Abstract
Multicolor flow cytometric (MFC) immunophenotyping is one of the basic test that is needed in the evaluation of hematolymphoid malignancies. Previously, there has been some reluctance in the use of MFC in plasma cell disorders (PCD). It was mainly due tolack of standardization, inadequate experience and detection of the lower number of plasma cells by MFC as compared to morphology. However, MFC has gone through many technological advancements in the last few years and a wide variety of reagents are now commercially available which worldwide allowed the establishment of standardized sensitive MFC-based immunophenotypic assay for PCD. Various studies have proven that MFC has a high clinical relevance in the diagnosis and risk stratification of multiple myeloma, its precursor conditions and other PCDs. Moreover, recent studies have shown that MFC is a highly sensitive and reliable technique for the monitoring of clinical response in the era of novel therapies. In this review, we have discussed the various applications of MFC in the management of PCD and their clinical relevance.
Keywords: Flow cytometry, Plasma cell disorders, Multiple myeloma
Introduction
Plasma cell dyscrasia (PCD) are a heterogeneous group of plasma cell disorders characterized by the accumulation of clonal plasma cells in the bone marrow and production of the excessive amount of monoclonal proteins that eventually leads to end-organ damage [1]. This group includes a spectrum of plasma cell neoplasms from precursor conditions like monoclonal gammopathies of undermined significance (MGUS) and smoldering multiple myeloma (SMM) to the full-blown multiple myeloma (MM) as well as localized disease, i.e. plasmacytoma. MGUS and SMM are two forms of asymptomatic precursor plasma cell disorders that may eventually progress to multiple myeloma MM [2]. MGUS affects approximately 3% of Caucasians over the age of 50 years [3], and MM is the second commonest hematological malignancy of elderly that accounts for 1% of all cancers and 10% of hematologic neoplasms [4]. Currently, several laboratory and radiological modalities are being used in the diagnosis and management of the PCD, including multicolor flow cytometry (MFC).
Historically, there has been some reluctance on the application of MFC in PCD, mainly because the lack of plasma cell (PC)—specific markers and the lower number of plasma cells usually detected in bone marrow aspirates (BMA) by MFC when compared to morphology [5–7]. In addition, usage of different methodologies, including different staining protocol, different monoclonal antibody (MoAb)—fluorochrome conjugates and lack of standardization had given rise to inconsistent results in the past [8, 9]. However, it is now well established that a well-standardized MFC has many unique advantages such as: (1) simultaneous analysis of multiple parameters, (2) study of high numbers of cells within a relatively short period, (3) study of low level of plasma cells, (4) quantitative analysis of antigen density, (5) simultaneous study of surface and intracellular antigens, (6) DNA-ploidy analysis and (7) concurrent study of non-PC bone marrow compartment [5, 10]. Thus, MFC is a fast, affordable, well-established, and widely available technique [11, 12]. Furthermore, MFC is easily amenable to multi-centre standardization [13–16]. Therefore, MFC has become an indispensable technique in the diagnosis, prognosis, and therapeutic response-monitoring of a majority of hematological neoplasms, including PCD [17, 18]. In this review, we briefly discuss the role of MFC in the diagnosis, prognostication, and monitoring of the PCD along with the recent advances. The highlights of this review are mentioned in Table 1.
Table 1.
Highlights of the article: MFC-based parameters in PCD and their clinical relevance
| MFC based parameters | Clinical relevance in the management of MM and other PCDs |
|---|---|
| Immunophenotyping in BM and peripheral blood | Useful in the diagnosis of PCDs: MM or plasma cell leukemia with unusual morphology, involvement by plasmacytoma [5, 19] |
| NPC > 5% of total PC in BM | An accurate parameter for the discrimination between MGUS and MM cases at diagnosis. >80% of MGUS harbours >5% NPC within total BM PC and only 15% of MM >5% NPC [25, 26, 33, 34] |
| Pattern of expression of CD38, CD19, CD45, CD20, CD56 and surface light chains | B-cells and plasma cells show typical pattern of maturation of clonal B cells to clonal PCs in Waldenstrom macroglobulinemia [35–37] |
| MM with MGUS-like immunophenotype | These MM has better prognosis with a greater response rate to HDT/ASCT and longer progression free survival (PFS) and overall survival (OS) rates [33] |
| MGUS & SMM > 95% APC out of total PC in BM | Predicts high-risk MGUS and SMM with shorter of time to progression (TTP) rate to MM [5, 33, 34] |
| CD117-/CD28+ MM | Indicates poor prognosis in MM with significantly shorter PFS and OS rates and less benefit from HDT/ ASCT [5, 8, 52, 53] |
| CD81+ MM | |
| MFC-based total PC > 15% | MM demonstrates inferior outcome as compared to MM <15% total PC in BM immunophenotyping [51] |
| Circulating PC of ≥400 cPCs/µl | Indicates MM with high PC proliferation, adverse cytogenetics and inferior time-to-next-treatment and OS [60] |
| MRD | MRD as an independent strong prognostic factor and superior treatment response evaluator in MM with hazard ratio 17.3 Persistent MRD by MFC at day 100 after HDT/ASCT (hazard ratio 8.0) were the only independent factors that predicted unsustained CR Immunophenotypic CR has significantly long PFS and TTP (hazard ratio = 4.1) [61–70] |
| DNA ploidy | Hyperdiploidy (DNA index >1.06) better prognosis [72, 76] |
| PC proliferation rate with >2% of PC in S phase | Associated with shorter disease free survival (DFS) and OS [77–81] |
| Reduced cytotoxic T-cells/NK cells and increased T-regs and TH17 cells | Causes impairment of anti-tumor immune effect Associated with shorter disease free survival (DFS) and OS [80, 84–91] |
Identification of Plasma Cells
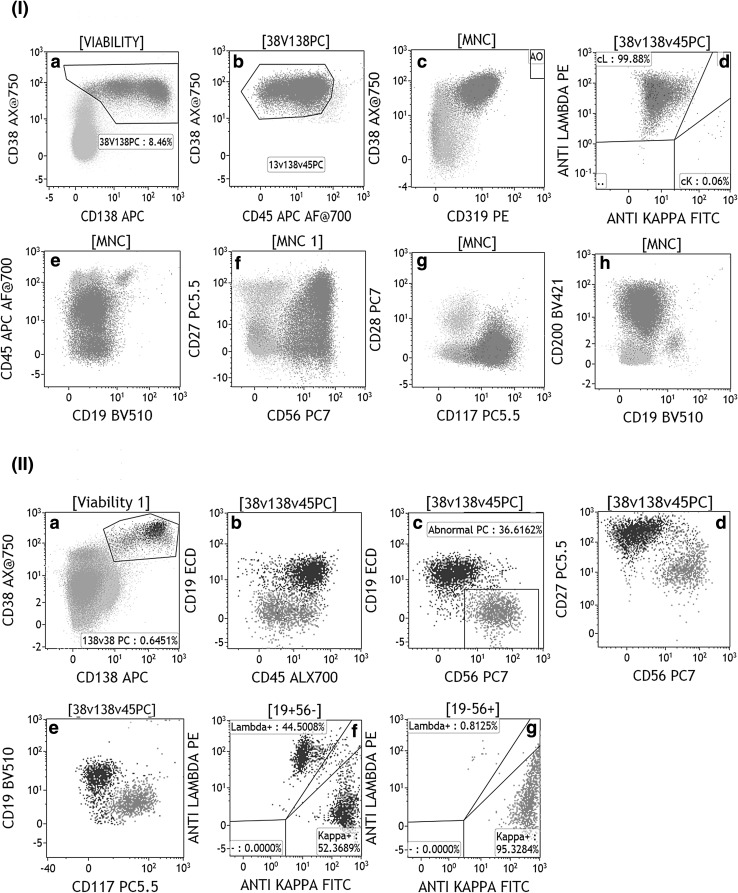
In MFC analysis, PCs are identified using its unique, strong expression of CD38 and intermediate to strong expression of CD138 [19]. CD138 (Syndecan-1) is an adhesion glycoprotein expressed by only PCs in all hematopoietic cells [20]. PCs also demonstrate intermediate CD45 expression and relatively higher light scatter than lymphocytes. Thus, the combination of CD38, CD138, and CD45 along with light scatter characteristics usually gives the most satisfactory discrimination of PCs on MFC (refer to Fig. 1I) [5, 19]. Nevertheless, it is important to note that relapsed or multi-therapy resistant cases of MM can show variable loss of CD138 and decreased expression of CD38. Loss of surface CD138 is also known following prolonged exposure to frigid temperature. Additionally, a novel anti-CD38 monoclonal-antibody therapy (daratumumab) and anti-CD138-IFNα14 fusion protein can also cause loss of CD38 and CD138 expression in plasma cells [21, 22]. This can lead to the suboptimal identification of PCs, and hence, new plasma cell-specific markers have been studied. Of them, signaling lymphocytic activation molecules (SLAM) i.e. CD229 and CD319 are shown to be extremely useful for distinguishing PCs from other hematopoietic cells [23, 24]. It is important to note that CD229 also shows relatively strong expression in plasmacytoid dendritic cells and lymphocytes; similarly, CD319 shows relatively strong expression in monocytes and heterogeneous expression in myeloid cells and lymphocytes [24]. Hence, these new markers can be best used in combination with side scatter and or CD45 to improve the gating of PCs.
Fig. 1.
I) Bivariate plots from “a to h” demonstrate the FC immunophenotypic analysis in a typical bone marrow sample from the patient of multiple myeloma. Plasma cells (red dots) are gated using strong CD38, heterogenous CD138 and weak to negative CD45 expression in plots a, b. The abnormal plasma cells (APC) showed bright expression of CD319 (a newly investigated PC gating marker), strong CD56, weak heterogenous CD27, weak CD117, moderate CD200 and cytoplasmic kappa light chain expression. PC is negative for CD19, CD28 and cytoplasmic lambda light chain expression. II) Bivariate plots from “a to g” demonstrate the FC immunophenotypic analysis in a bone marrow sample from the patient of monoclonal gammopathy of undetermined significance (MGUS). Plasma cells are gated using strong CD38 and heterogenous CD138 expression in plots A. Of the total plasma cells, 36.6% PCs are abnormal and clonal (red dots) and rest of PCs are normal PC (NPC, “purple dots”). In dot plots “b–g”, the APC showed weak to negative CD45, strong CD56, weak CD27, weak CD117, and cytoplasmic kappa light chain expression but they are negative for CD19 and cytoplasmic lambda light chain expression. NPC are positive for CD19, moderate CD45, strong CD27 and negative for CD56 and CD117. NPC show polyclonal expression of immunoglobulin light chains (plot g) (color figure online)
Immunophenotypic Characterization of Abnormal Plasma Cells (APC)
The multidirectional application of flowcytometry in diagnosis, prognosis, and monitoring of MM and precursor diseases relies on the unique ability of MFC to accurately isolate and quantitate clonal APCs verses NPCs [5, 10, 19]. Several studies have clearly demonstrated that APCs have unique antigen expression pattern [5, 25–29]. APCs typically exhibit (1) relatively dim expression of CD38 compared to NPCs; (2) under-expression of CD19, CD45, CD27 and CD81; (3) aberrant overexpression of CD56, CD20, CD117, CD200, CD28 and CD33; and (4) restriction of cytoplasmic light chain (kappa or lambda) expression (refer to Fig. 1-I; Tables 1, 2, & 3). The combination of CD19, CD45, CD38 and CD56 in a four-color combination can reliably distinguish APCs from NPCs in almost 90% cases, both at diagnosis and post-therapy [8, 28]. However, the studies from both the Salamanca group as well as the group from NIH have shown that loss of CD19 and CD45 can be seen in NPCs in as high as 50 and 41% of cases, thereby establishing the need for an extended panel [5, 25]. Tembhare et al. [25] have found that loss of CD19 followed by loss of CD81 were the most sensitive markers to identify APCs. Aberrant expression of CD56 was present in 69% of APCs but was also seen in 26% of NPCs. In fact, a subpopulation of NPCs with CD45 dim, CD19 negative and CD56 positive expression represent a terminally differentiated stage in PC maturation. This subpopulation can give rise to a potential miscalculation in APC percentage, particularly when a high number of events are acquired like in the setting of minimal residual disease (MRD) detection. The inclusion of cytoplasmic kappa and lambda in the panel can prove the clonal/ polyclonal nature of a target population in this scenario. In our experience, loss of CD19 and loss of CD81 is the most sensitive markers, whereas overexpression of CD20, CD28 and CD117 are the most specific markers to identify APCs. Tembhare et al. [25] concluded that the combination of CD19 and CD45 loss and CD56 overexpression identified APCs in 100% of cases, but had a specificity of only 32% whereas the combination of CD19 and CD81 loss is very useful in identifying APCs. More recently, Paiva et al. [30] demonstrated the PC differentiation using CD19 and CD81 in healthy individuals demonstrating three groups of normal PCs i.e. CD19(+)/CD81(+) less-differentiated PCs, CD19(−)/CD81(+) intermediate-differentiated PCs and CD19(−)/CD81(−) fully-differentiated PCs. They found that intermediate-differentiated and fully-differentiated PCs were almost absent in young individuals and their percentages increased with age of an individual. Thus, these studies have demonstrated that markers usually considered as abnormal can be seen on a significant number of normal PCs and one has to be very careful while interpreting the expression of these markers. Therefore, for the correct identification and accurate quantitation of APCs, a combination of multiple markers like CD19, CD20, CD45, CD28, CD56, CD81, and CD117 along with light chain restriction pattern is extremely important.
Table 2.
Expression pattern of a variety of commonly used immunophenotypic markers in normal and abnormal plasma cells [5, 9, 10, 17–19, 25, 44]
| Markers | Normal plasma cells (NPCs) | Abnormal plasma cells (APCs) |
|---|---|---|
| CD19 | Intermediate to weak positive | Negative |
| CD20 | Negative | Positive |
| CD27 | Strong to intermediate positive | Weak positive/negative |
| CD28 | Negative | Positive |
| CD33 | Negative | Positive |
| CD38 | Strong positive | Intermediate or weak positive |
| CD45 | Intermediate positive | Weak positive/negative |
| CD56 | Negative or subset-weak positive | Strong positive |
| CD81 | Strong or intermediate positive | Weak positive/negative |
| CD117 | Negative | Positive |
| CD200 | Negative or subset weak positive | Strong positive |
| Cytoplasmic light chains | Polyclonal | Clonal |
a small proportion of NPCs can show the weak expression of CD56 or loss of CD19, CD27, and CD45 and hence, a combination of multiple abnormalities along with demonstration of clonality is reliable approach in the identification of APCs
Table 3.
Expression pattern of immunophenotypic markers in APCs of MM, PCL, WM and AL Amyloidosis [5, 19, 25, 35–37, 39, 41–47, 58, 75]
| Expression of markers | APCs in MM | APCs in PCL | APCs in WM | APCs in AL amyloidosis |
|---|---|---|---|---|
| CD38 | Usually strong | Usually strong | Strong and heterogenous | Relatively weak in 42% |
| CD19 | Negative 96% | Negative | Heterogenous | Negative in 92% |
| CD20 | Usually negative but can be positive in 15–30% | Positive up to 50% | Heterogenous | Positive in 38–42% |
| CD27 | Negative in up to 68% | Negative in up to 40% | Heterogenous | Weak to negative in 67% |
| CD28 | Positive in 45% | Positive in 33% | Negative | Positive in 50–60% |
| CD33 | Positive in 18% | Not known | Usually negative | Not known |
| CD45 | Negative in 73% | Negative | Intermediate | Negative in 83% |
| CD56 | Positive in 75% | Negative >70% | Negative | Positive in 50% |
| CD81 | Negative in 60% | Not known | Heterogenous | Negative in 48% |
| CD117 | Positive in 30% | Positive in 30% | Negative | Positive in 29% |
| CD200 | Positive in ≥70% | Not known | Intermediate | Not known |
| Surface light chain restriction | Absent | Absent | Usually present | Absent |
| Clonal B cells | Absent | Absent | Present | Absent (positive in an occasional case) |
| Additional comments | APCs show down regulation of CD11a/CD18 | B-cells are CD25+/CD27+/IgM+/weak CD22+ | CD79a+ in 86%, CD32B+ in 99%, CD319+ in 98% & CD52+ in 25% |
Role of MFC in the Diagnosis of PCD
Diagnosis of MM is typically based on the findings of BM biopsy, laboratory parameters like amount of M-protein, free light chain ratio (FLC-R), haemoglobin levels, creatinine and calcium levels and radiological examination [31]. The role MFC in the diagnosis of PCD is currently limited to MM with unusual morphology and precursor condition of MM i.e. MGUS. Moreover, MFC is more useful in distinguishing plasmacytoma, plasmablastic lymphoma, lymphoplasmacytic lymphoma (LPL) and B-cell lymphoma with plasmacytic differentiation from MM.
MGUS and SMM
MGUS was first time described by Robert Kyle from Mayo Clinic, (Minnesota, USA) in 1978 and since then it is intensively studied worldwide [32]. Recently, international myeloma working group (IMWG) has refined the criteria for MGUS, SMM and MM [31]. For cases that have low PC percentages on the bone marrow, proving clonality by MFC helps to establish the diagnosis. The coexistence of NPC and APC together in BM is a consistent finding in MGUS. Studies have shown that presence of >5% NPC among all BM PC at diagnosis is an accurate parameter for discrimination of MGUS vs MM (refer to Fig. 1-II) [26, 33, 34]. Also, the presence of CD56 negative APC is a more consistent finding in MGUS whereas the presence of completely CD45 negative APC correlates well with the diagnosis of MM [25].
MM with Unusual Morphology
Although routine MM cases with significantly increased PC in the BM may not need MFC for establishing the diagnosis, MFC can be helpful in cases presenting with unusual morphology like those with a plasmablastic or anaplastic morphology. MFC is also very useful in establishing the polyclonal nature of plasma cells in cases with reactive plasmacytosis.
Differential Diagnosis of MM and Plasmacytoma Versus Waldenstrom Macroglobulinemia (WM) Versus B-Lineage Non-Hodgkin Lymphoma (B-NHL) (Refer to Table 3)
Plasmacytomas usually display similar immunophenotypic features [19] to MM (CD19 negative, CD45 dim and CD56 positive PC). MFC is also very helpful in the differential diagnosis of WM and MGUS or MM since PC from WM display a distinct immunophenotypic profile with a CD45+ CD19+ CD56− phenotype and surface light chain restriction [35–37]. Similarly, PCs from B-NHL with plasmacytic differentiation also express a CD19+ CD45+ CD56− phenotype and are readily distinguishable from MGUS, SMM or MM [38]. In our experience, WM characteristically shows clonal B lymphocytes exhibiting a heterogeneous expression of CD38 reaching to the level of plasma cells and PCs shows expression of surface CD19, CD20 and CD45 with a heterogeneous spectrum from weak to negative. These MFC findings show immunophenotypic pathway of differentiation of clonal B-cells to the clonal PCs.
Plasma Cell Leukemia (PCL) (Also Refer to Table 3)
PCL is a rare form of PCD accounting for 1–2% of cases [19]. PCL patients have a worse prognosis and adverse outcome compared to MM cases [39]. Immunophenotypically APCs from PCL are more likely to express CD20 and they are more frequently negative for CD56, CD117, CD19 and HLA-DR [39, 40]. Likewise, it has been suggested that CD28 could be used to differentiate primary from secondary PCL [39].
Systemic Light Chain Amyloidosis (AL Amyloidosis) (also refer to Table 3): a very few studies have been published focusing on the immunophenotyping in AL amyloidosis. These studies have revealed that APCs in amyloidosis show down-regulation of CD19, CD27, CD38, CD45 and CD81 [41–44]. The expression of CD20, CD28, CD32B, CD52, CD56, CD79a, CD117 and SLAMF7 (CD319) was noted in 38–42%, 50%, 99%, 25%, 50%, 86%, 29% and 98% of cases, respectively [43–47]
Role of MFC in the Prognostication of PCD
MGUS and SMM
The average rate of progression of MGUS and SMM to full-blown MM are 1% per year and 10% per year respectively. Hence, it is highly desirable to identify the patients with high risk of progression who might be eligible for and benefit from early therapeutic intervention [48]. Two risk-stratification models have been proposed by the Mayo Clinic group and the Spanish Myeloma group [26, 49]. The Mayo clinic model uses M protein levels and the extent of BM involvement, whereas the Spanish model relies on the criteria of >95% APC out of all BM-PC together with DNA aneuploidy using MFC and immunoparesis for MGUS and SMM respectively. MFC determination of >95% APC/ BM-PC predicts risk of transformation with time to progression (TTP) rates at 5 years of 25% vs. 5% and 64% vs. 8% respectively for MGUS and SMM [26]. More recently, innovative software-based automated analysis of MFC data allowed categorisation of patients having either MGUS-like signature or MM-like signature and MGUS patients with MM-like signature have a shorter median period of progression (TTP of 85 vs 234 months) as compared to the standard MGUS cases [33]. Similarly, MGUS-like SMM cases had a median TTP not reached (NR) with a 5% risk of progression at 2 years; the intermediate group had a median TTP of 108 months with a 28% risk of progression at 2 years, whereas MM-like SMM cases had a median TTP of 15 months with a 53% risk of progression at 2 years. These findings demonstrate the fact that MFC based determination of APC/BM-PC provides strong prognostic parameters in the risk-stratification of MGUS and SMM.
Identifying Distinct Subgroups of Symptomatic MM
MM is known for heterogenicity in the clinical outcome and disease-free survival. A distinct subgroup of MM patients has an indolent clinical course with very long TTP while not achieving complete response (CR) as defined by traditional criteria. It has been shown [5, 34] that MM patients with >5% NPC/ BM-PC have a lower frequency of immune paresis, (42 vs 83%, P = 0.003) and high-risk cytogenetic abnormalities like t(4;14), t(14;16), and del(17p)—(3% vs. 26%, P = 0.006), and displays a greater response rate to HDT/autologous stem cell transplantation (ASCT) (rate of complete remission (CR) after HDT/ASCT of 64% vs. 33%, P < 0.001), along with significant longer progression-free survival (PFS) and overall survival (OS) rates (5 years rates of 44% vs 33%, P < 0.001 and 71% vs 62%, P = 0.04% respectively). These findings demonstrated that MFC-based cut-off of <95% APC or >5% NPC is a useful indicator of better clinical outcome. Moreover, automated analysis of MFC data have shown that MM with MGUS like signature shows a significantly superior clinical outcome (TTP median NR vs 44 months, P < 0.001; hazard ratio: 3.27; and OS median NR vs 67 months, P < 0.001, hazard ratio: 2.54) [33]. In fact, no significant differences for median TTP and OS were noted between cases in CR versus those that did not attain CR after HDT/ASCT in this subgroup of MM patients. These findings suggest that MFC is very useful in defining a subgroup of MM patients with excellent prognosis irrespective of whether CR is achieved or not.
Enumeration of APCs in Bone Marrow
Accurate morphological enumeration of BM PC is mandatory for proper characterization of PCD [31]; however, due to the patchy and irregular distribution of PCs in MM, it is subjected to variance and is an inconsistent prognostic factor [50]. Although, MFC enumeration of BM-PC yields consistently lower number to morphology Paiva et al. [51] have shown that MFC enumeration of BM-PC provides prognostic information of higher value than morphological counts (>15% PC indicates poor prognosis compared to <15%) and remains an independent prognostic factor for OS in multivariate analysis.
Immune Profile of APCs
Many studies have tried to correlate the expression of immunophenotypic markers with prognosis in PCD [5, 8, 52, 53]. It has been shown that CD19 and CD28 positivity and absence of CD117 are associated with significantly shorter PFS and OS rates in MM patients. In fact, a subgroup of CD28+ CD117− MM patients were described who would not benefit from HDT/ ASCT. Further, the phenotypic-genotypic correlation has also been suggested in the form of association of CD28 expression and absence of CD117 with non-hyperdiploid MM, t(4;14) and del(17p) [8, 53]. Paiva et al has validated that CD81 expression is an independent prognostic factor for PFS (hazard ratio = 1.9, P = 0.003) and OS (hazard ratio = 2.0, P = 0.02). Moreover, CD81+ SMM patients had a shorter TTP to MM (P = 0.02) [52]. Very recently, the same group has also shown that MM with CD19/CD81 positive APCs corresponding to the less-differentiated stage had a poor outcome as compared to others. This study indicated that APCs differentiation status based on CD19/CD81 expression is an independent prognostic indicator for progression-free survival (hazard ratio = 1.7; P = .005) and overall survival (hazard ratio = 2.1; P = .006) in MM [30]. Studies have also highlighted the clinical significance of few other markers such as lack of CD27 or CD45 expression and aberrant expression of CD56 or CD200 to be associated with poor clinical outcome [44]. Robillard et al. [44, 54] suggested the association of CD20 expression with small mature plasma cell morphology as well as t(11;14) but did not reveal any prognostic significance.
Identification and Enumeration of Circulating APCs in Peripheral Blood (PB)
MFC approaches can also be applied to identify and quantitate circulating APCs in PB and PB-derived leukapheresis products. Circulating PCs (cPCs) have been observed in as high as 80% of newly diagnosed MM patients and 25% and 50% of MGUS and SMM patients [55–60]. The Mayo group have demonstrated that circulating PC of ≥400 cPCs/µl was associated with high PC proliferation, adverse cytogenetics and inferior time-to-next-treatment and OS [60]. Thus, MFC-based quantification of cPCs is claimed to be a valuable risk stratifying factor MM patients treated with novel agents. Studies have also suggested that the presence of APCs in PB-derived leukapheresis products before ASCT could be a predictor of OS [5]. Another attractive possibility is to determine MRD from PB. However, further studies in larger series are required to clarify further the role of circulating APC in PCD.
MFC in the Monitoring of MRD in MM (Fig. 2)
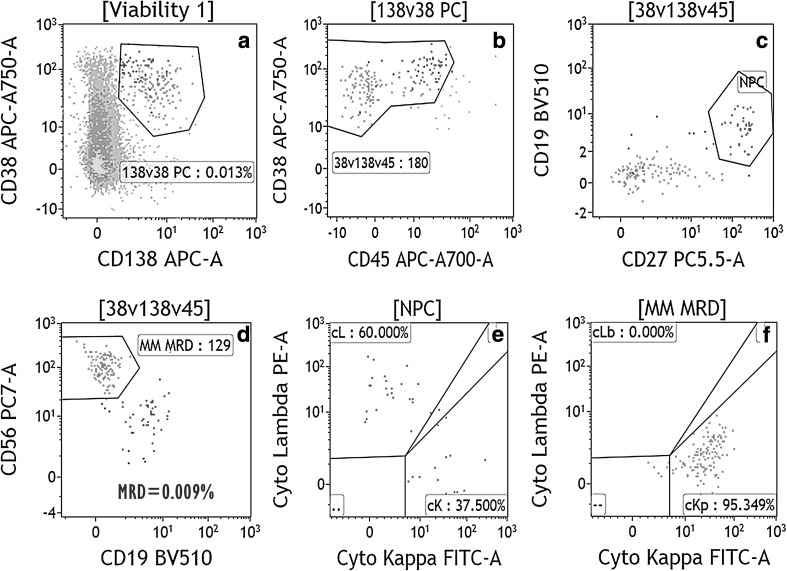
Fig. 2.
Bivariate plots from “a to f” demonstrates the MFC-based MRD analysis in a day 100 post ASCT bone marrow sample from the patient of MM. Of the 1.4 million event cells acquired, PC gated using strong CD38, moderate CD138 and heterogenous CD45 expression were 0.013% (plots a, b). Of the total plasma cells, 71% PCs are clonal abnormal PC (red dots) and rest of PCs are normal PC (NPC, “blue dots”). In dot plots “b–d”, the APC showed weak to negative CD27, strong CD56, and cytoplasmic kappa light chain expression, but negative for CD19, CD45 and cytoplasmic lambda light chain expression. NPC are positive for CD19, moderate CD45, strong CD27 and negative for CD56. NPC show polyclonal expression of immunoglobulin light chains (plot g). The MRD calculated in the all viable cells was 0.009% (color figure online)
MM is going through the era of dynamic therapeutic advancement where on one side HDT/ASCT has become the standard of care for eligible MM patients and on other side introduction of novel drugs like proteasome inhibitors (PI) and immunomodulators (IMiDs) have markedly increased survival rates within the last 10 years and produces a high rate of CR [61]. However, the disease-free and overall survival are still variable and unpredictable and disease recurrence remains the leading cause of death. Residual tumor cells are a well-known cause of such recurrence in any malignancy. The traditional criteria for complete remission (CR) are inadequate to exclude the possibility of such residual disease and hence, recent IMWG criterion has incorporated new criteria i.e. “stringent CR” (sCR) [62]. sCR has been defined incorporating serum free light chain ratio (sFLC) and immunohistochemistry/ immunofluorescence on BM biopsy. However, studies showing the superiority of sCR over CR are still limited [61–63]. These findings suggest the need for newer, more sensitive technique for treatment response monitoring and prediction of early relapse.
MFC-based MRD is now a well-established predictor of relapse in several hematological malignancies. In the last few years, few studies have established the role of MRD as an important prognostic factor and superior treatment response evaluator in MM also [51, 53–59] . The Spanish PETHEMA trial has shown that the presence of baseline high-risk cytogenetics by FISH(hazard ratio 17.3; P = 0.002) and persistent MRD by MFC at day 100 after HDT/ASCT (hazard ratio 8.0; P = 0.005) were the only independent factors that predicted unsustained CR [64]. Paiva et al. found that patients with immunophenotypic response (IR) had significantly long PFS and TTP when compared to sCR and CR on multivariate analysis (hazard ratio = 4.1, P = 0.01) [65]. Recent advances in the MFC allow more than eight color immunophenotyping which has increased sensitivity (1 in 105) of abnormal cell detection making MFC a suitable technique for MRD detection [61]. The most recent IMWG guidelines recommend the use of MRD monitoring using a protocol developed by Euroflow [62]. Furthermore, Rawstron et al. [63] have shown that there was a significant improvement in OS for each log depletion in MRD level (median OS was 1 year for >10%, 4 years for 1 to <10%, 5.9 years for 0.1 to <1%, 6.8 years for 0.01 to <0.1%, and more than 7.5 years for <0.01% MRD). MRD level as a continuous variable determined by flow cytometry independently predicted both PFS and OS, with approximately 1 year median OS benefit per log depletion. This indicates that quantitative analysis of tumor depletion could be more informative than just positive or negative status. These findings suggest that evaluation of MRD kinetics rather than a single-time-point MRD analysis could give a more robust assessment of treatment response and can form the basis of tailored therapy [61, 62].
Ploidy Analysis and DNA Proliferative Index (Fig. 3)
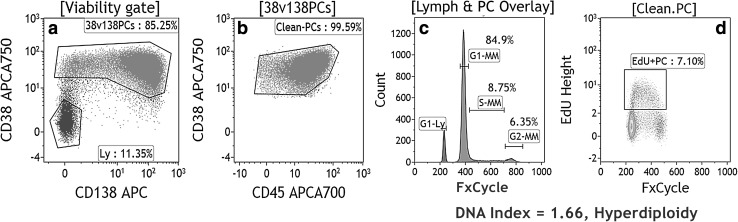
Fig. 3.
Plots from “a to d” demonstrates the MFC-based DNA ploidy and cell cycle analysis using FxCycle Violet dye in a bone marrow sample from the patient of MM. PC were gated using strong CD38, moderate CD138 and heterogenous CD45 expression (plot a, b). Histogram “c” showed an overlay of cell cycle analysis of lymphocytes (blue peak) and plasma cells (purple peak) from the same sample. This histogram showed “G1, S and G2” phases of the cell cycle of myeloma cells and G1 peak of internal lymphocytes. DNA index was calculated as a ratio of geometric mean of G1-peak of MM cells to geometric mean of G1-peak of lymphocytes and was revealed to be 1.66 indicating “hyperdiploidy”. Plot c also showed increased proportion of MM cells in S-phase (8.75%) indicating the highly proliferative nature of tumor. The increased proportion of S-phase is further confirmed with EdU which was 7.10% in plot “d” (color figure online)
In addition to the above application, MFC has long been utilized for measurement of PC DNA contents simultaneously using PC gating markers. It has been shown DNA index >1.06 indicating DNA hyperdiploidy is associated with better clinical outcome [66, 67] and contrarily, hypodiploidy is a poor prognostic parameter [68]. It could also be used as a tumor marker for MRD analysis [18]. Similarly, distribution of APCs in different cell cycle phases has great clinical relevance. Cases which show >2% of PC in S phase are associated with shorter disease-free survival (DFS) and OS [66, 69–71]. We have recently described an easy and cost effective MFC-based method of simultaneous DNA-ploidy and six-color surface marker assessment that allows APC-specific gating and ploidy analysis [72].
Role of MFC in Solitary Plasmacytoma and Systemic Light-Chain (AL) Amyloidosis
Solitary plasmacytoma (SP) are a rare type of plasma cell neoplasm (PCN) characterized by local plasma cell infiltration that renders high-risk of progression to multiple myeloma (MM). Paiva et al. and Quentin et al. [73, 74] have demonstrated that BM involvement using MFC is a new independent risk factor in determination of progression to MM (hazard risk of 17.4 and P < 0.001).
Similarly, recent studies have demonstrated that quantification of normal and abnormal bone marrow plasma cells (BMPCs) using MFC could be valuable risk stratification factor for OS in light chain amyloidosis [42, 75]. It has also been suggested that loss of CD27, and CD81 was associated with higher rate of very good partial response (VGPR) and expression CD27/CD56 was associated with poor OS [41]
Role of Tumor Microenvironment in PCD
Besides the factors associated with genetics of MM, tumor microenvironment also plays the vital role in the pathogenesis, and progression of precursor condition i.e. monoclonal gammopathy of undetermined significance (MGUS) to symptomatic myeloma [76–78]. In hematological malignancies like MM, peripheral blood and bone marrow both forms the tumor microenvironment. Immune cells comprised of T-cells (conventional T-cells, cytotoxic T-cells, regulatory T-cells, TH1, TH2, TH17 and γδ T cells) and NK cells forms the substantial component of microenvironment of MM [27, 76–83]. These immune cells show quantitative and functional variation in different stages of myeloma. Precisely, as the myeloma advances from early stages to aggressive forms, these cells, especially cytotoxic T cells and NK cells decrease in number and show functional impairment and conversely, regulatory T cells and TH17 cells increase [80, 84]. Also, the potential significance of the expression of several markers involved in the interaction between clonal PC and BM stromal cells is currently under investigation. Markers like VLA-1, ICAM-1, CD44 and Annexin—V seem promising in this regard [85–88].
Predictive Role of MFC in Upcoming Monoclonal Antibody (mAb) Therapies
Recent clinical trials have shown highly promising results of anti-CD38 (daratumumab) and within no time it may be seen in the clinics. Clinical trials are also targeting other antigens like CD74 or SLAM antigens [21, 89–91]. For the selection of eligible patients in these therapies, the basic requirement is the knowledge of the expression-pattern of these antigens and their levels on APC. MFC provides this information most accurately and thus provides predictive biomarkers in such therapies.
Standardization and Need for a Validated MFC Approach
In the past, inconsistent results have been obtained by MFC mainly due to inconsistencies in methodologies performed [5, 10]. Recently, many standardized approaches have been developed to meet this issue [61, 92, 93]. BMA, PB or fine needle aspirate specimens are suitable for MFC assay for PCD. Specimen age is of utmost importance for MFC assay and date and time of collection must be mentioned [93]. A cutoff of maximum 48 h’ specimen age is usually acceptable. The sample viability should be assessed and sample with >15% non-viable cells should have a line mentioned in the report that “sample viability is low and findings should be interpreted cautiously [93]. Euroflow recommends a bulk lysis method for high event acquisition [92]. In our institute, we follow a bulklyse-wash-stain approach. In diagnostic MM cases, the recommendation is acquisition of 1 × 105 cells or minimum 3000 PCs; however in cases of dilute BM, an acquisition of minimum 5 × 105 cells or 100 PCs is desirable [5]. For MRD analysis, it has been recommended to acquire at least 5 × 106 cells to achieve a sensitivity of 1 in 105 [61, 62, 93]. In our institute, we routinely acquire 5 × 105 cells for diagnostic samples and 3–5 × 106 cells for MRD analysis as well as in low PC samples to achieve a sensitivity of 1 × 105. For both diagnosis and MRD purpose, we use two-tube ten color PCD panel developed by TMH (Table 4). Also, the unique ability of MFC to assess simultaneously other bone marrow cells allow an assessment of the quality of the samples (CD19+ B cell precursors, CD56+ NK cells and CD117+ mast cells). Hemodilution and non-representative samples should have a line in report mentioning the quality of the sample.
Table 4.
Flow cytometric antibody panel for plasma cell disorders used in Tata Memorial Centre
| Fluor-chromes | BV510 | BV421 | FITC | PE | ECD | PC5.5 | PECy7 | APC | AF700 | AF750 |
|---|---|---|---|---|---|---|---|---|---|---|
| Tube 1 | CD20 | CD36 | Cykappa | Cylambda | CD19 | CD27 | CD56 | CD138 | CD45 | CD38 |
| Tube 2 | CD20 | CD200 | CD38 | CD319 | CD19 | CD117 | CD28 | CD138 | CD45 | CD81 |
Conclusion
In the past application of MFC in PCD has not gained as the widespread acceptance as in other hematological neoplasms. Nevertheless, recent studies have conclusively established the multidirectional application of MFC in PCD. The unique features of MFC including it being fast, easy to perform and widely applicable with simultaneous assessment of multiple parameters on multiple cell lines makes it a well-suitable method to carry out both at diagnosis as well as post-therapy. A well-standardized and adequately sensitive MFC based MRD assay could well lay the path of tailored therapy in MM in recent future.
Compliance with Ethical Standards
Conflict of interest
All Authors declare that they have no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Contributor Information
Gaurav Chatterjee, Phone: 022 24177000, Email: gauravchatnobel@gmail.com.
Sumeet Gujral, Phone: 022 24177000, Email: s_gujral@outlook.com.
Papagudi G. Subramanian, Phone: 022 24177000, Email: pgs_mani@yahoo.com
Prashant R. Tembhare, Email: docprt@gmail.com
References
- 1.Fonseca R, Bergsagel PL, Drach J, Shaughnessy J, Gutierrez N, Stewart AK, et al. International Myeloma Working Group molecular classification of multiple myeloma: spotlight review. Leukemia. 2009;23(12):2210–2221. doi: 10.1038/leu.2009.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rajkumar SV. MGUS and smoldering multiple myeloma: update on pathogenesis, natural history, and management. Hematol Am Soc Hematol Educ Program. 2005;1:340–345. doi: 10.1182/asheducation-2005.1.340. [DOI] [PubMed] [Google Scholar]
- 3.Agarwal A, Ghobrial IM. Monoclonal gammopathy of undetermined significance and smoldering multiple myeloma: a review of the current understanding of epidemiology, biology, risk stratification, and management of myeloma precursor disease. Clin Cancer Res. 2013;19(5):985–994. doi: 10.1158/1078-0432.CCR-12-2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davies FE, Rawstron AC, Owen RG, Morgan GJ. Minimal residual disease monitoring in multiple myeloma. Best Pract Res Clin Haematol. 2002;15(1):197–222. doi: 10.1053/beha.2002.0192. [DOI] [PubMed] [Google Scholar]
- 5.Paiva B, Almeida J, Perez-Andres M, Mateo G, Lopez A, Rasillo A, et al. Utility of flow cytometry immunophenotyping in multiple myeloma and other clonal plasma cell-related disorders. Cytometry B Clin Cytom. 2010;78(4):239–252. doi: 10.1002/cyto.b.20512. [DOI] [PubMed] [Google Scholar]
- 6.Nadav L, Katz BZ, Baron S, Yossipov L, Polliack A, Deutsch V, et al. Diverse niches within multiple myeloma bone marrow aspirates affect plasma cell enumeration. Br J Haematol. 2006;133(5):530–532. doi: 10.1111/j.1365-2141.2006.06068.x. [DOI] [PubMed] [Google Scholar]
- 7.Ng AP, Wei A, Bhurani D, Chapple P, Feleppa F, Juneja S. The sensitivity of CD138 immunostaining of bone marrow trephine specimens for quantifying marrow involvement in MGUS and myeloma, including samples with a low percentage of plasma cells. Haematologica. 2006;91(7):972–975. [PubMed] [Google Scholar]
- 8.Mateo G, Montalban MA, Vidriales MB, Lahuerta JJ, Mateos MV, Gutierrez N, et al. Prognostic value of immunophenotyping in multiple myeloma: a study by the PETHEMA/GEM cooperative study groups on patients uniformly treated with high-dose therapy. J Clin Oncol. 2008;26(16):2737–2744. doi: 10.1200/JCO.2007.15.4120. [DOI] [PubMed] [Google Scholar]
- 9.Mateo Manzanera G, San Miguel Izquierdo JF, Orfao de Matos A. Immunophenotyping of plasma cells in multiple myeloma. Methods Mol Med. 2005;113:5–24. doi: 10.1385/1-59259-916-8:5. [DOI] [PubMed] [Google Scholar]
- 10.Raja KR, Kovarova L, Hajek R. Review of phenotypic markers used in flow cytometric analysis of MGUS and MM, and applicability of flow cytometry in other plasma cell disorders. Br J Haematol. 2010;149(3):334–351. doi: 10.1111/j.1365-2141.2010.08121.x. [DOI] [PubMed] [Google Scholar]
- 11.Karawajew L, Dworzak M, Ratei R, Rhein P, Gaipa G, Buldini B, et al. Minimal residual disease analysis by eight-color flow cytometry in relapsed childhood acute lymphoblastic leukemia. Haematologica. 2015;100(7):935–944. doi: 10.3324/haematol.2014.116707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weng XQ, Shen Y, Sheng Y, Chen B, Wang JH, Li JM, et al. Prognostic significance of monitoring leukemia-associated immunophenotypes by eight-color flow cytometry in adult B-acute lymphoblastic leukemia. Blood Cancer J. 2013;3:e133. doi: 10.1038/bcj.2013.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fossat C, Roussel M, Arnoux I, Asnafi V, Brouzes C, Garnache-Ottou F, et al. Methodological aspects of minimal residual disease assessment by flow cytometry in acute lymphoblastic leukemia: a French multicenter study. Cytometry B Clin Cytom. 2015;88(1):21–29. doi: 10.1002/cyto.b.21195. [DOI] [PubMed] [Google Scholar]
- 14.Luria D, Rosenthal E, Steinberg D, Kodman Y, Safanaiev M, Amariglio N, et al. Prospective comparison of two flow cytometry methodologies for monitoring minimal residual disease in a multicenter treatment protocol of childhood acute lymphoblastic leukemia. Cytometry B Clin Cytom. 2010;78(6):365–371. doi: 10.1002/cyto.b.20532. [DOI] [PubMed] [Google Scholar]
- 15.Braham Jmili N, Nsaibia S, Jacob MC, Omri H, Laatiri MA, Yacoub S, et al. Immunophenotypic analysis of bone marrow B lymphocyte precursors (hematogones) by flow cytometry. Clin Lab Sci. 2009;22(4):208–215. [PubMed] [Google Scholar]
- 16.Irving J, Jesson J, Virgo P, Case M, Minto L, Eyre L, et al. Establishment and validation of a standard protocol for the detection of minimal residual disease in B lineage childhood acute lymphoblastic leukemia by flow cytometry in a multi-center setting. Haematologica. 2009;94(6):870–874. doi: 10.3324/haematol.2008.000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gertz MA, Buadi FK. Utility of immunophenotyping of plasma cells in multiple myeloma. Leuk Lymphoma. 2015;57(2):252–253. doi: 10.3109/10428194.2015.1068310. [DOI] [PubMed] [Google Scholar]
- 18.Almeida J, Orfao A, Mateo G, Ocqueteau M, Garcia-Sanz R, Moro MJ, et al. Immunophenotypic and DNA content characteristics of plasma cells in multiple myeloma and monoclonal gammopathy of undetermined significance. Pathol Biol (Paris) 1999;47(2):119–127. [PubMed] [Google Scholar]
- 19.Rawstron AC, Orfao A, Beksac M, Bezdickova L, Brooimans RA, Bumbea H, et al. Report of the European Myeloma Network on multiparametric flow cytometry in multiple myeloma and related disorders. Haematologica. 2008;93(3):431–438. doi: 10.3324/haematol.11080. [DOI] [PubMed] [Google Scholar]
- 20.O’Connell FP, Pinkus JL, Pinkus GS. CD138 (syndecan-1), a plasma cell marker immunohistochemical profile in hematopoietic and nonhematopoietic neoplasms. Am J Clin Pathol. 2004;121(2):254–263. doi: 10.1309/617DWB5GNFWXHW4L. [DOI] [PubMed] [Google Scholar]
- 21.Lonial S. Monoclonal antibodies for the treatment of myeloma: targeting SLAMF7 and CD38. Cancer J. 2016;22(1):3–6. doi: 10.1097/PPO.0000000000000172. [DOI] [PubMed] [Google Scholar]
- 22.Vasuthasawat A, Yoo EM, Trinh KR, Lichtenstein A, Timmerman JM, Morrison SL. Targeted immunotherapy using anti-CD138-interferon alpha fusion proteins and bortezomib results in synergistic protection against multiple myeloma. MAbs. 2016;8(7):1386–1397. doi: 10.1080/19420862.2016.1207030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muccio VE, Saraci E, Gilestro M, Gattei V, Zucchetto A, Astolfi M, et al. Multiple myeloma: new surface antigens for the characterization of plasma cells in the era of novel agents. Cytometry B Clin Cytom. 2016;90(1):81–90. doi: 10.1002/cyto.b.21279. [DOI] [PubMed] [Google Scholar]
- 24.Pojero F, Flores-Montero J, Sanoja L, Perez JJ, Puig N, Paiva B, et al. Utility of CD54, CD229, and CD319 for the identification of plasma cells in patients with clonal plasma cell diseases. Cytometry B Clin Cytom. 2016;90(1):91–100. doi: 10.1002/cyto.b.21269. [DOI] [PubMed] [Google Scholar]
- 25.Tembhare PR, Yuan CM, Venzon D, Braylan R, Korde N, Manasanch E, et al. Flow cytometric differentiation of abnormal and normal plasma cells in the bone marrow in patients with multiple myeloma and its precursor diseases. Leuk Res. 2014;38(3):371–376. doi: 10.1016/j.leukres.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perez-Persona E, Vidriales MB, Mateo G, Garcia-Sanz R, Mateos MV, de Coca AG, et al. New criteria to identify risk of progression in monoclonal gammopathy of uncertain significance and smoldering multiple myeloma based on multiparameter flow cytometry analysis of bone marrow plasma cells. Blood. 2007;110(7):2586–2592. doi: 10.1182/blood-2007-05-088443. [DOI] [PubMed] [Google Scholar]
- 27.Perez-Andres M, Almeida J, Martin-Ayuso M, Moro MJ, Martin-Nunez G, Galende J, et al. Clonal plasma cells from monoclonal gammopathy of undetermined significance, multiple myeloma and plasma cell leukemia show different expression profiles of molecules involved in the interaction with the immunological bone marrow microenvironment. Leukemia. 2005;19(3):449–455. doi: 10.1038/sj.leu.2403647. [DOI] [PubMed] [Google Scholar]
- 28.Ocqueteau M, Orfao A, Almeida J, Blade J, Gonzalez M, Garcia-Sanz R, et al. Immunophenotypic characterization of plasma cells from monoclonal gammopathy of undetermined significance patients. Implications for the differential diagnosis between MGUS and multiple myeloma. Am J Pathol. 1998;152(6):1655–1665. [PMC free article] [PubMed] [Google Scholar]
- 29.Bataille R, Jego G, Robillard N, Barille-Nion S, Harousseau JL, Moreau P, et al. The phenotype of normal, reactive and malignant plasma cells. Identification of “many and multiple myelomas” and of new targets for myeloma therapy. Haematologica. 2006;91(9):1234–1240. [PubMed] [Google Scholar]
- 30.Paiva B, Puig N, Cedena MT, de Jong BG, Ruiz Y, Rapado I, et al. Differentiation stage of myeloma plasma cells: biological and clinical significance. Leukemia. 2017;31(2):382–392. doi: 10.1038/leu.2016.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rajkumar SV, Dimopoulos MA, Palumbo A, Blade J, Merlini G, Mateos MV, et al. International myeloma working group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538–e548. doi: 10.1016/S1470-2045(14)70442-5. [DOI] [PubMed] [Google Scholar]
- 32.Kyle RA. Monoclonal gammopathy of undetermined significance. Blood Rev. 1994;8(3):135–141. doi: 10.1016/0268-960X(94)90073-Q. [DOI] [PubMed] [Google Scholar]
- 33.Paiva B, Vidriales MB, Rosinol L, Martinez-Lopez J, Mateos MV, Ocio EM, et al. A multiparameter flow cytometry immunophenotypic algorithm for the identification of newly diagnosed symptomatic myeloma with an MGUS-like signature and long-term disease control. Leukemia. 2013;27(10):2056–2061. doi: 10.1038/leu.2013.166. [DOI] [PubMed] [Google Scholar]
- 34.Paiva B, Vidriales MB, Mateo G, Perez JJ, Montalban MA, Sureda A, et al. The persistence of immunophenotypically normal residual bone marrow plasma cells at diagnosis identifies a good prognostic subgroup of symptomatic multiple myeloma patients. Blood. 2009;114(20):4369–4372. doi: 10.1182/blood-2009-05-221689. [DOI] [PubMed] [Google Scholar]
- 35.Ocio EM, Hernandez JM, Mateo G, Sanchez ML, Gonzalez B, Vidriales B, et al. Immunophenotypic and cytogenetic comparison of Waldenstrom’s macroglobulinemia with splenic marginal zone lymphoma. Clin Lymphoma. 2005;5(4):241–245. doi: 10.3816/CLM.2005.n.007. [DOI] [PubMed] [Google Scholar]
- 36.Paiva B, Montes MC, Garcia-Sanz R, Ocio EM, Alonso J, de Las HN, et al. Multiparameter flow cytometry for the identification of the Waldenstrom’s clone in IgM-MGUS and Waldenstrom’s macroglobulinemia: new criteria for differential diagnosis and risk stratification. Leukemia. 2014;28(1):166–173. doi: 10.1038/leu.2013.124. [DOI] [PubMed] [Google Scholar]
- 37.San Miguel JF, Vidriales MB, Ocio E, Mateo G, Sanchez-Guijo F, Sanchez ML, et al. Immunophenotypic analysis of Waldenstrom’s macroglobulinemia. Semin Oncol. 2003;30(2):187–195. doi: 10.1053/sonc.2003.50074. [DOI] [PubMed] [Google Scholar]
- 38.Seegmiller AC, Xu Y, McKenna RW, Karandikar NJ. Immunophenotypic differentiation between neoplastic plasma cells in mature B-cell lymphoma vs plasma cell myeloma. Am J Clin Pathol. 2007;127(2):176–181. doi: 10.1309/5EL22BH45PHUPM8P. [DOI] [PubMed] [Google Scholar]
- 39.Garcia-Sanz R, Orfao A, Gonzalez M, Tabernero MD, Blade J, Moro MJ, et al. Primary plasma cell leukemia: clinical, immunophenotypic, DNA ploidy, and cytogenetic characteristics. Blood. 1999;93(3):1032–1037. [PubMed] [Google Scholar]
- 40.Tembhare PR, Subramanian PG, Sehgal K, Yajamanam B, Kumar A, Gadge V, et al. Immunophenotypic profile of plasma cell leukemia: a retrospective study in a reference cancer center in India and review of literature. Indian J Pathol Microbiol. 2011;54(2):294–298. doi: 10.4103/0377-4929.81603. [DOI] [PubMed] [Google Scholar]
- 41.Filipova J, Rihova L, Vsianska P, Kufova Z, Kryukova E, Kryukov F, et al. Flow cytometry in immunoglobulin light chain amyloidosis: short review. Leukemia Research. 2015;39(11):1131–1136. doi: 10.1016/j.leukres.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 42.Paiva B, Vidriales MB, Perez JJ, Lopez-Berges MC, Garcia-Sanz R, Ocio EM, et al. The clinical utility and prognostic value of multiparameter flow cytometry immunophenotyping in light-chain amyloidosis. Blood. 2011;117(13):3613–3616. doi: 10.1182/blood-2010-12-324665. [DOI] [PubMed] [Google Scholar]
- 43.Paiva B, Martinez-Lopez J, Corchete LA, Sanchez-Vega B, Rapado I, Puig N, et al. Phenotypic, transcriptomic, and genomic features of clonal plasma cells in light-chain amyloidosis. Blood. 2016;127(24):3035–3039. doi: 10.1182/blood-2015-10-673095. [DOI] [PubMed] [Google Scholar]
- 44.Kumar S, Kimlinger T, Morice W. Immunophenotyping in multiple myeloma and related plasma cell disorders. Best Pract Res Clin Haematol. 2010;23(3):433–451. doi: 10.1016/j.beha.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Deshmukh M, Elderfield K, Rahemtulla A, Naresh KN. Immunophenotype of neoplastic plasma cells in AL amyloidosis. J Clin Pathol. 2009;62(8):724–730. doi: 10.1136/jcp.2009.065474. [DOI] [PubMed] [Google Scholar]
- 46.Zhou P, Comenzo RL, Olshen AB, Bonvini E, Koenig S, Maslak PG, et al. CD32B is highly expressed on clonal plasma cells from patients with systemic light-chain amyloidosis and provides a target for monoclonal antibody-based therapy. Blood. 2008;111(7):3403–3406. doi: 10.1182/blood-2007-11-125526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lisenko K, Schonland SO, Jauch A, Andrulis M, Rocken C, Ho AD, et al. Flow cytometry-based characterization of underlying clonal B and plasma cells in patients with light chain amyloidosis. Cancer Med. 2016;5(7):1464–1472. doi: 10.1002/cam4.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rajkumar SV, Landgren O, Mateos MV. Smoldering multiple myeloma. Blood. 2015;125(20):3069–3075. doi: 10.1182/blood-2014-09-568899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kyle RA, Remstein ED, Therneau TM, Dispenzieri A, Kurtin PJ, Hodnefield JM, et al. Clinical course and prognosis of smoldering (asymptomatic) multiple myeloma. N Engl J Med. 2007;356(25):2582–2590. doi: 10.1056/NEJMoa070389. [DOI] [PubMed] [Google Scholar]
- 50.Rajkumar SV, Fonseca R, Dispenzieri A, Lacy MQ, Lust JA, Witzig TE, et al. Methods for estimation of bone marrow plasma cell involvement in myeloma: predictive value for response and survival in patients undergoing autologous stem cell transplantation. Am J Hematol. 2001;68(4):269–275. doi: 10.1002/ajh.10003. [DOI] [PubMed] [Google Scholar]
- 51.Paiva B, Vidriales MB, Perez JJ, Mateo G, Montalban MA, Mateos MV, et al. Multiparameter flow cytometry quantification of bone marrow plasma cells at diagnosis provides more prognostic information than morphological assessment in myeloma patients. Haematologica. 2009;94(11):1599–1602. doi: 10.3324/haematol.2009.009100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Paiva B, Gutierrez NC, Chen X, Vidriales MB, Montalban MA, Rosinol L, et al. Clinical significance of CD81 expression by clonal plasma cells in high-risk smoldering and symptomatic multiple myeloma patients. Leukemia. 2012;26(8):1862–1869. doi: 10.1038/leu.2012.42. [DOI] [PubMed] [Google Scholar]
- 53.Mateo G, Castellanos M, Rasillo A, Gutierrez NC, Montalban MA, Martin ML, et al. Genetic abnormalities and patterns of antigenic expression in multiple myeloma. Clin Cancer Res. 2005;11(10):3661–3667. doi: 10.1158/1078-0432.CCR-04-1489. [DOI] [PubMed] [Google Scholar]
- 54.Robillard N, Avet-Loiseau H, Garand R, Moreau P, Pineau D, Rapp MJ, et al. CD20 is associated with a small mature plasma cell morphology and t(11;14) in multiple myeloma. Blood. 2003;102(3):1070–1071. doi: 10.1182/blood-2002-11-3333. [DOI] [PubMed] [Google Scholar]
- 55.Witzig TE, Gertz MA, Lust JA, Kyle RA, O’Fallon WM, Greipp PR. Peripheral blood monoclonal plasma cells as a predictor of survival in patients with multiple myeloma. Blood. 1996;88(5):1780–1787. [PubMed] [Google Scholar]
- 56.Rawstron AC, Owen RG, Davies FE, Johnson RJ, Jones RA, Richards SJ, et al. Circulating plasma cells in multiple myeloma: characterization and correlation with disease stage. Br J Haematol. 1997;97(1):46–55. doi: 10.1046/j.1365-2141.1997.72653.x. [DOI] [PubMed] [Google Scholar]
- 57.Peceliunas V, Janiulioniene A, Matuzeviciene R, Zvirblis T, Griskevicius L. Circulating plasma cells predict the outcome of relapsed or refractory multiple myeloma. Leuk Lymphoma. 2012;53(4):641–647. doi: 10.3109/10428194.2011.627481. [DOI] [PubMed] [Google Scholar]
- 58.Pardanani A, Witzig TE, Schroeder G, McElroy EA, Fonseca R, Dispenzieri A, et al. Circulating peripheral blood plasma cells as a prognostic indicator in patients with primary systemic amyloidosis. Blood. 2003;101(3):827–830. doi: 10.1182/blood-2002-06-1698. [DOI] [PubMed] [Google Scholar]
- 59.Nowakowski GS, Witzig TE, Dingli D, Tracz MJ, Gertz MA, Lacy MQ, et al. Circulating plasma cells detected by flow cytometry as a predictor of survival in 302 patients with newly diagnosed multiple myeloma. Blood. 2005;106(7):2276–2279. doi: 10.1182/blood-2005-05-1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gonsalves WI, Rajkumar SV, Gupta V, Morice WG, Timm MM, Singh PP, et al. Quantification of clonal circulating plasma cells in newly diagnosed multiple myeloma: implications for redefining high-risk myeloma. Leukemia. 2014;28(10):2060–2065. doi: 10.1038/leu.2014.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Paiva B, van Dongen JJ, Orfao A. New criteria for response assessment: role of minimal residual disease in multiple myeloma. Blood. 2015;125(20):3059–3068. doi: 10.1182/blood-2014-11-568907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kumar S, Paiva B, Anderson KC, Durie B, Landgren O, Moreau P, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016;17(8):e328–e346. doi: 10.1016/S1470-2045(16)30206-6. [DOI] [PubMed] [Google Scholar]
- 63.Rawstron AC, Gregory WM, de Tute RM, Davies FE, Bell SE, Drayson MT, et al. Minimal residual disease in myeloma by flow cytometry: independent prediction of survival benefit per log reduction. Blood. 2015;125(12):1932–1935. doi: 10.1182/blood-2014-07-590166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Paiva B, Gutierrez NC, Rosinol L, Vidriales MB, Montalban MA, Martinez-Lopez J, et al. High-risk cytogenetics and persistent minimal residual disease by multiparameter flow cytometry predict unsustained complete response after autologous stem cell transplantation in multiple myeloma. Blood. 2012;119(3):687–691. doi: 10.1182/blood-2011-07-370460. [DOI] [PubMed] [Google Scholar]
- 65.Paiva B, Martinez-Lopez J, Vidriales MB, Mateos MV, Montalban MA, Fernandez-Redondo E, et al. Comparison of immunofixation, serum free light chain, and immunophenotyping for response evaluation and prognostication in multiple myeloma. J Clin Oncol. 2011;29(12):1627–1633. doi: 10.1200/JCO.2010.33.1967. [DOI] [PubMed] [Google Scholar]
- 66.San Miguel JF, Garcia-Sanz R, Gonzalez M, Moro MJ, Hernandez JM, Ortega F, et al. A new staging system for multiple myeloma based on the number of S-phase plasma cells. Blood. 1995;85(2):448–455. [PubMed] [Google Scholar]
- 67.San Miguel JF, Garcia-Sanz R, Gonzalez M, Orfao A. Immunophenotype and DNA cell content in multiple myeloma. Baillieres Clin Haematol. 1995;8(4):735–759. doi: 10.1016/S0950-3536(05)80257-4. [DOI] [PubMed] [Google Scholar]
- 68.Smadja NV, Bastard C, Brigaudeau C, Leroux D, Fruchart C. Hypodiploidy is a major prognostic factor in multiple myeloma. Blood. 2001;98(7):2229–2238. doi: 10.1182/blood.V98.7.2229. [DOI] [PubMed] [Google Scholar]
- 69.Garcia-Sanz R, Gonzalez-Fraile MI, Mateo G, Hernandez JM, Lopez-Berges MC, de las Heras N, et al. Proliferative activity of plasma cells is the most relevant prognostic factor in elderly multiple myeloma patients. Int J Cancer. 2004;112(5):884–889. doi: 10.1002/ijc.20491. [DOI] [PubMed] [Google Scholar]
- 70.Girino M, Riccardi A, Luoni R, Ucci G, Cuomo A. Monoclonal antibody Ki-67 as a marker of proliferative activity in monoclonal gammopathies. Acta Haematol. 1991;85(1):26–30. doi: 10.1159/000204847. [DOI] [PubMed] [Google Scholar]
- 71.Minarik J, Scudla V, Ordeltova M, Bacovsky J, Pika T, Langova K. Monitoring of plasma cell proliferative and apoptotic indices in the course of multiple myeloma. Leuk Lymphoma. 2009;50(12):1983–1991. doi: 10.3109/10428190903291070. [DOI] [PubMed] [Google Scholar]
- 72.Tembhare P, Badrinath Y, Ghogale S, Patkar N, Dhole N, Dalavi P, et al. A novel and easy FxCycle violet based flow cytometric method for simultaneous assessment of DNA ploidy and six-color immunophenotyping. Cytometry A. 2016;89(3):281–291. doi: 10.1002/cyto.a.22803. [DOI] [PubMed] [Google Scholar]
- 73.Hill QA, Rawstron AC, de Tute RM, Owen RG. Outcome prediction in plasmacytoma of bone: a risk model utilizing bone marrow flow cytometry and light-chain analysis. Blood. 2014;124(8):1296–1299. doi: 10.1182/blood-2014-04-566521. [DOI] [PubMed] [Google Scholar]
- 74.Paiva B, Chandia M, Vidriales MB, Colado E, Caballero-Velazquez T, Escalante F, et al. Multiparameter flow cytometry for staging of solitary bone plasmacytoma: new criteria for risk of progression to myeloma. Blood. 2014;124(8):1300–1303. doi: 10.1182/blood-2014-04-567909. [DOI] [PubMed] [Google Scholar]
- 75.Muchtar E, Jevremovic D, Dispenzieri A, Dingli D, Buadi FK, Lacy MQ, et al. The prognostic value of multiparametric flow cytometry in AL amyloidosis at diagnosis and at the end of first-line treatment. Blood. 2017;129(1):82–87. doi: 10.1182/blood-2016-06-721878. [DOI] [PubMed] [Google Scholar]
- 76.Podar K, Chauhan D, Anderson KC. Bone marrow microenvironment and the identification of new targets for myeloma therapy. Leukemia. 2009;23(1):10–24. doi: 10.1038/leu.2008.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Caligaris-Cappio F, Gregoretti MG, Merico F, Gottardi D, Ghia P, Parvis G, et al. Bone marrow microenvironment and the progression of multiple myeloma. Leuk Lymphoma. 1992;8(1–2):15–22. doi: 10.3109/10428199209049813. [DOI] [PubMed] [Google Scholar]
- 78.Manier S, Sacco A, Leleu X, Ghobrial IM, Roccaro AM. Bone marrow microenvironment in multiple myeloma progression. J Biomed Biotechnol. 2012;2012:157496. doi: 10.1155/2012/157496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Perez-Andres M, Almeida J, Martin-Ayuso M, De Las HN, Moro MJ, Martin-Nunez G, et al. Soluble and membrane levels of molecules involved in the interaction between clonal plasma cells and the immunological microenvironment in multiple myeloma and their association with the characteristics of the disease. Int J Cancer. 2009;124(2):367–375. doi: 10.1002/ijc.23941. [DOI] [PubMed] [Google Scholar]
- 80.Romano A, Conticello C, Cavalli M, Vetro C, La Fauci A, Parrinello NL, et al. Immunological dysregulation in multiple myeloma microenvironment. Biomed Res Int. 2014;2014:198539. doi: 10.1155/2014/198539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Roodman GD. Role of the bone marrow microenvironment in multiple myeloma. J Bone Miner Res. 2002;17(11):1921–1925. doi: 10.1359/jbmr.2002.17.11.1921. [DOI] [PubMed] [Google Scholar]
- 82.Perez-Andres M, Almeida J, Martin-Ayuso M, Moro MJ, Garcia-Marcos MA, Moreno I, et al. Interaction between clonal plasma cells and the immune system in plasma cell dyscrasias. J Biol Regul Homeost Agents. 2004;18(2):161–165. [PubMed] [Google Scholar]
- 83.Perez-Andres M, Almeida J, Martin-Ayuso M, Moro MJ, Martin-Nunez G, Galende J, et al. Characterization of bone marrow T cells in monoclonal gammopathy of undetermined significance, multiple myeloma, and plasma cell leukemia demonstrates increased infiltration by cytotoxic/Th1 T cells demonstrating a squed TCR-Vbeta repertoire. Cancer. 2006;106(6):1296–1305. doi: 10.1002/cncr.21746. [DOI] [PubMed] [Google Scholar]
- 84.Dosani T, Carlsten M, Maric I, Landgren O. The cellular immune system in myelomagenesis: NK cells and T cells in the development of MM and their uses in immunotherapies. Blood Cancer J. 2015;5:e306. doi: 10.1038/bcj.2015.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Minarik J, Scudla V, Ordeltova M, Bacovsky J, Zemanova M. Evaluation of plasma cell propidium-iodide and annexin-V indices: their relation to prognosis in multiple myeloma. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2005;149(2):271–274. doi: 10.5507/bp.2005.039. [DOI] [PubMed] [Google Scholar]
- 86.Ohwada C, Nakaseko C, Koizumi M, Takeuchi M, Ozawa S, Naito M, et al. CD44 and hyaluronan engagement promotes dexamethasone resistance in human myeloma cells. Eur J Haematol. 2008;80(3):245–250. doi: 10.1111/j.1600-0609.2007.01014.x. [DOI] [PubMed] [Google Scholar]
- 87.Okada T, Hawley RG, Kodaka M, Okuno H. Significance of VLA-4-VCAM-1 interaction and CD44 for transendothelial invasion in a bone marrow metastatic myeloma model. Clin Exp Metastasis. 1999;17(7):623–629. doi: 10.1023/A:1006715504719. [DOI] [PubMed] [Google Scholar]
- 88.Vincent T, Mechti N. IL-6 regulates CD44 cell surface expression on human myeloma cells. Leukemia. 2004;18(5):967–975. doi: 10.1038/sj.leu.2403333. [DOI] [PubMed] [Google Scholar]
- 89.Einsele H, Schreder M. Treatment of multiple myeloma with the immunostimulatory SLAMF7 antibody elotuzumab. Ther Adv Hematol. 2016;7(5):288–301. doi: 10.1177/2040620716657993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.van de Donk NW, Moreau P, Plesner T, Palumbo A, Gay F, Laubach JP, et al. Clinical efficacy and management of monoclonal antibodies targeting CD38 and SLAMF7 in multiple myeloma. Blood. 2016;127(6):681–695. doi: 10.1182/blood-2015-10-646810. [DOI] [PubMed] [Google Scholar]
- 91.Kaufman JL, Niesvizky R, Stadtmauer EA, Chanan-Khan A, Siegel D, Horne H, et al. Phase I, multicentre, dose-escalation trial of monotherapy with milatuzumab (humanized anti-CD74 monoclonal antibody) in relapsed or refractory multiple myeloma. Br J Haematol. 2013;163(4):478–486. doi: 10.1111/bjh.12565. [DOI] [PubMed] [Google Scholar]
- 92.van Dongen JJ, Lhermitte L, Bottcher S, Almeida J, van der Velden VH, Flores-Montero J, et al. EuroFlow antibody panels for standardized n-dimensional flow cytometric immunophenotyping of normal, reactive and malignant leukocytes. Leukemia. 2012;26(9):1908–1975. doi: 10.1038/leu.2012.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Stetler-Stevenson M, Paiva B, Stoolman L, Lin P, Jorgensen JL, Orfao A, et al. Consensus guidelines for myeloma minimal residual disease sample staining and data acquisition. Cytometry B Clin Cytom. 2016;90(1):26–30. doi: 10.1002/cyto.b.21249. [DOI] [PMC free article] [PubMed] [Google Scholar]