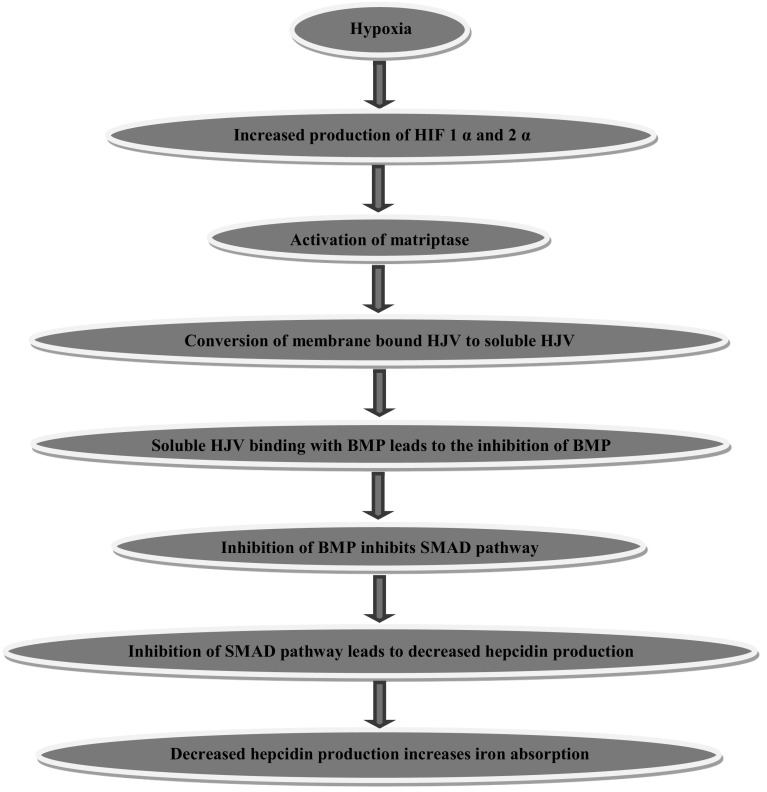
Iron deficiency anemia is associated with low levels of hepcidin in serum. This is brought about by a series of complex reactions resulting in reduced hepcidin production and increased iron absorption as explained below. Hypoxia inducible factor-1α (HIF-1) and factor-2α (HIF-2α) activate matriptase, a serine protease found in the liver [21]. Activated matriptase increases iron absorption through a series of reactions [22]; as illustrated in the following Fig. 1.
Fig. 1.
Hypoxia induced increased iron absorption. BMP bone morphogenic proteins, HJV hemojuvellin, SMAD (SMAD stands for sma and mad related proteins)
This study was undertaken with the object of assessing the hepcidin levels in iron deficient women in pregnancy and the effect of iron therapy on hepcidin levels in these patients. Iron was administered in the form of parenteral iron and the response of hepcidin was studied along with serum iron, serum ferritin and hemoglobin levels. Parenteral iron was chosen because it rapidly increases serum iron and to eliminate patient noncompliance.
It was postulated that serum hepcidin, serum iron, serum ferritin and hemoglobin levels which are usually low in pregnancy will increase after giving parenteral iron therapy. For this purpose, hemoglobin, serum iron, serum ferritin and serum hepcidin levels before and after giving iron therapy were assessed. CRP was also determined to exclude any inflammatory condition.
There was an increase in the hepcidin levels when iron was administered in the parenteral form in iron deficient anemic pregnant women. Mean ± SD values between pretreatment and post treatment serum iron, serum ferritin, serum hepcidin and hemoglobin in iron deficient pregnant females showed significant difference; p value being <0.001 as shown in Table 2.
Several studies confirm the findings of our study i.e. hepcidin levels along with serum iron, serum ferritin and hemoglobin increase when iron is given in parenteral or oral form. Moretti et al. [23] showed similar increase in serum hepcidin levels and various iron parameters in iron depleted but non anemic women of reproductive age group when given iron. Hwang et al. [24] found an increase in serum hepcidin and iron profile after single dose of oral iron in iron deficient as well as non-sideropenic healthy females. Berglund et al. [25] observed a rise in serum hepcidin levels when oral iron was given to iron deficient infants. Iron supplementation also increases iron level in the blood.
When difference of serum hepcidin, serum iron, serum ferritin and hemoglobin was taken into consideration and Pearson correlation was applied, no significant correlation was seen between serum hepcidin and hematological parameters as shown in Table 3. There was a positive correlation between serum iron and serum ferritin. It was found that the total increase in hepcidin values does not correlate with that of serum iron and serum ferritin, suggesting that serum hepcidin level is an independent marker and does not accurately predict the iron stores in iron deficient pregnant women after iron supplementation. Utilization of this correlation was to find out whether changes in hepcidin levels appropriately predict iron stores in pregnant women when given iron supplements. According to our findings hepcidin does have an association with iron but no mathematical correlation was observed between hepcidin, serum iron and serum ferritin.
Hepcidin levels are low during pregnancy as there is increased demand of iron in pregnancy and the demand increases in the second and the third trimester; therefore hepcidin level remains low in pregnancy, its level further decreases till term [26].
Simavali et al. [27] and Heddegran [28] support our observation that no correlation exist between serum hepcidin, serum ferritin and an inflammatory marker i.e. CRP in pregnancy. However, urinary hepcidin levels are positively correlated with ferritin levels in first trimester of pregnancy [29].
Limitations of our study include lack of serum transferrin receptor estimation, single stat dose of iron and absence of longer duration of patient follow up. Further studies are recommended to see the effect of iron supplementation on hepcidin level over longer duration and to establish standard values of hepcidin in normal pregnant women in each trimester.
Acknowledgements
First of all we would like to thank Allah. The authors would like to thank all the subjects participated in the study. We would like to thank Baqai medical university and Fatima hospital for their support; the nursing staff and doctors for their cooperation; all the technicians who helped us in running laboratory tests; Qamar Ayub who helped in the statistical work.
Compliance with Ethical Standards
Conflict of interest
Dr. Amtuz Zehra, Dr. Saleh Muhammad Saleh Abdullah, Dr. Muhammad Saboor and Dr. Moinuddin declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Baqai Medical University and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
References
- 1.U.S National Library of Medicine. Iron deficiency anemia. https://www.nlm.nih.gov/medlineplus/ency/article/000584.htm. Accessed 10 Mar 2014
- 2.Akhtar S, Ahmed A, Ahmad A, Ali Z, Riaz M, Ismail T. Iron status of the Pakistani population-current issues and strategies. Asia Pac J Clin Nutr. 2013;22:340–347. doi: 10.6133/apjcn.2013.22.3.17. [DOI] [PubMed] [Google Scholar]
- 3.Siddiqui MS, Siddiqui MK. Public health significance of iron deficiency anemia. Pak Armed Forces Med J. 2008;58:319–330. [Google Scholar]
- 4.Raza N, Sarwar I, Munazza B, Ayub M, Suleman M. Assessment of iron deficiency in pregnant women by determining iron status. J Ayub Med Coll Abbottabad. 2011;23:36–40. [PubMed] [Google Scholar]
- 5.Bothwell TH. Iron requirements in pregnancy and strategies to meet them. Am J Clin Nutr. 2016;72:257S–264S. doi: 10.1093/ajcn/72.1.257S. [DOI] [PubMed] [Google Scholar]
- 6.Cromell C. Hematologic changes in pregnancy. In: Hoffman R, Benz EJ, Silberstein LE, Heslop HE, Weitz JI, Anastasi J, editors. Hematology-basic principles and practice. 6. Philadelphia: Elsevier Saunders; 2013. pp. 2133–2144. [Google Scholar]
- 7.ACP guidelines.ACP releases new recommendations for iron deficiency anemia. http://emedicine.medscape.com/article/202333-overview. Accessed 10 Mar 2016
- 8.Goddard AF, James MW, McIntyre AS, Scott BB. Guidelines for the management of iron deficiency anemia. http://www.bsg.org.uk/pdf_word_docs/iron_def.pdf. Accessed 10 Mar 2014 [DOI] [PubMed]
- 9.Litton E, Xiao J, Ho KM. Safety and efficacy of intravenous iron therapy in reducing requirement for allogeneic blood transfusion: systematic review and meta-analysis of randomised clinical trials. BMJ. 2013;347:f4822. doi: 10.1136/bmj.f4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kriplani A, Mahey R, Dash B, Kulshrestha V, Agarwal N, Bhatla N. Intravenous iron sucrose therapy for moderate to severe anaemia in pregnancy. Indian J Med Res. 2013;138:78–82. [PMC free article] [PubMed] [Google Scholar]
- 11.Ganz T, Nemeth E. Hepcidin and regulation of body iron metabolism. Am J Physiol Gastrointest Liver Physiol. 2006;290:199–203. doi: 10.1152/ajpgi.00412.2005. [DOI] [PubMed] [Google Scholar]
- 12.Hoffbrand AV, Hershko C, Camaschella C. Iron metabolism, iron deficiency and disorders of haem synthesis. In: Hoffbrand A, Catovsky D, Tuddenham E, Green A, editors. Postgraduate haematology. 6. Oxford: Wiley-Blackwell; 2011. pp. 26–46. [Google Scholar]
- 13.Ward D, Kaplan J. Ferroportin-mediated iron transport: expression and regulation. Biochim Biophys Acta. 2012;1823(9):1426–1433. doi: 10.1016/j.bbamcr.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roetto A, Papanikolaou G, Politou M, Alberti F, Girelli D, Christakis J, et al. Mutant antimicrobial peptide hepcidin is associated with severe juvenile hemochromatosis. Nat Genet. 2002;33(1):21–22. doi: 10.1038/ng1053. [DOI] [PubMed] [Google Scholar]
- 15.Genetics Home Reference. HAMP gene. http://ghr.nlm.nih.gov/gene/HAMP. Accessed 12 May 2016
- 16.De Domenico I, Ward D, Langelier C, Vaughn M, Nemeth E, Sundquist W, et al. The molecular mechanism of hepcidin-mediated ferroportin down-regulation. Mol Biol Cell. 2007;18(7):2569–2578. doi: 10.1091/mbc.E07-01-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang H, Constante M, Layoun A, Santos M. Contribution of STAT3 and SMAD4 pathways to the regulation of hepcidin by opposing stimuli. Blood. 2009;113:3593–3599. doi: 10.1182/blood-2008-08-173641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoffbrand A, Moss P. Hypochromic anaemias. In: Hoffbrand A, Moss P, editors. Essential haematology. 6. Hoboken: Wiley-Blackwell; 2011. pp. 34–47. [Google Scholar]
- 19.Hoffbrand AV, Hershko C, Camaschella C (2011) Iron metabolism, iron deficiency and disorders of haemsynthesis. In: Postgraduate haematology, 6th edn. UK: Wiley-Blackwell, Hoboken, pp 26–46
- 20.Mleczko-Sanecka K, Casanovas G, Ragab A, Breitkopf K, Muller A, Boutros M, et al. SMAD7 controls iron metabolism as a potent inhibitor of hepcidin expression. Blood. 2010;115(13):2657–2665. doi: 10.1182/blood-2009-09-238105. [DOI] [PubMed] [Google Scholar]
- 21.Wang CY, Meynard D, Lin HY. The role of TMPRSS6/matriptase-2 in iron regulation and anemia. Front Pharmacol. 2014;5:114. doi: 10.3389/fphar.2014.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Du X, She E, Gelbart T, Truksa J, Lee P, Xia Y, Khovanath K, et al. The serine protease TMPRSS6 is required to sense iron deficiency. Science. 2008;320:1088–1092. doi: 10.1126/science.1157121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moretti D, Goede J, Zeder C, Jiskra M, Chatzinakou V, Tjalsma H, et al. Oral iron supplements increase hepcidin and decrease iron absorption from daily or twice-daily doses in iron-depleted young women. Blood. 2015;126:1981–1989. doi: 10.1182/blood-2015-05-642223. [DOI] [PubMed] [Google Scholar]
- 24.Hwang S, Lee Y, Park J, Norton H, Clemens E, Schrum L, et al. Effects of a single dose of oral iron on hepcidin concentrations in human urine and serum analyzed by a robust LC-MS/MS method. Clin Chim Acta. 2011;412:2241–2247. doi: 10.1016/j.cca.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berglund S, Lonnerdal B, Westrup B, Domellof M. Effects of iron supplementation on serum hepcidin and serum erythropoietin in low-birth-weight infants. Am J Clin Nutr. 2011;94:1553–1561. doi: 10.3945/ajcn.111.013938. [DOI] [PubMed] [Google Scholar]
- 26.Koenig M, Tussing-Humphreys L, Day J, Cadwell B, Nemeth E. Hepcidin and iron homeostasis during pregnancy. Nutrients. 2014;6:3062–3083. doi: 10.3390/nu6083062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simavli S, Derbent A, Uysal S, Turhan N. Hepcidin, iron status, and inflammation variables among healthy pregnant women in the Turkish population. J Matern Fetal Neonatal Med. 2014;27:75–79. doi: 10.3109/14767058.2013.804054. [DOI] [PubMed] [Google Scholar]
- 28.Hedengran KK, Nelson D, Andersen MR, Stender S, Szecsi PB. Hepcidin levels are low during pregnancy and increase around delivery in women without iron deficiency—a prospective cohort study. J Matern Fetal Neonatal Med. 2015;29:1506–1508. doi: 10.3109/14767058.2015.1052396. [DOI] [PubMed] [Google Scholar]
- 29.Schulze KJ, Christian P, Ray LA, Nath A, Wu L, Semba R. Hepcidin and iron status among pregnant women in Bangladesh. Asia Pac J Clin Nutr. 2016;17:451–456. [PMC free article] [PubMed] [Google Scholar]