Abstract
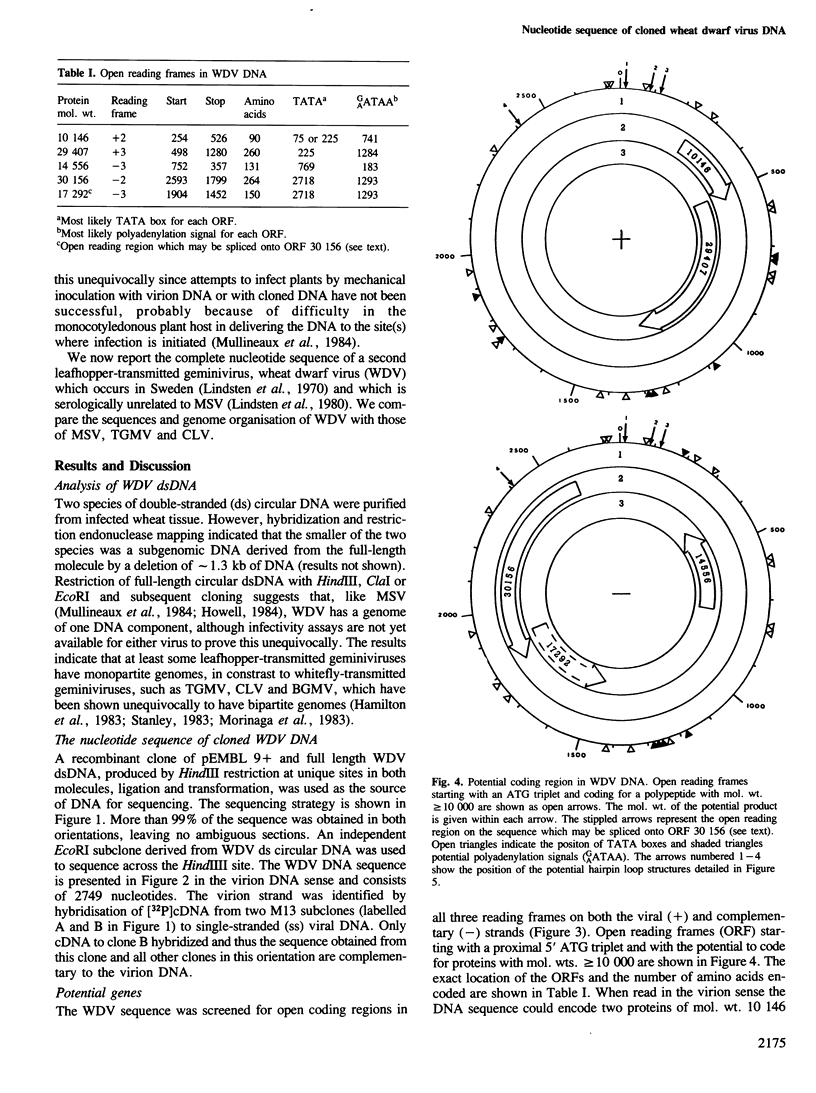
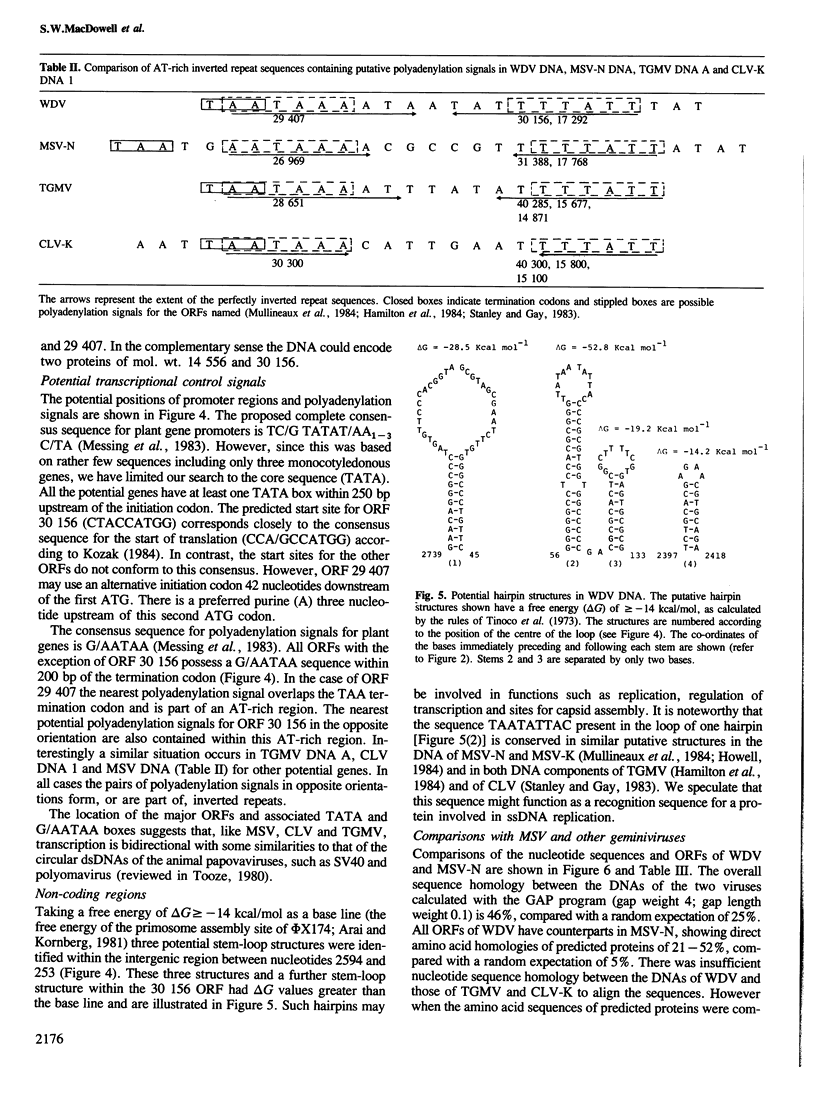
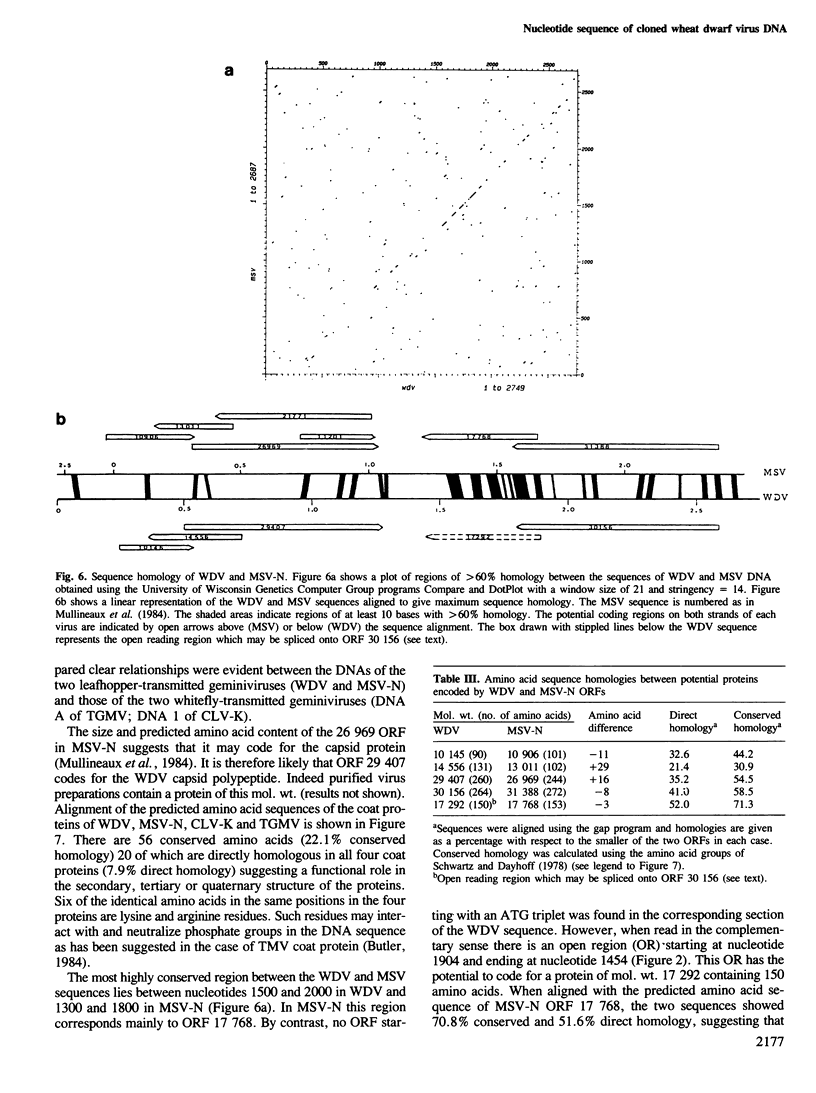
Restriction analysis and cloning of virus-specific double-stranded DNA isolated from plants infected with wheat dwarf virus (WDV) indicated that the virus genome, like that of maize streak virus (MSV), consists of a single DNA circle. The complete nucleotide sequence of cloned WDV DNA (2749 nucleotides) has been determined. Comparison of the potential coding regions in WDV DNA with those in the DNA of two strains of MSV suggests that these viruses encode at least two functional proteins, the coat protein read in the virion (+) DNA sense and a composite protein, formed from two open reading regions, in the complementary (−) DNA sense. Although WDV and MSV are serologically unrelated their coat proteins showed 35% direct amino acid sequence and their DNAs showed 46% nucleotide sequence homology. There was too little homology between the DNAs of WDV and those of two geminiviruses with bipartite genomes, cassava latent virus (CLV) and tomato golden mosaic virus (TGMV), to align the sequences. However comparison of the amino acid sequences of predicted proteins of WDV, MSV, TGMV and CLV revealed clear relationships between these viruses and suggested that the monopartite and the bipartite geminiviruses have a common ancestral origin. Four inverted repeat sequences which have the potential to form hairpin structures of △G≥-14 kcal/mol were detected in WDV DNA. The sequence TAATATTAC present in the loop of one of these hairpins is conserved in similar putative structures in MSV DNA and in both DNA components of CLV and TGMV and may function as a recognition sequence for a protein involved in virus DNA replication.
Keywords: geminivirus, nucleotide sequence, wheat dwarf virus, genome organisation, Triticum aestivum
Full text
PDF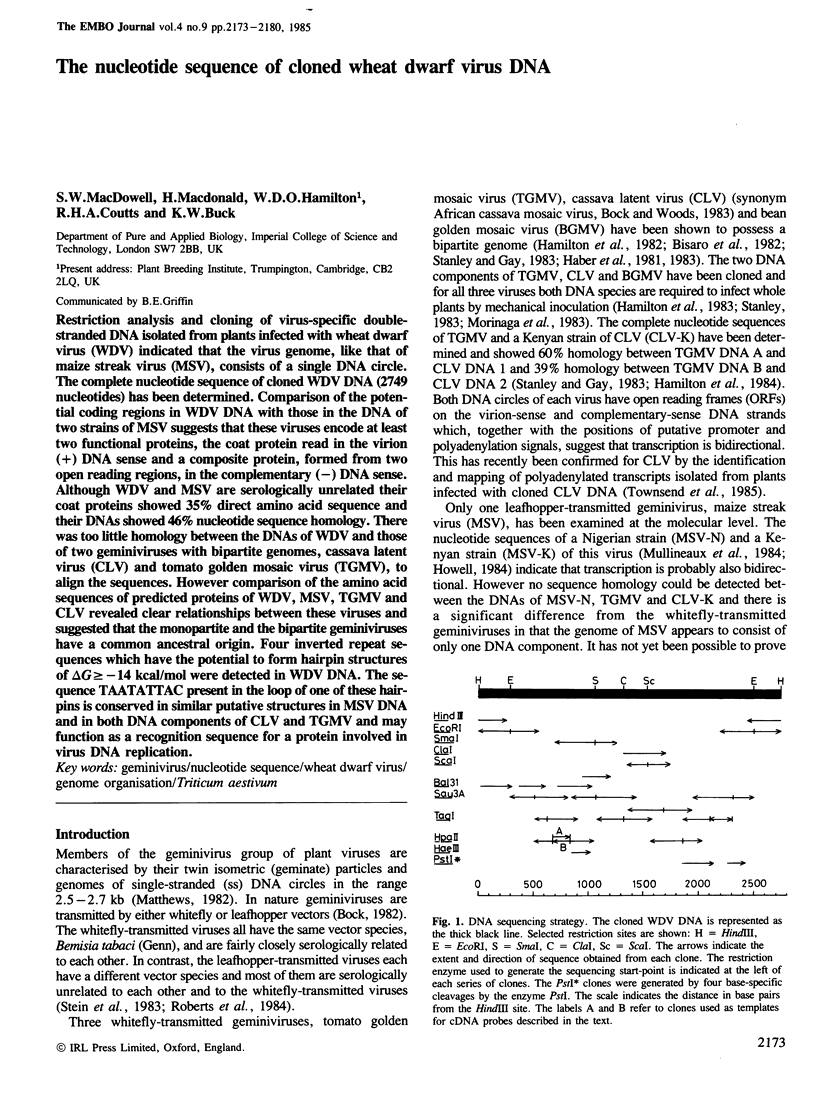




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arai K., Kornberg A. Unique primed start of phage phi X174 DNA replication and mobility of the primosome in a direction opposite chain synthesis. Proc Natl Acad Sci U S A. 1981 Jan;78(1):69–73. doi: 10.1073/pnas.78.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisaro D. M., Hamilton W. D., Coutts R. H., Buck K. W. Molecular cloning and characterisation of the two DNA components of tomato golden mosaic virus. Nucleic Acids Res. 1982 Aug 25;10(16):4913–4922. doi: 10.1093/nar/10.16.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler P. J. The current picture of the structure and assembly of tobacco mosaic virus. J Gen Virol. 1984 Feb;65(Pt 2):253–279. doi: 10.1099/0022-1317-65-2-253. [DOI] [PubMed] [Google Scholar]
- Classification and nomenclature of viruses. Fourth report of the International Committee on Taxonomy of Viruses. Intervirology. 1982;17(1-3):1–199. doi: 10.1159/000149278. [DOI] [PubMed] [Google Scholar]
- Dente L., Cesareni G., Cortese R. pEMBL: a new family of single stranded plasmids. Nucleic Acids Res. 1983 Mar 25;11(6):1645–1655. doi: 10.1093/nar/11.6.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garoff H., Ansorge W. Improvements of DNA sequencing gels. Anal Biochem. 1981 Aug;115(2):450–457. doi: 10.1016/0003-2697(81)90031-2. [DOI] [PubMed] [Google Scholar]
- Gay N. J., Walker J. E. Homology between human bladder carcinoma oncogene product and mitochondrial ATP-synthase. Nature. 1983 Jan 20;301(5897):262–264. doi: 10.1038/301262a0. [DOI] [PubMed] [Google Scholar]
- Hamilton W. D., Bisaro D. M., Buck K. W. Identification of novel DNA forms in tomato golden mosaic virus infected tissue. Evidence for a two component viral genome. Nucleic Acids Res. 1982 Aug 25;10(16):4901–4912. doi: 10.1093/nar/10.16.4901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton W. D., Bisaro D. M., Coutts R. H., Buck K. W. Demonstration of the bipartite nature of the genome of a single-stranded DNA plant virus by infection with the cloned DNA components. Nucleic Acids Res. 1983 Nov 11;11(21):7387–7396. doi: 10.1093/nar/11.21.7387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton W. D., Stein V. E., Coutts R. H., Buck K. W. Complete nucleotide sequence of the infectious cloned DNA components of tomato golden mosaic virus: potential coding regions and regulatory sequences. EMBO J. 1984 Sep;3(9):2197–2205. doi: 10.1002/j.1460-2075.1984.tb02114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell S. H. Physical structure and genetic organisation of the genome of maize streak virus (Kenyan isolate). Nucleic Acids Res. 1984 Oct 11;12(19):7359–7375. doi: 10.1093/nar/12.19.7359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegami M., Haber S., Goodman R. M. Isolation and characterization of virus-specific double-stranded DNA from tissues infected by bean golden mosaic virus. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4102–4106. doi: 10.1073/pnas.78.7.4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuno R., Toh H., Hayashida H., Miyata T. Sequence similarity between putative gene products of geminiviral DNAs. Nature. 1984 Apr 5;308(5959):562–562. doi: 10.1038/308562a0. [DOI] [PubMed] [Google Scholar]
- Kozak M. Comparison of initiation of protein synthesis in procaryotes, eucaryotes, and organelles. Microbiol Rev. 1983 Mar;47(1):1–45. doi: 10.1128/mr.47.1.1-45.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lycett G. W., Croy R. R., Shirsat A. H., Boulter D. The complete nucleotide sequence of a legumin gene from pea (Pisum sativum L.). Nucleic Acids Res. 1984 Jun 11;12(11):4493–4506. doi: 10.1093/nar/12.11.4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Mullineaux P. M., Donson J., Morris-Krsinich B. A., Boulton M. I., Davies J. W. The nucleotide sequence of maize streak virus DNA. EMBO J. 1984 Dec 20;3(13):3063–3068. doi: 10.1002/j.1460-2075.1984.tb02258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R. The use of thin acrylamide gels for DNA sequencing. FEBS Lett. 1978 Mar 1;87(1):107–110. doi: 10.1016/0014-5793(78)80145-8. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. I., Blattner F. R., Davies J. The isolation and partial characterization of a new restriction endonuclease from Providencia stuartii. Nucleic Acids Res. 1976 Feb;3(2):343–353. doi: 10.1093/nar/3.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunter G., Coutts R. H., Buck K. W. Negatively supercoiled DNA from plants infected with a single-stranded DNA virus. Biochem Biophys Res Commun. 1984 Feb 14;118(3):747–752. doi: 10.1016/0006-291x(84)91458-x. [DOI] [PubMed] [Google Scholar]
- Tinoco I., Jr, Borer P. N., Dengler B., Levin M. D., Uhlenbeck O. C., Crothers D. M., Bralla J. Improved estimation of secondary structure in ribonucleic acids. Nat New Biol. 1973 Nov 14;246(150):40–41. doi: 10.1038/newbio246040a0. [DOI] [PubMed] [Google Scholar]
- Townsend R., Stanley J., Curson S. J., Short M. N. Major polyadenylated transcripts of cassava latent virus and location of the gene encoding coat protein. EMBO J. 1985 Jan;4(1):33–37. doi: 10.1002/j.1460-2075.1985.tb02313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varmus H. E. Reverse transcriptase rides again. Nature. 1985 Apr 18;314(6012):583–584. doi: 10.1038/314583a0. [DOI] [PubMed] [Google Scholar]
- Zassenhaus H. P., Butow R. A., Hannon Y. P. Rapid electroelution of nucleic acids from agarose and acrylamide gels. Anal Biochem. 1982 Sep 1;125(1):125–130. doi: 10.1016/0003-2697(82)90392-x. [DOI] [PubMed] [Google Scholar]



