Abstract
Background and Purpose
Individualizing mean arterial blood pressure (MAP) targets to a patient’s cerebral blood flow (CBF) autoregulatory range might prevent brain ischemia for patients undergoing cardiopulmonary bypass (CPB). This study compares the accuracy of real-time CBF autoregulation monitoring using near infra-red spectroscopy (NIRS) with that of transcranial Doppler (TCD).
Methods
Sixty adult patients undergoing CPB had TCD monitoring of middle cerebral artery blood flow velocity (MCA-FV) and NIRS monitoring. The mean velocity index (Mx) was calculated as a moving, linear correlation coefficient between slow waves of MCA-FV and MAP. The cerebral oximetry index (COx) was calculated as a similar coefficient between slow waves of cerebral oximetry and MAP. When CBF is autoregulated, Mx and COx vary around zero. Loss of autoregulation results in progressively more positive Mx and COx.
Results
Mx and COx showed significant correlation (r=0.55, P<0.0001) and good agreement (bias, 0.08 ± 0.18, 95% limits of agreement: −0.27 to 0.43) during CPB. Autoregulation was disturbed in this cohort during CPB (average Mx 0.38,95% CI 0.34 to 0.43). The lower CBF autoregulatory threshold (defined as incremental increase in Mx > 0.45) during CPB ranged from 45 to 80 mmHg.
Conclusions
CBF autoregulation can be monitored continuously with NIRS in adult patients undergoing CPB. Real-time autoregulation monitoring may have a role in preventing injurious hypotension during CPB.
Keywords: Surgery, cardiopulmonary bypass, cerebrovascular circulation
During cardiopulmonary bypass (CPB) mean arterial blood pressure (MAP) is empirically managed often to targets of 50 to 60 mmHg and transiently even lower. These low MAPs are not believed to result in cerebral injury because they are within the putative range of normal cerebral blood flow (CBF) autoregulation.1 This widespread view, however, fails to acknowledge that a growing proportion of surgical patients have cerebral vascular disease that may alter the limits of normal CBF autoregulation, predisposing them to cerebral ischemic injury.2, 3 Individualizing MAP targets during CPB to be within a patient’s autoregulatory range might prevent cerebral hypoperfusion from low MAP and cerebral edema from high MAP.
Clinically, the linear regression correlation coefficient between cerebral perfusion pressure and transcranial Doppler (TCD)-measured CBF velocity has been calculated to assess autoregulation in patients with neurological conditions.4–7 This method provides a means to monitor CBF autoregulation continuously at the bedside and has been recommended as an option to define the optimal cerebral perfusion pressure targets for patients with traumatic brain injury.5, 8 The routine use of TCD, however, has limitations including the need for frequent transducer repositioning and the inability to obtain a transcranial “window” in some patients.
Near-infrared spectroscopy (NIRS) is increasingly used during cardiac surgery to monitor cerebral O2 supply and demand balance. As changes in the main determinants of NIRS (e.g., arterial oxygenation, cerebral metabolic O2 demand) are relatively stable over short periods of time, NIRS provides a surrogate of fluctuations in CBF.9–12 Our group has reported that the lower CBF autoregulatory limits can be detected reliably by measuring a moving linear correlation coefficient between cerebral perfusion pressure and cerebral oximeter waveforms in a piglet model.11 Further, good correlation was recently reported between NIRS and TCD assessments of CBF autoregulation in 23 patients with sepsis.12 NIRS is non-invasive, continuous, and requires minimal caregiver manipulation compared to TCD, and, thus, could be applied in a broad range of clinical settings to monitor CBF autoregulation. The purpose of this proof of concept study was to compare the accuracy of a NIRS-derived index of CBF autoregulation with that of a validated TCD-based method in adult patients undergoing CPB for cardiac surgery. We hypothesized that during CPB, the middle cerebral artery CBF velocity would be coherent with cerebral oximetry waveforms at frequencies relevant to autoregulation.
Materials and Methods
All procedures were approved by The Johns Hopkins Medical Institutions Investigational Review Board and were performed after receiving written informed consent. Subjects aged ≥45 years undergoing coronary artery bypass graft surgery and/or valvular surgery requiring CPB were eligible for enrollment in this prospective pilot study. Sixty patients were enrolled in the study, but 5 patients were subsequently excluded because of inability to obtain TCD monitoring.
Perioperative Care
Patients received standard perioperative monitoring including direct radial artery blood pressure monitoring. Nasal temperature was recorded every 5 min. Anesthetic drugs included midazolam, fentanyl, and isoflurane. End-tidal isoflurane concentrations before and after CPB were between 0.2% and 1.2%. During CPB isoflurane concentrations were kept between 0.5% and 1.0% on a vaporizer connected to the oxygenator inflow. CPB was performed using non-pulsatile flow between 2.0 and 2.4 L/min/m2 and α-stat pH management. The CPB circuit included a membrane oxygenator and a 40μm arterial line filter. MAP targets during surgery were based on usual clinical practice. Arterial blood gases and hemoglobin level were measured after tracheal intubation, 10 min after initiation of CPB, and then hourly. Mechanical ventilation and CPB gas flow were altered to maintain normocarbia based on arterial PaCO2 results or continuous in-line arterial blood gas monitoring during CPB. There was no protocol for transfusion of packed red blood cells. The rate of re-warming was not standardized. Institutional policy is to maintain the temperature of the perfusate blood at ≤ 37°C.
CBF Autoregulation Monitoring
Patients were attached to either an INVOS (Somenetics, Inc., Troy, MI) or Foresight (CAS Medical Systems, Branford, CT) NIRS monitor, depending on availability. Electrodes for monitoring NIRS were placed on the right and left forehead using the respective manufacturer’s recommendations and after first cleaning the skin with an alcohol swab. Transcranial Doppler monitoring (Doppler Box, DWL, Compumedics, USA, Charlotte, NC) of the middle cerebral arteries was with two 2.5-MHz transducers fitted on a headband. The depth of insonation varied between 35 and 52 mm until representative spectral artery flow was identified.
Analog arterial pressure data from the operating room hemodynamic monitor, TCD, and NIRS signals were sampled with an analog-to-digital converter at 60 Hz and then processed with ICM+ software version 6.1 (University of Cambridge, Cambridge, UK). These signals were time-integrated as non-overlapping 10-second mean values, which is equivalent to applying a moving average filter with a 10-second time window and re-sampling at 0.1 Hz. This operation was used to eliminate high-frequency noise from the respiratory and pulse frequencies, while allowing detection of oscillations and transients that occur below 0.05 Hz. Doppler, oximetry, and arterial blood pressure waveforms were further high pass filtered with a DC cutoff set at 0.003 Hz. This step removed slow drifts associated with hemodilution at the onset of bypass, blood transfusions, cooling, and rewarming. A continuous, moving Pearson’s correlation coefficient was calculated between the MAP and TCD blood flow velocities and between MAP and NIRS data, rendering the variables Mx (mean velocity index) and COx (cerebral oximetry index), respectively. Of note, MAP is used in this calculation and not cerebral perfusion pressure since intracranial pressure data are not available and since central venous pressure is often negative as a result of suction assisted venous drainage to the CPB reservoir. Consecutive, paired, 10-second averaged values from 300 seconds duration were used for each calculation, incorporating 30 data points for each index. Intact CBF autoregulation is indicated by an Mx value of approximately zero (CBF and MAP are not correlated), and CBF dysautoregulation is indicated by an Mx value approaching +1 (CBF and MAP correlated). Similar findings occur experimentally with COx.13
Data Analysis
Blood pressure, TCD, and oximetry waveforms were all inspected for artifact, which was manually removed before analysis of the data. Causes of artifact included accessing the arterial line for blood samples, electrocautery interfering with TCD signal acquisition, movement of the TCD probes, which commonly occurred during sternal opening, and internal mammary artery dissection.
NIRS-TCD Coherence Analysis
Waveforms of TCD (sampled at 60 Hz) and cerebral oximetry obtained during CPB were analyzed for coherence by the Welsh method; TCD was used as the input and cerebral oximetry as the output. Periods of uninterrupted waveforms of TCD with a standard physiologic appearance were analyzed within a spectral range from 0.4 to 4 beats per minute by using a moving 12-minute window composed of 8 segments that had 50% overlap (see Figure 1). As each patient has a different fundamental frequency of slow-wave activity, the maximum coherence (after interpolation with zero padding) within the spectral band of slow waves was averaged across the moving time window to give the coherence result for each patient during CPB.
Figure 1.

Coherence analysis between middle cerebral artery flow velocity (MCA-FV) and cerebral oximetry. The top panel shows low-pass filtered MCA-FV across a 12-minute window. The middle panel shows cerebral oximetry across the same time window. The bottom panel shows coherence between MCA-FV and cerebral oximetry as a function of frequency from the 12-minute window shown. The maximum coherence at the frequency of slow waves (0.4 to 4 beats per minute) was averaged across the entire monitoring period to give the results reported.
Comparing Mx and COx
Right and left TCD recordings were combined for analysis. If only unilateral recordings were available, only NIRS data from the corresponding brain hemisphere were included in the analysis. Time-averaged values for Mx and COx obtained during CPB were evaluated with linear regression and Pearson correlation. Bland-Altman bias analysis was used to compare the differences in Mx and COx versus the average of these values.14 Analysis was performed with GraphPad Prism software (GraphPad Software, Inc, La Jolla,CA) or Stata software (version 9.0, Stata Corp, College Station, TX).
Quantifying the effect of hypotension
To show the effect of MAP on autoregulation, all values of COx and Mx were categorized and averaged in bins of MAP spanning 5 mmHg for each patient. These individual histograms were then combined in a single histogram showing the autoregulatory indices as a function of MAP. Average values of COx and Mx were compared separately across the categorized MAP via a multiple linear regression model with generalized estimating equations and robust variance estimation (Stata version 10). This approach was used to account for within-individual correlations.15 Determining an absolute Mx cut-off indicating the lower autoregulatory threshold is difficult, but it is likely between 0.3 and 0.5.11, 16 Each individuals Mx data for each 5 mmHg MAP was further evaluated to assess for the lower CBF autoregulatory threshold defined as the MAP (in 5 mmHg increments) where Mx incrementally increased to > 0.45. The average COx value at the lower CBF autoregulatory was determined and the number of individuals with an increase in COx ≥ this value determined.
Results
Transcranial Doppler recordings with adequate stability (i.e., > 30 min) were available for analysis from 53 during CPB while NIRS data were available from all patients. Demographic and medical data for the enrolled patients are listed in Table 1. Twenty-nine patients were monitored with INVOS and 31 patients with Foresight NIRS. Unilateral TCD monitoring was available from 7 patients; the remainder had bilateral monitoring. Monitoring was performed for 100.5±25.9 minutes during CPB. MAP was 72.2±11.1 mmHg during CPB. Physiologic data obtained during CPB are listed in Table 2. The temperature nadir and peak (mean±SD, [95% confidence interval]) during CPB was 31.1±3.2°C (30.3°C to 32.0°C) and 37.1±0.8 °C (36.9°C to 37.3°C), respectively.
Table 1.
Patient demographic and medical data. Data listed as number of patients with percentage in parenthesis.
| Parameter | Number of Patients N=60 |
|---|---|
| Age (mean±SD years) | 64±13 |
| Male/female | 49/11 |
| Prior stroke | 10 (17%) |
| Prior transient ischemic attack | 7 (12%) |
| Hypertension | 43 (72%) |
| Diabetes (insulin and non-insulin dependent) | 11(18%) |
| Prior myocardial infarction | 15 (25%) |
| Congestive heart failure | 10 (17%) |
| Peripheral vascular disease | 8 (13%) |
| Chronic obstructive lung disease | 7 (12%) |
| Current smoker | 7 (12%) |
| Prior cardiac surgery | 2 (3%) |
| Preoperative echocardiogram function | |
| Left ventricular ejection fraction ≥50% | 41 (69%) |
| Left ventricular ejection fraction <50% | 14 (23%) |
| Left ventricular ejection Fraction <30% | 5 (8%) |
| Preoperative Medication | |
| Statins | 45 (75%) |
| Aspirin | 39 (65%) |
| Beta-blockers | 33 (55%) |
| ACE inhibitors | 15 (25%) |
| Ca++ channel blockers | 9 (15%) |
| Surgical procedure | |
| CABG only | 36 (60%) |
| CABG with aortic valve replacement | 13 (22%) |
| CABG with mitral valve replacement/repair | 6 (10%) |
| Aortic valve replacement | 3 (5%) |
| Mitral valve replacement | 2 (3%) |
CABG, coronary artery bypass graft; CPB; cardiopulmonary bypass
Table 2.
Physiologic data during cardiopulmonary bypass.
| Variable | Result |
|---|---|
|
| |
| pH | 7.40±0.03 |
|
| |
| PaCO2 (mmHg) | 39.1± 2.9 |
|
| |
| PaO2 (mmHg) | 253.8±30.7 |
|
| |
| Hemoglobin (gm/dL) | 9.5±1.4 |
|
| |
| MCA CBF velocity (cm/s) | |
| Left | 37.7±9.1 |
| Right | 36.5±9.9 |
|
| |
| Left Frontal NIRS O2 Saturation | |
| SctO2 % (n=31) | 64.2±6.9 |
| rSO2 % (n=29) | 50.9±10.6 |
|
| |
| Right Frontal NIRS O2 Saturation | |
| SctO2 % (n=31) | 62.8±6.9 |
| rSO2 % (n=29) | 48.1±15.8 |
Values are listed as mean±SD. MCA CBF= middle cerebral artery cerebral blood flow; NIRS=near infra-red spectroscopy. SctO2 = regional cerebral tissue oxygen saturation measured with Foresight™ (CAS Medical Systems, Branford, CT); rSO2 = regional cerebral oxygen saturation measured with Invos™ (Somenetics, Inc., Troy, MI)
A representative example of data used for coherence analysis is shown in Figure 1. Coherence between middle cerebral artery blood flow velocity and cerebral oximetry values averaged 0.72 ± 0.11 during CPB. Linear regression and Bland-Altman analysis results are shown in Figures 2A and 2B. There was a correlation (r=0.55, P<0.0001) between COx and Mx for the time-averaged data. There was also good agreement with limited bias between Mx and COx (95% limits of agreement: −0.27 to 0.43, bias 0.08 ± 0.18). The correlation between Mx and COx during CPB for the Foresight device was r=0.49, p<0.0001 and the bias −0.034 (95% limits of agreement −0.404 to 0.34). The correlation between Mx and COx during CPB for the Somentics device was r=0.57, p< 0.0001 and the bias −0.05 (95% limits of agreement −0.34 to 0.25).
Figure 2.

Average Mx and COx values obtained during CPB were compared by linear correlation (A) and the Bland-Altman method (B). The dashed lines represent the 95% confidence band of the regression line and the 95% limits of agreement (−0.27 to 0.43) for the bias analysis.
Values for Mx and COx at different MAP values are shown in Figures 3A and 3B. Both, Mx and COx increased with decreasing MAP, indicating CBF autoregulation dysfunction (P<0.0001). MAP changes recorded were those that occurred during the course of surgery and were not intentionally induced for the purpose of the study. Not all individuals experienced a MAP < 50 mmHg; 3 patients had a MAP as low as 30 mmHg. Further, the time spent at each MAP was not evenly distributed. Low MAPs were typically transient, particularly during initiation of CPB or during re-warming from hypothermia.
Figure 3.

Distribution of COx and Mx over mean arterial blood pressure (MAP) during CPB for the study cohort. A, COx values (mean + SD) are binned to the corresponding MAP at the time of the measurement. B, Simultaneous reading of Mx (mean + SD) are also binned to the corresponding MAP. Note that a wide range of MAP is covered during CPB, with a significant increase (impaired autoregulation) in both the COx and Mx at lower MAP.
Autoregulation as measured by Mx was disturbed in multiple subjects during CPB. In this cohort, the average Mx observed during CPB was 0.38 (95% CI 0.34 to 0.43). Although the absolute threshold of Mx indicating disturbed autoregulation is not known, an average Mx greater than 0.3 was also found in a cohort of patients with sepsis and delirium. By comparison, septic patients not suffering from delirium, had an average Mx less than 0.2.12
Forty-six patients demonstrated an incremental increase in Mx >0.45 with declining MAP during CPB (Figure 4). The average COx at the MAP where Mx increased > 0.45 was 0.38±0.27. A representative CBF autoregulation monitoring study for a patient during CPB is shown in Figure 5. Forty-two (91%) patients had an increase in COx > 0.38 within 10 mmHg of the MAP where Mx increased to > 0.45. All but 1 patient had an increase in COx to > 0.30 at the MAP where Mx increased > 0.45.
Figure 4.
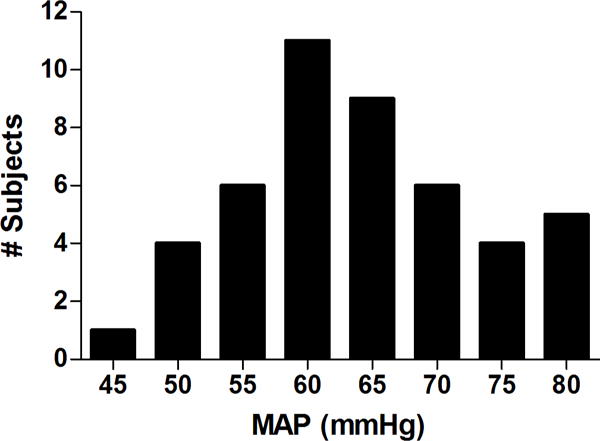
Histogram showing the number of subjects versus the mean arterial pressure during cardiopulmonary bypass where the mean velocity index (Mx) increased to > 0.45 indicating a putative lower cerebral blood flow autoregulatory limit.
Figure 5.

Representative graph of cerebral blood flow autoregulation monitoring during cardiopulmonary bypass in a single patient. A) The top panel shows mean arterial pressure (MAP), the middle panel shows the middle cerebral artery flow velocity (MCA FV), and the lower panel shows the near infrared spectroscopy (NIRS) measurements of frontal lobe oximetry, all synchronously measured on the same time scale. B) Values of mean velocity index (Mx) and C), values of cerebral oximetry index (COx) obtained from the time window in A are sorted according to MAP and show a similar putative lower limit of autoregulation at 75 mmHg, where Mx and COx values increase precipitously. A horizontal line at Mx and COx values of 0.4 is shown for reference.
Discussion
Our results suggest that CBF autoregulation can be monitored reliably and continuously by NIRS in patients undergoing CPB. We document high Mx in patients undergoing CPB consistent with a state of impaired CBF autoregulation. These data suggest that for many individuals, CBF is pressure passive during CPB, placing patients at risk of delirium and neurologic injury. Our data further suggest a wide range of MAP at the lower CBF autoregulatory limit (45 to 80 mmHg).
Assessment of CBF autoregulation is increasingly used in neurosurgical ICU patients for optimizing MAP.8, 17 Impaired autoregulation was associated with poor outcome after traumatic brain injury whereas outcomes were better when MAP was optimized within the autoregulatory range.5, 18 Application of this technology to the CPB arena may be similarly informative. We evaluated the MAP where Mx increased to > 0.45 as an indicator of the lower CBF autoregulatory threshold. The exact Mx where CBF becomes pressure passive is not known and may occur at Mx values lower or higher than 0.45.11, 16 It is likely that the patient specific autoregulatory curves for Mx and COx (Figure 5), provide more useful information regarding the MAP where CBF autoregulation is optimal. Regardless, our data showing a wide range of MAP at the lower CBF autoregulation range suggests that for many patients CBF may be pressure passive during portions of CPB using current MAP targets.
There is precedence for using NIRS to monitor CBF autoregulation. Tsuji et al10 found high coherence between slow waves in NIRS and blood pressure in premature neonates, and this was associated with a high incidence of neurologic injury. The frequency-domain technique used in that study is analogous to the time-domain method reported here (COx). Using cerebral oximetry, Steiner et al12 recently reported that slow waves with periodicity of 20 seconds to 2 minutes were observed with NIRS that had high coherence with similar slow waves of TCD in patients with sepsis. The present study confirms this result and extends it to the clinical scenario of CPB. Our finding of high coherence between slow waves of CBF velocity and NIRS supports the assumption that slow changes in cerebral oximetry at this frequency are the result of changes in CBF. These slow fluctuations in CBF velocity and NIRS signals, which represent autoregulatory compensations for slow hemodynamic oscillations, have been previously observed.9, 12, 18
Monitoring COx in a vascular territory distinct from the middle cerebral artery where Mx was calculated might explain the lower correlation between these methods in our study compared with the results of Steiner et al12 (r=0.81) and our experimental findings (r=0.67)11. Error might be introduced conceivably in monitoring NIRS in the frontal lobe in an individual with significant stenosis of the middle cerebral artery. Maintaining MAP above the lower CBF autoregulatory threshold, even if estimated from vascular territories with functional autoregulation, would likely ensure an adequate cerebral perfusion to pressure-dependent brain regions.19 The responsiveness of Mx and COx to declining MAP may vary during CPB compared with that observed in ICU patients. Regardless, limitations associated with comparing two measurements with simple correlation are well described.14 We, therefore, performed bias analysis finding the mean difference on average between Mx and COx was 0.08 and the 95% confidence intervals for the limits of agreement between −0.27 to 0.43. The correlation and agreement was significant for both NIRS devices used in our study. The small number of comparisons and the fact that both devices (INVOS and Foresight) were not used simultaneously in the same patient precludes drawing conclusions regarding the accuracy of one device versus the other.
Prior studies of CBF-pressure autoregulation in patients undergoing CPB were derived by intermittently measuring CBF (e.g., 133Xe methodology).1, 20,21 These studies revealed a slightly positive slope to the CBF autoregulation plateau. This finding might explain, in part, the positive Mx and COx we observed during CPB. In combination, these data show that in most patients, CBF is pressure passive during CPB. Thus, simply raising MAP targets during CPB to reduce the risk of cerebral hypoperfusion might inadvertently increase the risk for cerebral injury in some patients by increasing cerebral embolic load.22 Further, increased CBF that results from higher MAP could lead to cerebral edema if a patient is experiencing enhanced inflammation from CPB or possibly intra-cerebral hemorrhage.23 The cause of increased pressure passivity of CBF during CPB is not known but it might result simply from MAP below an individual’s autoregulatory threshold or possibly endothelial dysfunction resulting from hypothermic CPB.12, 24
Acknowledgments
Funded in part by Grant-In-Aid Number 103363 from the Mid-Atlantic Affiliate of the American Heart Association and Grant R01HL092259 from the National Institutes of Health (to Dr. Hogue); Mentored Research Training Grant from the Foundation for Anesthesia Education and Research (FAER) (to Dr. Brady); Swiss National Science Foundation number PBBSP3-125550 (to Dr. Zweifel)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
ICM+ software is licensed by the University of Cambridge, Cambridge Enterprise Ltd. Drs. Smielewski and Czosnyka have a financial interest in a part of licensing fee.
Clinical Trials Registration at www.clinicaltrials.gov (NCT00769691)
Under a licensing agreement with Somanetics, Dr. Brady is entitled to a share of fees and royalty received by The Johns Hopkins University on the monitoring technology described in this manuscript. The terms of this arrangement are being managed by the Johns Hopkins University in accordance with its conflict of interest policies.
References
- 1.Schell R, Kern F, Greeley W, Schulman S, Frasco P, Croughwell N, Newman M, Reves J. Cerebral blood flow and metabolism during cardiopulmonary bypass. Anesth Analg. 1993;76:849–865. doi: 10.1213/00000539-199304000-00029. [DOI] [PubMed] [Google Scholar]
- 2.Moraca R, Lin E, Holmes J, IV, Fordyce D, Campbell W, Ditkoff M, Hill M, Gutyon S, Paull D, Hall R. Impaired baseline regional cerebral perfusion in patients referred for coronary artery bypass. J Thorac Cardiovasc Surg. 2006;131:540–546. doi: 10.1016/j.jtcvs.2005.10.046. [DOI] [PubMed] [Google Scholar]
- 3.Gottesman R, Sherman P, Grega M, Yousem D, Borowicz LJ, Selnes O, Baumgartner W, McKhann G. Watershed strokes after cardiac surgery: Diagnosis, etiology, and outcome. Stroke. 2006;37:2306–2311. doi: 10.1161/01.STR.0000236024.68020.3a. [DOI] [PubMed] [Google Scholar]
- 4.Steiner L, Coles J, Johnston A, Chatfield D, Smielewski P, Fryer T, Aigbirhio F, Clark J, Pickard J, Menon D, Czosnyka M. Assessment of cerebrovascular autoregulation in head-injured patients: A validation study. Stroke. 2003;34:2404–2409. doi: 10.1161/01.STR.0000089014.59668.04. [DOI] [PubMed] [Google Scholar]
- 5.Steiner L, Czosnyka M, Piechnik S, Smielewski P, Chatfield D, Menon D, Pickard J. Continuous monitoring of cerebrovascular pressure reactivity allows determination of optimal cerebral perfusion pressure in patients with traumatic brain injury. Crit Care Med. 2002;30:733–738. doi: 10.1097/00003246-200204000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Minhas PS, Smielewski P, Kirkpatrick PJ, Pickard JD, Czosnyka M. Pressure autoregulation and positron emission tomography-derived cerebral blood flow acetazolamide reactivity in patients with carotid artery stenosis. Neurosurg. 2004;55:63–68. doi: 10.1227/01.neu.0000126876.10254.05. [DOI] [PubMed] [Google Scholar]
- 7.Reinhard M, Roth M, Guschlbauer B, Harloff A, Timmer J, Czosnyka M, Hetzel A. Dynamic cerebral autoregulation in acute ischemic stroke assessed from spontaneous blood pressure fluctuations. Stroke. 2005;36:1684–1689. doi: 10.1161/01.STR.0000173183.36331.ee. [DOI] [PubMed] [Google Scholar]
- 8.Bratton S, Chestnut R, Ghajar J, McConnell F, Harris O, Hartl R, Manley G, Nemecek A, Newell D, Rosenthal G, Schouten J, Shutter L, Timmons S, Ullman J, Videtta W, Wilberger J, Wright D. Guidelines for the management of severe traumatic brain injury. Ix. Cerebral perfusion thresholds. J Neurotrauma. 2007;24(Suppl 1):S59–S64. doi: 10.1089/neu.2007.9987. [DOI] [PubMed] [Google Scholar]
- 9.Smielewski P, Kirkpatrick P, Minhas P, Pickard JD, Czosnyka M. Can cerebrovascular reactivity be measured with near-infrared spectroscopy? Stroke. 1995;26:2285–2292. doi: 10.1161/01.str.26.12.2285. [DOI] [PubMed] [Google Scholar]
- 10.Tsuji M, Saul J, du Plessis A, Eichenwald E, Sobh J, Crocker R, Volpe J. Cerebral intravascular oxygenation correlates with mean arterial pressure in critically ill premature infants. Pediatrics. 2000;106:625–632. doi: 10.1542/peds.106.4.625. [DOI] [PubMed] [Google Scholar]
- 11.Brady K, Lee J, Kibler K, Smielewski P, Czosnyka M, Easley R, Koehler R, Shaffner D. Continuous time-domain analysis of cerebrovascular autoregulation using near-infrared spectroscopy. Stroke. 2007;38:2818–2825. doi: 10.1161/STROKEAHA.107.485706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steiner L, Pfister D, Strebel S, Radolovich D, Smielewski P, Czonsnyka M. Near-infared spectroscopy can monitor dynamic cerebral autoregulation in adults. Neurocrit Care. 2008 Sep; doi: 10.1007/s12028-008-9140-5. Epub. [DOI] [PubMed] [Google Scholar]
- 13.Brady K, Lee JK, Kibler KK, Smielewski P, Czosnyka M, Easley B, Koehler RC, DH S. Continuous time-domain analysis of cerebrovascular autoregulation using near-infrared spectroscopy. Stroke. 2007;38:2818–2825. doi: 10.1161/STROKEAHA.107.485706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bland J, Altman D. Statistical methods fo rassessing agreement between two methods of clinical measurement. Lancet. 1986;8(1(8476)):307–310. [PubMed] [Google Scholar]
- 15.Zeger S, Liang K. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–130. [PubMed] [Google Scholar]
- 16.Czonsnyka M, Brady K, Reinhard M, Smielewski P, Steiner L. Monitoring of cerebrovascular autoregulation: Facts, myths, and missing links. Neurocrit Care. 2009;10:373–386. doi: 10.1007/s12028-008-9175-7. [DOI] [PubMed] [Google Scholar]
- 17.Consonni F, Abate M, Galli D, Citerio G. Feasibility of a continuous computerized monitoring of cerebral autoregulation in neurointensive care. Neurocrit Care. 2008 doi: 10.1007/s12028-008-9151-2. ePublication. [DOI] [PubMed] [Google Scholar]
- 18.Czosnyka M, Smielewski P, Kirkpatrick P, Menon D. Monitoring of cerebral autoregulation in head-injured patients. Stroke. 1996;27:1829–1834. doi: 10.1161/01.str.27.10.1829. [DOI] [PubMed] [Google Scholar]
- 19.Vorstrup S, Brun B, Lassen N. Evaluation of the cerebral vasodilatory capacity by the acetazolamide test before ec-ic bypass surgery in patients with occlusion of the internal carotid artery. Stroke. 1986;17:1291–1298. doi: 10.1161/01.str.17.6.1291. [DOI] [PubMed] [Google Scholar]
- 20.Govier A, Reves J, McKay R, Karp R, Zorn G, Morawetz R, Smith L, Adams M, Freeman A. Factors and their influence on regional cerebral blood flow during nonpulsatile cardiopulmonary bypass. Ann Thorac Surg. 1984;38:592–600. doi: 10.1016/s0003-4975(10)62316-8. [DOI] [PubMed] [Google Scholar]
- 21.Newman M, Croughwell N, Blumenthal J, White W, Lewis J, Smith L, Frasco P, Towner E, Schell R, Hurwitz B, Reves J. Effect of aging on cerebral autoregulation during cardiopulmonary bypass. Association with postoperative cognitive dysfunction. Circulation. 1994;90(part 2):II-243–I-249. [PubMed] [Google Scholar]
- 22.Hogue C, Jr, Palin C, Arrowsmith J. Cardiopulmonary bypass management and neurologic outcomes: An evidence-based appraisal of current practices. Anesth Analg. 2006;103:21–37. doi: 10.1213/01.ANE.0000220035.82989.79. [DOI] [PubMed] [Google Scholar]
- 23.Laffey J, Boylan J, Cheng D. The systemic inflammatory response to cardiac surgery. Anesthesiology. 2002;97:215–252. doi: 10.1097/00000542-200207000-00030. [DOI] [PubMed] [Google Scholar]
- 24.Wagerle L, Russo P, Dahdah N, Kapadia N, Davis D. Endothelial dysfunction in cerebral microcirulation during hypothermic cardiopulmonary bypass in newborn lambs. J Thorac Cardiovasc Surg. 1998;115:1047–1054. doi: 10.1016/S0022-5223(98)70404-0. [DOI] [PubMed] [Google Scholar]


