Abstract
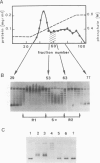
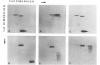
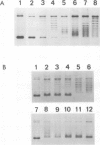
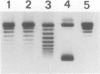
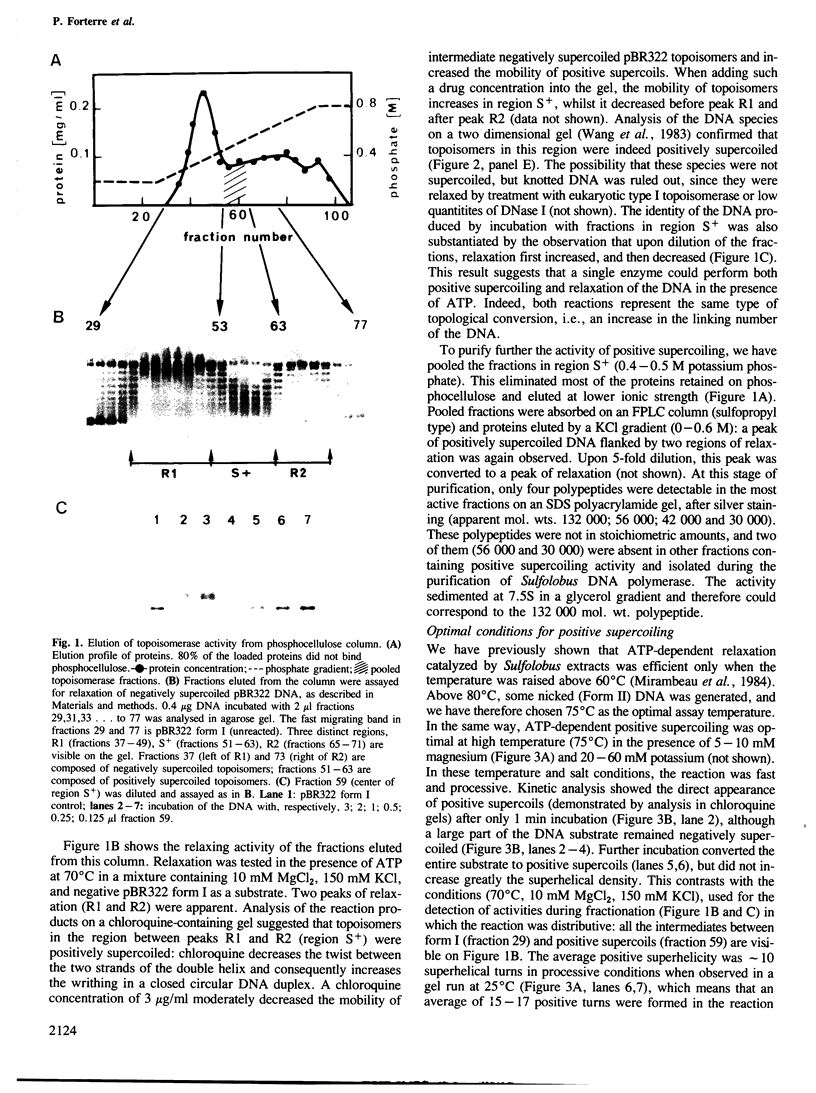
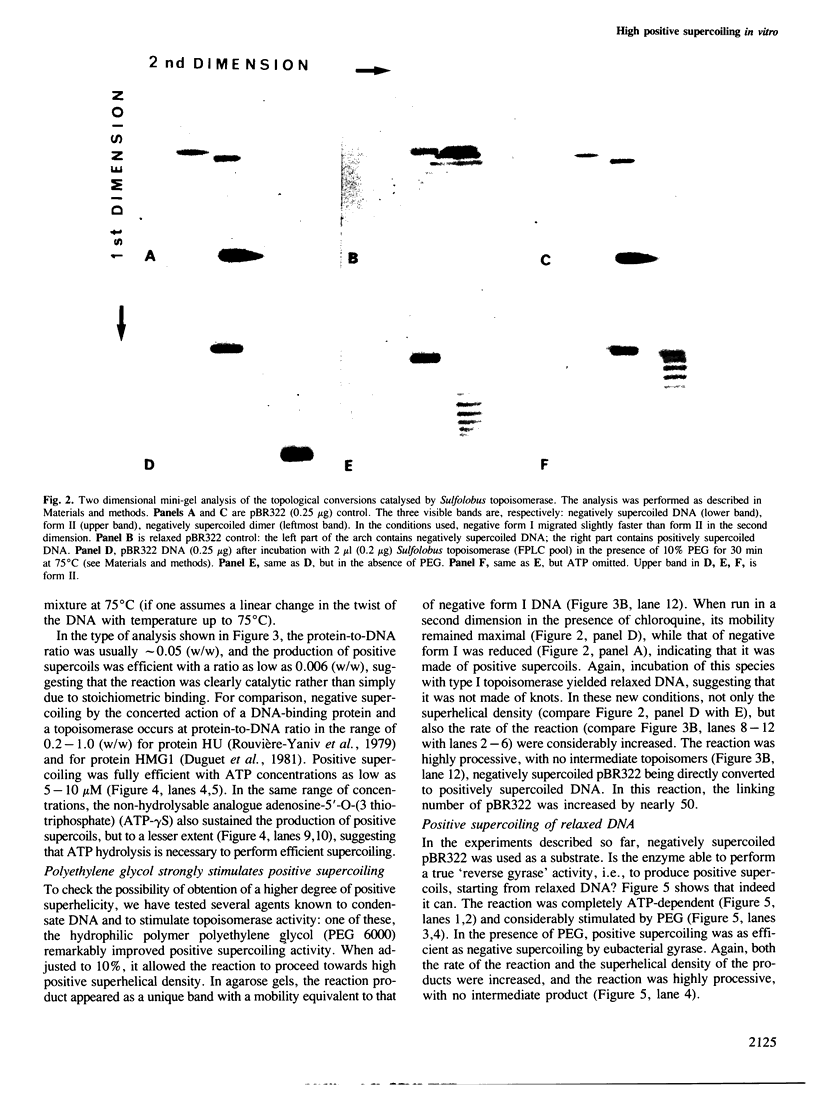
A topoisomerase able to introduce positive supercoils in a closed circular DNA, has been isolated from the archaebacterium Sulfolobus acidocaldarius. This enzyme, fully active at 75 degrees C, performed in vitro positive supercoiling either from negatively supercoiled, or from relaxed DNA in a catalytic reaction. In the presence of polyethylene glycol (PEG 6000), this reaction became very fast and highly processive, and the product was positively supercoiled DNA with a high superhelical density (form I+). Very low (5 - 10 micromoles) ATP concentrations were sufficient to support full supercoiling; the nonhydrolyzable analogue adenosine-5' -0-(3-thiotriphosphate) also sustained the production of positive supercoils, but to a lesser extent, suggesting that ATP hydrolysis was necessary for efficient activity. Nevertheless, low residual of positive supercoiling occurred, even in the absence of ATP, when the substrate was negatively supercoiled. Finally, the different ATP-driven topoisomerizations observed, i.e., relaxation of negative supercoils and positive supercoiling, in all cases increased the linking number of DNA in steps of 1, suggesting the action of a type I, rather than a type II topoisomerase.=
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Badet B., Hughes P., Kohiyama M., Forterre P. Inhibition of DNA replication in vitro by pefloxacin. FEBS Lett. 1982 Aug 23;145(2):355–359. doi: 10.1016/0014-5793(82)80199-3. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brock T. D., Brock K. M., Belly R. T., Weiss R. L. Sulfolobus: a new genus of sulfur-oxidizing bacteria living at low pH and high temperature. Arch Mikrobiol. 1972;84(1):54–68. doi: 10.1007/BF00408082. [DOI] [PubMed] [Google Scholar]
- Chen G. L., Yang L., Rowe T. C., Halligan B. D., Tewey K. M., Liu L. F. Nonintercalative antitumor drugs interfere with the breakage-reunion reaction of mammalian DNA topoisomerase II. J Biol Chem. 1984 Nov 10;259(21):13560–13566. [PubMed] [Google Scholar]
- Cozzarelli N. R. DNA gyrase and the supercoiling of DNA. Science. 1980 Feb 29;207(4434):953–960. doi: 10.1126/science.6243420. [DOI] [PubMed] [Google Scholar]
- Drlica K. Biology of bacterial deoxyribonucleic acid topoisomerases. Microbiol Rev. 1984 Dec;48(4):273–289. doi: 10.1128/mr.48.4.273-289.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duguet M., Bonne C., de Recondo A. M. Single-strand deoxyribonucleic acid binding protein from rat liver changes the helical structure of deoxyribonucleic acid. Biochemistry. 1981 Jun 9;20(12):3598–3603. doi: 10.1021/bi00515a045. [DOI] [PubMed] [Google Scholar]
- Duguet M., Lavenot C., Harper F., Mirambeau G., De Recondo A. M. DNA topoisomerases from rat liver: physiological variations. Nucleic Acids Res. 1983 Feb 25;11(4):1059–1075. doi: 10.1093/nar/11.4.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher L. M. DNA supercoiling and gene expression. Nature. 1984 Feb 23;307(5953):686–687. doi: 10.1038/307686a0. [DOI] [PubMed] [Google Scholar]
- Foglesong P. D., Bauer W. R. Effects of ATP and inhibitory factors on the activity of vaccinia virus type I topoisomerase. J Virol. 1984 Jan;49(1):1–8. doi: 10.1128/jvi.49.1.1-8.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellert M. DNA topoisomerases. Annu Rev Biochem. 1981;50:879–910. doi: 10.1146/annurev.bi.50.070181.004311. [DOI] [PubMed] [Google Scholar]
- Goto T., Laipis P., Wang J. C. The purification and characterization of DNA topoisomerases I and II of the yeast Saccharomyces cerevisiae. J Biol Chem. 1984 Aug 25;259(16):10422–10429. [PubMed] [Google Scholar]
- Harrison B., Zimmerman S. B. Polymer-stimulated ligation: enhanced ligation of oligo- and polynucleotides by T4 RNA ligase in polymer solutions. Nucleic Acids Res. 1984 Nov 12;12(21):8235–8251. doi: 10.1093/nar/12.21.8235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javaherian K., Tse Y. C., Vega J. Drosophila topoisomerase I: isolation, purification and characterization. Nucleic Acids Res. 1982 Nov 11;10(21):6945–6955. doi: 10.1093/nar/10.21.6945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi A., Asai K. Reverse gyrase--a topoisomerase which introduces positive superhelical turns into DNA. Nature. 1984 Jun 21;309(5970):677–681. doi: 10.1038/309677a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Miller K. G., Liu L. F., Englund P. T. A homogeneous type II DNA topoisomerase from HeLa cell nuclei. J Biol Chem. 1981 Sep 10;256(17):9334–9339. [PubMed] [Google Scholar]
- Mirambeau G., Duguet M., Forterre P. ATP-dependent DNA topoisomerase from the archaebacterium Sulfolobus acidocaldarius. Relaxation of supercoiled DNA at high temperature. J Mol Biol. 1984 Nov 5;179(3):559–563. doi: 10.1016/0022-2836(84)90080-9. [DOI] [PubMed] [Google Scholar]
- Rouvière-Yaniv J., Yaniv M., Germond J. E. E. coli DNA binding protein HU forms nucleosomelike structure with circular double-stranded DNA. Cell. 1979 Jun;17(2):265–274. doi: 10.1016/0092-8674(79)90152-1. [DOI] [PubMed] [Google Scholar]
- Ryoji M., Worcel A. Chromatin assembly in Xenopus oocytes: in vivo studies. Cell. 1984 May;37(1):21–32. doi: 10.1016/0092-8674(84)90297-6. [DOI] [PubMed] [Google Scholar]
- Shelton E. R., Osheroff N., Brutlag D. L. DNA topoisomerase II from Drosophila melanogaster. Purification and physical characterization. J Biol Chem. 1983 Aug 10;258(15):9530–9535. [PubMed] [Google Scholar]
- Snounou G., Malcolm A. D. Supercoiling and the mechanism of restriction endonucleases. Eur J Biochem. 1984 Jan 16;138(2):275–280. doi: 10.1111/j.1432-1033.1984.tb07912.x. [DOI] [PubMed] [Google Scholar]
- Sugino A., Higgins N. P., Brown P. O., Peebles C. L., Cozzarelli N. R. Energy coupling in DNA gyrase and the mechanism of action of novobiocin. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4838–4842. doi: 10.1073/pnas.75.10.4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Switzer R. C., 3rd, Merril C. R., Shifrin S. A highly sensitive silver stain for detecting proteins and peptides in polyacrylamide gels. Anal Biochem. 1979 Sep 15;98(1):231–237. doi: 10.1016/0003-2697(79)90732-2. [DOI] [PubMed] [Google Scholar]
- Villeponteau B., Lundell M., Martinson H. Torsional stress promotes the DNAase I sensitivity of active genes. Cell. 1984 Dec;39(3 Pt 2):469–478. doi: 10.1016/0092-8674(84)90454-9. [DOI] [PubMed] [Google Scholar]
- Wang J. C., Peck L. J., Becherer K. DNA supercoiling and its effects on DNA structure and function. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 1):85–91. doi: 10.1101/sqb.1983.047.01.011. [DOI] [PubMed] [Google Scholar]
- Zimmerman S. B., Pheiffer B. H. Macromolecular crowding allows blunt-end ligation by DNA ligases from rat liver or Escherichia coli. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5852–5856. doi: 10.1073/pnas.80.19.5852. [DOI] [PMC free article] [PubMed] [Google Scholar]