Abstract
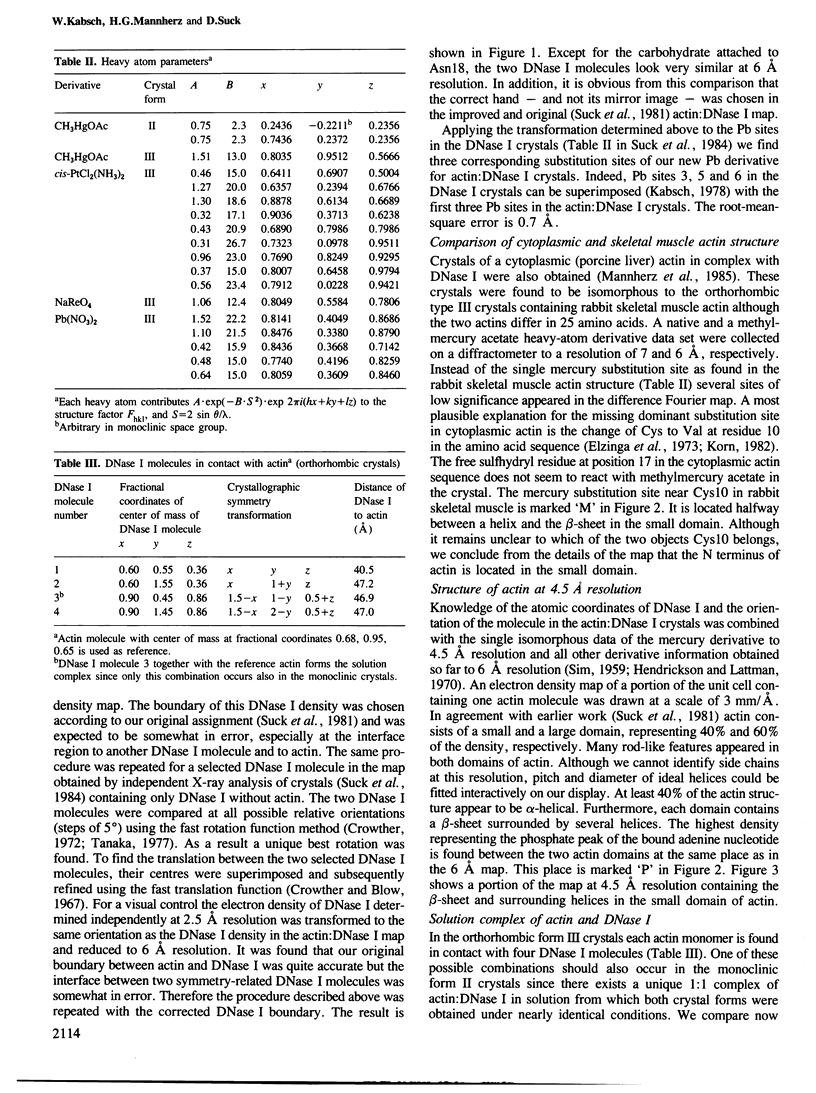
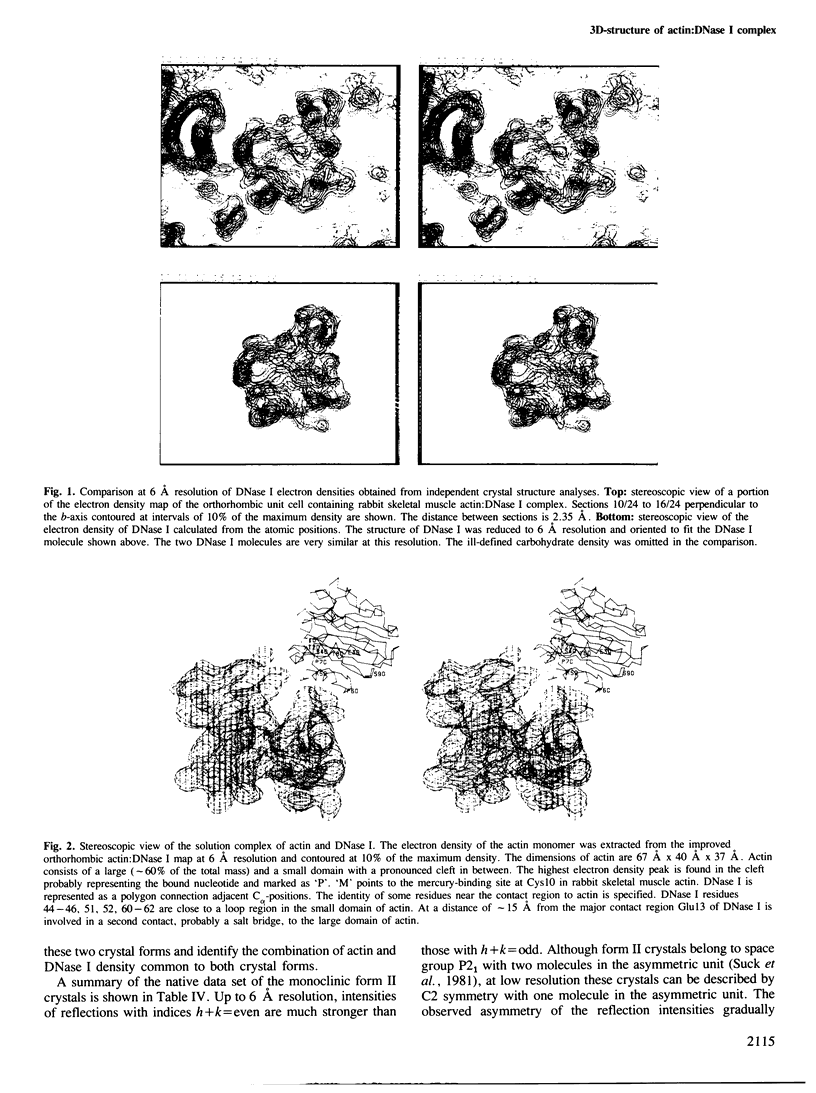
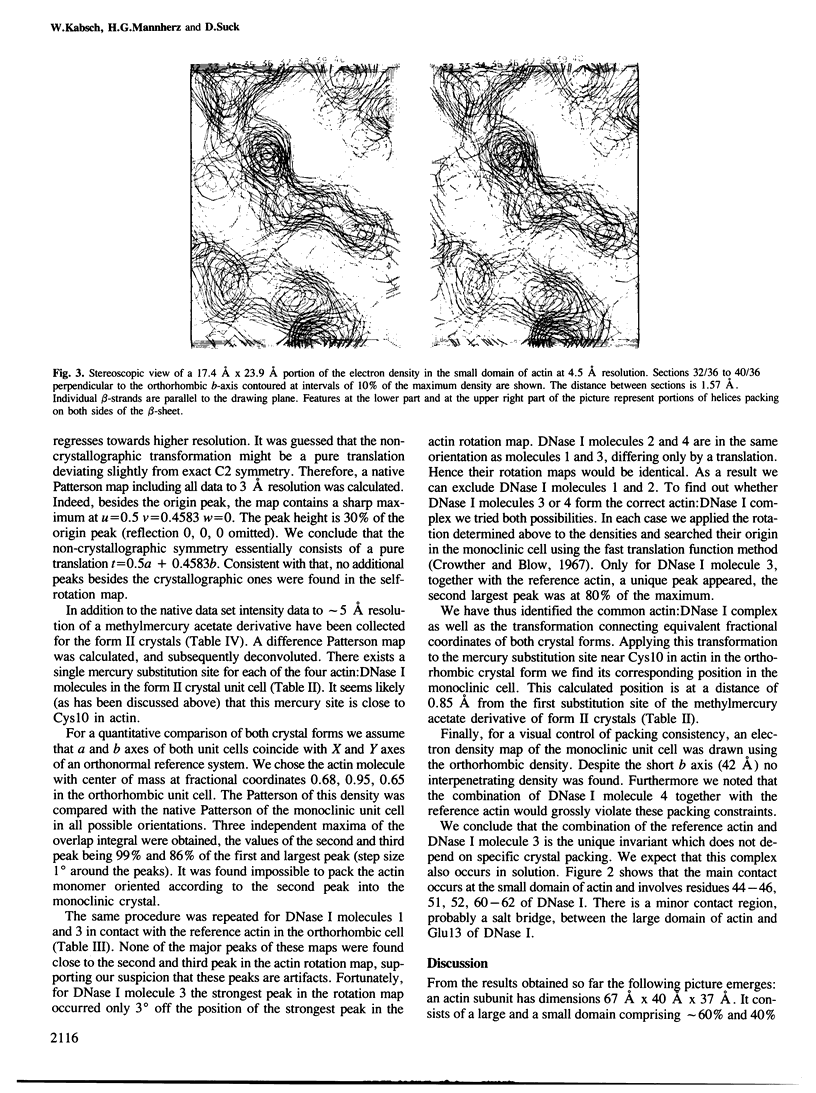
The shape of an actin subunit has been derived from an improved 6 A map of the complex of rabbit skeletal muscle actin and bovine pancreatic DNase I obtained by X-ray crystallographic methods. The three-dimensional structure of DNase I determined independently at 2.5 A resolution was compared with the DNase I electron density in the actin:DNase map. The two structures are very similar at 6 A resolution thus leading to an unambiguous identification of actin as well as DNase I electron density. Furthermore the correct hand of the actin structure is determined from the DNase I atomic structure. The resolution of the actin structure was extended to 4.5 A by using a single heavy-atom derivative and the knowledge of the atomic coordinates of DNase I. The dimensions of an actin subunit are 67 A X 40 A X 37 A. It consists of a small and a large domain, the small domain containing the N terminus. Actin is an alpha,beta-protein with a beta-pleated sheet in each domain. These sheets are surrounded by several alpha-helices, comprising at least 40% of the structure. The phosphate peak of the adenine nucleotide is located between the two domains. The complex of actin and DNase I as found in solution (i.e., the actin:DNase I contacts which do not depend on crystal packing) was deduced from a comparison of monoclinic with orthorhombic crystals. Residues 44-46, 51, 52, 60-62 of DNase I are close to a loop region in the small domain of actin. At a distance of approximately 15 A there is a second contact in the large domain in which Glu13 of DNase I is involved. A possible binding region for myosin is discussed.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Elzinga M., Collins J. H., Kuehl W. M., Adelstein R. S. Complete amino-acid sequence of actin of rabbit skeletal muscle. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2687–2691. doi: 10.1073/pnas.70.9.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elzinga M., Phelan J. J. F-actin is intermolecularly crosslinked by N,N'-p-phenylenedimaleimide through lysine-191 and cysteine-374. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6599–6602. doi: 10.1073/pnas.81.21.6599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock-De Gregori S. E., Mandala S., Sachs G. A. Changes in actin lysine reactivities during polymerization detected using a competitive labeling method. J Biol Chem. 1982 Nov 10;257(21):12573–12580. [PubMed] [Google Scholar]
- Korn E. D. Actin polymerization and its regulation by proteins from nonmuscle cells. Physiol Rev. 1982 Apr;62(2):672–737. doi: 10.1152/physrev.1982.62.2.672. [DOI] [PubMed] [Google Scholar]
- Lazarides E., Lindberg U. Actin is the naturally occurring inhibitor of deoxyribonuclease I. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4742–4746. doi: 10.1073/pnas.71.12.4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R. C., Szilagyi L. Change of reactivity of lysine residues upon actin polymerization. Biochemistry. 1981 Sep 29;20(20):5914–5919. doi: 10.1021/bi00523a040. [DOI] [PubMed] [Google Scholar]
- Mabuchi I. An actin-depolymerizing protein (depactin) from starfish oocytes: properties and interaction with actin. J Cell Biol. 1983 Nov;97(5 Pt 1):1612–1621. doi: 10.1083/jcb.97.5.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannherz H. G., Goody R. S., Konrad M., Nowak E. The interaction of bovine pancreatic deoxyribonuclease I and skeletal muscle actin. Eur J Biochem. 1980 Mar;104(2):367–379. doi: 10.1111/j.1432-1033.1980.tb04437.x. [DOI] [PubMed] [Google Scholar]
- Mannherz H. G., Kabsch W., Suck D., Friebel K., Frimmer M. Crystallization of cytoplasmic actin in complex with deoxyribonuclease I. Biochem J. 1985 Jan 15;225(2):517–522. doi: 10.1042/bj2250517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannherz H. G., Schenck H., Goody R. S. Synthesis of ATP from ADP and inorganic phosphate at the myosin-subfragment 1 active site. Eur J Biochem. 1974 Oct 1;48(1):287–295. doi: 10.1111/j.1432-1033.1974.tb03767.x. [DOI] [PubMed] [Google Scholar]
- Miki M., Wahl P. Fluorescence energy transfers between points in acto-subfragment-1 rigor complex. Biochim Biophys Acta. 1984 Nov 9;790(3):275–283. doi: 10.1016/0167-4838(84)90032-3. [DOI] [PubMed] [Google Scholar]
- Mornet D., Bertrand R., Pantel P., Audemard E., Kassab R. Structure of the actin-myosin interface. Nature. 1981 Jul 23;292(5821):301–306. doi: 10.1038/292301a0. [DOI] [PubMed] [Google Scholar]
- Naharro G., Robbins K. C., Reddy E. P. Gene product of v-fgr onc: hybrid protein containing a portion of actin and a tyrosine-specific protein kinase. Science. 1984 Jan 6;223(4631):63–66. doi: 10.1126/science.6318314. [DOI] [PubMed] [Google Scholar]
- Suck D., Kabsch W., Mannherz H. G. Three-dimensional structure of the complex of skeletal muscle actin and bovine pancreatic DNAse I at 6-A resolution. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4319–4323. doi: 10.1073/pnas.78.7.4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suck D., Oefner C., Kabsch W. Three-dimensional structure of bovine pancreatic DNase I at 2.5 A resolution. EMBO J. 1984 Oct;3(10):2423–2430. doi: 10.1002/j.1460-2075.1984.tb02149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutoh K. Actin-actin and actin-deoxyribonuclease I contact sites in the actin sequence. Biochemistry. 1984 Apr 24;23(9):1942–1946. doi: 10.1021/bi00304a009. [DOI] [PubMed] [Google Scholar]
- Sutoh K. Identification of myosin-binding sites on the actin sequence. Biochemistry. 1982 Jul 20;21(15):3654–3661. doi: 10.1021/bi00258a020. [DOI] [PubMed] [Google Scholar]
- Sutoh K. Mapping of actin-binding sites on the heavy chain of myosin subfragment 1. Biochemistry. 1983 Mar 29;22(7):1579–1585. doi: 10.1021/bi00276a009. [DOI] [PubMed] [Google Scholar]
- Szilagyi L., Lu R. C. Changes of lysine reactivities of actin in complex with myosin subfragment-1, tropomyosin and troponin. Biochim Biophys Acta. 1982 Dec 20;709(2):204–211. doi: 10.1016/0167-4838(82)90462-9. [DOI] [PubMed] [Google Scholar]
- Taylor D. L., Reidler J., Spudich J. A., Stryer L. Detection of actin assembly by fluorescence energy transfer. J Cell Biol. 1981 May;89(2):362–367. doi: 10.1083/jcb.89.2.362. [DOI] [PMC free article] [PubMed] [Google Scholar]





