Abstract
Objectives
To determine the repeatability and response to therapy of dynamic contrast-enhanced (DCE) MRI biomarkers of synovitis in the hand and wrist of rheumatoid arthritis (RA) patients, and in particular the performance of the transfer constant K trans, in a multicentre trial setting.
Methods
DCE-MRI and RA MRI scoring (RAMRIS) were performed with meticulous standardisation at baseline and 6 and 24 weeks in a substudy of fostamatinib monotherapy in reducing synovitis compared with placebo or adalimumab. Analysis employed statistical shape modelling to avoid biased regions-of-interest, kinetic modelling and heuristic analyses. Repeatability was also evaluated.
Results
At early study termination, DCE-MRI data had been acquired from 58 patients in 19 imaging centres. K trans intra-subject coefficient of variation (N = 14) was 30%. K trans change demonstrated inferiority of fostamatinib (N = 11) relative to adalimumab (N = 10) after 6 weeks (treatment ratio = 1.92, p = 0.003), and failed to distinguish fostamatinib from placebo (N = 10, p = 0.79). RAMRIS showed superiority of fostamatinib relative to placebo at 6 weeks (p = 0.023), and did not distinguish fostamatinib from adalimumab at either 6 (p = 0.175) or 24 (p = 0.230) weeks.
Conclusion
This demonstrated repeatability of K trans and its ability to distinguish treatment groups show that DCE-MRI biomarkers are suitable for use in multicentre RA trials.
Key Points
• DCE-MRI biomarkers are feasible in large multicentre studies of joint inflammation.
• DCE-MRI K trans showed fostamatinib inferior to adalimumab after 6 weeks.
• K trans repeatability coefficient of variation was 30% multicentre.
Electronic supplementary material
The online version of this article (doi:10.1007/s00330-017-4736-9) contains supplementary material, which is available to authorized users.
Keywords: Magnetic resonance imaging; Arthritis, rheumatoid; Fostamatinib; Adalimumab; Biological markers
Introduction
MRI with gadolinium-based contrast agents (Gd-CAs) provides biomarkers dependent on perfusion, vascular volume, capillary endothelial permeability and interstitial volume, all of which increase in inflammation. MRI is widely available, sensitive, a low risk to patients and amenable to quantitation. OMERACT (Outcome Measures in Rheumatology) RAMRIS (Rheumatoid Arthritis MRI scoring) [1, 2] synovitis score is well established [3], but, as an ordinal variable, is theoretically less sensitive than a continuous variable [4] as a biomarker. Also, RAMRIS reports amount (an ‘extensive’ variable), but not severity (an ‘intensive’ variable), of synovitis, and cannot distinguish the importance of extent versus intensity of inflammation in RA, which is currently unknown.
Dynamic contrast enhanced (DCE) MRI [5] characterises regional uptake and washout of Gd-CA. It has been extensively used in oncology [6] and other diseases, and in RA provides biomarkers of synovial inflammation [7]. Despite over 60 DCE-MRI RA studies (over 1,000 patients) in PubMed, DCE-MRI RA studies until recently [8] were performed only in single expert centres, or occasionally [9] in two centres with identical equipment. RAMRIS, however, is routinely employed in large multicentre studies using different vendors’ MRI equipment [3]. A likely reason for failure to exploit DCE-MRI in multicentre RA studies is that the heuristic variables commonly used to characterise synovial Gd-CA uptake curves are inherently scanner-dependent, and therefore unlikely to provide biomarker values comparable between centres and studies. Also, as with any intensive variable, DCE-MRI biomarkers depend on how their region-of-interest (ROI) is defined, and because of variations in patient positioning and other technical factors, it is difficult to ensure that ROIs correspond between time points and subjects.
We reasoned that with rigorous site qualification and scanner monitoring, objective definition of ROIs by statistical shape modelling and robustly quantified compartmental modelling, we could reliably measure DCE-MRI biomarkers even in a large multicentre study using a variety of MRI equipment in centres with little or no previous quantitative DCE-MRI experience.
Here we present multicentre DCE-MRI, repeatability and response to treatment, in a study [10] of fostamatinib [11].
Methods (see Supplementary Material for detail)
Patients and treatment
The MRI substudy to OSKIRA-4 (Oral SYK Inhibition in Rheumatoid Arthritis) [10] (ClinicalTrials.gov Identifier: NCT02092961) was a Phase IIB, multicentre, randomised, double-blind, placebo-controlled, parallel-group study of efficacy and safety of fostamatinib disodium (a spleen tyrosine kinase inhibitor) monotherapy, compared with placebo or adalimumab monotherapy in patients with active RA. The primary substudy objective was to assess the efficacy of fostamatinib in reducing joint synovial disease activity as measured by change from baseline to week 6 (vs. placebo) in OMERACT RAMRIS synovitis score. Exploratory objectives included assessment of efficacy of fostamatinib in reducing joint synovial disease activity as measured by change from baseline to week 6 (vs. placebo and adalimumab) and week 24 (vs. adalimumab) in certain DCE-MRI biomarkers including K trans.
The findings of the full OSKIRA-4 clinical trial are reported elsewhere [10]. All patients gave written informed consent. Patients were DMARD-naïve, intolerant to DMARDs or had had inadequate response to maximally two DMARDs. Patients were randomised to one of three treatments: fostamatinib (100 mg bid for 24 weeks plus placebo subcutaneous injection every 2 weeks); adalimumab (40 mg subcutaneous injection every 2 weeks for 24 weeks, plus placebo to fostamatinib bid); placebo bid for 6 weeks followed by switch to 100 mg fostamatinib bid up to week 24, plus placebo subcutaneous injection every 2 weeks.
The more clinically active hand and wrist was imaged at screening, week 6 and week 24 using 3.0 T or 1.5 T whole-body MRI, with knee coils to allow simultaneous scanning of MCP and wrist joints. An acrylic frame ensured reproducible hand/wrist positioning. Some patients provided an additional baseline scan before the first dose of randomised treatment. All randomised patients were to have contrast-enhanced MRI (CE-MRI) assessments at the scheduled time points. Where participating sites could demonstrate acceptable DCE-MRI performance, this more technically demanding acquisition (subject of this report) was also performed. Approximately 20 patients in each dosing regimen were planned to have DCE-MRI evaluable at baseline, week 6 and week 24. On 4 June 2013, AstraZeneca announced results from Phase III trials of fostamatinib, and its decision not to proceed with regulatory filings, following which this study was terminated early.
DCE-MRI biomarkers
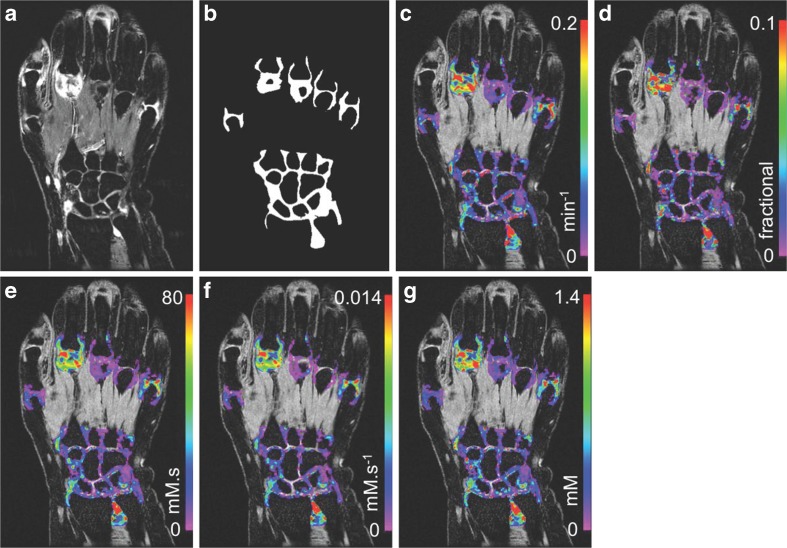
Pre-specified DCE-MRI biomarkers (Fig. 1c–g), in priority order, were:
K trans/min-1: volume transfer constant for Gd-CA between blood plasma and extravascular extracellular space from extended Tofts [5] compartmental model.
IRE/ mM.s-1: initial rate (gradient) of enhancement following Gd-CA over 60 s post-arrival in tissue.
IAUC 60, IAUC 120/mM.s: initial area under Gd-CA concentration curve over 60 s or 120 s post-arrival in tissue.
VEP/mL: volume of enhancing pannus [12].
ME/mM: maximum enhancement of Gd-CA concentration curve during DCE-MRI series.
v e, v p: volumes respectively of extravascular extracellular space, and blood plasma volume, per unit volume tissue (dimensionless).
Fig. 1.
Post-contrast high resolution T 1-weighted spoiled gradient-recalled echo (SPGR) image with fat saturation shows predominant disease in the second metacarpal joint (MCP) and isolated areas of disease in MCP-5, the distal radio-ulnar and radio-carpal joints (a). Segmented joint voxel masks were produced for each joint and used in the DCE-MRI analysis (b). Pre-contrast images with DCE-MRI parameterisation overlays for: K trans (c), v p (d), IAUC 120 (e), IRE (f) and ME (g)
Each biomarker was measured voxelwise over a ROI defined objectively using statistical shape modelling.
Statistical analysis
Analyses followed a predetermined statistical analysis plan, finalised and signed prior to locking the database and unblinding. Fostamatinib was compared pairwise with placebo (week 6) and adalimumab (weeks 6 and 24). Endpoints were tested at a two-sided significance level of 10%. Double baseline DCE-MRI repeatability was assessed using a mixed model of the two baseline results only.
Results
No new safety findings inconsistent with the known profiles of adalimumab, fostamatinib or any of the contrast agents were reported in the substudy. Prone MRI, with arm over head, may be uncomfortable for patients, but most tolerated the procedure well, providing images without unacceptable motion artefact (Fig. 1). Although the DCE-MRI protocol is not longer than the CE-MRI protocol, for DCE-MRI patients must remain immobile for the entire 26- to 28-min scan. We did not prospectively seek to evaluate relative tolerability of DCE-MRI and CE-MRI; informal records, however, indicate that of 64 who had both, two were unable to remain still during the dynamic scans because of discomfort, resulting in excessive motion artefact, and transferred to the CE-MRI-only cohort after baseline. In addition, four whose DCE-MRI failed quality control at baseline declined repeat scans because of discomfort and unwillingness to repeat MRI so quickly. No other patient withdrew for reasons connected with MRI.
Fourteen patients from six centres provided two valid DCE-MRI datasets and RAMRIS scores at baseline allowing repeatability to be determined (Table 1). Demography was generally balanced across treatment arms (Table S1). Because of early study termination, fewer data were accrued than planned: 58 patients (from 19 centres) provided technically valid DCE-MRI (Fig. S6); 45 were randomised and treated; 31 (52% of target) provided DCE-MRI and RAMRIS scores at week 6, and 19 (32% of target) at week 24 (Table 2). At week 6, K trans was 66% of baseline (geometric mean, N = 10) with adalimumab (range 27–154%); 104% of baseline (N = 11) (66–240%) with fostamatinib; and 124% of baseline (N = 10) (66–517%) with placebo (Fig. S2).
Table 1.
Repeatability and range, averaging over diseased and non-diseased joints
| MR biomarker | Patients with two baseline scans | Treated patients at baseline | ||||
|---|---|---|---|---|---|---|
| N for repeatability | Repeatability (intra-subject CoV %) | Variability (inter-subject CoV %) | N for range | Geometric mean | Range | |
| K trans (min-1) | 14 | 30.0% | 59.3% | 45 | 0.069 | 0.025–0.273 |
| IRE (mM.s-1) | 14 | 29.5% | 51.3% | 45 | 0.003 | 0.001–0.013 |
| IAUC 60 (mM.s) | 14 | 31.4% | 58.3% | 45 | 7.69 | 1.92–28.17 |
| IAUC 120 (mM.s) | 14 | 29.3% | 55.7% | 45 | 18.23 | 5.58–70.27 |
| VEP (mL) | 15 | 22.0% | 41.3% | 45 | 1.06 | 0.20–2.48* |
| ME (mM) | 14 | 27.5% | 52.4% | 45 | 0.32 | 0.11–1.31 |
| v e (%) | 0 | n/a | n/a | 0 | n/a | n/a |
| v p (%) | 14 | 53.4% | 60.1% | 45 | 1.2 | 0.3–4.5 |
| RAMRIS synovitis score** | 13 | 1.30 (SD) | 6.20 (SD) | 31 | 6.95 (arithmetic mean) | 0.0–21.8 |
*VEP excludes non-enhancing pannus. The range for total (non-enhancing plus enhancing) pannus was 3.29-8.30 ml
** VEP and the DCE-MRI endpoints are log-normally distributed but RAMRIS synovitis score is not. Therefore geometric mean and intra-subject CoV were calculated for VEP and the DCE-MRI endpoints, while arithmetic mean and intra-subject SD were calculated for RAMRIS synovitis score. The intra-class correlation coefficients were respectively 0.958 for RAMRIS synovitis score and 0.777 for K trans. RAMRIS synovitis score is based on an ordinal scale (0–24) so there is a restriction on how many values the synovitis score can actually take, therefore increasing the chance of a repeatable result. Given this, a direct comparison in repeatability with K trans is difficult
CoV coefficient of variation, DCE-MRI dynamic contrast-enhanced MRI, IAUC 60 IAUC 120/mM.s initial area under the Gd-CA concentration curve over 60 s or 120 s post-arrival in tissue, IRE/ mM.s-1 initial rate (gradient) of enhancement following Gd-CA over 60 s post-arrival in tissue, K trans/min-1 volume transfer constant for Gd-CA between blood plasma and extravascular extracellular space, ME/mM maximum enhancement of Gd-CA concentration curve during DCE-MRI series, RAMRIS RA MRI score, SD standard deviation, T 1 /s longitudinal relaxation time, v e volume of extravascular extracellular space per unit volume tissue, v p volume of blood plasma volume per unit volume tissue, VEP/ml volume of enhancing pannus
Table 2.
Change from baseline of the synovial MRI biomarkers in response to intervention
| Biomarker | Fostamatinib (N = 11) vs. placebo (N = 10) at 6 weeks | Fostamatinib (N = 11) vs. adalimumab (N = 10) at 6 weeks | Fostamatinib (N = 6) vs. adalimumab (N = 5) at 24 weeks | |||
|---|---|---|---|---|---|---|
| Treatment ratio* (tr) or difference (td) (90% CI) | Two-sided p-value | Treatment ratio* (tr) or difference (td) (90% CI) | Two-sided p-value | Treatment ratio* (tr) or difference (td) (90% CI) | Two-sided p-value | |
| K trans | tr = 0.95 (0.68–1.33) | 0.794 | tr = 1.92 (1.36–2.72) | 0.003 | tr = 1.59 (0.95–2.68) | 0.137 |
| IRE | tr = 0.86 (0.62–1.18) | 0.417 | tr = 1.55 (1.12–2.15) | 0.031 | tr = 1.60 (1.06–2.42) | 0.064 |
| IAUC 60 | tr = 0.91 (0.66–1.24) | 0.603 | tr = 1.67 (1.21–2.30) | 0.012 | tr = 1.57 (0.97–2.54) | 0.120 |
| IAUC 120 | tr = 0.88 (0.65–1.19) | 0.478 | tr = 1.67 (1.22–2.28) | 0.010 | tr = 1.60 (0.98–2.61) | 0.116 |
| VEP | tr = 0.85 (0.66–1.08) | 0.130 | tr = 0.77 (0.60–1.00) | 0.053 | tr = 1.23 (0.62–2.11) | 0.508 |
| ME | tr = 0.95 (0.70–1.28) | 0.756 | tr = 1.64 (1.20–2.25) | 0.012 | tr = 1.57 (1.05–2.36) | 0.070 |
| v e | n/a | n/a | n/a | n/a | n/a | n/a |
| v p | tr = 0.90 (0.60–1.29) | 0.610 | tr = 1.75 (1.21–2.54) | 0.016 | tr = 1.75 (1.21–2.54) | 0.065 |
| RAMRIS synovitis score | td = -2.00 (-3.25–-0.75) | 0.023 | td = -1.50 (-2.50–0.00) | 0.175 | td = 2.00 (-0.50–5.00) | 0.230 |
| DAS-28 CRP | td = 0.65 (-0.11–1.41) | 0.155 | td = -0.13 (-0.89–0.64) | 0.780 | td = -1.48 (-3.43–0.47) | 0.200 |
Based on the response at 6 weeks in the adalimumab group, the standardised response mean for K trans was -0.64
DAS-28 CRP Disease Activity Score 28 based on C-reactive protein
*tr is used for VEP and the DCE-MRI endpoints as they are log-normally distributed but td for the RAMRIS synovitis score and DAS-28 CRP which are not:
tr <1 or td < 0 indicate an effect in favour of fostamatinib
Discussion
MRI biomarkers [13] pose different challenges to soluble biomarkers. Biomarker quality and validity depends on operation of an MRI device not primarily designed for quantitative work, perhaps in a manner unfamiliar to users in trial sites. Encouraging measures of repeatability and response to therapy in small studies in single expert centres may not translate to real-world multicentre trials. It is therefore necessary to evaluate [14] these biomarkers specifically in the multicentre setting.
Previously, various MRI biomarkers have been derived from Gd-CA-enhanced images. The rationale for selecting our preferred DCE-MRI biomarker, K trans, and our exploratory biomarkers, is described in detail in the Supplementary Material. While many previous DCE-MRI studies in RA used variants of the heuristic parameters IRE and ME, in this work we followed international standardisation projects and guidelines [15] in employing K trans. Unlike the case with CT or nuclear medicine, in DCE-MRI the signal intensity has a non-linear relationship to Gd-CA concentration [16] which depends in a complex way on baseline T 1, B 1 heterogeneity, flow artefacts, pulse sequence parameters and post-processing. Metrics dependent on the native signal intensities necessarily incorporate these dependencies, and while there should little effect on repeatability when patients are imaged in the same scanner, they make values difficult to compare between scanners (or even between upgrades of the same scanner). Thus while the MR signal intensity-based heuristic biomarkers can exhibit good repeatability single-centre [17] they were inappropriate for the present study.
For our preferred DCE-MRI biomarker, K trans, the multicentre intra-subject repeatability coefficient of variation (CoV) of 30% was similar to a previous single-centre RA report [18] but worse than typically seen in oncology studies [6] where repeatability CoV of 15% is more typical. This likely reflects our choice to average over all joints including those with little (or no) synovitis. At week 6 K trans clearly distinguished the inferior effect of fostamatinib from adalimumab on synovial inflammation, but failed to distinguish fostamatinib from placebo. This is interpreted as an early effect of adalimumab, but not fostamatinib, on synovial capillary blood flow and/or capillary endothelial permeability. The main OSKIRA-4 [10] study (N = 279) concluded fostamatinib at the two higher dose regimens was more efficacious than placebo at week 6 but less than adalimumab at week 24 in terms of RA signs and symptoms. In this substudy, RAMRIS findings are consistent with the main study at week 6, but the number of substudy patients at week 24 was too small to draw firm conclusions. Heuristic biomarkers IAUC 120, IAUC 60, IRE and ME exhibited similar repeatability to K trans, and similarly distinguished fostamatinib from adalimumab. We derived IRE and ME in absolute not arbitrary (machine-dependent) units, which probably reduced scanner-related variation from what would be expected from signal intensity-based heuristics. v p is challenging to measure, as blood plasma constitutes only a small volume fraction of the synovitis (here around 1.5%) but it did distinguish fostamatinib from adalimumab despite worse repeatability than the other biomarkers.
Given this variability in K trans, a future parallel group DCE-MRI study of 20 patients per arm would give 80% power to detect a treatment ratio of 1.67 at a two-sided significance level of 10%.
Due to early study termination, comparisons between groups should be interpreted with caution. All biomarkers were exploratory without correction for multiple comparisons. The study was not designed to compare adalimumab with placebo, nor to compare DCE-MRI with RAMRIS, nor to test whether early MRI changes forecast clinical outcome. However, our findings from this truncated study do demonstrate that DCE-MRI biomarkers are feasible and sensitive in a large multicentre trial setting.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(DOCX 4896 kb)
Acknowledgments
We thank the 64 patients who volunteered for this study, and particularly those 15 patients who gave up their time to provide the double-baseline data.
Abbreviations and acronyms
- bid
Twice daily
- CE-MRI
Contrast-enhanced MRI
- CoV
Coefficient of variation
- DAS-28 CRP
Disease Activity Score calculated from 28 joints and using C-reactive protein
- DCE-MRI
Dynamic contrast-enhanced MRI
- DMARD
Disease-modifying anti-rheumatic drug
- Gd-CA
Gadolinium-based contrast agent
- IAUC60IAUC120/mM.s
Initial area under the Gd-CA concentration curve over 60 s or 120 s post-arrival in tissue
- IRE/ mM.s-1
Initial rate (gradient) of enhancement following Gd-CA over 60 s post-arrival in tissue
- Ktrans/min-1
Volume transfer constant for Gd-CA between blood plasma and extravascular extracellular space
- MCP
Metacarpophalangeal
- ME/mM
Maximum enhancement of Gd-CA concentration curve during DCE-MRI series
- MRI
Magnetic resonance imaging
- OMERACT
Outcome measures in rheumatology initiative
- OSKIRA
Oral SYK inhibition in RA
- QC
Quality control
- RA
Rheumatoid arthritis
- RAMRIS
RA MRI score
- ROI
Region of interest
- SD
Standard deviation
- SPGR
Spoiled gradient-recalled echo
- SYK
Spleen tyrosine kinase
- T1 /s
Longitudinal relaxation time
- td
Treatment difference
- tr
Treatment ratio
- ve
Volume of extravascular extracellular space per unit volume tissue
- vp
Volume of blood plasma volume per unit volume tissue
- VEP/ml
Volume of enhancing pannus
Compliance with ethical standards
Guarantor
The scientific guarantor of this publication is Professor John Charles Waterton MA PhD CSci FRSC(UK), Manchester Academic Health Sciences Centre, University of Manchester, Stopford building, Oxford Road, MANCHESTER M13 9PT, UK.
Conflict of interest
The authors of this manuscript declare relationships with the following companies:
John Waterton is a former employee of, and has stock in, AstraZeneca, a for-profit company engaged in the discovery, development, manufacture and marketing of proprietary therapeutics. He does not consider that this creates any conflict of interest with the subject-matter of the present manuscript, as fostamatinib is no longer a development product of AstraZeneca.
Meilien Ho (deceased) is a former employee of AstraZeneca, a for-profit company engaged in the discovery, development, manufacture and marketing of proprietary therapeutics.
Lars Nordenmark has employment and stock in AstraZeneca, a for-profit company engaged in the discovery, development, manufacture and marketing of proprietary therapeutics. He does not consider that this creates any conflict of interest with the subject-matter of the present manuscript, as fostamatinib is no longer a development product of AstraZeneca.
Martin Jenkins has employment and stock in AstraZeneca, a for-profit company engaged in the discovery, development, manufacture and marketing of proprietary therapeutics. He does not consider that this creates any conflict of interest with the subject-matter of the present manuscript, as fostamatinib is no longer a development product of AstraZeneca.
Julie DiCarlo has employment in Spire Sciences, Inc., a for-profit company providing centralized image analysis services to multiple pharmaceutical, biotechnology and medical device companies. She also has received consulting fees from Abbvie, Amgen, AstraZeneca, BMS, Five Prime Therapeutics, Genentech, Roche, Janssen, Lilly, Merck, Novartis, Samsung, Salix-Santarus and UCB.
Gwenael Guillard has employment and stock options in Imorphics, a for-profit company engaged in the discovery, development, and marketing of imaging biomarkers.
Caleb Roberts has employment and stock options in Bioxydyn, a for-profit company engaged in the discovery, development, and marketing of imaging biomarkers.
Giovanni Buonaccorsi has employment in Bioxydyn, a for-profit company engaged in the discovery, development, and marketing of imaging biomarkers.
Geoffrey Parker has employment and stock in, and is a director of, Bioxydyn, a for-profit company engaged in the discovery, development, and marketing of imaging biomarkers.
Michael Bowes has employment and stock in, and is a director of, Imorphics, a for-profit company engaged in the discovery, development, and marketing of imaging biomarkers.
Charles Peterfy has employment, and is owner and chief executive officer of, Spire Sciences, Inc., a for-profit company providing centralized image analysis services to multiple pharmaceutical, biotechnology and medical device companies. He is also on the speakers bureau for Amgen, and has received consulting fees from Abbvie, Amgen, AstraZeneca, BMS, Five Prime Therapeutics, Genentech, Roche, Janssen, Lilly, Merck, Novartis, Samsung, Salix-Santarus and UCB.
Peter Taylor has received grant/research support from UCB and is a consultant for AstraZeneca, Pfizer, Lilly and Celgene. He would like to acknowledge NIHR for funding of the Oxford Biomedical Research Unit.
Funding
This study has received funding by AstraZeneca.
Statistics and biometry
One of the authors, Dr Martin Jenkins, has significant statistical expertise. Dr Jenkins is employed by AstraZeneca as Principal Statistician.
Ethical approval
Institutional Review Board approval was obtained. As appropriate for a large international study, appropriate IRB approvals were secured at each of the recruiting centres. Specifically the following 20 investigators had appropriate local approvals for the 58 patients whose data are described in this report:
Dr Wahib Al-Allaf, New Cross Hospital, Wolverhampton, UK; Dr Dale Bramlet, Advent Clinical Research Centers, Inc., Pinellas Park, FL, USA; Dr Ian Bruce, Manchester Royal Infirmary, Kellgren Centre for Rheumatology, Manchester, UK; Dr Tina Bunch, Austin Regional Clinic, Austin TX, USA; Dr Anna Dudek, Niepubliczny Zakład Opieki Zdrowotnej Medica Pro Familia, Warszawa, Poland; Dr Danielle Gerlag, Academisch Medisch Centrum, Amsterdam, The Netherlands; Dr Joanna Hilt, Centrum Terapii Współczesnej J.M. Jasnorzewska s.k.a, Łódź, Poland; Dr Josette Johnson, Piedmont Arthritis Clinic, Greenville, SC, USA; Dr Eleni Kanakaridu, Synexus Magyarország Kft., Budapest, Hungary; Dr Herbert Kellner, Private practice and Division of Rheumatology KHl Neuwittelsbach, München, Germany; Dr Aleksandra Kołczewska, Niepubliczny Zakład Opieki Zdrowotnej Medicus, Sroda Wielkopolska, Poland; Dr Dennis Levinson, APEX Medical Research Inc, Chicago, IL, USA; Dr Savithree Nayiager, St Augustine's Hospital, Medical Centre, Durban, KwaZulu Natal, South Africa; Dr Tom Philipp, Fakultní Thomayerova nemocnice s poliklinikou, Praha, Czech Republic; Dr Peter Prouse, Basingstoke and North Hampshire Hospital, Basingstoke, UK; Dr Areena Swarup, Arizona Arthritis and Rheumatology Research, Scottsdale and Glendale, AZ, USA; Dr Peter Taylor, Charing Cross Hospital, London, UK; Dr Yong Tsai, Daytona Beach, FL, USA; Dr Leonore Unger, Städtisches Klinikum Dresden-Friedrichstadt, Dresden, Germany; Dr Jiří Vencovský, Revmatologický ústav, Praha, Czech Republic.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Study subjects or cohorts overlap
Some study subjects or cohorts have been previously reported:
The data presented here arise from the magnetic resonance imaging (MRI) substudy in OSKIRA-4 (Oral SYK Inhibition in Rheumatoid Arthritis), a Phase IIb monotherapy study of fostamatinib in RA.
OSKIRA-4 was a 6-month Phase IIb study evaluating improvements in signs and symptoms of RA in 280 patients who had never previously used a disease-modifying anti-rheumatic drug (DMARD), were DMARD-intolerant or had an inadequate response to DMARDs and were randomised to receive fostamatinib as a monotherapy, adalimumab as a monotherapy, or placebo. The findings of this study have recently been reported in Annals of the Rheumatic Diseases (http://dx.doi.org/10.1136/annrheumdis-2014-205361).
AstraZeneca terminated the development of fostamatinib in 2013 before the OSKIRA-4 MRI substudy was complete.
There was almost no overlap between the patients (only two patients overlap) between the patients in the MRI substudy and those patients in the main OSKIRA-4 study who were not examined by MRI. No data from the MRI substudy have previously been disclosed or submitted for publication.
Methodology
ᅟ
prospective
randomised controlled trial
multicentre study
Footnotes
Meilien Ho is deceased
Electronic supplementary material
The online version of this article (doi:10.1007/s00330-017-4736-9) contains supplementary material, which is available to authorized users.
References
- 1.Østergaard M, Peterfy C, Conaghan P, et al. OMERACT rheumatoid arthritis magnetic resonance imaging studies. Core set of MRI acquisitions, joint pathology definitions, and the OMERACT RA-MRI scoring system. J Rheumatol. 2003;6:1385–1386. [PubMed] [Google Scholar]
- 2.Haavardsholm EA, Østergaard M, Ejbjerg BJ, et al. Reliability and sensitivity to change of the OMERACT rheumatoid arthritis magnetic resonance imaging score in a multireader, longitudinal setting. Arthritis Rheum. 2005;52:3860–3867. doi: 10.1002/art.21493. [DOI] [PubMed] [Google Scholar]
- 3.Peterfy C, Østergaard M, Conaghan PG. MRI comes of age in RA clinical trials. Ann Rheum Dis. 2013;72:794–796. doi: 10.1136/annrheumdis-2012-202696. [DOI] [PubMed] [Google Scholar]
- 4.Kessler LG, Barnhart HX, Buckler AJ, et al. The emerging science of quantitative imaging biomarkers terminology and definitions for scientific studies and regulatory submissions. Stat Methods Med Res Published online first 11 June 2014 doi:10.1177/0962280214537333 [DOI] [PubMed]
- 5.Tofts PS, Brix G, Buckley DL, et al. Estimating kinetic parameters from dynamic contrast-enhanced T1 weighted MRI of a diffusable tracer: standardized quantities and symbols. J Magn Reson Imaging. 1999;10:223–232. doi: 10.1002/(SICI)1522-2586(199909)10:3<223::AID-JMRI2>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 6.O'Connor JPB, Jackson A, Parker GJM, Roberts C, Jayson GC. Dynamic contrast-enhanced MRI in clinical trials of antivascular therapies. Nat Rev Clin Onc. 2012;9:167–177. doi: 10.1038/nrclinonc.2012.2. [DOI] [PubMed] [Google Scholar]
- 7.Hodgson RJ, O'Connor P, Moots R. MRI of rheumatoid arthritis image quantitation for the assessment of disease activity, progression and response to therapy. Rheumatology (Oxford) 2008;47:13–21. doi: 10.1093/rheumatology/kem250. [DOI] [PubMed] [Google Scholar]
- 8.Beals C, Baumgartner R, Peterfy C et al (2013) Treatment effects measured by dynamic contrast enhanced MRI and RAMRIS for rheumatoid arthritis. Ann Rheum Dis 72:Suppl 3 A748
- 9.Reece RJ, Kraan MC, Radjenovic A, et al. Comparative assessment of leflunomide and methotrexate for the treatment of rheumatoid arthritis, by dynamic enhanced magnetic resonance imaging. Arthritis Rheum. 2002;46:366–372. doi: 10.1002/art.10084. [DOI] [PubMed] [Google Scholar]
- 10.Taylor PC, Genovese MC, Greenwood M, et al. OSKIRA-4: a phase IIb randomised, placebo-controlled study of the efficacy and safety of fostamatinib monotherapy. Ann Rheum Dis. 2015;74:2123–2129. doi: 10.1136/annrheumdis-2014-205361. [DOI] [PubMed] [Google Scholar]
- 11.Genovese MC, Kavanaugh A, Weinblatt ME, et al. An oral Syk kinase inhibitor in the treatment of rheumatoid arthritis: a three-month randomized, placebo-controlled, phase II study in patients with active rheumatoid arthritis that did not respond to biologic agents. Arthritis Rheum. 2011;63:337–345. doi: 10.1002/art.30114. [DOI] [PubMed] [Google Scholar]
- 12.Waterton JC, Rajanayagam V, Ross BD, Brown D, Whittemore A, Johnstone D. Magnetic resonance methods for measurement of disease progression in rheumatoid arthritis. Magn Reson Imaging. 1993;11:1033–1038. doi: 10.1016/0730-725X(93)90222-Y. [DOI] [PubMed] [Google Scholar]
- 13.Waterton JC. Translational magnetic resonance imaging and spectroscopy: opportunities and challenges. In: Garrido L, Beckmann N, editors. New applications of NMR in drug discovery and development. Cambridge: RSC press; 2013. pp. 333–360. [Google Scholar]
- 14.Eshed I, Krabbe S, Østergaard M, et al. Influence of field strength, coil type and image resolution on assessment of synovitis by unenhanced MRI: a comparison with contrast-enhanced MRI. Eur Radiol. 2015;25:1059–1067. doi: 10.1007/s00330-014-3470-9. [DOI] [PubMed] [Google Scholar]
- 15.Leach MO, Morgan B, Tofts PS, et al. Imaging vascular function for early stage clinical trials using dynamic contrast-enhanced magnetic resonance imaging. Eur Radiol. 2012;22:1451–1464. doi: 10.1007/s00330-012-2446-x. [DOI] [PubMed] [Google Scholar]
- 16.Buckley DL, Parker GJM. Measuring contrast agent concentration in T1-weighted dynamic contrast-enhanced MRI. In: Jackson A, Buckley DL, Parker GJM, editors. Dynamic contrast-enhanced magnetic resonance imaging in oncology. Heidelberg: Springer; 2005. pp. 69–79. [Google Scholar]
- 17.Malattia C, Damasio MB, Basso C, et al. Dynamic contrast-enhanced magnetic resonance imaging in the assessment of disease activity in patients with juvenile idiopathic arthritis. Rheumatol. 2010;49:178–185. doi: 10.1093/rheumatology/kep343. [DOI] [PubMed] [Google Scholar]
- 18.Hodgson RJ, Connolly S, Barnes T, Eyes B, Campbell RS, Moots R. Pharmacokinetic modeling of dynamic contrast-enhanced MRI of the hand and wrist in rheumatoid arthritis and the response to anti-tumor necrosis factor-alpha therapy. Magn Reson Med. 2007;58:482–489. doi: 10.1002/mrm.21349. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 4896 kb)