Abstract
Glutamate is the predominant excitatory neurotransmitter in the mammalian CNS. It mediates essentially all rapid excitatory signaling. Dysfunction of glutamatergic signaling contributes to developmental, neurologic, and psychiatric disease. Extracellular glutamate is cleared by a family of five Na+-dependent glutamate transporters. Two of these transporters (GLAST and GLT-1) are relatively selectively expressed in astrocytes. Other of these transporters (EAAC1) is expressed by neurons throughout the nervous system. Expression of the last two members of this family (EAAT4 and EAAT5) is almost exclusively restricted to specific populations of neurons in cerebellum and retina, respectively. In this review, we will discuss our current understanding of the mechanisms that control transcriptional regulation of the different members of this family. Over the last two decades our understanding of the mechanisms that regulate expression of GLT-1 and GLAST has advanced considerably; several specific transcription factors, cis-elements, and epigenetic mechanisms have been identified. For the other members of the family, little or nothing is known about the mechanisms that control their transcription. It is assumed that by defining the mechanisms involved, we will advance our understanding of the events that result in cell specific expression of these transporters and perhaps begin to define the mechanisms by which neurologic diseases are changing the biology of the cells that express these transporters. This approach might provide a pathway for developing new therapies for a wide-range of essentially untreatable and devastating diseases that kill neurons by an excitotoxic mechanism.
Keywords: Glu uptake, astrocytes, EAAT, GLT-1, GLAST, EAAC1, transcriptional regulation
1. Introduction
L-Glutamate (Glu) is the major excitatory neurotransmitter in the mammalian CNS and activates both ligand-gated ion channels and G-protein coupled receptors (Fagg, Mena & Cotman, 1983, Nakanishi, 1992, Nakanishi, 1994, Robinson & Coyle, 1987). Even before the receptors were cloned (Hollman & Heinemann, 1994), there was strong evidence that excessive activation of Glu receptors contributes to neuronal loss in a variety of neurological insults. This was based on the following observations: 1) Exogenous (and non-transported) receptor agonists are toxic to neurons in vitro and in vivo, and the potencies closely correlate with that observed for receptor activation (Schwarcz & Coyle, 1977, Schwarcz, Scholz & Coyle, 1978). 2) The patterns of damage caused by these agonists roughly paralleled those observed in humans with various disorders (Beal, Kowall, Ellison, Mazurek, Swartz & Martin, 1986, Schwarcz, Bennett & Coyle, 1977, Schwarcz, Whetsell & Mangano, 1983, Spencer, Nunn, Hugon, Ludolph, Ross, Roy et al, 1987). 3) Acute insults including stroke-like insults or traumatic brain injury were associated with increases in extracellular concentrations of Glu (Benveniste, Drejer, Schousboe & Diemer, 1984, Faden, Demediuk, Panter & Vink, 1989, Rossi, Oshima & Attwell, 2000, Rothman, 1984). 4) And finally, Glu receptor antagonists attenuate the damage caused by some of the acute insults (Gill, Foster & Woodruff, 1987). In the 1990’s, it became clear that Glu-mediated excitotoxicity involved an apoptotic-necrotic continuum (Cheung, Pascoe, Giardina, John & Beart, 1998). This process of excitotoxicity has been implicated in virtually every neurologic disorder (for reviews, see Choi, 1992, Coyle & Puttfarcken, 1993, Faden, Demediuk, Panter & Vink, 1989, Fontana, 2015, Greene & Greenamyre, 1996, McDonald & Johnston, 1990). In spite of this strong set of complementary observations that were reproduced in several different laboratories, drug companies have not been successful in targeting Glu receptors in spite of the billions of dollars that were spent (for discussions, see Nicoletti, Bruno, Ngomba, Gradini & Battaglia, 2015, Wieronska, Zorn, Doller & Pilc, 2015). It appears that the side-effects caused by blocking the N-methyl-D-aspartate subtype of Glu receptor (psychotic symptoms, cell death) and possibly the fact that Glu receptor activation is required for essentially all human actions may have limited the utility of this strategy (Olney, 1994, Olney, Labruyere, Wang, Wozniak, Price & Sesma, 1991).
If, in fact, excessive activation of Glu receptors contributes to neurodegeneration observed after acute insults and/or in chronic neurodegenerative diseases, then it becomes important to understand the mechanisms that control extracellular concentrations of potentially toxic amino acids, including Glu and aspartate. To date, there is still no evidence of extracellular metabolism of either amino acid (for reviews, see Danbolt, 1994, Schousboe, 1981). Instead extracellular Glu concentrations are maintained below those required to chronically activate Glu receptors (Herman & Jahr, 2007) by a family of Na+-dependent Glu transporters. This transport process was first identified and characterized in the early 1970s (Balcar & Johnston, 1972, Beart, 1976, Logan & Snyder, 1971). Then in the early 1990s, a family of five transporters that mediate sodium-dependent Glu uptake was cloned. The first three Glu transporters that were identified and cloned were named glutamate/aspartate transporter (GLAST), glutamate transporter 1 (GLT-1), and excitatory amino acid carrier 1 (EAAC1) (Kanai & Hediger, 1992, Pines, Danbolt, Bjørås, Zhang, Bendahan, Eide et al, 1992, Storck, Schulte, Hofmann & Stoffel, 1992). Shortly thereafter, the human homologues of these transporters were cloned and called excitatory amino acid transporters (EAAT1-3 respectively) (Arriza, Fairman, Wadiche, Murdoch, Kavanaugh & Amara, 1994). Two additional members of the family were also cloned; there were called EAAT4 and EAAT5 (Arriza, Eliasof, Kavanaugh & Amara, 1997, Fairman, Vandenberg, Arriza, Kavanaugh & Amara, 1995). The names of the genes that code for these transporters is different; they are called SLC1A3, 2, 1, 6, & 7, respectively with capital letters for the human homologs and lower case letters for the rodent homologs. These transporters co-transport 3 molecules of Na+ and 1 H+ with one molecule of Glu; the countertransport 1 K+ completes the cycle. This stoichiometry allows these transporters to generate up to a 1-million-fold concentration gradient across the membrane (Levy, Warr & Attwell, 1998, Owe, Marcaggi & Attwell, 2006, Wadiche, Arriza, Amara & Kavanaugh, 1995, Zerangue & Kavanaugh, 1996). Several reviews have discussed the pharmacology, localization, and biophysical properties of these transporters (Anderson & Swanson, 2000, Beart & O’Shea R, 2006, Danbolt, 2001, Gegelashvili & Schousboe, 1997, Kanner, 2006, Robinson, 1999, Robinson & Dowd, 1997, Ryan & Vandenberg, 2005, Seal & Amara, 1999, Shigeri, Seal & Shimamoto, 2004, Sims & Robinson, 1999, Tanaka, 2000, Trotti, Danbolt & Volterra, 1998, Vandenberg & Ryan, 2013). Therefore, in this review we will focus on their transcriptional regulation.
2. Differential localization of glutamate transporters
If one assumes that transcriptional mechanisms are the strongest driver of endogenous expression, it is important to first understand the expression patterns of the transporter subtypes. All five subtypes of the Glu transporters are enriched in different brain regions and different cell types. GLAST and GLT-1 are mainly expressed in astrocytes, while the other three subtypes are enriched in neurons (Chaudhry, Lehre, Campagne, Ottersen, Danbolt & Storm-Mathisen, 1995, Lehre, Levy, Ottersen, Storm-Mathisen & Danbolt, 1995, Pines et al, 1992, Regan, Huang, Kim, Dykes-Hoberg, Jin, Watkins et al, 2007, Rothstein, Martin, Levey, Dykes-Hoberg, Jin, Wu et al, 1994). GLAST, GLT-1 and EAAC1 are also found in oligodendroglia (DeSilva, Kabakov, Goldhoff, Volpe & Rosenberg, 2009, Domerq, Sánchez-Gómez, Areso & Matute, 1999, Martinez-Lozada, Waggener, Kim, Zou, Knapp, Hayashi et al, 2014, Pitt, Nagelmeier, Wilson & Raine, 2003), GLT-1 has also been observed in activated microglia (Lopez-Redondo, Nakajima, Honda & Kohsaka, 2000). GLT-1 is also expressed by neurons, but at much lower levels than those observed in astrocytes (for recent discussion, see Furness, Dehnes, Akhtar, Rossi, Hamann, Grutle et al, 2008, Petr, Sun, Frederick, Zhou, Dhamne, Hameed et al, 2015). GLAST is enriched in Bergmann glial cells of the cerebellum (Rothstein et al, 1994, Ruiz & Ortega, 1995), in Müller glial cells of the retina (Bringmann, Pannicke, Biedermann, Francke, Iandiev, Grosche et al, 2009), and in astrocytes in the olfactory bulb (Utsumi, Ohno, Onchi, Sato & Tohyama, 2001). GLT-1 is enriched in astrocytes in the cerebral cortex, hippocampus, lateral septum, striatum and spinal cord (Regan et al, 2007, Rothstein et al, 1994, Torp, Danbolt, Babaie, Bjoras, Seeberg, Storm-Mathisen et al, 1994). EAAC1 is observed in neurons in the forebrain, diencephalon, hindbrain, dorsal root ganglia and spinal cord (Bar-Peled, Ben-Hur, Biegon, Groner, Dewhurts, Furata et al, 1997, Furuta, Martin, Lin, Dykes-Hoberg & Rothstein, 1997). EAAT4 is almost exclusively expressed in Purkinje cells of the cerebellum (Dehnes, Chaudhry, Ullensvang, Lehre, Storm-Mathisen & Danbolt, 1998, Yamada, Watanabe, Shibata, Tanaka, Wada & Inoue, 1996). EAAT5 is almost exclusively expressed by photoreceptors and bipolar cells in the retina (Arriza et al, 1997, Pow & Barnett, 2000). Expression of these transporters is also differentially controlled during development. GLAST is found at relatively high levels early in development, while GLT-1 levels increase dramatically during development (Furuta, Rothstein & Martin, 1997). This suggests that GLT-1 may be a marker of astrocyte maturation.
Although many think of these transporters as molecules that clear the ‘neurotransmitter’ Glu, several studies show that virtually all cells express Na+-dependent Glu transporters. GLAST is expressed in heart, muscle, placenta, lung and liver (Gegelashvili & Schousboe, 1998). GLT-1 is expressed in pancreas and liver, but the levels are 100-fold or more higher in brain (Berger & Hediger, 2006). EAAC1 is expressed in intestine, kidney, heart, lung, placenta and liver (Nakayama, Kawakami, Tanaka & Nakamura, 1996). Low levels of EAAT4 mRNA are found in placenta, and EAAT5 is expressed in liver, kidney, intestine, heart, lung and skeletal muscle (Gegelashvili et al, 1998, Lee, Anderson, Stevens, Beasley, Barnett & Pow, 2013). These differential expression patterns strongly suggest that each of these transporters is under specific transcriptional regulation.
3. Why study transcriptional regulation of glutamate transporters?
Glu is a neurotransmitter, a source of energy through oxidation (Dienel & McKenna, 2014), a building block for the anti-oxidant glutathione (Brosnan & Brosnan, 2013, Had-Aissouni, 2012), the only precursor for the major inhibitory neurotransmitter (γ-aminobutyric acid- GABA), and is a building block for proteins. During development, Glutamatergic signaling participates in proliferation, migration, and differentiation (for reviews, see Jansson & Akerman, 2014, Lujan, Shigemoto & Lopez-Bendito, 2005, Nguyen, Rigo, Rocher, Belachew, Malgrange, Rogister et al, 2001). It also controls synapse formation and the shape of dendritic spines. Thus, it is critical to control extracellular Glu spatially and temporally during development and in the adult nervous system. In the adult nervous system, Glu transporters are found at such high levels (particularly GLT-1) that they function as buffers of the amount of Glu that is available for activation of receptors (Tong & Jahr, 1994) and/or they shape the excitatory post-synaptic currents at other synapses (for reviews, see Conti & Weinberg, 1999, Huang & Bergles, 2004, Marcaggi & Attwell, 2004, Otis, Brasnjo, Dzubay & Pratap, 2004, Tzingounis & Wadiche, 2007).
Decreased levels of Glu transporter proteins and/or mRNAs have been observed in animal models of stroke, head trauma, amyotrophic lateral sclerosis (ALS), Alzheimer’s disease, epilepsy, and others. In many cases, similar changes have been observed in post-mortem specimens from patients with these diseases (for reviews, see Dunlop, 2006, Fontana, 2015, Kim, Lee, Kegelman, Su, Das, Dash et al, 2011 Sattler & Rothstein, 2006, Sheldon & Robinson, 2007, Yi & Hazell, 2006). In fact, a loss of GLT-1 that is consistently observed in both animal models of ALS (for review, see Rattray & Bendotti, 2006) and humans with ALS, prompted Rothstein and his colleagues to screen for compounds that increase GLT-1 expression. They identified the antibiotic ceftriaxone and showed that it delayed the onset of motor symptoms and death in a mouse model of ALS (Rothstein, Patel, Regan, Haenggeli, Huang, Bergles et al, 2005). Although ceftriaxone did not show a therapeutic benefit in a phase 3 clinical trial (Cudkowicz, Titus, Kearney, Yu, Sherman, Schoenfeld et al, 2014), several groups have shown therapeutic benefits of ceftriaxone in animal models of a wide-range of neurologic and psychiatric conditions (Amin, Hajhashemi, Abnous & Hosseinzadeh, 2014, Cui, Cui, Gao, Sun, Wang, Wang et al, 2014, Fontana, 2015, Hsu, Hung, Chang, Liao, Ho & Ho, 2015, Inui, Alessandri, Heimann, Nishimura, Frauenknecht, Sommer et al, 2013, Soni, Reddy & Kumar, 2014). Glu transporters are regulated by a variety of mechanisms, including transcription, mRNA maturation and stabilization, post-translational modifications, trafficking to and from the plasma membrane (for reviews see Robinson, 2002, Robinson, 2006), and diffusion in the membrane (Benediktsson, Marrs, Tu, Worley, Rothstein, Bergles et al, 2012, Murphy-Royal, Dupuis, Varela, Panatier, Pinson, Baufreton et al, 2015, Shin, Nguyen, Pow, Knight, Buljan, Bennett et al, 2009).
In this review, we will focus on the mechanisms that control transcription of the transporters. This has mostly been approached by examining the effects of agents that activate cell surface receptors or by direct modulation of intracellular signals. In some cases, the effects of these agents have been linked to specific cis elements in the transporter genes and transcription factors that bind to these elements. There is some evidence to suggest that these regulatory events are influenced by epigenetic mechanisms (DNA methylation or histone acetylation), but this is still a relatively underexplored area.
One assumes that, as the field develops an understanding of the mechanisms that control transcription of these transporters, we will develop a better understanding of the specific signals and transcription factors that define populations of cells and/or subpopulations of cells in the brain. In some cases this information is starting to be used to define the mechanisms that contribute to decreases in transporter expression that accompany diverse neurologic insults. In spite of setbacks, it still seems appealing to consider the mechanisms that regulate Glu transporters as potential drug targets. It is also possible that developing an understanding of the transcriptional events that lead to altered expression of Glu transporters may lead to a broader mechanistic understanding of the pathogenesis of diverse neurologic and psychiatric diseases.
For the purposes of this review, we decided to simplify our discussion and include many studies in which steady state protein and/or mRNA levels change in response to an external stimulus. However there are examples of regulation of mRNA stability (Zelenaia, Gochenauer & Robinson, 1999), translation (Tian, Lai, Guo, Lin, Butchbach, Chang et al, 2007), or protein degradation (Wu, Xia, Lin, Cao, Chen, Liu et al, 2013). Therefore it seems possible that our simplification will end up being incorrect at least in some cases.
3.1 Transcriptional Regulation of SLC1A3/GLAST/EAAT1
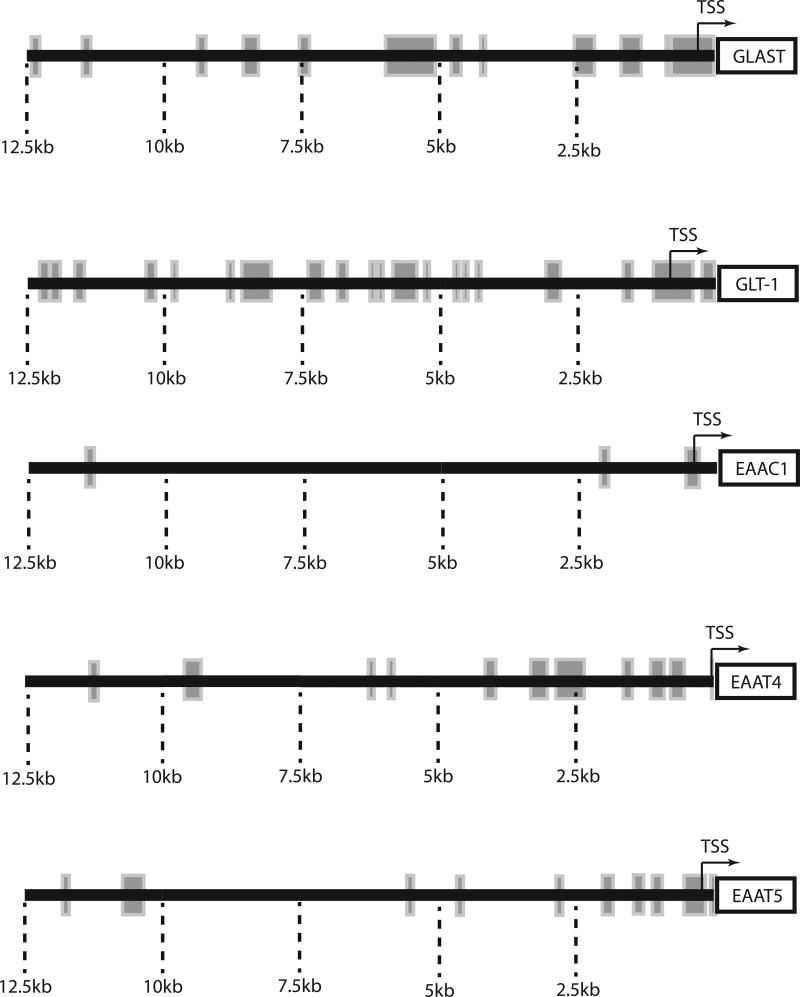
The mouse GLAST gene was mapped to chromosome 15A2 (Hagiwara, Tanaka, Takai, Maeno-Hikichi, Mukainaka & Wada, 1996). Although the human gene was originally mapped to 5p13 (Takai, Yamada, Kawakami, Tanaka & Nakamura, 1995), one year latter it was re-mapped to chromosome 5p11–12 (Stoffel, Sasse, Duker, Muller, Hofmann, Fink et al, 1996). With the complete sequencing of both genomes, these locations have been verified. As the cellular, developmental, and regional expression patterns are shared between mouse/rodent and human (Bar-Peled et al, 1997, Furuta et al, 1997, Regan et al, 2007, Schmitt, Asan, Puschel & Kugler, 1997), it seems that core promoter elements are likely to be conserved through evolution. The fact that transgenic mice that utilize the entire human SLC1A3 gene to control discosoma red fluorescent protein (dsRFP) display complete overlap of GLAST with dsRFP further supports the notion that similar elements control GLAST expression in mice and humans (Regan et al, 2007). Therefore, evolutionarily conserved regions of the promoter are likely to provide insights into the cis elements that may be involved in transcriptional control; these are presented in Figure 1.
Figure 1. Schematic representation of evolutionary conserved domains in the promoter regions of Glu transporters.
The mouse and human homologs of the genes encoding GLAST, GLT-1, EAAC1, EAAT4 or EAAT5 were aligned using an online resources (DCODE database; http://ecrbase.dcode.org). Rectangular boxes represent evolutionary conserved domains, defined as regions of ≥70% homology for at least 100 nucleotides. It is important to remember that enhancer elements can be outside the regions aligned and that some of the distal conserved regions may not be involved in transport regulation and may instead, regulate the neighboring gene.
Almost 20 years ago, the structures of both the mouse and human GLAST promoters were defined (Hagiwara et al, 1996, Stoffel et al, 1996). As might be expected, the proximal 2 kb of the promoter are highly conserved and all studies to date have focused on this region. Neither the mouse nor the human promoters contain a TATA box in this region but they both contain a GC box and the human gene also contains an E box. As is observed with several housekeeping genes, the transcription factors stimulating proteins 1 and 3 (Sp1, Sp3) bind to the GC box and upstream stimulating factor (USF1) binds to the E box in electrophoretic mobility assays (EMSA) (Kim, Choi, Chao & Volsky, 2003) (see figure 2A). Mutation of either the GC or E box dramatically reduces promoter reporter expression in human fetal astrocytes, suggesting that binding to both sites is required for transcription.
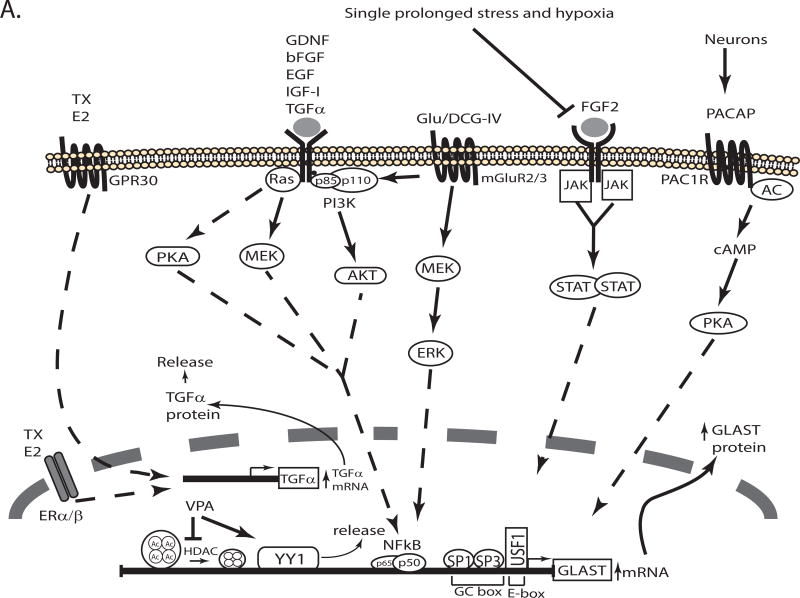
Figure 2. Schematic representation of the signaling pathways that regulate transcription of GLAST/EAAT1/SLC1A3.
Several signaling pathways activated in response to extracellular molecules regulate the expression of GLAST by activating transcription factors that interact with cis elements in the promoter. The pathways that increase or decrease GLAST transcription are depicted in different schematics (Panel A & B, respectively). For a detailed description see the text. TX: Tamoxifen, E2: Estrogen, Glu: Glu, AC: adenylate cyclase, ET1: endothelin-1, ETA, ETB: Endothelin receptors A and B.
While working from the gene sequence has yielded some information about the cis and trans factors involved in controlling GLAST expression, an alternative strategy has been to identify agents that regulate GLAST protein and/or mRNA levels when applied to cells (generally astrocytes). Using this strategy, it has been possible to identify extracellular stimuli that either increase or decrease GLAST expression/transcription. We will first discuss the pathways that have been implicated in transcriptional activation (Figure 2A) and then the pathways that have been implicated in transcriptional repression (Figure 2B).
Astrocytes in culture have been an important model system to study transcriptional regulation of GLAST and in this system neurons increase astrocytic expression of GLAST (Schlag, Vondrasek, Munir, Kalandadze, Zelenaia, Rothstein et al, 1998, Swanson, Liu, Miller, Rothstein, Farrell, Stein et al, 1997). This effect is at least in part caused by secreted molecules (Gegelashvili, Danbolt & Schousboe, 1997, Schlag et al, 1998, Swanson et al, 1997). In earlier studies, it had been shown that dibutyryl-cyclic AMP (dbcAMP) increases glutamate uptake in astrocytes (Hertz, Bock & Schousboe, 1978) and three groups essentially simultaneously demonstrated that dbcAMP increases the levels of GLAST mRNA and protein in primary cultures of astrocytes from forebrain or retina (Eng, Lee & Lal, 1997, Schluter, Figiel, Rozyczka & Engele, Swanson et al, 1997). The effect of dbcAMP is blocked by protein-kinase A (PKA) inhibitors (Schlag et al, 1998). Shortly thereafter, Figuel and colleagues demonstrated that pituitary adenylate cyclase-activating polypeptide (PACAP) mimics the effects of neurons and that PACAP-directed antibodies block the effects of neuron-conditioned media (Figiel & Engele, 2000). Furthermore, inhibitors of a PACAP receptor (PAC1R) or PKA antagonists also blocked the effects of neuron-conditioned media. Together these studies are consistent with the notion that neurons use a PACAP, cAMP, PKA-dependent pathway to increase GLAST expression (see Figure 2A). Although these data are consistent with the activation of the transcription factor cAMP-response element binding protein (CREB), the transcription factor(s) or the cis element(s) of the promoter responsible for this effect have not been identified.
Steroids are neuroprotective in animal models of both acute insults and chronic neurodegenerative diseases (Baudry, Bi & Aguirre, 2013, Scott, Zhang, Wang, Vadlamudi & Brann, 2012). Estrogen or tamoxifen increase GLAST mRNA and protein (Karki, Webb, Zerguine, Choi, Son & Lee, 2014, Lee, Sidoryk, Jiang, Yin & Aschner, 2009, Pawlak, Brito, Kuppers & Beyer, 2005). These effects are dependent upon activation of both the G protein-coupled receptor 30 (GPR30) and the nuclear receptors ERα and ERß (Karki et al, 2014, Lee, Sidoryk-Wegrzynowicz, Wang, Webb, Son, Lee et al, 2012), but there is some evidence that ERα may be more important for this effect (Sato, Matsuki, Ohno & Nakazawa, 2003). The effects of estrogen on GLAST are indirect and are mediated by estrogen-dependent induction of transforming growth factor – α (TGFα) (Dhandapani, Wade, Mahesh & Brann, 2005, Karki et al, 2014, Lee et al, 2009). Steroids or tamoxifen are thought to attenuate manganese-dependent neurotoxicity by a mechanism that depends on up-regulation of GLAST (Karki, Smith, Johnson & Lee, 2014, Lee et al, 2009).
In addition to TGFα, several other growth and neurotrophic factors increase expression of GLAST. Epidermal growth factor (EGF), basic fibroblast growth factor (bFGF), insulin-like growth factor-1 (IGF-1) and glial cell line-derived neurotrophic factor (GDNF) also increase GLAST mRNA and protein levels (Bonde, Sarup, Schousboe, Gegelashvili, Noraberg & Zimmer, 2003, Suzuki, Ikegaya, Matsuura, Kanai, Endou & Matsuki, 2001, Zelenaia, Schlag, Gochenauer, Ganel, Song, Beesley et al, 2000). Growth factors induce GLAST expression through Ras/Mitogen-extracellular signal regulated kinase (Ras/MEK), phosphatidylinositol-4,5-bisphosphate 3 kinase/Akt (PI3K/Akt) and PKA pathways (Dhandapani et al, 2005, Figiel, Maucher, Rozyczka, Bayatti & Engele, 2003, Lee et al, 2009) with a consequent activation of nuclear factor kappa B (NFκB)(Figiel et al, 2003, Karki et al, 2014) (see Figure 2A). Although inhibitors of NFκB signaling block these effects and exogenous expression of the active subunits of NFκB (p50 and p65) mimic these effects, a direct interaction between NFκB and the GLAST promoter has not been described (Figiel et al, 2003, Karki et al, 2014, Lin, You, Wei & Gean, 2014).
The effects of growth factors on GLAST expression also seem to depend on activation of the Janus kinase/signal transducer activator of transcription (JAK/STAT) pathway. Using an in vivo model, Raymond and colleagues demonstrated that fibroblast growth factor-2 (FGF2) blocks the damage caused by hypoxia (Raymond, Li, Mangin, Huntsman & Gallo, 2011). They show that hypoxia causes decreases in GLAST, phospho-JAK, and phospho-STAT. They also show that these effects are blocked by FGF2 and that the effects of FGF2 are blocked by an inhibitor of JAK/STAT signaling. Prolonged stress also causes a decrease in GLAST protein and an increase in Glu in cerebrospinal fluid (Feng, Guo, Liu, Wang, Wang, Gao et al, 2015). These effects were also strongly linked to inhibition of JAK/STAT signaling. At present, it is not clear if STAT directly interacts with the GLAST promoter, but these studies strongly suggest that the JAK/STAT pathway contributes to maintenance of GLAST levels in vivo. Together these studies also suggest that several different signaling pathways may function downstream of growth factors to regulate transcription of GLAST; it will be important to learn if these pathways converge or function in parallel/independently.
As these transporters play an important role in regulating a potential toxin (Glu itself), it should not be surprising that Glu receptors are also linked to transcriptional regulation of GLAST. In fact, some of the subtypes of Glu receptors are linked to increases in GLAST, while others decrease GLAST. A selective group II metabotropic Glu receptor (mGluR) agonist increases GLAST mRNA and protein (Aronica, Gorter, Ljlst-Keizsers, Rozemuller, Yankaya, Leenstra et al, 2003, Gegelashvili, Dehnes, Danbolt & Schousboe, 2000). Using pharmacological strategies, this effect was linked to the ERK/PI3K/NFkB signaling pathway (Lin et al, 2014)(Figure 2A). The in vivo relevance of this effect is supported by the observation that mice deleted of one of the members of the group II receptors, mGluR3, have lower levels of GLAST protein (Lyon, Kew, Corti, Harrison & Burnet, 2008). This suggests that tonic activation of mGluR3 maintains GLAST expression in vivo.
In contrast to the effects of mGluR3 activation, agonists of group I mGluRs decrease GLAST expression (Aronica et al, 2003, Gegelashvili et al, 2000). This effect has only been studied using a group I mGluRs selective agonist and an antagonist. Therefore more studies are needed to identify the downstream signaling pathways and transcription factors involved.
The ionotropic Glu receptors (iGluRs) also regulate GLAST expression in cerebellar Bergmann glia. Lopez-Bayghen and Ortega demonstrated that Glu and the Glu receptor agonist, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), decrease GLAST mRNA and protein levels (Lopez-Bayghen, Espinoza-Rojo & Ortega, 2003). This variant of Glu receptor is Ca2+ permeable, and the consequent increase in intracellular Ca2+ is known to activate protein kinase C (PKC) (Burnashev, Khodorova, Jonas, Helm, Wisden, Monyer et al, 1992). Inhibition of PKC blocks the effect of Glu and exogenous expression of constitutively active PKCα mimics the effects of Glu, strongly suggesting that activation of PKC is necessary and sufficient for this effect (Lopez-Bayghen et al, 2003, Lopez-Bayghen & Ortega, 2004). They showed that exogenous expression of c-jun or c-fos, subunits of the transcription factor activator protein-1 (AP1), decrease GLAST levels. They also demonstrated that Glu, AMPA, or a PKC activator increase AP-1 binding to a sequence in the proximal GLAST promoter using EMSAs (Lopez-Bayghen et al, 2004). Together these studies demonstrate that Glu down-regulates GLAST expression through AMPA receptors that are coupled to PKC and AP-1 (see figure 2B). In a subsequent study, this same group showed that Glu and AMPA also increases the interaction of the transcription factor Ying Yang 1 (YY1) with the GLAST promoter, and overexpression of this transcription factor decreases GLAST expression (Rosas, Vargas, Lopez-Bayghen & Ortega, 2007). These studies suggest that the effects of AMPA may depend on both AP-1 and YY1. Using the same model, Poblete-Naredo and her colleagues demonstrated that insulin increases YY1 binding to the GLAST promoter by EMSA and decreases GLAST expression (Poblete-Naredo, Angulo, Hernandez-Kelly, Lopez-Bayghen, Aguilera & Ortega, 2009).
The cytokine, tumor necrosis factor α (TNFα), decreases GLAST protein levels (Korn, Magnus & Jung, 2005). However astrocytes grown in media containing dbcAMP are resistant to the effects of TNFα (Tilleux & Hermans, 2008). As dbcAMP simulates some aspects of astrocyte maturation, these results suggest that regulation of GLAST transcription may vary at different stages of astrocyte maturation. This effect of TNFα does not generalize to all molecules that decrease GLAST expression as endothelin 1 (ET1) decreases GLAST protein levels even in the presence of dbcAMP, PACAP, EGF or TGFα (Rozyczka, Figiel & Engele, 2004). Although a role for ET1-dependent regulation of GLAST has not been defined in vivo, increases in ET1 are correlated with decreases in GLAST levels observed in Alzheimer’s disease (Luo & Grammas, 2010).
Environmental toxins also decrease GLAST expression. For example, chronic manganese exposure has been associated with a Parkinsonian-like disease (Kwakye, Paoliello, Mukhopadhyay, Bowman & Aschner, 2015). In astrocytes in culture, Mn causes a decrease in GLAST expression that is associated with an increase in TNFα. The decrease is blocked by an inhibitor of TNFα synthesis or a receptor antagonist (Lee et al, 2009). Arsenic exposure has also been associated with neurological dysfunction (e.g. impaired learning and memory, mood disorders and diminished IQ) (for a review see Tyler & Allan, 2014). Arsenite decreases GLAST expression, transport activity, and increases the binding of the transcription factors Nrf2 and AP-1 to the GLAST promoter (Castro-Coronel, Del Razo, Huerta, Hernandez-Lopez, Ortega & Lopez-Bayghen, 2011).
In summary, several different extrinsic signals regulate GLAST protein and mRNA. It is assumed that these effects are dependent upon increased transcription, but in most cases this has not been formally demonstrated. Several different signaling pathways have been implicated in this regulation (see Figure 2), but it is not clear if these pathways function independently. There is also a need to carefully define the specific transcription factors involved and the cis promoter elements required. At least two different sets of evidence suggest that different populations of astrocytes employ different mechanisms to control expression of GLAST (Gegelashvili, Civenni, Racagni, Danbolt, Schousboe & Schousboe, 1996, Schluter et al, 2002). Therefore it is likely that as different subpopulations of astrocytes are molecularly characterized (for discussion see Matyash & Kettenmann, 2010, Rusnakova, Honsa, Dzamba, Stahlberg, Kubista & Anderova, 2013, Schitine, Nogaroli, Costa & Hedin-Pereira, 2015, Walz, 2000, Zhang & Barres, 2010), it will be possible to link differential control of GLAST expression to these subtypes.
3.2 Transcriptional Regulation of SLC1A2/GLT-1/EAAT2
The human GLT1 gene (SLC1A2) was mapped to chromosome 11 bands p13-p12 (Li & Francke, 1995). The mouse GLT-1 gene (Slc1a2) was mapped to the middle region of chromosome 2 (Kirschner, Copeland, Gilbert, Jenkins & Amara, 1994). As is observed with GLAST, the gene contains 10 exons that range from 127 bp to 251 bp and there is no TATA box in the proximal promoter. There is a GC box with five Sp1 binding sites (Su, Leszczyniecka, Kang, Sarkar, Chao, Volsky et al, 2003), but it is not known if these sites are required for GLT-1 expression. As is true for GLAST, when a bacterial artificial chromosome containing the human SLCA2 gene is used to control expression of enhanced green fluorescent protein (eGFP), expression of reporter closely correlates with GLT-1 expression in transgenic mice (Regan et al, 2007). This suggests that transcriptional control is similar between the two species, and it suggests that studies of evolutionarily conserved domains in the promoter region may be informative.
While GLAST and GLT-1 are both enriched in astrocytes, GLT-1 expression uniquely correlates with synaptogenesis (Furuta et al, 1997), suggesting that GLT-1 is a marker of astrocyte maturation. When astrocytes are maintained in culture they assume a polygonal (fibroblast-like) shape. Almost forty years ago dbcAMP was demonstrated to induce a dramatic change in astrocyte morphology to a more stellate shape that is somewhat similar to that observed with mature astrocytes in vivo (Moonen, Heinen & Goessens, 1976). Two groups essentially simultaneously realized that rat astrocytes in culture express little or no GLT-1 protein, but co-culturing astrocytes with neurons induces expression of GLT-1 in astrocytes (Schlag et al, 1998, Swanson et al, 1997). In fact, several subsequent studies have documented low levels of GLT-1 in mouse astrocyte cultures, but neurons also increase GLT-1 transcription in this system (Apricó, Beart, Crawford & O’Shea, 2004, Ghosh, Lane, Krizman, Sattler, Rothstein & Robinson, 2015, O’Shea, Lau, Farso, Diwakarla, Zagami, Svendsen et al, 2006). The effect of neurons is at least in part dependent upon soluble factors but it may also depend on contact (Drejer, Meier & Schousboe, 1983, Gegelashvili et al, 1997, Gegelashvili et al, 2000, Yang, Gozen, Watkins, Lorenzini, Lepore, Gao et al, 2009, Zelenaia et al, 2000). dbcAMP mimics this effect of neurons (Eng et al, 1997, Schlag et al, 1998, Swanson et al, 1997). Interestingly, inducing stellation using inhibitors of Rho kinase inhibitors also increases GLAST and GLT-1 protein, although the effect on GLT-1 is much larger (Lau, O’Shea, Broberg, Bischof & Beart, 2011).
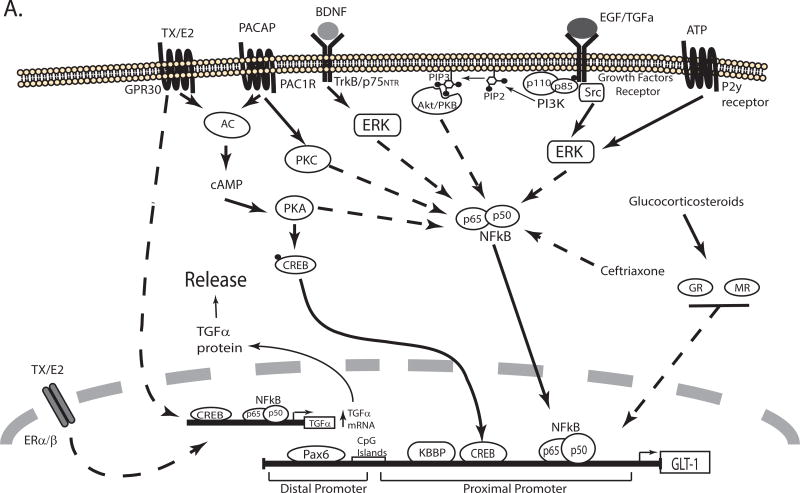
Figiel and colleagues tested PACAP as a potential mediator of the effect of neurons because it was known that neurons release PACAP and that PACAP activates adenylate cyclase (Figiel et al, 2000). They showed that anti-PACAP directed antibodies or a PACAP receptor (PAC1 receptor) antagonist block the effects of neuron-conditioned media (Figiel et al, 2000). Using pharmacological approaches, they also demonstrated that blocking either PKA or PKC attenuate the effects of PACAP, but a PKC inhibitor had a bigger effect. Inhibitors of NFκB also block PACAP-dependent induction of GLT-1 (Figiel et al, 2003). Expression of dominant-negative inhibitors of NFκB in astrocytes blocks neuron-dependent induction of GLT-1 (or eGFP under the control of a bacterial artificial chromosome GLT-1 promoter) (Ghosh, Yang, Rothstein & Robinson, 2011). Exogenous expression of either of two different NFκB subunits, p65 or p50, induce expression of GLT-1 and both subunits interact with the GLT-1 promoter in vivo as demonstrated with ChIP. This interaction was not observed in a tissue that does not express GLT-1 protein. While it seems logical that activation of cAMP and PKA would signal through CREB there is no evidence that CREB is activated by PACAP in astrocytes, however, neuron-conditioned media or cAMP increase CREB phosphorylation (activation) in astrocytes (Gegelashvili et al, 2000, Schluter et al, 2002). The cAMP/PKA/CREB pathway has also been linked to expression of GLT-1 in vivo. Using chronic unpredictable stress to create an animal model of depression, Liu and colleagues observe decreases in cAMP, the catalytic subunit of PKA, phospho-CREB and GLT-1 levels (Liu, 2015). An inhibitor of phosphodiesterase type 4, prevents all of these changes, strongly implicating this pathway in the regulation of GLT-1 in vivo. They also find that inhibition of phosphodiesterase partially corrects the ‘depression phenotype’ suggesting that down-regulation of GLT-1 may contribute to the pathology of this disease. Together these studies suggest that the cAMP/PKA/CREB signaling pathway contributes to GLT-1 regulation in vitro and in vivo, but there is also dependence of NFκB signaling (see Figure 3A). It is not known if these two transcription factors function together or independently.
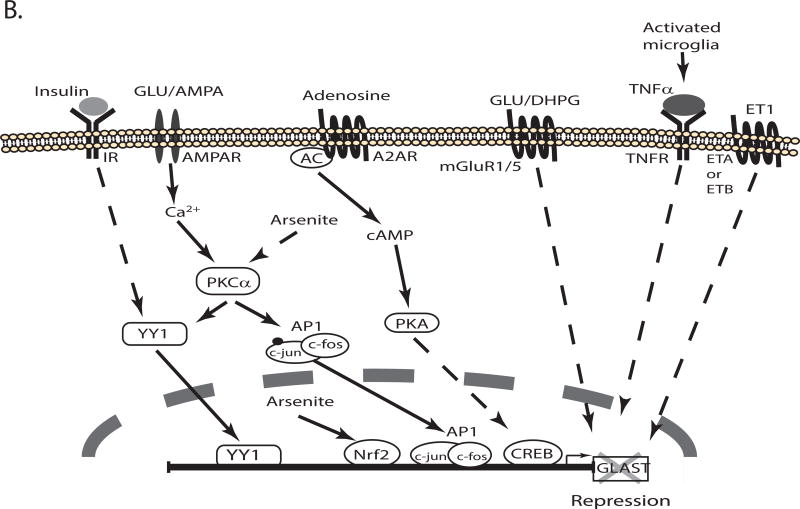
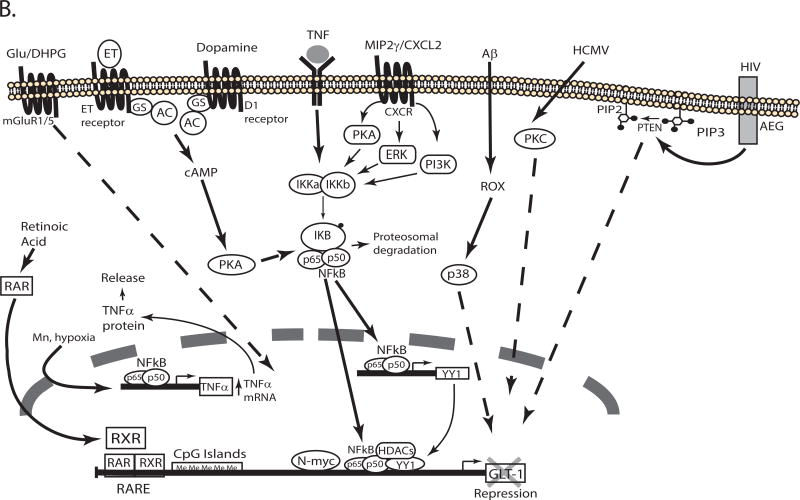
Figure 3. Schematic representation of the signaling pathways that regulate transcription of GLT-1/EAAT2/SLC1A2.
Several signaling pathways activated in response to extracellular molecules regulate the expression of GLT-1 by activating transcription factors that interact with cis elements in the promoter. The pathways that increase or decrease GLT-1 transcription are depicted in different schematics (Panel A & B, respectively). For a detail description see the text. TX: tamoxifen, E2: estrogen, AC: adenylate cyclase, GR: glucocorticoid receptor, MR: mineralocorticoid receptor.
As is observed with GLAST, growth factors also increase GLT-1 expression. EGF or TGFα increase GLT-1 mRNA, protein levels, and GLT-1-mediated uptake in cultured cortical astrocytes. These effects are blocked by inhibitors of receptor tyrosine kinase, PI3K, or NFκB (Zelenaia et al, 2000). In a later study, we demonstrated that expression of a dominant-negative variant of Akt kinase blocks the effect of EGF and expression of a constitutively active form of Akt mimics the effect of EGF (Li, Toan, Zelenaia, Watson, Wolfe, Rothstein et al, 2006). These studies are consistent with a growth factor receptor/PI3K/Akt/NFκB pathway regulating transcription of GLT-1 (see Figure 3A). The effects of EGF and TGFα on GLT-1 expression have been replicated by others (Figiel et al, 2003). We initially found that platelet-derived growth factor (PDGF) increases GLT-1 protein levels in astrocyte-enriched cultures, but these effects were associated with an increase in the number of A2B5+ oligodendroglial precursor cells that express GLT-1 (Zelenaia et al, 2000). We observed no effect of PDGF on GLT-1 expression in astrocyte cultures devoid of these cells (Zelenaia et al, 2000), but others have reported that PDGF increases GLT-1 protein levels (Figiel et al, 2003). There are also differences in the effects of GDNF and brain-derived neurotrophic factor (BDNF). While neither GDNF nor BDNF have an effect on GLT-1 in one study (Figiel et al, 2003), other groups have shown that GDNF (Bonde et al, 2003) or BDNF (Rodriguez-Kern, Gegelashvili, Schousboe, Zhang, Sung & Gegelashvili, 2003) increase expression of GLT-1. The effects of BDNF are blocked by pharmacological inhibition of the ERK/NFκB signaling pathway (Rodriguez-Kern et al, 2003). In addition, the work from Lau and colleagues shows that in stellated astrocytes GLT-1 and BDNF are co-regulated (Lau, Kovacevic, Tingleff, Forsythe, Cate, Merlo et al, 2014, Lau, Perreau, Chen, Cate, Merlo, Cheung et al, 2012). These differences may reflect differential regulation of GLT-1 in different populations of astrocytes. It is also possible that the presence of neurons changes the response of astrocytes to these stimuli. Cortical astrocytes were used in our studies and those of Rodriguez-Kern, while Figiel and colleagues used forebrain astrocytes. Bonde and colleagues used organotypic slice cultures (Bonde et al, 2003, Figiel et al, 2003, Rodriguez-Kern et al, 2003, Zelenaia et al, 2000). It is not clear if all of the effects of PDGF are related to increased proliferation of the A2B5+ cells or if PDGF also has a direct effect on GLT-1 expression in astrocytes.
Estrogen also increases GLT-1 expression (Pawlak et al, 2005). The effects of estrogen are mediated through both nuclear receptors (ERα, ERß) and G-protein coupled receptor, GPR30. Activation of estrogen receptors increases binding of the transcription factors CREB and NFκB to GLT-1 promoter in EMSA and chromatin immunoprecipitation (ChIP) assays (Karki, Webb, Smith, Lee, Son, Aschner et al, 2013, Karki et al, 2014). Mutation of the putative binding site for CREB (−308), or mutation of all three NFκB binding sites (−251, −272 and −583) in the GLT-1 promoter block estrogen- or dbcAMP-dependent activation of promoter reporter constructs (Lee et al, 2012). The effects of estrogen are, at least in part dependent on estrogen-dependent up-regulation of TGFα that in turn serves as an autocrine factor to regulate GLT-1 expression by the MEK/ERK and PI3K/Akt signaling pathways (Karki et al, 2014, Lee et al, 2012). Other selective estrogen receptor modulators (tamoxifen and raloxifene) also increase TGFα mRNA and protein levels (Karki et al, 2013). Exogenous/over-expression of CREB, p65, or p50 activate the TGFα and the GLT-1 promoters in promoter-reporter assays. Together these studies suggest that estrogen regulates GLT-1 expression through two mechanisms. First, it up-regulates expression of TGFα which in turn activates PI3K/Akt/NFκB and MEK/ERK/NFκB signaling pathways. It also appears that estrogen activates GLT-1 expression through GPR30/cAMP/PKA/CREB signaling pathway (see Figure 3A). Several neuroprotective roles had been attributed to estrogen (for discussions see Karki et al, 2014, Simpkins, Singh, Brock & Etgen, 2012), the previous results suggest that some of these neuroprotective roles may be associated with the induction of GLT-1 expression.
Glucocorticoids also increase GLT-1 mRNA and protein levels (Autry, Grillo, Piroli, Rothstein, McEwen & Reagan, 2006, Zschocke, Bayatti, Clement, Witan, Figiel, Engele et al, 2005). The effect of the synthetic glucocorticoid, dexamethasone is blocked by antagonists of either the glucocorticoid or the mineralocorticoid receptors (GR and MR, respectively)(Figure 3A). The downstream signals or transcription factors involved in the regulation have not been identified, but it is thought that chronic stress results in glucocorticoid-dependent up-regulation of GLT-1 (Autry et al, 2006, Reagan, Rosell, Wood, Spedding, Munoz, Rothstein et al, 2004).
Both ATP (Frizzo, Frizzo, Amadio, Rodrigues, Perry, Bernardi et al, 2007) and adenosine (Wu, Lee, Kim, Johng, Rohrback, Kang et al, 2011) increase GLT-1 expression. Pharmacological approaches demonstrated that P2Y (ATP receptors) and A1 (adenosine receptors) mediate these effects. An inhibitor of ERK signaling blocks the effects of ATP. As described above, the effects of ERK activation are blocked by inhibitors of NFκB. This suggests that the effects of ATP depend upon NFκB, but this has not been examined.
As an alternate approach to identifying substances that activate cell surface receptors or manipulate intracellular signaling molecules, we and others have identified evolutionarily conserved domains with the 5’ non-coding region of Slc1a2 and used this information to identify cis-elements or transcription factors that control transcription. As indicated above, this strategy has been validated with bacterial artificial chromosome mice. For example, we identified sequences within the proximal promoter region (which is highly conserved, see Figure 1) that are required for neuron-dependent expression of reporter in astrocytes (Yang et al, 2009). Through sequential deletion and site-directed mutagenesis a region (−688 to −679) that contains cis-elements essential for GLT-1 promoter activity was identified in the proximal promoter. Using this sequence as ‘bait’, mass spectrometry was used to identify kappa B-motif binding phosphoprotein (KBBP) as a transcription factor that binds to this region. Knock down of KBBP was shown to reduce GLT-1 or reporter activity in mice engineered to express eGFP under the control of the complete human GLT-1 gene. Decreased expression of KBBP correlates with the loss of eGFP observed in ricin-induced lesions or in an animal model of ALS.
Allritz and colleagues examined basal promoter activity upon transduction of rat or fetal human astrocytes (Allritz, Bette, Figiel & Engele, 2010). They found that deletions of nucleotides -216 through -502 in human promoter or -399 through -557 of the rat promoter sequence dramatically reduce reporter activity (Allritz et al, 2010). It is somewhat unclear how to interpret these results because cultured rat astrocytes, unlike cultured mice astrocytes, don’t normally express much GLT-1. Therefore, these elements may or may not be important for the increase in GLT-1 that is observed upon astrocyte maturation.
While the proximal 2.5kb promoter of GLT-1 gene is highly conserved and has been well characterized, there are several additional evolutionary conserved domains distal to this region out to ~12.5kb from the translation start site (Figure 1) (Ghosh et al, 2015). Analyses of promoter reporter mice generated by Rothstein and his colleagues have revealed that the proximal 7.9 kb of the promoter is not sufficient to direct astrocytic expression of reporter protein, reporter is observed mostly in neurons (Rothstein unpublished observations; for discussion, see Ghosh et al, 2015). When 8.3 kb of promoter is used to control expression, reporter protein is essentially exclusively found in astrocytes, but not all astrocytes express the reporter (Yang, Vidensky, Jin, Jie, Lorenzini, Frankl et al, 2011). These studies have three implications. First, they suggest that the region between 7.9 and 8.3 kb is required to direct astrocytic expression. In fact, we recently showed that Pax6 interacts with this region in vitro (EMSA) and in vivo (ChIP). ShRNA directed knockdown of Pax6 attenuates neuron-dependent induction of GLT-1 and exogenous expression of Pax6 increases GLT-1 expression (Ghosh et al, 2015). Secondly, these data suggest that different subtypes of astrocytes engage different mechanisms to control expression of GLT-1 in vivo. A similar conclusion has been drawn from in vitro analyses (Drejer et al, 1983, Gegelashvili et al, 1996, Schluter et al, 2002). Finally, these studies suggest that the evolutionarily conserved domains that are distal to 8.3 kb are important for expression of GLT-1 in a subtype of astrocytes. This has not been explored.
Rothstein and colleagues used a screen of 1,040 FDA-approved drugs, to identify β-lactam antibiotics that increase GLT-1 levels. To understand the mechanism, they used reporter promoter assays in-vitro and in-vivo, and found that this promoter was activated by the β-lactam antibiotics ceftriaxone and amoxicillin (Rothstein, Patel, Regan, Haenggeli, Huang, Bergles et al, 2005). They also demonstrated that ceftriaxone induces neuroprotection in mouse models of oxygen glucose deprivation, threo-hydroxyaspartate-induced motor neuron loss, and in a mouse model of ALS with the gene of superoxide dismutase 1 mutated (Rothstein et al, 2005). Since this time over 100 papers have been published most of them demonstrate a neuroprotective role of ceftriaxone and other β-lactam antibiotics (for review see Fontana, 2015, Soni et al, 2014). However the molecular mechanism responsible the effect are not well understood. Lee and colleagues demonstrated that NFκB inhibitors block ceftriaxone-dependent GLT-1 expression (Lee, Su, Emdad, Gupta, Sarkar, Borjabad et al, 2008). They showed that ceftriaxone increases the binding of NFκB to GLT-1 promoter using EMSA. They demonstrated that mutation of GLT-1 promoter at −272 blocks the effect of ceftriaxone but is also reduces basal activity. Finally, they demonstrated that ceftriaxone induces translocation of p65 to the nucleus and the degradation of IkBa (Lee et al, 2008). These results strongly suggest that NFκB is responsible for the effect of ceftriaxone, however it is still not known how ceftriaxone activates NFκB.
Besides ceftriaxone, several other drugs that increase GLT-1 expression are also neuroprotective. A similar screening assay was used to identify harmine, a β -carboline alkaloid, which increases GLT-1 protein and mRNA levels (Li, Sattler, Yang, Nunes, Ayukawa, Akhtar et al, 2011). Riluzole, an anti-convulsant agent, also increases GLT-1 protein and Glu uptake (Azbill, Mu & Springer, 2000, Carbone, Duty & Rattray, 2012). Together these data suggest that increasing the expression of GLT-1 may be neuroprotective, but there is evidence that over-expression of GLT-1 can also exacerbate the damage observed with certain acute insults (Li, Hala, Seetharam, Poulsen, Wright & Lepore, 2015, Poulsen, Schousboe, Sarup, White & Schousboe, 2006).
Not surprisingly, GLT-1 transcription is also regulated in other subtypes of glia. In some cases the regulation appears to parallel that observed in astrocytes. For example, neuron conditioned medium increases Glu uptake and GLT-1 protein in primary cultures of microglia (Nakajima, Yamamoto, Kohsaka & Kurihara, 2008). In other cases, the regulation is opposite to that observed in astrocytes at least in vitro. While TNFα decreases GLT-1 expression in most experiments using astrocytes (Boycott, Wilkinson, Boyle, Pearson & Peers, 2008, Sitcheran, Gupta, Fisher & Baldwin, 2005, Su et al, 2003), it increases expression of GLT-1 in activated microglia (Persson, Brantefjord, Hansson & Ronnback, 2005). In oligodendroglia, TNFα decreases GLT-1 expression (Pitt et al, 2003). In patients, with multiple sclerosis, the levels of GLT-1 in oligodendrocytes are decreased in areas of active lesions (Pitt et al, 2003). The fact that TNFα levels are increased in these same lesions suggests that TNFα may contribute to the loss of GLT-1 in these patients.
Some of the transcription factor(s) that underlie suppression of GLT-1 expression in astrocytes have been identified. Although NFκB binding to the promoter contributes to activation (see above), Sitcheran and colleagues used both EMSA and DNA-based affinity purification to demonstrate that TNFα increases NFκB binding to GLT-1 promoter (Sitcheran et al, 2005). These results support a bidirectional regulation of GLT-1 by NFκB. When it is activated by EGF/TGFα (or presumably neurons), it increases GLT-1 expression, however when activated by TNFα, it decreases GLT-1 expression. Sitcheran and colleagues found that TNFα increases the binding of N-myc to the GLT-1 promoter. This transcription factor also contributes to GLT-1 repression, as its overexpression decreases basal and NFκB-induced activation of GLT-1 (Sitcheran et al, 2005). Together these studies suggest that the interaction of NFκB with other transcription factors may regulate the direction of the effect of NFκB. The TNFα-dependent repression of GLT-1 has been associated with the decrease in GLT-1 expression observed after hypoxia (Boycott et al, 2008). The interaction of N-myc with the promoter is high at post-natal day 0 and decreases as GLT-1 expression increases during development (Gupta & Prasad, 2014). Thus, it is possible that repression of the GLT-1 promoter may contribute to the low expression observed early in development.
In addition to NFκB and N-myc, TNFα also increases the binding of the transcription factor YY1 to the GLT-1 promoter. Exogenous expression of YY1 decreases GLT-1 promoter activity. Furthermore, mutation of the ‘putative’ YY1 binding site in the GLT-1 promoter or expression of siRNA directed against YY1 increases GLT-1 promoter activity, suggesting that YY1 represses basal GLT-1 expression (Karki, Webb, Smith, Johnson, Lee, Son et al, 2014). Interestingly NFκB is itself a regulator of YY1 expression as exogenous expression of p65 activates the promoter of YY1 (Karki et al, 2014)(Figure 3B). Exogenous expression of p65 increases GLT-1 promoter activity, however when p65 is expressed with YY1 there is a decrease in GLT-1 promoter activity (Karki et al, 2014). Thus as is observed with N-myc, binding of YY1 to the GLT-1 promoter switches the effect of NFκB from activation to suppression.
TNFα also increases the expression of the chemokine, macrophage inflammatory protein-2γ (MIP2γ) in astrocytes (Fang, Han, Hong, Tan & Tian, 2012). Exogenous expression of MIP2γ decreases GLT-1 mRNA and protein, localization of GLT-1 in raft domains and Glu uptake. In fact, inhibition of signaling pathways that normally activate the GLT-1 promotor (e.g. NFκB, PI3K, PKA, MEK/ERK), block MIP2γ-dependent suppression of the promoter (Fang et al, 2012)(see Figure 3). This suggests that the ability of TNFα to switch activation to repression may extend to other signals.
Other extracellular stimuli also seem to switch signals that normally result in promoter activation to signals that suppress the GLT-1 promoter. For example, endothelins decrease GLT-1 protein levels and this effect is blocked by an inhibitor of PKA (Rozyczka et al, 2004). Dopamine also decreases GLT-1 protein and mRNA in astrocytes isolated from striatum (Brito, Rozanski, Beyer & Kuppers, 2009). Using pharmacological tools the authors demonstrate that D1 receptors mediate this effect. Although the downstream signaling components responsible for GLT-1 repression have not been identified, D1 receptors are normally coupled to increased cAMP and might be expected to activate the PKA signaling pathway (Figure 3B).
Several signals suppress GLT-1 expression. For example, retinoic acid or a specific retinoid × receptor (RXR) agonist decrease GLT-1 levels (Chan, Her, Liaw, Chen & Tzeng, 2012). The authors demonstrate that retinoic acid increases the binding of RXR to the retinoic acid response element (RARE) at −632 to −612 of GLT-1 promoter using EMSA (Chan et al, 2012). Aronica and colleagues demonstrated that DHPG, a specific agonist of group 1 mGluRs, decreases GLT-1 protein levels (Aronica et al, 2003). Antagonists of mGluR1 block the loss of GLT-1 observed after transient global ischemia, suggesting the mGluR1 activation may stimulate the loss of GLT-1 observed with these insults (Chen, Hsu-Chou, Lu, Chiang, Huang, Wang et al, 2005).
Amyloid-β (Aβ) peptides, the major component of amyloid plaques observed in Alzheimer’s disease, decrease Glu uptake and GLT-1 protein levels. Aβ increases the phosphorylation/activation of ERK, JNK and p38 MAPK. p38 MAPK is activated by oxidative stress, accordingly trolox, an antioxidant, blocks the Aβ-dependent decrease in Glu uptake (Matos, Augusto, Oliveira & Agostinho, 2008). Human cytomegalovirus infection can result in birth defects that affect primarily the CNS. Infection of astrocytes with this virus decreases expression of GLT-1, GLAST and glutamine synthetase. Except for the fact that inhibition of PKC blocks these effects, nothing is known about the mechanisms involved (Zhang, Li, Wang, Qian, Song & Hu, 2014). Down-regulation of GLT-1 also has been implicated in human immunodeficiency virus (HIV)-associated dementia (Wang, Pekarskaya, Bencheikh, Chao, Gelbard, Ghorpade et al, 2003). An HIV-inducible gene, astrocyte-elevated gene (AEG), decreases GLT-1 promoter activity. The phosphatase and tensin homolog (PTEN), a negative regulator of PI3K/Akt signaling, mimics the effect of AEG (Kang, Su, Sarkar, Emdad, Volsky & Fisher, 2005).
From these analyses, it has become clear that many different signals can increase transcription of GLT-1 and under certain circumstances these signals can switch from induction to suppression. Many of these signals have been implicated in the loss of GLT-1 that is observed in various neurologic insults. Although no unifying concepts have emerged, it seems likely that these studies will provide mechanistic insights into the pathogenesis of various disease processes. Several groups are also focused on therapeutically targeting GLT-1 expression (for recent review, see Takahashi, Foster & Lin, 2015).
3.3 Transcriptional Regulation of SLC1A1/EAAC1/EAAT3
The gene that encodes human EAAC1 (SLC1A1) was localized to chromosome 9 band p24 using fluorescence in situ hybridization (Smith, Weremowicz, Kanai, Stelzner, Morton & Hediger, 1994). The mouse homolog is located in chromosome 19 at the centromere (http://www.ncbi.nlm.nih.gov/gene). In comparison to GLAST and GLT-1, there is much less evolutionarily conserved sequence in the 5’ non-coding region for the EAAC1 gene (Figure 1). To date, no bacterial artificial chromosome EAAC1 reporter mice have been generated. Therefore, it is possible that the elements required for in vivo expression are relatively small. It is also possible that the control of EAAC1 expression is different in mice and humans. In fact, there is one example of differential expression (see below).
Several studies have suggested that EAAC1 may be more important for the synthesis of the anti-oxidant glutathione than for the clearance of neurotransmitter pools of Glu by importing cysteine (and possibly Glu) (Aoyama & Nakaki, 2013). For example, in mice deleted of EAAC1 there is a delayed neuronal degeneration that is associated with decreased glutathione; this damage is blocked by N-acetylcysteine (Aoyama, Suh, Hamby, Liu, Chan, Chen et al, 2006, Berman, Chan, Brennan, Reyes, Adler, Suh et al, 2011). Consistent with this general role of EAAC1, evolutionarily conserved antioxidant responsive elements (ARE) are found in the promoter (Escartin, Won, Malgorn, Auregan, Berman, Chen et al, 2011). One of the transcription factors that bind to these elements is nuclear factor (erythroid-derived 2)-like 2 (Nrf2). In fact, activators of Nrf2 or exogenous expression of Nrf2 increase EAAC1 expression in C6 glioma cells that endogenously express EAAC1 and not the other transporters (Escartin et al, 2011). They also demonstrated that Nrf2 binds to the ARE sequence in the EAAC1 promoter in vivo (Figure 4). Selective expression of Nrf2 in neurons in vivo increases both EAAC1 and glutathione levels (Escartin et al, 2011).
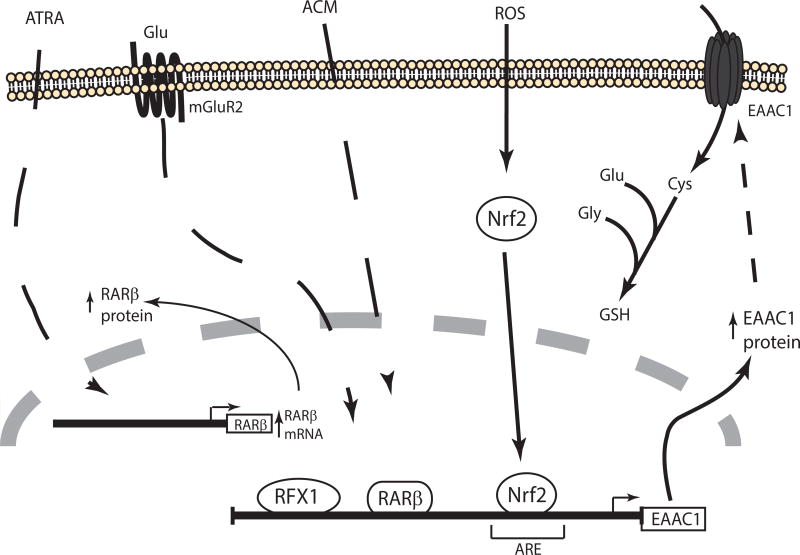
Figure 4. Schematic representation of the signaling pathways that regulate transcription of EAAC1/EAAT3/SLC1A1.
Extracellular signals and transcription factors that have been associated with an increase in EAAC1 expression are shown. For a detail description see the text. ACM: astrocyte conditioned media, ROS: reactive oxygen species.
Ma and colleagues identified a binding site for the regulatory factor X1 (RFX1) in human EAAC1 promoter. Using C6 and SH-SY5Y cell lines, the authors demonstrated that transfection of RFX1 increases both EAAC1 protein levels and activates a promoter reporter. In addition knockdown of RFX1 decreases EAAC1 expression in cultured rat cortical neurons (Ma, Zheng & Zuo, 2006).
Bianchi and colleagues demonstrated that all trans retinoic acid (ATRA) treatment increases EAAC1 mRNA and protein levels in C6 glioma (Bianchi, Gazzola, Tognazzi & Bussolati, 2008)(Figure 4). An agonist for the retinoic acid receptor β (RARβ) or exogenous expression of this receptor mimics the effect of ATRA. It appears that this effect is dependent on synthesis of an intermediary protein as a protein synthesis inhibitor blocks the ATRA-dependent increase in mRNA. RARβ expression increases after ATRA treatment, suggesting that RARβ may be the intermediate of ATRA-dependent EAAC1 increase. Accordingly they identified two putative-binding sites for RARβ (at −191 and −2696) in EAAC1 rat promoter (Bianchi, Gazzola, Cagnin, Kagechika & Bussolati, 2009). This site is not evolutionarily conserved; therefore it is not clear if these effects will extend to humans.
As mentioned above, neurons regulate expression of the astrocytic transporters. Although essentially nothing is known about the mechanism, there is a reciprocal interaction; astrocyte-conditioned media increases expression EAAC1 (Canolle, Masmejean, Melon, Nieoullon, Pisano & Lortet, 2004). It is also interesting to note that the circadian rhythm changes EAAC1 expression in a region-dependent fashion (Cagampang, Rattray, Powell, Chong, Campbell & Coen, 1996). The signaling pathway (s), transcription factor(s) or cis-elements responsible of this regulation have not been identified. Finally, EAAC1 levels are decreased in mice deleted of mGluR2, suggesting that mGluR may regulate basal expression of EAAC1 (Lyon et al, 2008).
3.4 Transcriptional Regulation of SLC1A6/EAAT4 and SLC1A7/EAAT5
The human EAAT4 gene (SLC1A6) localizes to chromosome 19 band 13.12. The mouse gene (Slc1a6) is mapped in chromosome 10 in the centromeric region (http://www.ncbi.nlm.nih.gov/gene). Gincel and colleagues generated promoter reporter mice using a bacterial artificial chromosome containing the human EAAT4 gene plus 107kb of upstream sequence and 54kb downstream of the last exon (Gincel, Regan, Jin, Watkins, Bergles & Rothstein, 2007). The expression of reporter protein correlates with EAAT4 protein, suggesting that there is evolutionary conservation of transcriptional regulation. Essentially nothing is known about the transcriptional regulation of EAAT4, except that subjecting rats to chronic restraint stress lowers EAAT4 protein levels (Zink, Vollmayr, Gebicke-Haerter & Henn, 2010).
The human EAAT5 gene (SLC1A7) localizes to chromosome 1 band 32.3, and the mouse gene (Slc1a7) is mapped in the centromere of chromosome 4 (http://www.ncbi.nlm.nih.gov/gene). Nothing is known about the events that control transcriptional regulation of EAAT5.
3.5 Epigenetic Regulation
As is true for transcriptional regulation, most studies of epigenetic regulation have focused on just two transporters, GLAST and GLT-1. There is evidence that methylation contributes to the different expression patterns and localization during brain development (Danbolt, 2001, Freeman, 2010, Furuta et al, 1997, Perisic, Holsboer, Rein & Zschocke, 2012).
DNA methylation is mediated by a family of DNA methyltransferases (DNMT), these enzymes transfer a methyl group from S-adenosyl-L-methionine to the carbon 5 of cytosine. Normally this methylation occurs in CpG islands which are defined by repeats of the nucleotides cytosine and guanine that occur 10–20 times more frequently than would be expected to occur by chance (e.g. 1 in 16). Generally it is thought that hyper-methylation reduces transcription and hypo-methylation increases transcription (Robertson & Wolffe, 2000); methylation may preclude binding of transcription factors (Perisic et al, 2012).
The GLT-1 promoter has several CpG islands (−1473 to −1146, −685 to −491, −247 to −20, etc) that are methylated (Yang, Gozen, Vidensky, Robinson & Rothstein, 2010, Zschocke, Allritz, Engele & Rein, 2007), and as expected there are several evolutionarily conserved putative transcription factor binding sites in these regions (Su et al, 2003). There are a couple of studies to suggest that demethylation of the GLT-1 promoter is required for transcriptional activation. First, co-culturing neurons with astrocytes reduces methylation of the GLT-1 promoter and differential methylation is associated with different gel shifts by EMSA (Yang et al, 2010). Second, differential methylation of the GLT-1 promoter also contributes to the region-specific effects of glucocorticoids; in forebrain, where the promoter is hypo-methylated, they up-regulate GLT-1 expression and in brainstem/cerebellum, where the region is hyper-methylated, they have no effect (Zschocke et al, 2005)(Figure 3).
Several diseases that result in lower transporter expression are associated with changes in methylation. For example, hypermethylation of the GLT-1 promoter is observed in brain tumors. It has been suggested that this contributes to the decreased expression of GLT-1 observed with some of these tumors and that this decrease allows for excitotoxic expansion of the tumor (Groot, Liu, Fuller & Yung, 2005). Abnormal control of methylation underlies the basis of Rett syndrome, a neurodevelopmental disorder caused by mutations in the DNMT methyl-CpG-binding protein 2 (MeCP2), that results in dysregulation of both GLAST and GLT-1 (Amir, Van den Veyver, Wan, Tran, Francke & Zoghbi, 1999, Dunn & MacLeod, 2001, Guy, Hendrich, Holmes, Martin & Bird, 2001, Okabe, Takahashi, Mitsumasu, Kosai, Tanaka & Matsuishi, 2012). In patients who have died of ALS, there is evidence that hypermethylation of the GLT-1 promoter correlates with the decrease of GLT-1 expression (Yang et al, 2010).
Histone modifications also play an important role in epigenetic regulation. Two enzymes carry out histone modifications: histone acetyltransferases (HATs) and histone deacetylases (HDACs). HATs acetylate the ε-amino group of lysine residues in histones, and HDACs remove this acetyl group (for review see Kuo & Allis, 1998).
HDAC inhibitors like trichostatin A and valproic acid (VPA) increase the expression of EAATs. For example, VPA increases the levels of GLAST, GLT-1 and EAAC1 mRNA and protein in astrocytes and oligodendrocytes (Bianchi, Franchi-Gazzola, Reia, Allegri, Uggeri, Chiu et al, 2012, Hassel, Iversen, Gjerstad & Tauboll, 2001, Rosas et al, 2007). The fact that only 1 to 7% of all genes are thought to be regulated by HDACs (Butler & Bates, 2006) and that all the EAATs that had been studied to date are regulated by HDACs suggest that epigenetic regulation of this family of transporters may be important.
In addition to modification of histones, HATs and HDAC also acetylate and de-acetylate transcription factors. VPA decreases binding of the transcription factor YY1 to the GLAST promoter (Aguirre, Rosas, Lopez-Bayghen & Ortega, 2008) and increases GLAST mRNA and protein levels in cerebellum and hippocampus (Hassel et al, 2001, Rosas et al, 2007, Ueda & Willmore, 2000)(Figure 2A). There is also evidence that the HATs, p300 or p300/CBP associated factor, acetylate YY1, while HDACs de-acetylate YY1 (Yao, Yang & Seto, 2001). Although VPA is not a particularly selective drug, it is tempting to speculate that VPA regulates GLAST expression by modulating acetylation of YY1. This has not been directly tested.
VPA also regulates the expression of GLT-1, but the direction of the effect is region dependent. It increases expression in cortex and hippocampus and decreases expression in cerebellum (Perisic et al, 2012). There is evidence that this effect is influenced by methylation of the promoter; thus interactions between methylation and acetylation likely regulate GLT-1 expression.
4. Conclusions and summary
During the last 15 years, several groups examined the signals that regulate expression of the two astroglial Glu transporters. Together these studies identify a complex web of signals that either up- or down-regulate expression of these transporters. In some cases, these signals converge on seemingly common transcription factors. In other cases, the direction of an effect caused by one signal can be switched by the presence of a second signal. While many of the signals regulate both transporters, there are differences that may underlie the unique maturation-associated increases in GLT-1. Although it has not been the topic of extensive analysis, it seems likely that different populations of astrocytes engage different mechanisms to control expression of these transporters. Developing an understanding of the mechanisms involved may lead to new insights into the mechanisms that generate astrocyte heterogeneity. Epigenetic modifications seem likely to contribute to this differential control but this is still relatively unexplored. Virtually every neurologic disease is associated with altered expression of one or both of these transporters. Several recent studies have implicated specific pathways in the loss of transporters observed in various models of disease. While there is hope that this approach will lead to new therapies, it will certainly help define pathways that are dysregulated and thereby presumably lead to a better mechanistic understanding of the pathogenesis of disease. Remarkably, there have been relatively few analyses of the other three transporters. It is somewhat surprising that there have been so few analyses of EAAC1. This might be an ideal target given its known role in limiting oxidant-mediated damage, however the low evolutionary conservation in the promoter region may be hindering the identification of cis and trans elements involved in the regulation of transcription. This low conservation may also have implications for extensions to humans.
Acknowledgments
ZML is partially supported by a fellowship from Conacyt-Mexico. ZML and MBR are also supported by NIH Grant R01 NS092067. The authors would like to apologize to those authors whose work was not cited in this review article; we tried to fit in as much as possible.
I (Michael Robinson) would also like to thank Dr. Joseph Coyle for his mentorship and friendship. In early 1985, I accepted a post-doctoral position in his laboratory. Upon sharing this information with colleagues, I was immediately impressed by the fact that I repeatedly heard “What a great guy”. Of course, I also knew that I had been fortunate to land a position with an outstanding scientist. Joe and his group (including one of the editors of this special volume) were among the first investigators to demonstrate that excessive activation of Glu receptors is toxic to neurons; they also defined many of the mechanisms involved in this cell loss. Joe had also been involved in pioneering analyses of the monoamine transporters, the targets of many psychoactive molecules. I joined Joe’s laboratory to study the acidic dipeptide N-acetylaspartylglutamate (a topic of two chapters in this volume). During this time, many lasting friendships were formed. When a faculty position came along, I decided to establish a laboratory focused on analyses of Glu transporters because it was becoming clear that ‘excitotoxicity’ was likely involved in virtually all neurologic diseases. By this time, it had also become clear that Glu transporters were the only mechanism to clear extracellular Glu. Joe, our kids, and a group of colleagues have been enjoying an annual ‘retreat’ for just about 20 years doing the same thing that he and I did our Dads. I couldn’t have made a better choice for my post-doctoral position. I am grateful for his friendship and his unassuming inspirational style.
Abbreviations
- Aβ
Amyloid beta
- AEG
Astrocyte elevated gene-1
- ALS
Amyotrophic Lateral Sclerosis
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- AP-1
Activating protein-1
- ARE
Antioxidant response element
- ATRA
All trans retinoic acid
- BDNF
Brain derived neurotrophic factor
- bFGF
Basic fibroblast growth factor
- ChIP
Chromatin immunoprecipitation
- CREB
cAMP response element binding protein
- dbcAMP
dibutyryl-cyclic AMP
- DNMT
DNA methyltransferase
- dsRFP
Discosoma red fluorescent protein
- EAAC1
excitatory amino acid carrier-1
- EAATs
excitatory amino acid transporters
- EGF
Epidermal growth factor
- eGFP
enhanced green fluorescent protein
- EMSA
Electrophoretic mobility shift assay
- ERK
Extracellular signal-regulated kinase
- ET1
Endothelin 1
- FGF
Fibroblast growth factor
- GDNF
Glial cell line-derived neurotrophic factor
- GLAST
Glutamate/aspartate transporter
- GLT1
Glutamate transporter 1
- Glu
L-Glutamate
- GPR30
G protein-coupled receptor 30
- HAT
Histone acetyltransferase enzyme
- HDAC
Histone deacetylases
- HIV
Human immunodeficiency virus
- IGF-1
Insulin-like growth factor-1
- iGluRs
Ionotropic glutamate receptors
- JAK
Janus kinase
- KBBP
kappa-B motif-binding phosphoprotein
- LPS
lipopolysaccharide
- MeCP2
Methyl CpG binding protein 2
- mGluRs
Metabotropic glutamate receptors
- NFκB
Nuclear factor kappa-B
- Nrf2
Nuclear factor (erythroid-derived)-like 2
- MEK
Mitogen-extracellular signal regulated kinase
- PACAP
Pituitary adenylate cyclase-activating polypeptide
- PDGF
Platelet-derived growth factor
- PI3K
Phosphatidylinositol-4,5-bisphosphate 3 kinase
- PKA
Protein kinase A
- PKC
Protein kinase C
- PTEN
Phosphatase and tensin homolog
- RARβ
Retinoic acid receptor β
- RARE
Retinoic acid response element
- RFX1
Regulatory factor X 1
- RXR
Receptor X retinoide
- Sp1,Sp3
Stimulating protein 1 and 3
- STAT
Signal Transducer and Activator of Transcription
- TGFα
Transforming growth factor – α
- TNFα
Tumor necrosis factor α
- USF1
Upstream stimulating factor
- VPA
Valproic acid
- YY1
Ying Yang 1
Footnotes
Conflict of interest statement
The authors have no conflicts to declare.
References
- Aguirre G, Rosas S, Lopez-Bayghen E, Ortega A. Valproate-dependent transcriptional regulation of GLAST/EAAT1 expression: c-Yang 1. Neurochem Int. 2008;52:1322–1331. doi: 10.1016/j.neuint.2008.01.015. [DOI] [PubMed] [Google Scholar]
- Allritz C, Bette S, Figiel M, Engele J. Comparative structural and functional analysis of the GLT-1/EAAT-2 promoter from man and rat. J Neurosci Res. 2010;88:1234–1241. doi: 10.1002/jnr.22303. [DOI] [PubMed] [Google Scholar]
- Amin B, Hajhashemi V, Abnous K, Hosseinzadeh H. Ceftriaxone, a beta-lactam antibiotic, modulates apoptosis pathways and oxidative stress in a rat model of neuropathic pain. Biomed Res Int. 2014;2014:937568. doi: 10.1155/2014/937568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir RE, Van den Veyver IB, Wan M, Tran CQ, Francke U, Zoghbi HY. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet. 1999;23:185–188. doi: 10.1038/13810. [DOI] [PubMed] [Google Scholar]
- Anderson CM, Swanson RA. Astrocyte glutamate transport: review of properties, regulation, and physiological functions. Glia. 2000;32:1–14. [PubMed] [Google Scholar]
- Aoyama K, Nakaki T. Neuroprotective properties of the excitatory amino acid carrier 1 (EAAC1) Amino Acids. 2013;45:133–142. doi: 10.1007/s00726-013-1481-5. [DOI] [PubMed] [Google Scholar]
- Aoyama K, Suh SW, Hamby AM, Liu J, Chan WY, Chen Y, Swanson RA. Neuronal glutathione deficiency and age-dependent neurodegeneration in the EAAC1 deficient mouse. Nat Neurosci. 2006;9:119–126. doi: 10.1038/nn1609. [DOI] [PubMed] [Google Scholar]
- Apricó K, Beart PM, Crawford D, O’Shea RD. Binding and transport of [3H](2S,4R)-4-methylglutamate, a new ligand for glutamate transporters, demonstrate labeling of EAAT1 in cultured astrocytes. Journal of Neuroscience Research. 2004;75:751–759. doi: 10.1002/jnr.20013. [DOI] [PubMed] [Google Scholar]
- Aronica E, Gorter JA, Ljlst-Keizsers H, Rozemuller AJ, Yankaya B, Leenstra S, Troost D. Expression and functional role of mGluR3 and mGluR5 in human astrocytes and glioma cells: opposite regulation of glutamate transporter proteins. European Journal of Neuroscience. 2003;17:2106–2118. doi: 10.1046/j.1460-9568.2003.02657.x. [DOI] [PubMed] [Google Scholar]
- Arriza JL, Eliasof S, Kavanaugh MP, Amara SG. Excitatory amino acid transporter 5, a retinal glutamate transporter coupled to a chloride conductance. Proceedings of the National Academy of Sciences USA. 1997;94:4155–4160. doi: 10.1073/pnas.94.8.4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arriza JL, Fairman WA, Wadiche JI, Murdoch GH, Kavanaugh MP, Amara SG. Functional comparisons of three glutamate transporter subtypes cloned from human motor cortex. Journal of Neuroscience. 1994;14:5559–5569. doi: 10.1523/JNEUROSCI.14-09-05559.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autry AE, Grillo CA, Piroli GG, Rothstein JD, McEwen BS, Reagan LP. Glucocorticoid regulation of GLT-1 glutamate transporter isoform expression in the rat hippocampus. Neuroendocrinology. 2006;83:371–379. doi: 10.1159/000096092. [DOI] [PubMed] [Google Scholar]
- Azbill RD, Mu X, Springer JE. Riluzole increases high-affinity glutamate uptake in rat spinal cord synaptosomes. Brain Res. 2000;871:175–180. doi: 10.1016/s0006-8993(00)02430-6. [DOI] [PubMed] [Google Scholar]
- Balcar VJ, Johnston GAR. The structural specificity of the high affinity uptake of L-glutamate and L-aspartate by rat brain slices. Journal of Neurochemistry. 1972;19:2657–2666. doi: 10.1111/j.1471-4159.1972.tb01325.x. [DOI] [PubMed] [Google Scholar]
- Bar-Peled O, Ben-Hur H, Biegon A, Groner Y, Dewhurts S, Furata A, Rothstein JD. Distribution of glutamate transporter subtypes during human brain development. Journal of Neuro chemistry. 1997;69:2571–2580. doi: 10.1046/j.1471-4159.1997.69062571.x. [DOI] [PubMed] [Google Scholar]
- Baudry M, Bi X, Aguirre C. Progesterone-estrogen interactions in synaptic plasticity and neuroprotection. Neuroscience. 2013;239:280–294. doi: 10.1016/j.neuroscience.2012.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beal MF, Kowall NW, Ellison DW, Mazurek MF, Swartz KJ, Martin JB. Replication of the neurochemical characteristics of Huntingtons disease by quinolinic acid. Nature. 1986;321:168–171. doi: 10.1038/321168a0. [DOI] [PubMed] [Google Scholar]
- Beart PM. The autoradiographic localization of L-(3H) glutamate in synaptosomal preparations. Brain research. 1976;103:350–355. doi: 10.1016/0006-8993(76)90804-0. [DOI] [PubMed] [Google Scholar]
- Beart PM, O’Shea R D. Transporters for L-glutamate: An update on their molecular pharmacology and pathological involvement. Br J Pharmacol. 2006;150:5–17. doi: 10.1038/sj.bjp.0706949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benediktsson AM, Marrs GS, Tu JC, Worley PF, Rothstein JD, Bergles DE, Dailey ME. Neuronal activity regulates glutamate transporter dynamics in developing astrocytes. Glia. 2012;60:175–188. doi: 10.1002/glia.21249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste H, Drejer J, Schousboe A, Diemer NH. Elevation of the extracellular concentrations of glutamate and aspartate in rat hippocampus during transient cerebral ischemia monitored by intracerebral microdialysis. J Neurochem. 1984;43:1369–1374. doi: 10.1111/j.1471-4159.1984.tb05396.x. [DOI] [PubMed] [Google Scholar]
- Berger UV, Hediger MA. Distribution of the glutamate transporters GLT-1 (SLC1A2) and GLAST (SLC1A3) in peripheral organs. Anat Embryol (Berl) 2006;211:595–606. doi: 10.1007/s00429-006-0109-x. [DOI] [PubMed] [Google Scholar]
- Berman AE, Chan WY, Brennan AM, Reyes RC, Adler BL, Suh SW, Kauppinen TM, Edling Y, Swanson RA. N-acetylcysteine prevents loss of dopaminergic neurons in the EAAC1−/− mouse. Ann Neurol. 2011;69:509–520. doi: 10.1002/ana.22162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi MG, Franchi-Gazzola R, Reia L, Allegri M, Uggeri J, Chiu M, Sala R, Bussolati O. Valproic acid induces the glutamate transporter excitatory amino acid transporter-3 in human oligodendroglioma cells. Neuroscience. 2012;227:260–270. doi: 10.1016/j.neuroscience.2012.09.055. [DOI] [PubMed] [Google Scholar]
- Bianchi MG, Gazzola GC, Cagnin S, Kagechika H, Bussolati O. The ATRA- dependent overexpression of the glutamate transporter EAAC1 requires RARbeta induction. Biochim Biophys Acta. 2009;1788:1861–1868. doi: 10.1016/j.bbamem.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Bianchi MG, Gazzola GC, Tognazzi L, Bussolati O. C6 glioma cells differentiated by retinoic acid overexpress the glutamate transporter excitatory amino acid carrier 1 (EAAC1) Neuroscience. 2008;151:1042–1052. doi: 10.1016/j.neuroscience.2007.11.055. [DOI] [PubMed] [Google Scholar]
- Bonde C, Sarup A, Schousboe A, Gegelashvili G, Noraberg J, Zimmer J. GDNF pre-treatment aggravates neuronal cell loss in oxygen-glucose deprived hippocampal slice cultures: a possible effect of glutamate transporter up-regulation. Neurochem Int. 2003;43:381–388. doi: 10.1016/s0197-0186(03)00025-1. [DOI] [PubMed] [Google Scholar]
- Boycott HE, Wilkinson JA, Boyle JP, Pearson HA, Peers C. Differential involvement of TNF alpha in hypoxic suppression of astrocyte glutamate transporters. Glia. 2008;56:998–1004. doi: 10.1002/glia.20673. [DOI] [PubMed] [Google Scholar]
- Bringmann A, Pannicke T, Biedermann B, Francke M, Iandiev I, Grosche J, Wiedemann P, Albrecht J, Reichenbach A. Role of retinal glial cells in neurotransmitter uptake and metabolism. Neurochem Int. 2009;54:143–160. doi: 10.1016/j.neuint.2008.10.014. [DOI] [PubMed] [Google Scholar]
- Brito VI, Rozanski VE, Beyer C, Kuppers E. Dopamine regulates the expression of the glutamate transporter GLT1 but not GLAST in developing striatal astrocytes. J Mol Neurosci. 2009;39:372–379. doi: 10.1007/s12031-009-9273-9. [DOI] [PubMed] [Google Scholar]
- Brosnan JT, Brosnan ME. Glutamate: a truly functional amino acid. Amino Acids. 2013;45:413–418. doi: 10.1007/s00726-012-1280-4. [DOI] [PubMed] [Google Scholar]
- Burnashev N, Khodorova A, Jonas P, Helm PJ, Wisden W, Monyer H, Seeburg PH, Sakmann B. Calcium-permeable AMPA-kainate receptors in fusiform cerebellar glial cells. Science. 1992;256:1566–1570. doi: 10.1126/science.1317970. [DOI] [PubMed] [Google Scholar]
- Butler R, Bates GP. Histone deacetylase inhibitors as therapeutics for polyglutamine disorders. Nat Rev Neurosci. 2006;7:784–796. doi: 10.1038/nrn1989. [DOI] [PubMed] [Google Scholar]
- Cagampang FRA, Rattray M, Powell JF, Chong NWS, Campbell IC, Coen CW. Circadian variation of EAAC1 glutamate transporter messenger RNA in the rat suprachaismatic nuclei. Molecular Brain Research. 1996;35:190–196. doi: 10.1016/0169-328x(95)00203-5. [DOI] [PubMed] [Google Scholar]
- Canolle B, Masmejean F, Melon C, Nieoullon A, Pisano P, Lortet S. Glial soluble factors regulate the activity and expression of the neuronal glutamate transporter EAAC1: implication of cholesterol. J Neurochem. 2004;88:1521–1532. doi: 10.1046/j.1471-4159.2003.02301.x. [DOI] [PubMed] [Google Scholar]
- Carbone M, Duty S, Rattray M. Riluzole elevates GLT-1 activity and levels in striatal astrocytes. Neurochem Int. 2012;60:31–38. doi: 10.1016/j.neuint.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Coronel Y, Del Razo LM, Huerta M, Hernandez-Lopez A, Ortega A, Lopez-Bayghen E. Arsenite exposure downregulates EAAT1/GLAST transporter expression in glial cells. Toxicol Sci. 2011;122:539–550. doi: 10.1093/toxsci/kfr126. [DOI] [PubMed] [Google Scholar]
- Chan TJ, Her LS, Liaw HJ, Chen MC, Tzeng SF. Retinoic acid mediates the expression of glutamate transporter-1 in rat astrocytes through genomic RXR action and non-genomic protein kinase C signaling pathway. J Neurochem. 2012;121:537–550. doi: 10.1111/j.1471-4159.2012.07715.x. [DOI] [PubMed] [Google Scholar]
- Chaudhry FA, Lehre KP, Campagne MVL, Ottersen OP, Danbolt NC, Storm-Mathisen J. Glutamate transporters in glial plasma membranes: Highly differentiated localizations revealed by quantitative ultrastructural immunocytochemistry. Neuron. 1995;15:711–720. doi: 10.1016/0896-6273(95)90158-2. [DOI] [PubMed] [Google Scholar]
- Chen JC, Hsu-Chou H, Lu JL, Chiang YC, Huang HM, Wang HL, Wu T, Liao JJ, Yeh TS. Down-regulation of the glial glutamate transporter GLT-1 in rat hippocampus and striatum and its modulation by a group III metabotropic glutamate receptor antagonist following transient global forebrain ischemia. Neuropharmacology. 2005;49:703–714. doi: 10.1016/j.neuropharm.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Cheung NS, Pascoe CJ, Giardina SF, John CA, Beart PM. Micromolar L-glutamate induces extensive apoptosis in an apoptotic-necrotic continuum of insult-dependent, excitotoxic injury in cultured cortical neurones. Neuropharmacology. 1998;37:1419–1429. doi: 10.1016/s0028-3908(98)00123-3. [DOI] [PubMed] [Google Scholar]
- Choi DW. Excitotoxic cell death. Journal of Neurobiology. 1992;23:1261–1276. doi: 10.1002/neu.480230915. [DOI] [PubMed] [Google Scholar]
- Conti F, Weinberg RJ. Shaping excitation at glutamatergic synapses. Trends in Neurosciences. 1999;22:451–458. doi: 10.1016/s0166-2236(99)01445-9. [DOI] [PubMed] [Google Scholar]
- Coyle JT, Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science. 1993;262:689–695. doi: 10.1126/science.7901908. [DOI] [PubMed] [Google Scholar]
- Cudkowicz ME, Titus S, Kearney M, Yu H, Sherman A, Schoenfeld D, Hayden D, Shui A, Brooks B, Conwit R, Felsenstein D, Greenblatt DJ, Keroack M, Kissel JT, Miller R, Rosenfeld J, Rothstein JD, Simpson E, Tolkoff-Rubin N, Zinman L, Shefner JM, Ceftriaxone Study I. Safety and efficacy of ceftriaxone for amyotrophic lateral sclerosis: a multi-stage, randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2014;13:1083–1091. doi: 10.1016/S1474-4422(14)70222-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui C, Cui Y, Gao J, Sun L, Wang Y, Wang K, Li R, Tian Y, Song S, Cui J. Neuroprotective effect of ceftriaxone in a rat model of traumatic brain injury. Neurol Sci. 2014;35:695–700. doi: 10.1007/s10072-013-1585-4. [DOI] [PubMed] [Google Scholar]
- Danbolt NC. The high affinity uptake system for excitatory amino acids in the brain. Progress in Neurobiology. 1994;44:377–396. doi: 10.1016/0301-0082(94)90033-7. [DOI] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Progress in Neurobiology. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Dehnes Y, Chaudhry FA, Ullensvang K, Lehre KP, Storm-Mathisen J, Danbolt NC. The glutamate transporter EAAT4 in rat cerebellar Purkinje cells: a glutamate- gated chloride channel concentrated near the synapse in the parts of the dendritic membrane facing astroglia. Journal of Neuroscience. 1998;18:3606–3610. doi: 10.1523/JNEUROSCI.18-10-03606.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSilva TM, Kabakov AY, Goldhoff PE, Volpe JJ, Rosenberg PA. Regulation of glutamate transport in developing rat oligodendrocytes. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29:7898–7908. doi: 10.1523/JNEUROSCI.6129-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhandapani KM, Wade FM, Mahesh VB, Brann DW. Astrocyte-derived transforming growth factor-{beta} mediates the neuroprotective effects of 17{beta}- estradiol: involvement of nonclassical genomic signaling pathways. Endocrinology. 2005;146:2749–2759. doi: 10.1210/en.2005-0014. [DOI] [PubMed] [Google Scholar]
- Dienel GA, McKenna MC. A dogma-breaking concept: glutamate oxidation in astrocytes is the source of lactate during aerobic glycolysis in resting subjects. J Neurochem. 2014;131:395–398. doi: 10.1111/jnc.12835. [DOI] [PubMed] [Google Scholar]
- Domerq M, Sánchez-Gómez MV, Areso P, Matute C. Expression of glutamate transporters in rat opitc nerve oligodendrocytes. European Journal of Neuroscience. 1999;11:2226–2236. doi: 10.1046/j.1460-9568.1999.00639.x. [DOI] [PubMed] [Google Scholar]
- Drejer J, Meier E, Schousboe A. Novel neuron-related regulatory mechanisms for astrocytic glutamate and GABA high affinity uptake. Neuroscience letters. 1983;37:301–306. doi: 10.1016/0304-3940(83)90448-2. [DOI] [PubMed] [Google Scholar]
- Dunlop J. Glutamate-based therapeutic approaches: targeting the glutamate transport system. CurrOpin Pharmacol. 2006;6:103–107. doi: 10.1016/j.coph.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Dunn HG, MacLeod PM. Rett syndrome: review of biological abnormalities. Can J Neurol Sci. 2001;28:16–29. doi: 10.1017/s0317167100052513. [DOI] [PubMed] [Google Scholar]
- Eng DL, Lee YL, Lal PG. Expression of glutamate uptake transporters after dibutyryl cyclic AMP differentiation and traumatic injury in cultured astrocytes. Brain Research. 1997;778:215–221. doi: 10.1016/s0006-8993(97)01093-7. [DOI] [PubMed] [Google Scholar]
- Escartin C, Won SJ, Malgorn C, Auregan G, Berman AE, Chen PC, Deglon N, Johnson JA, Suh SW, Swanson RA. Nuclear factor erythroid 2-related factor 2 facilitates neuronal glutathione synthesis by upregulating neuronal excitatory amino acid transporter 3 expression. J Neurosci. 2011;31:7392–7401. doi: 10.1523/JNEUROSCI.6577-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faden AI, Demediuk P, Panter SS, Vink R. The role of excitatatory amino acids and NMDA receptors in traumatic brain injury. Science. 1989;244:798–800. doi: 10.1126/science.2567056. [DOI] [PubMed] [Google Scholar]
- Faden AI, Demediuk P, Panter SS, Vink R. The role of excitatory amino acids and NMDA receptors in traumatic brain injury. Science. 1989;244:798–800. doi: 10.1126/science.2567056. [DOI] [PubMed] [Google Scholar]
- Fagg GE, Mena EE, Cotman CW. L-glutamate receptor populations in synaptic membranes: effects of ions and pharmacological characteristics. Adv Biochem Psychopharmacol. 1983;37:199–209. [PubMed] [Google Scholar]
- Fairman WA, Vandenberg RJ, Arriza JL, Kavanaugh MP, Amara SG. An excitatory amino acid transporter with properties of a ligand-gated chloride channel. Nature (Lond.) 1995;375:599–603. doi: 10.1038/375599a0. [DOI] [PubMed] [Google Scholar]
- Fang J, Han D, Hong J, Tan Q, Tian Y. The chemokine, macrophage inflammatory protein-2gamma, reduces the expression of glutamate transporter-1 on astrocytes and increases neuronal sensitivity to glutamate excitotoxicity. J Neuroinflammation. 2012;9:267. doi: 10.1186/1742-2094-9-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng D, Guo B, Liu G, Wang B, Wang W, Gao G, Qin H, Wu S. FGF2 alleviates PTSD symptoms in rats by restoring GLAST function in astrocytes via the JAK/STAT pathway. Eur Neuropsychopharmacol. 2015;25:1287–1299. doi: 10.1016/j.euroneuro.2015.04.020. [DOI] [PubMed] [Google Scholar]
- Figiel M, Engele J. Pituitary adenylate cyclase-activating polypeptide (PACAP), a neuron-derived peptide regulating glial glutamate transport and metabolism. Journal of Neuroscience. 2000;20:3596–3605. doi: 10.1523/JNEUROSCI.20-10-03596.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figiel M, Maucher T, Rozyczka J, Bayatti N, Engele J. Regulation of glial glutamate transporter expression by growth factors. Exp Neurol. 2003;183:124–135. doi: 10.1016/s0014-4886(03)00134-1. [DOI] [PubMed] [Google Scholar]
- Fontana AC. Current approaches to enhance glutamate transporter function and expression. J Neurochem. 2015;134:982–1007. doi: 10.1111/jnc.13200. [DOI] [PubMed] [Google Scholar]
- Freeman MR. Specification and morphogenesis of astrocytes. Science. 2010;330:774–778. doi: 10.1126/science.1190928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frizzo ME, Frizzo JK, Amadio S, Rodrigues JM, Perry ML, Bernardi G, Volonte C. Extracellular adenosine triphosphate induces glutamate transporter-1 expression in hippocampus. Hippocampus. 2007;17:305–315. doi: 10.1002/hipo.20269. [DOI] [PubMed] [Google Scholar]
- Furness DN, Dehnes Y, Akhtar AQ, Rossi DJ, Hamann M, Grutle NJ, Gundersen V, Holmseth S, Lehre KP, Ullensvang K, Wojewodzic M, Zhou Y, Attwell D, Danbolt NC. A quantitative assessment of glutamate uptake into hippocampal synaptic terminals and astrocytes: new insights into a neuronal role for excitatory amino acid transporter 2 (EAAT2) Neuroscience. 2008;157:80–94. doi: 10.1016/j.neuroscience.2008.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta A, Martin LJ, Lin C-LG, Dykes-Hoberg M, Rothstein JD. Cellular and synaptic localization of the neuronal glutamate transporters EAAT3 and EAAT4. Neuroscience. 1997;81:1031–1042. doi: 10.1016/s0306-4522(97)00252-2. [DOI] [PubMed] [Google Scholar]
- Furuta A, Rothstein JD, Martin LJ. Glutamate transporter protein subtypes are expressed differentially during rat central nervous system development. Journal of Neuroscience. 1997;17:8363–8375. doi: 10.1523/JNEUROSCI.17-21-08363.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gegelashvili G, Civenni G, Racagni G, Danbolt NC, Schousboe I, Schousboe A. Glutamate receptor agonists up-regulate glutamate transporter GLAST in astrocytes. NeuroReport. 1996;8:261–265. doi: 10.1097/00001756-199612200-00052. [DOI] [PubMed] [Google Scholar]
- Gegelashvili G, Danbolt NC, Schousboe A. Neuronal soluble factors differentially regulate the expression of the GLT1 and GLAST glutamate transporters in cultured astroglia. J Neurochem. 1997;69:2612–2615. doi: 10.1046/j.1471-4159.1997.69062612.x. [DOI] [PubMed] [Google Scholar]
- Gegelashvili G, Dehnes Y, Danbolt NC, Schousboe A. The high-affinity glutamate transporters GLT1, GLAST, and EAAT4 are regulated via different signalling mechanisms. Neurochemistry International. 2000;37:163–170. doi: 10.1016/s0197-0186(00)00019-x. [DOI] [PubMed] [Google Scholar]
- Gegelashvili G, Schousboe A. High affinity glutamate transporters: regulation of expression and activity. Mol Pharmacol. 1997;52:6–15. doi: 10.1124/mol.52.1.6. [DOI] [PubMed] [Google Scholar]
- Gegelashvili G, Schousboe A. Cellular distribution and kinetic properties of high-affinity glutamate transporters. Brain Res Bull. 1998;45:233–238. doi: 10.1016/s0361-9230(97)00417-6. [DOI] [PubMed] [Google Scholar]
- Ghosh M, Lane M, Krizman E, Sattler R, Rothstein JD, Robinson MB. Transcription Factor Pax6 Contributes to Induction of GLT-1 Expression in Astrocytes Through an Interaction with a Distal Enhancer Element. J Neurochem. 2015 doi: 10.1111/jnc.13406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh M, Yang Y, Rothstein JD, Robinson MB. Nuclear Factor-kB contributes to neuron-dependent induction of GLT-1 expression in astrocytes. Journal of Neuroscience. 2011;31:9159–9169. doi: 10.1523/JNEUROSCI.0302-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill R, Foster AC, Woodruff GN. Systemic administration of MK-801 protects against ischemia-induced hippocampal neurodegeneration in the gerbil. The Journal of neuroscience: the official journal ofthe Society for Neuroscience. 1987;7:3343–3349. doi: 10.1523/JNEUROSCI.07-10-03343.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gincel D, Regan MR, Jin L, Watkins AM, Bergles DE, Rothstein JD. Analysis of cerebellar Purkinje cells using EAAT4 glutamate transporter promoter reporter in mice generated via bacterial artificial chromosome-mediated transgenesis. Exp Neurol. 2007;203:205–212. doi: 10.1016/j.expneurol.2006.08.016. [DOI] [PubMed] [Google Scholar]
- Greene JG, Greenamyre JT. Bioenergetics and glutamate excitotoxicity. Progress in Neurobiology. 1996;48:613–634. doi: 10.1016/0301-0082(96)00006-8. [DOI] [PubMed] [Google Scholar]
- Groot JFd, Liu TJ, Fuller G, Yung WKA. The excitatory amino acid transporter-2 induces apoptosis and decreases glioma growth in vitro and in vivo. Cancer Research. 2005;65:1934–1940. doi: 10.1158/0008-5472.CAN-04-3626. [DOI] [PubMed] [Google Scholar]
- Gupta RK, Prasad S. Differential regulation of GLT-1/EAAT2 gene expression by NF-kappaB and N-myc in male mouse brain during postnatal development. Neurochem Res. 2014;39:150–160. doi: 10.1007/s11064-013-1200-3. [DOI] [PubMed] [Google Scholar]
- Guy J, Hendrich B, Holmes M, Martin JE, Bird A. A mouse Mecp2-null mutation causes neurological symptoms that mimic Rett syndrome. Nat Genet. 2001;27:322–326. doi: 10.1038/85899. [DOI] [PubMed] [Google Scholar]
- Had-Aissouni L. Toward a new role for plasma membrane sodium-dependent glutamate transporters of astrocytes: maintenance of antioxidant defenses beyond extracellular glutamate clearance. Amino Acids. 2012;42:181–197. doi: 10.1007/s00726-011-0863-9. [DOI] [PubMed] [Google Scholar]
- Hagiwara T, Tanaka K, Takai S, Maeno-Hikichi Y, Mukainaka Y, Wada K. Genomic organization, promoter analysis, and chromosomal localization of the gene for the mouse glial high-affinity glutamate transporter Slc1a3. Genomics. 1996;33:508–515. doi: 10.1006/geno.1996.0226. [DOI] [PubMed] [Google Scholar]
- Hassel B, Iversen EG, Gjerstad L, Tauboll E. Up-regulation of hippocampal glutamate transport during chronic treatment with sodium valproate. J Neurochem. 2001;77:1285–1292. doi: 10.1046/j.1471-4159.2001.00349.x. [DOI] [PubMed] [Google Scholar]
- Herman MA, Jahr CE. Extracellular glutamate concentration in hippocampal slice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:9736–9741. doi: 10.1523/JNEUROSCI.3009-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz L, Bock E, Schousboe A. GFA Content, Glutamate Uptake and Activity of Glutamate Metabolizing Enzymes in Differentiating Mouse Astrocytes in Primary Cultures. Developmental Neuroscience. 1978;1:226–238. [Google Scholar]
- Hollman M, Heinemann S. Cloned glutamate receptors. Annual Review of Neuroscience. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- Hsu CY, Hung CS, Chang HM, Liao WC, Ho SC, Ho YJ. Ceftriaxone prevents and reverses behavioral and neuronal deficits in an MPTP-induced animal model of Parkinson’s disease dementia. Neuropharmacology. 2015;91:43–56. doi: 10.1016/j.neuropharm.2014.11.023. [DOI] [PubMed] [Google Scholar]
- Huang YH, Bergles DE. Glutamate transporters bring competition to the synapse. Current Opinion in Neurobiology. 2004;14:346–352. doi: 10.1016/j.conb.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Inui T, Alessandri B, Heimann A, Nishimura F, Frauenknecht K, Sommer C, Kempski O. Neuroprotective effect of ceftriaxone on the penumbra in a rat venous ischemia model. Neuroscience. 2013;242:1–10. doi: 10.1016/j.neuroscience.2013.03.018. [DOI] [PubMed] [Google Scholar]
- Jansson LC, Akerman KE. The role of glutamate and its receptors in the proliferation, migration, differentiation and survival of neural progenitor cells. J Neurael Transm (Vienna) 2014;121:819–836. doi: 10.1007/s00702-014-1174-6. [DOI] [PubMed] [Google Scholar]
- Kanai Y, Hediger MA. Primary structure and functional characterization of a high-affinity glutamate transporter. Nature. 1992;360:467–471. doi: 10.1038/360467a0. [DOI] [PubMed] [Google Scholar]
- Kang DC, Su ZZ, Sarkar D, Emdad L, Volsky DJ, Fisher PB. Cloning and characterization of HIV-1-inducible astrocyte elevated gene-1, AEG-1. Gene. 2005;353:8–15. doi: 10.1016/j.gene.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Kanner BI. Structure and function of sodium-coupled GABA and glutamate transporters. J Membr Biol. 2006;213:89–100. doi: 10.1007/s00232-006-0877-5. [DOI] [PubMed] [Google Scholar]
- Karki P, Smith K, Johnson J, Jr, Lee E. Astrocyte-derived growth factors and estrogen neuroprotection: role of transforming growth factor-alpha in estrogen-induced upregulation of glutamate transporters in astrocytes. Mol Cell Endocrinol. 2014;389:58–64. doi: 10.1016/j.mce.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karki P, Webb A, Smith K, Johnson J, Jr, Lee K, Son DS, Aschner M, Lee E. Yin Yang 1 is a repressor of glutamate transporter EAAT2, and it mediates manganese-induced decrease of EAAT2 expression in astrocytes. Mol Cell Biol. 2014;34:1280–1289. doi: 10.1128/MCB.01176-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karki P, Webb A, Smith K, Lee K, Son DS, Aschner M, Lee E. cAMP response element-binding protein (CREB) and nuclear factor kappaB mediate the tamoxifen-induced up-regulation of glutamate transporter 1 (GLT-1) in rat astrocytes. J Biol Chem. 2013;288:28975–28986. doi: 10.1074/jbc.M113.483826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karki P, Webb A, Zerguine A, Choi J, Son DS, Lee E. Mechanism of raloxifene-induced upregulation of glutamate transporters in rat primary astrocytes. Glia. 2014;62:1270–1283. doi: 10.1002/glia.22679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Lee SG, Kegelman TP, Su ZZ, Das SK, Dash R, Dasgupta S, Barral PM, Hedvat M, Diaz P, Reed JC, Stebbins JL, Pellecchia M, Sarkar D, Fisher PB. Role of excitatory amino acid transporter-2 (EAAT2) and glutamate in neurodegeneration: opportunities for developing novel therapeutics. J Cell Physiol. 2011;226:2484–2493. doi: 10.1002/jcp.22609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S-Y, Choi S-Y, Chao W, Volsky DJ. Transcriptional regulation of human excitatory amino acid transporter 1 (EAAT1): cloning of the EAAT1 promoter and characterization of its basal and inducible activity in human astrocytes. journal of Neurochemistry. 2003;87:1485–1498. doi: 10.1046/j.1471-4159.2003.02128.x. [DOI] [PubMed] [Google Scholar]
- Kirschner MA, Copeland NG, Gilbert DJ, Jenkins NA, Amara SG. Mouse excitatory amino acid transporter EAAT2: isolation, characterization, and proximity to neuroexcitability loci on mouse chromosome 2. Genomics. 1994;24:218–224. doi: 10.1006/geno.1994.1609. [DOI] [PubMed] [Google Scholar]
- Korn T, Magnus T, Jung S. Autoantigen specific T cells inhibit glutamate uptake in astrocytes by decreasing expression of astrocytic glutamate transporter GLAST: a mechanism mediated by tumor necrosis factor-alpha. FASEB J. 2005;19:1878–1880. doi: 10.1096/fj.05-3748fje. [DOI] [PubMed] [Google Scholar]
- Kuo MH, Allis CD. Roles of histone acetyltransferases and deacetylases in gene regulation. Bioessays. 1998;20:615–626. doi: 10.1002/(SICI)1521-1878(199808)20:8<615::AID-BIES4>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Kwakye GF, Paoliello MM, Mukhopadhyay S, Bowman AB, Aschner M. Manganese-Induced Parkinsonism and Parkinson’s Disease: Shared and Distinguishable Features. Int J Environ Res Public Health. 2015;12:7519–7540. doi: 10.3390/ijerph120707519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau CL, Kovacevic M, Tingleff TS, Forsythe JS, Cate HS, Merlo D, Cederfur C, Maclean FL, Parish CL, Horne MK, Nisbet DR, Beart PM. 3D Electrospun scaffolds promote a cytotrophic phenotype of cultured primary astrocytes. Journal of neurochemistry. 2014;130:215–226. doi: 10.1111/jnc.12702. [DOI] [PubMed] [Google Scholar]
- Lau CL, O’Shea RD, Broberg BV, Bischof L, Beart PM. The Rho kinase inhibitor Fasudil up-regulates astrocytic glutamate transport subsequent to actin remodelling in murine cultured astrocytes. Br J Pharmacol. 2011;163:533–545. doi: 10.1111/j.1476-5381.2011.01259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau CL, Perreau VM, Chen MJ, Cate HS, Merlo D, Cheung NS, OShea RD, Beart PM. Transcriptomic profiling of astrocytes treated with the Rho kinase inhibitor fasudil reveals cytoskeletal and pro-survival responses. Journal of cellular physiology. 2012;227:1199–1211. doi: 10.1002/jcp.22838. [DOI] [PubMed] [Google Scholar]
- Lee A, Anderson AR, Stevens M, Beasley S, Barnett NL, Pow DV. Excitatory amino acid transporter 5 is widely expressed in peripheral tissues. European journal of histochemistry. 2013;57:e11. doi: 10.4081/ejh.2013.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E, Sidoryk-Wegrzynowicz M, Wang N, Webb A, Son DS, Lee K, Aschner M. GPR30 regulates glutamate transporter GLT-1 expression in rat primary astrocytes. J Biol Chem. 2012;287:26817–26828. doi: 10.1074/jbc.M112.341867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee ES, Sidoryk M, Jiang H, Yin Z, Aschner M. Estrogen and tamoxifen reverse manganese-induced glutamate transporter impairment in astrocytes. J Neurochem. 2009;110:530–544. doi: 10.1111/j.1471-4159.2009.06105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SG, Su ZZ, Emdad L, Gupta P, Sarkar D, Borjabad A, Volsky DJ, Fisher PB. Mechanism of ceftriaxone induction of excitatory amino acid transporter-2 expression and glutamate uptake in primary human astrocytes. J Biol Chem. 2008;283:13116–13123. doi: 10.1074/jbc.M707697200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehre KP, Levy LM, Ottersen OP, Storm-Mathisen J, Danbolt NC. Differential expression of two glial glutamate transporters in the rat brain: Quantitative and immunocytochemical observations. Journal of Neuroscience. 1995;15:1835–1853. doi: 10.1523/JNEUROSCI.15-03-01835.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy LM, Warr O, Attwell D. Stoichiometry of the glial glutamte transporter GLT-1 expressed inducibly in a chinese hamster ovary cell line selected for low endogenous Na+-dependent glutamate uptake. Journal of Neuroscience. 1998;18:9620–9628. doi: 10.1523/JNEUROSCI.18-23-09620.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K, Hala TJ, Seetharam S, Poulsen DJ, Wright MC, Lepore AC. GLT1 overexpression in SOD1(G93A) mouse cervical spinal cord does not preserve diaphragm function or extend disease. Neurobiol Dis. 2015;78:12–23. doi: 10.1016/j.nbd.2015.03.010. [DOI] [PubMed] [Google Scholar]
- Li L-B, Toan SV, Zelenaia O, Watson DJ, Wolfe JH, Rothstein JD, Robinson MB. Regulation of astrocytic glutamate transporter expression by Akt: Evidence for a selective transcriptional effect on the GLT-1/EAAT2 subtype. Journal of Neuro chemistry. 2006;97:759–771. doi: 10.1111/j.1471-4159.2006.03743.x. [DOI] [PubMed] [Google Scholar]
- Li X, Francke U. Assignment of the gene SLC1A2 coding for the human glutamate transporter EAAT2 to human chromosome 11 bands p13-p12. Cytogenet Cell Genet. 1995;71:212–213. doi: 10.1159/000134111. [DOI] [PubMed] [Google Scholar]
- Li Y, Sattler R, Yang EJ, Nunes A, Ayukawa Y, Akhtar S, Ji G, Zhang PW, Rothstein JD. Harmine, a natural beta-carboline alkaloid, upregulates astroglial glutamate transporter expression. Neuropharmacology. 2011;60:1168–1175. doi: 10.1016/j.neuropharm.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CH, You JR, Wei KC, Gean PW. Stimulating ERK/PI3K/NFkappaB signaling pathways upon activation of mGluR2/3 restores OGD-induced impairment in glutamate clearance in astrocytes. Eur J Neurosci. 2014;39:83–96. doi: 10.1111/ejn.12383. [DOI] [PubMed] [Google Scholar]
- Liu XG, Sayed H, Lu MDS, Yang Y, Zhou T, Chen D, Wang Z, Wang H, XuJ C. cAMP/PKA/CREB/GLT1 signaling involved in the antidepressant-like effects of phosphodiesterase 4D inihibitor (GEBR-7b) in rats. Neuropsychiatric Disease and Treatment. 2015:1–9. doi: 10.2147/NDT.S90960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan WJ, Snyder SH. Unique high affinity uptake systems for glycine, glutamic and aspartic acids in central nervous tissue of the rat. Nature. 1971;234:297–299. doi: 10.1038/234297b0. [DOI] [PubMed] [Google Scholar]
- Lopez-Bayghen E, Espinoza-Rojo M, Ortega A. Glutamate down-regulates GLAST expression through AMPA receptors in Bergmann glial cells. Molecular Brain Research. 2003;115:1–9. doi: 10.1016/s0169-328x(03)00136-0. [DOI] [PubMed] [Google Scholar]
- Lopez-Bayghen E, Ortega A. Glutamate-dependent transcriptional regulation of GLAST: role of PKC. J Neurochem. 2004;91:200–209. doi: 10.1111/j.1471-4159.2004.02706.x. [DOI] [PubMed] [Google Scholar]
- Lopez-Redondo F, Nakajima K, Honda S, Kohsaka S. Glutamate transporter GLT-1 is highly expressed in activated microglia following facial nerve axotomy. Brain Res Mol Brain Res. 2000;76:429–435. doi: 10.1016/s0169-328x(00)00022-x. [DOI] [PubMed] [Google Scholar]
- Lujan R, Shigemoto R, Lopez-Bendito G. Glutamate and GABA receptor signalling in the developing brain. Neuroscience. 2005;130:567–580. doi: 10.1016/j.neuroscience.2004.09.042. [DOI] [PubMed] [Google Scholar]
- Luo J, Grammas P. Endothelin-1 is elevated in Alzheimers disease brain microvessels and is neuroprotective. J Alzheimers Dis. 2010;21:887–896. doi: 10.3233/JAD-2010-091486. [DOI] [PubMed] [Google Scholar]
- Lyon L, Kew JN, Corti C, Harrison PJ, Burnet PW. Altered hippocampal expression of glutamate receptors and transporters in GRM2 and GRM3 knockout mice. Synapse. 2008;62:842–850. doi: 10.1002/syn.20553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma K, Zheng S, Zuo Z. The transcription factor regulatory factor X1 increases the expression of neuronal glutamate transporter type 3. J Biol Chem. 2006;281:21250–21255. doi: 10.1074/jbc.M600521200. [DOI] [PubMed] [Google Scholar]
- Marcaggi P, Attwell D. Role of glial amino acid transporters in synaptic transmission and brain energetics. Glia. 2004;47:217–225. doi: 10.1002/glia.20027. [DOI] [PubMed] [Google Scholar]
- Martinez-Lozada Z, Waggener CT, Kim K, Zou S, Knapp PE, Hayashi Y, Ortega A, Fuss B. Activation of sodium-dependent glutamate transporters regulates the morphological aspects of oligodendrocyte maturation via signaling through calcium/calmodulin-dependent kinase IIbetas actin-binding/-stabilizing domain. Glia. 2014;62:1543–1558. doi: 10.1002/glia.22699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matos M, Augusto E, Oliveira CR, Agostinho P. Amyloid-beta peptide decreases glutamate uptake in cultured astrocytes: involvement of oxidative stress and mitogen-activated protein kinase cascades. Neuroscience. 2008;156:898–910. doi: 10.1016/j.neuroscience.2008.08.022. [DOI] [PubMed] [Google Scholar]
- Matyash V, Kettenmann H. Heterogeneity in astrocyte morphology and physiology. Brain Res Rev. 2010;63:2–10. doi: 10.1016/j.brainresrev.2009.12.001. [DOI] [PubMed] [Google Scholar]
- McDonald JW, Johnston MV. Physiological and pathophysiological roles of excitatory amino acids during central nervous system development. Brain Research Reviews. 1990;15:41–70. doi: 10.1016/0165-0173(90)90011-c. [DOI] [PubMed] [Google Scholar]
- Moonen G, Heinen E, Goessens G. Comparative ultrastructural study of the effects of serum-free medium and dibutyryl-cyclic AMP on newborn rat astroblasts. Cell Tissue Res. 1976;167:221–227. doi: 10.1007/BF00224329. [DOI] [PubMed] [Google Scholar]
- Murphy-Royal C, Dupuis JP, Varela JA, Panatier A, Pinson B, Baufreton J, Groc L, Oliet SH. Surface diffusion of astrocytic glutamate transporters shapes synaptic transmission. Nat Neurosci. 2015;18:219–226. doi: 10.1038/nn.3901. [DOI] [PubMed] [Google Scholar]
- Nakajima K, Yamamoto S, Kohsaka S, Kurihara T. Neuronal stimulation leading to upregulation of glutamate transporter-1 (GLT-1) in rat microglia in vitro. Neurosci Lett. 2008;436:331–334. doi: 10.1016/j.neulet.2008.03.058. [DOI] [PubMed] [Google Scholar]
- Nakanishi S. Molecular diversity of glutamate receptors and implications for brain function. Science. 1992;258:597–603. doi: 10.1126/science.1329206. [DOI] [PubMed] [Google Scholar]
- Nakanishi S. Metabotropic glutamate receptors: Synaptic transmission, modulation, and plasticity. Neuron. 1994;13:1031–1037. doi: 10.1016/0896-6273(94)90043-4. [DOI] [PubMed] [Google Scholar]
- Nakayama T, Kawakami H, Tanaka K, Nakamura S. Expression of three glutamate transporter subtype mRNAs in human brain regions and peripheral tissues. Brain Res Mol Brain Res. 1996;36:189–192. doi: 10.1016/0169-328x(95)00297-6. [DOI] [PubMed] [Google Scholar]
- Nguyen L, Rigo JM, Rocher V, Belachew S, Malgrange B, Rogister B, Leprince P, Moonen G. Neurotransmitters as early signals for central nervous system development. Cell Tissue Res. 2001;305:187–202. doi: 10.1007/s004410000343. [DOI] [PubMed] [Google Scholar]
- Nicoletti F, Bruno V, Ngomba RT, Gradini R, Battaglia G. Metabotropic glutamate receptors as drug targets: whats new? Curr Opin Pharmacol. 2015;20:89–94. doi: 10.1016/j.coph.2014.12.002. [DOI] [PubMed] [Google Scholar]
- O’Shea RD, Lau CL, Farso MC, Diwakarla S, Zagami CJ, Svendsen BB, Feeney SJ, Callaway JK, Jones NM, Pow DV, Danbolt NC, Jarrott B, Beart PM. Effects of lipopolysaccharide on glial phenotype and activity of glutamate transporters: Evidence for delayed up-regulation and redistribution of GLT-1. Neurochem Int. 2006;48:604–610. doi: 10.1016/j.neuint.2005.12.028. [DOI] [PubMed] [Google Scholar]
- Okabe Y, Takahashi T, Mitsumasu C, Kosai K, Tanaka E, Matsuishi T. Alterations of gene expression and glutamate clearance in astrocytes derived from an MeCP2-null mouse model of Rett syndrome. PLoS One. 2012;7:e35354. doi: 10.1371/journal.pone.0035354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olney JW. Neurotoxicity of NMDA receptor antagonists: an overview. PsychopharmacolBull. 1994;30:533–540. [PubMed] [Google Scholar]
- Olney JW, Labruyere J, Wang G, Wozniak DF, Price MT, Sesma MA. NMDA antagonist neurotoxicity: mechanism and prevention. Science. 1991;254:1515–1518. doi: 10.1126/science.1835799. [DOI] [PubMed] [Google Scholar]
- Otis TS, Brasnjo G, Dzubay JA, Pratap M. Interactions between glutamate transporters and metabotropic glutamate receptors at excitatory synapses in the cerebellar cortex. Neurochemistry International. 2004;45:537–544. doi: 10.1016/j.neuint.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Owe SG, Marcaggi P, Attwell D. The ionic stoichiometry of the GLAST glutamate transporter in salamander retinal glia. J Physiol. 2006;577:591–599. doi: 10.1113/jphysiol.2006.116830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlak J, Brito V, Kuppers E, Beyer C. Regulation of glutamate transporter GLAST and GLT-1 expression in astrocytes by estrogen. Brain Res Mol Brain Res. 2005;138:1–7. doi: 10.1016/j.molbrainres.2004.10.043. [DOI] [PubMed] [Google Scholar]
- Perisic T, Holsboer F, Rein T, Zschocke J. The CpG island shore of the GLT-1 gene acts as a methylation-sensitive enhancer. Glia. 2012;60:1345–1355. doi: 10.1002/glia.22353. [DOI] [PubMed] [Google Scholar]
- Persson M, Brantefjord M, Hansson E, Ronnback L. Lipopolysaccharide increases microglial GLT-1 expression and glutamate uptake capacity in vitro by a mechanism dependent on TNF-alpha. Glia. 2005;51:111–120. doi: 10.1002/glia.20191. [DOI] [PubMed] [Google Scholar]
- Petr GT, Sun Y, Frederick NM, Zhou Y, Dhamne SC, Hameed MQ, Miranda C, Bedoya EA, Fischer KD, Armsen W, Wang J, Danbolt NC, Rotenberg A, Aoki CJ, Rosenberg PA. Conditional deletion of the glutamate transporter GLT-1 reveals that astrocytic GLT-1 protects against fatal epilepsy while neuronal GLT-1 contributes significantly to glutamate uptake into synaptosomes. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2015;35:5187–5201. doi: 10.1523/JNEUROSCI.4255-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pines G, Danbolt NC, Bjørås M, Zhang Y, Bendahan A, Eide L, Koepsell H, Storm- Mathisen J, Seeberg E, Kanner BI. Cloning and expression of a rat brain L- glutamate transporter. Nature. 1992;360:464–467. doi: 10.1038/360464a0. [DOI] [PubMed] [Google Scholar]
- Pitt D, Nagelmeier IE, Wilson HC, Raine CS. Glutamate uptake by oligodendrocytes: Implications for excitotoxicity in multiple sclerosis. Neurology. 2003;61:1113–1120. doi: 10.1212/01.wnl.0000090564.88719.37. [DOI] [PubMed] [Google Scholar]
- Poblete-Naredo I, Angulo C, Hernandez-Kelly L, Lopez-Bayghen E, Aguilera J, Ortega A. Insulin-dependent regulation of GLAST/EAAT1 in Bergmann glial cells. Neurosci Lett. 2009;451:134–138. doi: 10.1016/j.neulet.2008.12.049. [DOI] [PubMed] [Google Scholar]
- Poulsen CF, Schousboe I, Sarup A, White HS, Schousboe A. Effect of topiramate and dBcAMP on expression of the glutamate transporters GLAST and GLT-1 in astrocytes cultured separately, or together with neurons. Neurochem Int. 2006;48:657–661. doi: 10.1016/j.neuint.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Pow DV, Barnett NL. Developmental expression of excitatory amino acid transporter 5: a photoreceptor and bipolar cell glutamate transporter in rat retina. Neuroscience Letters. 2000;280:21–24. doi: 10.1016/s0304-3940(99)00988-x. [DOI] [PubMed] [Google Scholar]
- Rattray M, Bendotti C. Does excitotoxic cell death of motor neurons in ALS arise from glutamate transporter and glutamate receptor abnormalities? Exp Neurol. 2006;201:15–23. doi: 10.1016/j.expneurol.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Raymond M, Li P, Mangin JM, Huntsman M, Gallo V. Chronic perinatal hypoxia reduces glutamate-aspartate transporter function in astrocytes through the Janus kinase/signal transducer and activator of transcription pathway. J Neurosci. 2011;31:17864–17871. doi: 10.1523/JNEUROSCI.3179-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reagan LP, Rosell DR, Wood GE, Spedding M, Munoz C, Rothstein J, McEwen BS. Chronic restraint stress up-regulates GLT-1 mRNA and protein expression in the rat hippocampus: reversal by tianeptine. Proc Natl Acad Sci USA. 2004;101:2179–2184. doi: 10.1073/pnas.0307294101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan MR, Huang YH, Kim YS, Dykes-Hoberg MI, Jin L, Watkins AM, Bergles DE, Rothstein JD. Variations in promoter activity reveal a differential expression and physiology of glutamate transporters by glia in the developing and mature CNS. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2007;27:6607–6619. doi: 10.1523/JNEUROSCI.0790-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson KD, Wolffe AP. DNA methylation in health and disease. Nat Rev Genet. 2000;1:11–19. doi: 10.1038/35049533. [DOI] [PubMed] [Google Scholar]
- Robinson MB. The family of sodium-dependent glutamate transporters: A focus on the GLT-1/EAAT2 subtype. Neurochemistry International. 1999;33:479–491. doi: 10.1016/s0197-0186(98)00055-2. [DOI] [PubMed] [Google Scholar]
- Robinson MB. Regulated trafficking of neurotransmitter transporters: Common notes different melodies. Journal of Neurochemistry. 2002;80:1–11. doi: 10.1046/j.0022-3042.2001.00698.x. [DOI] [PubMed] [Google Scholar]
- Robinson MB. Acute regulation of sodium-dependent glutamate transporters: A focus on constitutive and regulated trafficking. Handbook of Experimental Pharmacology. 2006;175:251–275. doi: 10.1007/3-540-29784-7_13. [DOI] [PubMed] [Google Scholar]
- Robinson MB, Coyle JT. Glutamate and related acidic excitatory neurotransmitters: from basic science to clinical application. FasebJ. 1987;1:446–455. doi: 10.1096/fasebj.1.6.2890549. [DOI] [PubMed] [Google Scholar]
- Robinson MB, Dowd LA. Heterogeneity and functional properties of subtypes of sodium-dependent glutamate transporters in the mammalian central nervous system. Advances in Pharmacology. 1997;37:69–115. doi: 10.1016/s1054-3589(08)60948-5. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Kern A, Gegelashvili M, Schousboe A, Zhang J, Sung L, Gegelashvili G. Beta-amyloid and brain-derived neurotrophic factor, BDNF, up-regulate the expression of glutamate transporter GLT-1/EAAT2 via different signaling pathways utilizing transcription factor NF-kappaB. Neurochem Int. 2003;43:363–370. doi: 10.1016/s0197-0186(03)00023-8. [DOI] [PubMed] [Google Scholar]
- Rosas S, Vargas MA, Lopez-Bayghen E, Ortega A. Glutamate-dependent transcriptional regulation of GLAST/EAAT1: a role for YY1. J Neurochem. 2007;101:1134–1144. doi: 10.1111/j.1471-4159.2007.04517.x. [DOI] [PubMed] [Google Scholar]
- Rossi DJ, Oshima T, Attwell D. Glutamate release in severe brain ischaemia is mainly by reversed uptake. Nature. 2000;403:316–321. doi: 10.1038/35002090. [DOI] [PubMed] [Google Scholar]
- Rothman S. Synaptic release of excitatory amino acid neurotransmitter mediates anoxic neuronal death. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1984;4:1884–1891. doi: 10.1523/JNEUROSCI.04-07-01884.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein JD, Martin L, Levey AI, Dykes-Hoberg M, Jin L, Wu D, Nash N, Kuncl RW. Localization of neuronal and glial glutamate transporters. Neuron. 1994;13:713–725. doi: 10.1016/0896-6273(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Patel S, Regan MR, Haenggeli C, Huang YH, Bergles DE, Jin L, Dykes Hoberg M, Vidensky S, Chung DS, Toan SV, Bruijn LI, Su ZZ, Gupta P, Fisher PB. Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 2005;433:73–77. doi: 10.1038/nature03180. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Patel S, Regan MR, Haenggeli C, Huang YH, Bergles DE, Jin L, Hoberg MD, Vidensky S, Chung DS, Toan SV, Bruijn LI, Su Z-Z, Gupta P, Fisher PB. B-Lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 2005;433:73–77. doi: 10.1038/nature03180. [DOI] [PubMed] [Google Scholar]
- Rozyczka J, Figiel M, Engele J. Endothelins negatively regulate glial glutamate transporter expression. Brain Pathol. 2004;14:406–414. doi: 10.1111/j.1750-3639.2004.tb00084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz M, Ortega A. Characterization of an Na(+)-dependent glutamate/aspartate transporter from cultured Bergmann glia. Neuroreport. 1995;6:2041–2044. doi: 10.1097/00001756-199510010-00021. [DOI] [PubMed] [Google Scholar]
- Rusnakova V, Honsa P, Dzamba D, Stahlberg A, Kubista M, Anderova M. Heterogeneity of astrocytes: from development to injury - single cell gene expression. PLoS One. 2013;8:e69734. doi: 10.1371/journal.pone.0069734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan RM, Vandenberg RJ. A channel in a transporter. Clin Exp Pharmacol Physiol. 2005;32:1–6. doi: 10.1111/j.1440-1681.2005.04164.x. [DOI] [PubMed] [Google Scholar]
- Sato K, Matsuki N, Ohno Y, Nakazawa K. Estrogens inhibit l-glutamate uptake activity of astrocytes via membrane estrogen receptor alpha. J Neurochem. 2003;86:1498–1505. doi: 10.1046/j.1471-4159.2003.01953.x. [DOI] [PubMed] [Google Scholar]
- Sattler R, Rothstein JD. Regulation and dysregulation of glutamate transporters. Handb Exp Pharmacol. 2006:277–303. doi: 10.1007/3-540-29784-7_14. [DOI] [PubMed] [Google Scholar]
- Schitine C, Nogaroli L, Costa MR, Hedin-Pereira C. Astrocyte heterogeneity in the brain: from development to disease. Front Cell Neurosci. 2015;9:76. doi: 10.3389/fncel.2015.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlag BD, Vondrasek JR, Munir M, Kalandadze A, Zelenaia OA, Rothstein JD, Robinson MB. Regulation of the glial Na+-dependent glutamate transporters by cyclic AMP analogs and neurons. Molecular Pharmacology. 1998;53:355–369. doi: 10.1124/mol.53.3.355. [DOI] [PubMed] [Google Scholar]
- Schluter K, Figiel M, Rozyczka J, Engele J. CNS region-specific regulation of glial glutamate transporter expression. Eur J Neurosci. 2002;16:836–842. doi: 10.1046/j.1460-9568.2002.02130.x. [DOI] [PubMed] [Google Scholar]
- Schmitt A, Asan E, Puschel B, Kugler P. Cellular and regional distribution of the glutamate transporter GLAST in the CNS of rats: nonradioactive in situ hybridization and comparative immunocytochemistry. Journal of Neuroscience. 1997;17:1–10. doi: 10.1523/JNEUROSCI.17-01-00001.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schousboe A. Transport and metabolism of glutamate and GABA in neurons and glial cells. International Review of Neurobiology. 1981;22:1–45. doi: 10.1016/s0074-7742(08)60289-5. [DOI] [PubMed] [Google Scholar]
- Schwarcz R, Bennett JP, Jr, Coyle JT., Jr Loss of striatal serotonin synaptic receptor binding induced by kainic acid lesions: correlations with Huntingtons Disease. J Neurochem. 1977;28:867–869. doi: 10.1111/j.1471-4159.1977.tb10641.x. [DOI] [PubMed] [Google Scholar]
- Schwarcz R, Coyle JT. Striatal lesions with kainic acid: neurochemical characteristics. Brain Res. 1977;127:235–249. doi: 10.1016/0006-8993(77)90538-8. [DOI] [PubMed] [Google Scholar]
- Schwarcz R, Scholz D, Coyle JT. Structure-activity relations for the neurotoxicity of kainic acid derivatives and glutamate analogues. Neuropharmacology. 1978;17:145–151. doi: 10.1016/0028-3908(78)90127-2. [DOI] [PubMed] [Google Scholar]
- Schwarcz R, Whetsell WO, Jr, Mangano RM. Quinolinic acid: an endogenous metabolite that produces axon-sparing lesions in rat brain. Science. 1983;219:316–318. doi: 10.1126/science.6849138. [DOI] [PubMed] [Google Scholar]
- Scott E, Zhang QG, Wang R, Vadlamudi R, Brann D. Estrogen neuroprotection and the critical period hypothesis. FrontNeuroendocrinol. 2012;33:85–104. doi: 10.1016/j.yfrne.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seal RP, Amara SG. Excitatory amino acid transporters: A family in flux. Annual Reviews in Pharmacology and Toxicology. 1999;39:431–456. doi: 10.1146/annurev.pharmtox.39.1.431. [DOI] [PubMed] [Google Scholar]
- Sheldon AL, Robinson MB. The role of glutamate transporters in neurodegenerative diseases and potential opportunities for intervention. Neurochem Int. 2007;51:333–355. doi: 10.1016/j.neuint.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigeri Y, Seal RP, Shimamoto K. Molecular pharmacology of glutamate transporters, EAATs and VGLUTs. Brain Research Reviews. 2004;45:250–265. doi: 10.1016/j.brainresrev.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Shin JW, Nguyen KT, Pow DV, Knight T, Buljan V, Bennett MR, Balcar VJ. Distribution of glutamate transporter GLAST in membranes of cultured astrocytes in the presence of glutamate transport substrates and ATP. Neurochem Res. 2009;34:1758–1766. doi: 10.1007/s11064-009-9982-z. [DOI] [PubMed] [Google Scholar]
- Simpkins JW, Singh M, Brock C, Etgen AM. Neuroprotection and estrogen receptors. Neuroendocrinology. 2012;96:119–130. doi: 10.1159/000338409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims KD, Robinson MB. Expression patterns and regulation of glutamate transporters in the developing and adult nervous system. Critical Reviews in Neurobiology. 1999;13:169–197. doi: 10.1615/critrevneurobiol.v13.i2.30. [DOI] [PubMed] [Google Scholar]
- Sitcheran R, Gupta P, Fisher PB, Baldwin AS. Positive and negative regulation of EAAT2 by NF-kappaB: a role for N-myc in TNFalpha-controlled repression. EMBOJournal. 2005;24:510–520. doi: 10.1038/sj.emboj.7600555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CP, Weremowicz S, Kanai Y, Stelzner M, Morton CC, Hediger MA. Assignment of the gene coding for the human high-affinity glutamate transporter EAAC1 to 9p24: potential role in dicarboxylic aminoaciduria and neurodegenerative disorders. Genomics. 1994;20:335–336. doi: 10.1006/geno.1994.1183. [DOI] [PubMed] [Google Scholar]
- Soni N, Reddy BV, Kumar P. GLT-1 transporter: an effective pharmacological target for various neurological disorders. Pharmacol Biochem Behav. 2014;127:70–81. doi: 10.1016/j.pbb.2014.10.001. [DOI] [PubMed] [Google Scholar]
- Spencer PS, Nunn PB, Hugon J, Ludolph AC, Ross SM, Roy DN, Robertson RC. Guam amyotrophic lateral sclerosis-parkinsonism-dementia linked to a plant excitant neurotoxin. Science. 1987;237:517–522. doi: 10.1126/science.3603037. [DOI] [PubMed] [Google Scholar]
- Stoffel W, Sasse J, Duker M, Muller R, Hofmann K, Fink T, Lichter P. Human high affinity, Na(+)-dependent L-glutamate/L-aspartate transporter GLAST-1 (EAAT-1): gene structure and localization to chromosome 5p11-p12. FEBS Lett. 1996;386:189–193. doi: 10.1016/0014-5793(96)00424-3. [DOI] [PubMed] [Google Scholar]
- Storck T, Schulte S, Hofmann K, Stoffel W. Structure, expression, and functional analysis of a Na+-dependent glutamate/aspartate transporter from rat brain. Proceedings of the National Academy of Sciences USA. 1992;89:10955–10959. doi: 10.1073/pnas.89.22.10955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su ZZ, Leszczyniecka M, Kang DC, Sarkar D, Chao W, Volsky DJ, Fisher PB. Insights into glutamate transport regulation in human astrocytes: cloning of the promoter for excitatory amino acid transporter 2 (EAAT2) Proceedings of the National Academy of Sciences USA. 2003;100:1955–1960. doi: 10.1073/pnas.0136555100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Ikegaya Y, Matsuura S, Kanai Y, Endou H, Matsuki N. Transient upregulation of the glial glutamate transporter GLAST in response to fibroblast growth factor, insulin-like growth factor and epidermal growth factor in cultured astrocytes. Journal of cell science. 2001;114:3717–3725. doi: 10.1242/jcs.114.20.3717. [DOI] [PubMed] [Google Scholar]
- Swanson RA, Liu J, Miller JW, Rothstein JD, Farrell K, Stein BA, Longuemare MC. Neuronal regulation of glutamate transporter subtype expression in astrocytes. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1997;17:932–940. doi: 10.1523/JNEUROSCI.17-03-00932.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Foster JB, Lin CL. Glutamate transporter EAAT2: regulation, function, and potential as a therapeutic target for neurological and psychiatric disease. Cellular and molecular life sciences: CMLS. 2015;72:3489–3506. doi: 10.1007/s00018-015-1937-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai S, Yamada K, Kawakami H, Tanaka K, Nakamura S. Localization of the gene (SLC1A3) encoding human glutamate transporter (GluT-1) to 5p13 by fluorescence in situ hybridization. Cytogenet Cell Genet. 1995;69:209–210. doi: 10.1159/000133965. [DOI] [PubMed] [Google Scholar]
- Tanaka K. Functions of glutamate transporters in the brain. Neurosci Res. 2000;37:15–19. doi: 10.1016/s0168-0102(00)00104-8. [DOI] [PubMed] [Google Scholar]
- Tian G, Lai L, Guo H, Lin Y, Butchbach ME, Chang Y, Lin CL. Translational control of glial glutamate transporter EAAT2 expression. J Biol Chem. 2007;282:1727–1737. doi: 10.1074/jbc.M609822200. [DOI] [PubMed] [Google Scholar]
- Tilleux S, Hermans E. Down-regulation of astrocytic GLAST by microglia- related inflammation is abrogated in dibutyryl cAMP-differentiated cultures. J Neurochem. 2008;105:2224–2236. doi: 10.1111/j.1471-4159.2008.05305.x. [DOI] [PubMed] [Google Scholar]
- Tong G, Jahr CE. Block of glutamate transporters potentiates postsynaptic excitation. Neuron. 1994;13:1195–1203. doi: 10.1016/0896-6273(94)90057-4. [DOI] [PubMed] [Google Scholar]
- Torp R, Danbolt NC, Babaie E, Bjoras M, Seeberg E, Storm-Mathisen J, Ottersen OP. Differential expression of two glial glutamate transporters in the rat brain: An in situ hybridization study. European Journal of Neuroscience. 1994;6:936–942. doi: 10.1111/j.1460-9568.1994.tb00587.x. [DOI] [PubMed] [Google Scholar]
- Trotti D, Danbolt NC, Volterra A. Glutamate transporters are oxidant- vulnerable: a molecular link between oxidative and excitotoxic neurodegeneration. Trends in Pharmacological Sciences. 1998;19:328–334. doi: 10.1016/s0165-6147(98)01230-9. [DOI] [PubMed] [Google Scholar]
- Tyler CR, Allan AM. The Effects of Arsenic Exposure on Neurological and Cognitive Dysfunction in Human and Rodent Studies: A Review. Curr Environ Health Rep. 2014;1:132–147. doi: 10.1007/s40572-014-0012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzingounis AV, Wadiche JI. Glutamate transporters: confining runaway excitation by shaping synaptic transmission. Nat Rev Neurosci. 2007;8:935–947. doi: 10.1038/nrn2274. [DOI] [PubMed] [Google Scholar]
- Ueda Y, Willmore LJ. Molecular regulation of glutamate and GABA transporter proteins by valproic acid in rat hippocampus during epileptogenesis. Exp Brain Res. 2000;133:334–339. doi: 10.1007/s002210000443. [DOI] [PubMed] [Google Scholar]
- Utsumi M, Ohno K, Onchi H, Sato K, Tohyama M. Differential expression patterns of three glutamate transporters (GLAST, GLT1 and EAAC1) in the rat main olfactory bulb. Brain Res Mol Brain Res. 2001;92:1–11. doi: 10.1016/s0169-328x(01)00098-5. [DOI] [PubMed] [Google Scholar]
- Vandenberg RJ, Ryan RM. Mechanisms of glutamate transport. Physiol Rev. 2013;93:1621–1657. doi: 10.1152/physrev.00007.2013. [DOI] [PubMed] [Google Scholar]
- Wadiche JI, Arriza JL, Amara SG, Kavanaugh MP. Kinetics of a human glutamate transporter. Neuron. 1995;14:1019–1027. doi: 10.1016/0896-6273(95)90340-2. [DOI] [PubMed] [Google Scholar]
- Walz W. Controversy surrounding the existence of discrete functional classes of astrocytes in adult gray matter. Glia. 2000;31:95–103. doi: 10.1002/1098-1136(200008)31:2<95::aid-glia10>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Wang Z, Pekarskaya O, Bencheikh M, Chao W, Gelbard HA, Ghorpade A, Rothstein JD, Volsky DJ. Reduced expression of glutamate transporter EAAT2 and impaired glutamate transport in human primary astrocytes exposed to HIV-1 or gp120. Virology. 2003;312:60–73. doi: 10.1016/s0042-6822(03)00181-8. [DOI] [PubMed] [Google Scholar]
- Wieronska JM, Zorn SH, Doller D, Pilc A. Metabotropic glutamate receptors as targets for new antipsychotic drugs: Historical perspective and critical comparative assessment. Pharmacol Ther. 2015 doi: 10.1016/j.pharmthera.2015.10.007. [DOI] [PubMed] [Google Scholar]
- Wu J, Lee MR, Kim T, Johng S, Rohrback S, Kang N, Choi DS. Regulation of ethanol-sensitive EAAT2 expression through adenosine A1 receptor in astrocytes. Biochem Biophys Res Commun. 2011;406:47–52. doi: 10.1016/j.bbrc.2011.01.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Xia S, Lin J, Cao D, Chen W, Liu L, Fu Y, Liang J, Cao M. Effects of the altered activity of delta-opioid receptor on the expression of glutamate transporter type 3 induced by chronic exposure to morphine. J Neurol Sci. 2013;335:174–181. doi: 10.1016/j.jns.2013.09.026. [DOI] [PubMed] [Google Scholar]
- Yamada K, Watanabe M, Shibata T, Tanaka K, Wada K, Inoue Y. EAAT4 is a post-synaptic glutamate transporters in Purkinje cell synapses. Neuroreport. 1996;7:2013–2017. doi: 10.1097/00001756-199608120-00032. [DOI] [PubMed] [Google Scholar]
- Yang Y, Gozen O, Vidensky S, Robinson MB, Rothstein JD. Epigenetic regulation of neuron-dependent induction of astroglial synaptic protein GLT1. Glia. 2010;58:277–286. doi: 10.1002/glia.20922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Gozen O, Watkins A, Lorenzini I, Lepore A, Gao Y, Vidensky S, Brennan J, Poulsen D, Won Park J, Li Jeon N, Robinson MB, Rothstein JD. Presynaptic regulation of astroglial excitatory neurotransmitter transporter GLT1. Neuron. 2009;61:880–894. doi: 10.1016/j.neuron.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Vidensky S, Jin L, Jie C, Lorenzini I, Frankl M, Rothstein JD. Molecular comparison of GLT1+ and ALDH1L1+ astrocytes in vivo in astroglial reporter mice. Glia. 2011;59:200–207. doi: 10.1002/glia.21089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao YL, Yang WM, Seto E. Regulation of transcription factor YY1 by acetylation and deacetylation. Mol Cell Biol. 2001;21:5979–5991. doi: 10.1128/MCB.21.17.5979-5991.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi JH, Hazell AS. Excitotoxic mechanisms and the role of astrocytic glutamate transporters in traumatic brain injury. Neurochem Int. 2006;48:394–403. doi: 10.1016/j.neuint.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Zelenaia O, Schlag BD, Gochenauer GE, Ganel R, Song W, Beesley JS, Grinspan JB, Rothstein JD, Robinson MB. Epidermal growth factor receptor agonists increase expression of glutamate transporter GLT-1 in astrocytes through pathways dependent on phosphatidylinositol 3-kinase and transcription factor NF-κB. Molecular Pharmacology. 2000;57:667–678. doi: 10.1124/mol.57.4.667. [DOI] [PubMed] [Google Scholar]
- Zelenaia OA, Gochenauer GE, Robinson MB. Glutamate transporter mRNA stability in cultured astrocytes is regulated by a factor (s) dependent on RNA and protein synthesis. Society for Neuroscience Abstracts. 1999;25:427. [Google Scholar]
- Zerangue N, Kavanaugh MP. Flux coupling in a neuronal glutamate transporter. Nature. 1996;383:634–637. doi: 10.1038/383634a0. [DOI] [PubMed] [Google Scholar]
- Zhang L, Li L, Wang B, Qian DM, Song XX, Hu M. HCMV induces dysregulation of glutamate uptake and transporter expression in human fetal astrocytes. Neurochem Res. 2014;39:2407–2418. doi: 10.1007/s11064-014-1445-5. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Barres BA. Astrocyte heterogeneity: an underappreciated topic in neurobiology. Curr Opin Neurobiol. 2010;20:588–594. doi: 10.1016/j.conb.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Zink M, Vollmayr B, Gebicke-Haerter PJ, Henn FA. Reduced expression of glutamate transporters vGluT1, EAAT2 and EAAT4 in learned helpless rats, an animal model of depression. Neuropharmacology. 2010;58:465–473. doi: 10.1016/j.neuropharm.2009.09.005. [DOI] [PubMed] [Google Scholar]
- Zschocke J, Allritz C, Engele J, Rein T. DNA methylation dependent silencing of the human glutamate transporter EAAT2 gene in glial cells. Glia. 2007;55:663–674. doi: 10.1002/glia.20497. [DOI] [PubMed] [Google Scholar]
- Zschocke J, Bayatti N, Clement AM, Witan H, Figiel M, Engele J, Behl C. Differential promotion of glutamate transporter expression and function by glucocorticoids in astrocytes from various brain regions. J Biol Chem. 2005;280:34924–34932. doi: 10.1074/jbc.M502581200. [DOI] [PubMed] [Google Scholar]