Abstract
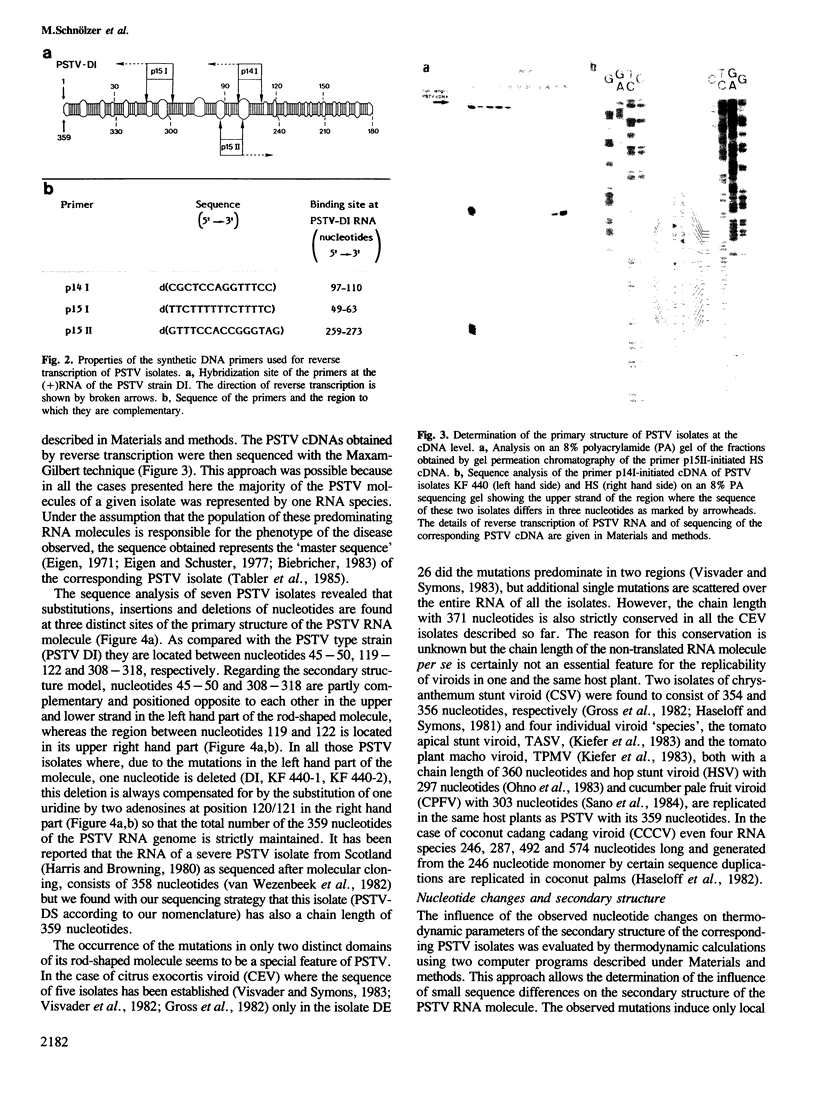
Sequence analysis by primer-extension at the level of their cDNA showed that the RNA genomes of various field isolates of potato spindle tuber viroid (PSTV) of different virulence differ from each other only in a few nucleotides in two distinct regions of the rod-shaped molecule. Despite insertions and deletions the chain length of 359 nucleotides is strictly conserved in all the isolates studied. Thermodynamic calculations revealed that due to the observed sequence differences the region located at the left hand part of the rod-like secondary structure of the PSTV molecule, denoted `virulence modulating (VM) region', becomes increasingly unstable with the increasing virulence of the corresponding isolate. Based on these data we propose in molecular terms a model for the mechanism of viroid pathogenicity. It implies that the nucleotides of the VM region specify and modulate the binding- and hence the competition-potential of the PSTV RNA molecule for a still unknown host factor(s) and thus determine the virulence of PSTV.
Keywords: cDNA sequencing, model for viroid pathogenesis, reverse transcription, thermodynamic analysis, viroid virulence
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Branch A. D., Robertson H. D., Dickson E. Longer-than-unit-length viroid minus strands are present in RNA from infected plants. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6381–6385. doi: 10.1073/pnas.78.10.6381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho Henriquez A., Sänger H. L. Analysis of acid-extractable tomato leaf proteins after infection with a viroid, two viruses and a fungus and partial purification of the "pathogenesis-related" protein p 14. Arch Virol. 1982;74(2-3):181–196. doi: 10.1007/BF01314711. [DOI] [PubMed] [Google Scholar]
- Camacho Henriquez A., Sänger H. L. Gelelectrophoretic analysis of phenol-extractable leaf proteins from different viroid/host combinations. Arch Virol. 1982;74(2-3):167–180. doi: 10.1007/BF01314710. [DOI] [PubMed] [Google Scholar]
- Camacho Henriquez A., Sänger H. L. Purification and partial characterization of the major "pathogenesis-related" tomato leaf protein P14 from potato spindle tuber viroid (PSTV)-infected tomato leaves. Arch Virol. 1984;81(3-4):263–284. doi: 10.1007/BF01309998. [DOI] [PubMed] [Google Scholar]
- Carey J., Cameron V., de Haseth P. L., Uhlenbeck O. C. Sequence-specific interaction of R17 coat protein with its ribonucleic acid binding site. Biochemistry. 1983 May 24;22(11):2601–2610. doi: 10.1021/bi00280a002. [DOI] [PubMed] [Google Scholar]
- Chow F., Kempe T., Palm G. Synthesis of oligodeoxyribonucleotides on silica gel support. Nucleic Acids Res. 1981 Jun 25;9(12):2807–2817. doi: 10.1093/nar/9.12.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conejero V., Semancik J. S. Exocortis viroid: alteration in the proteins of Gynura aurantiaca accompanying viroid infection. Virology. 1977 Mar;77(1):221–232. doi: 10.1016/0042-6822(77)90420-2. [DOI] [PubMed] [Google Scholar]
- Davies J. W., Kaesberg P., Diener T. O. Potato spindle tuber viroid. XII. An investigation of viroid RNA as a messenger for protein synthesis. Virology. 1974 Sep;61(1):281–286. doi: 10.1016/0042-6822(74)90262-1. [DOI] [PubMed] [Google Scholar]
- Dickson E. A model for the involvement of viroids in RNA splicing. Virology. 1981 Nov;115(1):216–221. doi: 10.1016/0042-6822(81)90104-5. [DOI] [PubMed] [Google Scholar]
- Dickson E., Robertson H. D. Potential regulatory roles for RNA in cellular development. Cancer Res. 1976 Sep;36(9 Pt 2):3387–3393. [PubMed] [Google Scholar]
- Diener T. O. Are viroids escaped introns? Proc Natl Acad Sci U S A. 1981 Aug;78(8):5014–5015. doi: 10.1073/pnas.78.8.5014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diener T. O. Portraits of viruses: the viroid. Intervirology. 1984;22(1):1–16. doi: 10.1159/000149528. [DOI] [PubMed] [Google Scholar]
- Diener T. O. Potato spindle tuber "virus". IV. A replicating, low molecular weight RNA. Virology. 1971 Aug;45(2):411–428. doi: 10.1016/0042-6822(71)90342-4. [DOI] [PubMed] [Google Scholar]
- Diener T. O. Viroids. Adv Virus Res. 1983;28:241–283. doi: 10.1016/s0065-3527(08)60725-3. [DOI] [PubMed] [Google Scholar]
- Diener T. O. Viroids: autoinducing regulatory RNAs? Brookhaven Symp Biol. 1977 May 12;(29):50–61. [PubMed] [Google Scholar]
- Domingo E., Dávila M., Ortín J. Nucleotide sequence heterogeneity of the RNA from a natural population of foot-and-mouth-disease virus. Gene. 1980 Nov;11(3-4):333–346. doi: 10.1016/0378-1119(80)90073-6. [DOI] [PubMed] [Google Scholar]
- Domingo E., Sabo D., Taniguchi T., Weissmann C. Nucleotide sequence heterogeneity of an RNA phage population. Cell. 1978 Apr;13(4):735–744. doi: 10.1016/0092-8674(78)90223-4. [DOI] [PubMed] [Google Scholar]
- Donis-Keller H. Site specific enzymatic cleavage of RNA. Nucleic Acids Res. 1979 Sep 11;7(1):179–192. doi: 10.1093/nar/7.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eigen M., Schuster P. The hypercycle. A principle of natural self-organization. Part A: Emergence of the hypercycle. Naturwissenschaften. 1977 Nov;64(11):541–565. doi: 10.1007/BF00450633. [DOI] [PubMed] [Google Scholar]
- Eigen M. Selforganization of matter and the evolution of biological macromolecules. Naturwissenschaften. 1971 Oct;58(10):465–523. doi: 10.1007/BF00623322. [DOI] [PubMed] [Google Scholar]
- Fields S., Winter G. Nucleotide-sequence heterogeneity and sequence rearrangements in influenza virus cDNA. Gene. 1981 Nov;15(2-3):207–214. doi: 10.1016/0378-1119(81)90130-x. [DOI] [PubMed] [Google Scholar]
- Goelet P., Lomonossoff G. P., Butler P. J., Akam M. E., Gait M. J., Karn J. Nucleotide sequence of tobacco mosaic virus RNA. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5818–5822. doi: 10.1073/pnas.79.19.5818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gralla J., Steitz J. A., Crothers D. M. Direct physical evidence for secondary structure in an isolated fragment of R17 bacteriophage mRNA. Nature. 1974 Mar 15;248(445):204–208. doi: 10.1038/248204a0. [DOI] [PubMed] [Google Scholar]
- Gross H. J., Domdey H., Lossow C., Jank P., Raba M., Alberty H., Sänger H. L. Nucleotide sequence and secondary structure of potato spindle tuber viroid. Nature. 1978 May 18;273(5659):203–208. doi: 10.1038/273203a0. [DOI] [PubMed] [Google Scholar]
- Gross H. J., Krupp G., Domdey H., Raba M., Jank P., Lossow C., Alberty H., Ramm K., Sänger H. L. Nucleotide sequence and secondary structure of citrus exocortis and chrysanthemum stunt viroid. Eur J Biochem. 1982 Jan;121(2):249–257. doi: 10.1111/j.1432-1033.1982.tb05779.x. [DOI] [PubMed] [Google Scholar]
- Gross H. J., Liebl U., Alberty H., Krupp G., Domdey H., Ramm K., Sänger H. L. A severe and a mild potato spindle tuber viroid isolate differ in three nucleotide exchanges only. Biosci Rep. 1981 Mar;1(3):235–241. doi: 10.1007/BF01114910. [DOI] [PubMed] [Google Scholar]
- Gross H. J., Riesner D. Viroids: a class of subviral pathogens. Angew Chem Int Ed Engl. 1980;19(4):231–243. doi: 10.1002/anie.198002313. [DOI] [PubMed] [Google Scholar]
- Hall T. C., Wepprich R. K., Davies J. W., Weathers L. G., Semancik J. S. Functional distinctions between the ribonucleic acids from citrus exocortis viroid and plant viruses: cell-free translation and aminoacylation reactions. Virology. 1974 Oct;61(2):486–492. doi: 10.1016/0042-6822(74)90284-0. [DOI] [PubMed] [Google Scholar]
- Haseloff J., Symons R. H. Chrysanthemum stunt viroid: primary sequence and secondary structure. Nucleic Acids Res. 1981 Jun 25;9(12):2741–2752. doi: 10.1093/nar/9.12.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay E., Bambara R., Padmanabhan R., Wu R. DNA sequence analysis: a general, simple and rapid method for sequencing large oligodeoxyribonucleotide fragments by mapping. Nucleic Acids Res. 1974 Mar;1(3):331–353. doi: 10.1093/nar/1.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaper J. M. Rapid synthesis of double-stranded cucumber mosaic virus-associated RNA 5: mechanism controlling viral pathogenesis? Biochem Biophys Res Commun. 1982 Apr 14;105(3):1014–1022. doi: 10.1016/0006-291x(82)91071-3. [DOI] [PubMed] [Google Scholar]
- Kaper J. M., Tousignant M. E. Viral satellites: parasitic nucleic acids capable of modulating disease expression. Endeavour. 1984;8(4):194–200. doi: 10.1016/0160-9327(84)90084-x. [DOI] [PubMed] [Google Scholar]
- Kaper J. M., Waterworth H. E. Cucumber mosaic virus associated RNA 5: causal agent for tomato necrosis. Science. 1977 Apr 22;196(4288):429–431. doi: 10.1126/science.196.4288.429. [DOI] [PubMed] [Google Scholar]
- Kiefer M. C., Owens R. A., Diener T. O. Structural similarities between viroids and transposable genetic elements. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6234–6238. doi: 10.1073/pnas.80.20.6234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krug M., de Haseth P. L., Uhlenbeck O. C. Enzymatic synthesis of a 21-nucleotide coat protein binding fragment of R17 ribonucleic acid. Biochemistry. 1982 Sep 14;21(19):4713–4720. doi: 10.1021/bi00262a030. [DOI] [PubMed] [Google Scholar]
- Lerner M. R., Boyle J. A., Mount S. M., Wolin S. L., Steitz J. A. Are snRNPs involved in splicing? Nature. 1980 Jan 10;283(5743):220–224. doi: 10.1038/283220a0. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Ohno T., Takamatsu N., Meshi T., Okada Y. Hop stunt viroid: molecular cloning and nucleotide sequence of the complete cDNA copy. Nucleic Acids Res. 1983 Sep 24;11(18):6185–6197. doi: 10.1093/nar/11.18.6185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rackwitz H. R., Rohde W., Sänger H. L. DNA-dependent RNA polymerase II of plant origin transcribes viroid RNA into full-length copies. Nature. 1981 May 28;291(5813):297–301. doi: 10.1038/291297a0. [DOI] [PubMed] [Google Scholar]
- Randles J. W., Rillo E. P., Diener T. O. The viroidlike structure and cellular location of anomalous RNA associated with the cadang-cadang disease. Virology. 1976 Oct 1;74(1):128–139. doi: 10.1016/0042-6822(76)90135-5. [DOI] [PubMed] [Google Scholar]
- Riesner D., Colpan M., Goodman T. C., Nagel L., Schumacher J., Steger G., Hofmann H. Dynamics and interactions of viroids. J Biomol Struct Dyn. 1983 Dec;1(3):669–688. doi: 10.1080/07391102.1983.10507474. [DOI] [PubMed] [Google Scholar]
- Riesner D., Henco K., Rokohl U., Klotz G., Kleinschmidt A. K., Domdey H., Jank P., Gross H. J., Sänger H. L. Structure and structure formation of viroids. J Mol Biol. 1979 Sep 5;133(1):85–115. doi: 10.1016/0022-2836(79)90252-3. [DOI] [PubMed] [Google Scholar]
- Robertson H. D., Dickson E. RNA processing and the control of gene expression. Brookhaven Symp Biol. 1975 Jul;(26):240–266. [PubMed] [Google Scholar]
- Rohde W., Schnölzer M., Rackwitz H. R., Haas B., Seliger H., Sänger H. L. Specifically primed synthesis in vitro of full-length DNA complementary to potato-spindle-tuber viroid. Eur J Biochem. 1981 Aug;118(1):151–157. doi: 10.1111/j.1432-1033.1981.tb05498.x. [DOI] [PubMed] [Google Scholar]
- Rohde W., Schnölzer M., Sänger H. L. Sequence-specific priming of the in vitro synthesis of DNA complementary to citrus exocortis viroid. FEBS Lett. 1981 Aug 3;130(2):208–212. doi: 10.1016/0014-5793(81)81121-0. [DOI] [PubMed] [Google Scholar]
- Rohde W., Sänger H. L. Detection of complementary RNA intermediates of viroid replication by Northern blot hybridization. Biosci Rep. 1981 Apr;1(4):327–336. doi: 10.1007/BF01114872. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger H. L., Klotz G., Riesner D., Gross H. J., Kleinschmidt A. K. Viroids are single-stranded covalently closed circular RNA molecules existing as highly base-paired rod-like structures. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3852–3856. doi: 10.1073/pnas.73.11.3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano T., Uyeda I., Shikata E., Ohno T., Okada Y. Nucleotide sequence of cucumber pale fruit viroid: homology to hop stunt viroid. Nucleic Acids Res. 1984 Apr 25;12(8):3427–3434. doi: 10.1093/nar/12.8.3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher J., Randles J. W., Riesner D. A two-dimensional electrophoretic technique for the detection of circular viroids and virusoids. Anal Biochem. 1983 Dec;135(2):288–295. doi: 10.1016/0003-2697(83)90685-1. [DOI] [PubMed] [Google Scholar]
- Schumacher J., Sänger H. L., Riesner D. Subcellular localization of viroids in highly purified nuclei from tomato leaf tissue. EMBO J. 1983;2(9):1549–1555. doi: 10.1002/j.1460-2075.1983.tb01622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semancik J. S., Conejero V., Gerhart J. Citrus exocortis viroid: survey of protein synthesis in Xenopus laevis oocytes following addition of viroid RNA. Virology. 1977 Jul 1;80(1):218–221. doi: 10.1016/0042-6822(77)90395-6. [DOI] [PubMed] [Google Scholar]
- Semancik J. S., Weathers L. G. Exocortis disease: evidence for a new species of "infectious" low molecular weight RNA in plants. Nat New Biol. 1972 Jun 21;237(77):242–244. doi: 10.1038/newbio237242a0. [DOI] [PubMed] [Google Scholar]
- Spiesmacher E., Mühlbach H. P., Schnölzer M., Haas B., Sänger H. L. Oligomeric forms of potato spindle tuber viroid (PSTV) and of its complementary RNA are present in nuclei isolated from viroid-infected potato cells. Biosci Rep. 1983 Aug;3(8):767–774. doi: 10.1007/BF01120988. [DOI] [PubMed] [Google Scholar]
- Spiesmacher E., Mühlbach H. P., Tabler M., Sänger H. L. Synthesis of (+) and (-) RNA molecules of potato spindle tuber viroid (PSTV) in isolated nuclei and its impairment by transcription inhibitors. Biosci Rep. 1985 Mar;5(3):251–265. doi: 10.1007/BF01119595. [DOI] [PubMed] [Google Scholar]
- Steger G., Hofmann H., Förtsch J., Gross H. J., Randles J. W., Sänger H. L., Riesner D. Conformational transitions in viroids and virusoids: comparison of results from energy minimization algorithm and from experimental data. J Biomol Struct Dyn. 1984 Dec;2(3):543–571. doi: 10.1080/07391102.1984.10507591. [DOI] [PubMed] [Google Scholar]
- Symons R. H. Avocado sunblotch viroid: primary sequence and proposed secondary structure. Nucleic Acids Res. 1981 Dec 11;9(23):6527–6537. doi: 10.1093/nar/9.23.6527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabler M., Schnölzer M., Sänger H. L. Molecular cloning of potato spindle tuber viroid (PSTV) cDNA synthesized by enzymatic elongation of PSTV-specific DNA primers: a general strategy for viroid cloning. Biosci Rep. 1985 Feb;5(2):143–158. doi: 10.1007/BF01117061. [DOI] [PubMed] [Google Scholar]
- Tabler M., Sänger H. L. Cloned single- and double-stranded DNA copies of potato spindle tuber viroid (PSTV) RNA and co-inoculated subgenomic DNA fragments are infectious. EMBO J. 1984 Dec 20;3(13):3055–3062. doi: 10.1002/j.1460-2075.1984.tb02257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabler M., Sänger H. L. Infectivity studies on different potato spindle tuber viroid (PSTV) RNAs synthesized in vitro with the SP6 transcription system. EMBO J. 1985 Sep;4(9):2191–2199. doi: 10.1002/j.1460-2075.1985.tb03914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visvader J. E., Gould A. R., Bruening G. E., Symons R. H. Citrus exocortis viroid: nucleotide sequence and secondary structure of an Australian isolate. FEBS Lett. 1982 Jan 25;137(2):288–292. doi: 10.1016/0014-5793(82)80369-4. [DOI] [PubMed] [Google Scholar]
- Wolff P., Gilz R., Schumacher J., Riesner D. Complexes of viroids with histones and other proteins. Nucleic Acids Res. 1985 Jan 25;13(2):355–367. doi: 10.1093/nar/13.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaitlin M., Hariharasubramanian V. A gel electrophoretic analysis of proteins from plants infected with tobacco mosaic and potato spindle tuber viruses. Virology. 1972 Feb;47(2):296–305. doi: 10.1016/0042-6822(72)90265-6. [DOI] [PubMed] [Google Scholar]
- Zuker M., Stiegler P. Optimal computer folding of large RNA sequences using thermodynamics and auxiliary information. Nucleic Acids Res. 1981 Jan 10;9(1):133–148. doi: 10.1093/nar/9.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wezenbeek P., Vos P., van Boom J., van Kammen A. Molecular cloning and characterization of a complete DNA copy of potato spindle tuber viroid RNA. Nucleic Acids Res. 1982 Dec 20;10(24):7947–7957. doi: 10.1093/nar/10.24.7947. [DOI] [PMC free article] [PubMed] [Google Scholar]







