Abstract
Cytokines produced by dendritic cells (DCs) can largely determine the direction of immunity. Transcriptional analysis revealed that besides IL-15, IL-32 was the only other cytokine expressed by human Langerhans cells. IL-32 is a human cytokine that exists in four main isoforms. Currently, little is known about the regulation and function of the various IL-32 isoforms. In this study, we found that IL-15 is a potent inducer of IL-32α in DCs. Because IL-15 promotes NK cell activation, we investigated the interplay between IL-32 and IL-15 and their role in NK cell activity. We show that IL-32α acts on NK cells to inhibit IL-15–mediated STAT5 phosphorylation and to suppress their IL-15–induced effector molecule expression and cytolytic capacity. IL-32α also acted on DCs by downregulating IL-15–induced IL-18 production, an important cytokine in NK cell activity. Blocking IL-32α during DC:NK cell coculture enhanced NK cell effector molecule expression as well as their cytolytic capacity. Taken together, our findings suggest a feedback inhibition of IL-15–mediated NK cell activity by IL-32α.
Introduction
Dendritic cells (DCs) are professional APCs that upon activation are able to migrate to lymphoid organs and shape immune responses (1). DCs are known to induce a wide range of T cell responses, including Th1, Th2, Th22, Th17, and CTL responses (2, 3). Specific DC subtypes are specialized at inducing specific T cell responses. To achieve this, they use a unique set of costimulatory molecules and secrete specific cytokines (4). In human skin, four different DC subsets have been described: Langerhans cells (LCs) that reside in the epidermis and three dermal DC populations that express either CD1a at an intermediate level (CD1adim) or CD14. The CD1adim population is heterogeneous and contains CD141-expressing DCs (4). Each one of these subsets produces unique cytokines, which contribute to their ability to drive a specific T cell response. For instance, LCs produce IL-15, which supports their ability to prime CTL responses (4, 5). IL-15 was also shown to be important for Th17 induction by LCs (6, 7). Alternatively, IL-10 was shown to play a role in the induction of regulation of T cell responses by dermal CD14+ DCs (8, 9). IL-12, which is also produced by dermal CD14+ DCs, is important for the priming of naive B cells into IgM-secreting plasma cells (10) and for the generation of follicular Th cells (11).
In addition to directing lymphocytes, DCs provide positive and negative signals that are important for priming NK cell responses (12–16). For example, fractalkine promotes NK activation by DCs (17), IL-15 is important for the induction of effector molecules (18, 19), whereas IL-12, IL-18, and TNF-α are important for IFN-γ production by NK cells (20–22).
IL-32 (NK-4), which was initially cloned from human NK cells (23), is a recently identified human cytokine that exists in four main isoforms: α, β, γ, and δ (23). Each isoform of IL-32 seems to possess a different immune function. IL-32γ has been described to induce proinflammatory responses by promoting IL-1β, TNF-α, or IL-18 expression (24). However, IL-32α isoform inhibits the expression of IL-6 and TNF-α (25). IL-32 has been described in various diseases, including atopic dermatitis (26), Helicobacter pylori gastric inflammation (27), HIV infection (28), and esophageal cancer (29), and was correlated with either a good or bad prognosis. The preferential expression of a specific IL-32 isoform in these different diseases may help explain its role in pathogenesis. Very few studies have described the induction and regulation of IL-32 expression and its biological significance. Particularly, there have been limited studies on the roles of each specific isoform. One important study links IL-32 to IL-15–induced defense response against Mycobacterium tuberculosis in macrophages (30). Interestingly, we found that skin LCs and dermal CD1adimCD141− DCs express IL-15 and IL-32. In this work, we examine the interplay between IL-15 and IL-32, and its impact on DC and NK cell function.
Materials and Methods
DC subsets
Human skin specimens were obtained from donors who underwent cosmetic and plastic surgeries at Washington University School of Medicine and Barnes Jewish Hospital (St. Louis, MO) in accordance with Institutional Review Board guidelines. LCs, dermal CD1adimCD141−, CD1adimCD141+ DCs, and CD14+ DCs were purified from normal human skin, as previously described (31). In brief, specimens were incubated with the bacterial protease dispase type II for 18 h at 4°C. Epidermal and dermal layers were separated and placed in RPMI 1640 supplemented with 10% FBS. After 48 h, the cells that migrated into the medium were enriched using a Ficoll gradient. DCs were purified by cell sorting after staining with HLA-DR (G46.6; BD Biosciences), CD1a (NA1/34; Dako), CD141 (AD14H12; Miltenyi Biotec), and CD14 (Tük4; Thermo Fisher) mAbs.
To obtain monocyte-derived DCs (moDCs), CD14+ monocytes were isolated from PBMCs using microbeads (Miltenyi Biotec) or by adherence and incubated for 3 d in RPMI 1640 containing 10% FBS and 100 ng/ml GM-CSF (Leukine; Senofi). To generate IFN-α moDCs or IL-15 moDCs, 500 U/ml IFN-α (Schering) or 200 ng/ml IL-15 (R&D Systems) was added to the culture, respectively (32). To obtain IL-32α moDCs, 100 ng/ml IL-32α (R&D Systems) was added to the culture. Cytokines were replenished on day 1; cells were harvested on day 3.
NK cell isolation
Blood from healthy donors was provided by the Pheresis Center of Barnes Jewish Hospital. PBMCs from buffy coats were recovered from the Ficoll interface after a 400 × g centrifugation for 30 min. NK cells were isolated from PBMCs using magnetic beads coupled with anti-CD56 mAb (Miltenyi Biotec). Cell purity was higher than 98%.
Cytokines, agonists, and reagents
Neutralizing anti–IL-32α Ab (rabbit, polyclonal; Acris) was used at 0.5 μg/ml, as recommended by the manufacturer. A rabbit polyclonal Ab was used as a control. IL-32α, IL-32β, and IL-32γ (R&D Systems) were used at 100 ng/ml. IL-15 was used at 1 and 20 ng/ml (where indicated).
NK cells and DC cocultures
moDCs or dermal CD1adim DCs were stimulated with CD40L (100 ng/ml; R&D Systems), IL-32α (100 ng/ml; Sino Biological), IL-15 (1 ng/ml; R&D Systems), IL-32α, and CD40L for periods of 18 or 48 h, or left unstimulated. Neutralizing anti–IL-32α Ab (0.5 μg/ml, polyclonal; Acris) was added where indicated. Cells were then cocultured with purified NK cells at 1:10 DC:NK cell ratio. NK cell effector molecule expression or killing capacity was assessed, as described below.
Killing assay
Purified NK cells were stimulated with IL-15, IL-32α, or both cytokines for 24 h or cocultured with stimulated DCs for 4 d. Daudi cells, NK cell targets that lack class I MHC, were harvested and stained with 0.5 μM CFSE (Thermo Fisher). Target cells were added to NK cells at a ratio of 1:1 for 4 h. Cells were then stained with a viability dye (Aqua; Invitrogen) to determine specific killing of CFSE-labeled target cells by flow cytometry. Results are presented as percentage of Aqua+CFSE+ cells.
Flow cytometry
For intracellular cytokine staining, cells were incubated for 6 h with brefeldin A and monensin (BD Biosciences) to prevent cytokine secretion. Cells were stained for the extracellular expression of Aqua Live/Dead (Invitrogen), CD56-FITC (HCD56; BioLegend), CD3-AF700 (UCHT1; BD Biosciences), CD122-PE (IL-2R β-chain), and CD132-PE (IL-2R γ-chain), where indicated, washed, fixed, permeabilized, and then stained for intracellular expression of IL-32 (all isoforms; A11-C9; YbdY, IL-32α [373821; R&D Systems], IL-15 [34559; R&D Systems], or IL-18 using a polyclonal rabbit anti-human IL-18 Ab [1 μg/ml, polyclonal; MBL]) for 1 h. After washing, a secondary donkey anti-rabbit AF-647 Ab (1.2 μg/ml, polyclonal; Jackson ImmunoResearch) was used to detect intracellular IL-18 by flow cytometry. NK cells were analyzed for intracellular granzyme B (GB11; Invitrogen) and perforin (δ G9; eBioscience). Where indicated, NK cell (labeled as CD3− HLA-DR− CD56+) surface was stained for receptor-bound IL-15 using a unique Ab that is specific to the IL-15/IL-15R α-chain complex (clone 2G11; a gift of G. Zurawski, Baylor Institute for Immunology Research, Dallas, TX) (9) after culturing PBMCs in the presence or absence of IL-15 or IL-15 plus IL-32α for 1 h at 4°C.
Phospho-flow cytometry
NK cells were isolated and stimulated for 15 min with IL-15, IL-32α, or both cytokines in the indicated concentrations. NK cells were then fixed with 4% paraformaldehyde for another 15 min, washed, and stained for NK cell surface markers. After several washes, NK cells were incubated in True-Phos permeabilization buffer (BioLegend) at −20°C for 1 h and then stained for phosphorylated STAT5-AF488 (47/STAT5; BD Biosciences) for 30 min at room temperature. Results were analyzed by flow cytometry and represented as the percentage of viable phosphorylated STAT5-positive CD56+CD3− NK cells.
Real-time quantitative RT-PCR
mRNA expression of IL-32 isoforms was assessed by quantitative RT-PCR using SYBR Green Fast Master Mix (Roche Diagnostics) using ABI7900 Fast Real-Time PCR System (Thermo Fisher). In brief, 50 ng RNA was used per reaction to generate cDNA using oligo(dT) primers and Superscript III transcriptase (Thermo Fisher). IL-32 isoform expression was detected using the following specific primers: IL-32α forward, 5′-GCT GGA GGA CGA CTT CAA AGA-3′ and reverse, 5′-CAG TGG AGC TGG GTC ATC TCA-3′; IL-32β forward, 5′-CAG TGG AGC TGG GTC ATC TCA-3′ and reverse, 5′-GGG CCT TCA GCT TCT TCA TGT CAT CA-3′; IL-32γ forward, 5′-CCA CAG TGT CCT CAG TGT CAC A-3′ and reverse, 5′-AGG CCC GAA TGG TAA TGC T-3′; and IL-32δ forward, 5′-GCC TTG GCT CCT TGA ACT TTT G-3′ and reverse, 5′-TTT GAA GTC GTC CTG TCC ACG T-3′. The results were normalized to the housekeeping genes, as follows: β-actin (ACTB) forward, 5′-AGG CAC CAG GGC GTG AT-3′ and reverse, 5′-GCC CAC ATA GGA ATC CTT CTG AC-3′ and GAPDH forward, 5′-TGA TGA CAT CAA GAA GGT GGT GAA G-3′ and reverse, 5′-TCC TTG GAG GCC ATG TGG GCC AT-3′, and were expressed as the median of fold change = 2−ΔΔCt, where ΔΔ cycle threshold (Ct) = (CtTarget − Cthousekeeping gene)assay − (CtTarget − Cthousekeeping gene)control, as described previously (33).
CD107a mobilization assay
DCs were stimulated with CD40L, IL-32α, IL-15, and IL-32α plus CD40L, or remained unstimulated for 24 h. Cells were then washed extensively and cocultured with isolated NK cells (10 NK cells:1 DC) in the presence of an anti-human CD107a Ab or an isotype-matched control (H4A3; BD Biosciences) and monensin for 4 h. Alternatively, DCs were stimulated overnight, as indicated, and cocultured with isolated NK cells for another 24 h. Primed NK cells were cultured with Daudi target cells in the presence of anti-human CD107a Ab (H4A3; BD Biosciences) or an isotype-matched control and monensin for 4 h. The CD107a mobilization by NK cells was evaluated by flow cytometry.
Statistical analysis
Statistical significance was calculated using ANOVA. A significant p value was <0.05 and labeled as *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.005, and ****p ≤ 0.001. Results are shown as value ± SD.
Results
IL-32 is expressed by LCs and dermal CD1adim DCs
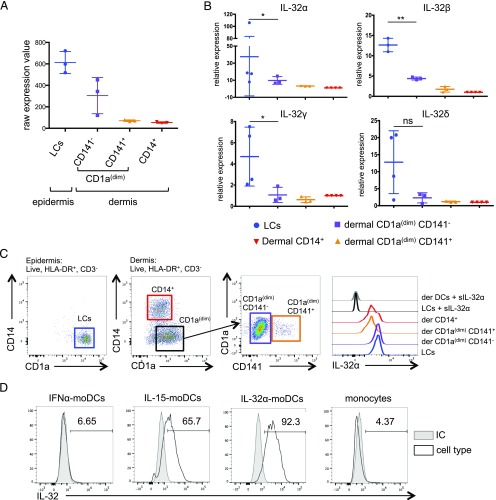
Cytokine production is a key component of DC-driven immune response. We investigated the cytokines that are expressed by the different subtypes of skin DCs, epidermal LCs, and dermal skin DCs: CD1adimCD141−, CD1adimCD141+, and CD14+ DCs. Microarray analysis of purified DCs (31) revealed that, in addition to the previously reported IL-15 that is expressed by both LCs and CD1adimCD141− dermal DCs, both of these subsets expressed IL-32. CD1adimCD141+ or CD14+ dermal DCs did not express these cytokines (Fig. 1A). The level of IL-32 expression was higher in LCs compared with dermal CD1adimCD141− (Fig. 1A). Although IL-15 expression and its role in skin DC function have been previously reported (34), the endogenous expression of IL-32 in skin DCs has not been reported. Thus, we thought to investigate the role of IL-32 in skin DCs.
FIGURE 1.
Expression of IL-32 in steady state DCs. (A) Raw expression value of IL32 gene expression from microarray data (31) (Gene Expression Omnibus accession no. GSE66355; https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE66355). Each dot represents a single donor (n = 3). (B) IL-32 isoforms α, β, γ, and δ expression was determined in sorted skin DCs by quantitative RT-PCR relative to the CD14+ dermal DCs. Each dot represents a single donor (n = 3–4). Values are calculated as average ± SD. *p ≤ 0.05, **p ≤ 0.01. (C) Left, Dot plots represent the gating strategy for epidermal and dermal DC subset. Right, IL-32α expression in skin DC subsets was assessed by flow cytometry. IL-32α was stained in the presence of an excess molar concentration of soluble IL-32α (1 μg/ml). Histograms show one representative experiment of three. (D) IL-32 expression in IFN-α moDCs, IL-15 moDCs, IL-32α moDCs, and monocytes was analyzed by flow cytometry. Histograms show IL-32 (all isoforms) expression (black line) or isotype control (IC; gray filled histogram). One representative experiment of three is shown.
Because IL-32 exists in four main isoforms (α, β, γ, δ), we designed a primer set specific to each isoform and measured their expression by quantitative RT-PCR. We found that LCs expressed higher levels of the four isoforms compared with the dermal DC subtypes. IL-32α was on average 37.5 ± 30-fold higher, IL-32β was 12.6 ± 4.5-fold, IL-32γ was 8 ± 0.3-fold, and IL-32δ was 12 ± 7.8-fold higher in LCs than in the dermal CD14+ DCs. Consistent with the microarray data, we found that dermal CD1adim DCs expressed lower levels of IL-32 isoforms than LCs did. We measured a 9.7 ± 4.5-fold increase in IL-32α, a 4.3 ± 0.36-fold increase in IL-32β, a 1.1 ± 0.7-fold increase in IL-32γ expression, and no significant change in IL-32δ compared with dermal CD14+ DCs (Fig. 1B). Next, the expression of IL-32α protein by skin DCs was measured, as this was the most abundantly expressed isoform. As predicted, LCs and dermal CD1adimCD141− DCs expressed higher levels of IL-32α than CD1adimCD141+ and CD14+ dermal DCs did (Fig. 1C). Taken together, these results show that, in addition to IL-15, IL-32 (particularly isoform α) is expressed in LCs and dermal CD1adimCD141− DCs.
IL-15 induces IL-32 expression in human DCs
Because both IL-15 and IL-32 were expressed in the same DC subtypes, we wondered whether these cytokines would regulate each other. Thus, we differentiated monocytes into DCs (moDCs) with GM-CSF and either IFN-α, IL-15, or IL-32α for 3 d. Cells were then stained for intracellular IL-32 using an Ab that recognizes all isoforms. As shown in Fig. 1D, IL-15 moDCs expressed higher levels of IL-32 as compared with IFN-α moDCs and nondifferentiated monocytes (65.7, 6.65, and 4.37%, respectively). IL-32α moDCs showed the highest IL-32 expression (Fig. 1D), which could result from an exogenous IL-32α uptake during the differentiation process.
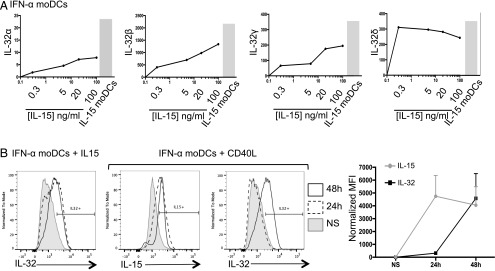
Furthermore, we assessed the mRNA expression of all IL-32 isoforms in moDCs. Cells were stimulated with 0.3, 5, 20, or 100 ng/ml IL-15, and the expression values of IL-32 isoforms were compared with those of unstimulated GM-CSF– and IFN-α–differentiated moDCs and GM-CSF– and IL-15–differentiated moDCs. Interestingly, we observed that the lowest amount of IL-15 (0.3 ng/ml) was sufficient to induce the various IL-32 isoforms in IFN-α moDCs (fold change [FC] = 2 for IL-32α, FC = 450 for IL-32β, FC = 90 for IL-32γ, and FC = 300 for IL-32δ; Fig. 2A). The expression of IL-32 isoforms α, β, and γ increased in a dose-dependent manner, and that of IL-32δ reached a plateau at 0.3 ng/ml IL-15. IL-15 moDCs showed the highest levels of all IL-32 isoforms and served as our positive control (Fig. 2A). Next, we stimulated moDCs with CD40L for 24 or 48 h and tested the expression of IL-15 and IL-32 by flow cytometry (Fig. 2B). Indeed, CD40L induced IL-15 expression in moDCs after 24 h (4746 ± 1618), and this persisted at 48 h (4052 ± 1437). However, IL-32, which was not detected in the cells at 24 h post-CD40 ligation (318 ± 112.6), was induced after 48 h (4583.3 ± 1926) (Fig. 2B). This suggests that IL-32 is induced indirectly by CD40L, via the production of IL-15. Overall, IL-15 is a potent inducer of IL-32 in DCs.
FIGURE 2.
IL-15 is a potent inducer of IL-32. (A) IFN-α moDCs were stimulated with different concentrations of IL-15 (0.3, 5, 20, or 100 ng/ml) for 6 h or not stimulated. IL-32 isoform expression was assessed by quantitative RT-PCR. Graphs show the expression level of IL-32α, IL-32β, IL-32γ, and IL-32δ (left to right) relative to unstimulated DCs. The gray bar chart represents the corresponding IL-32 isoform expression at steady state in IL-15 moDCs. One representative experiment of three is shown. (B) IFN-α moDCs were stimulated with IL-15 (1 ng/ml) or CD40L (200 ng/ml) for 24 h (dashed line) or 48 h (black line) or NS (gray filled histogram). Intracellular production of IL-32 and IL-15 was assessed by flow cytometry. One representative experiment of three performed is shown. Far right, Graph shows IL-32 (black) and IL-15 (gray) expression by moDCs at 24 or 48 h following stimulation with CD40L; n = 3.
IL-32a antagonizes IL-15–induced granzyme B and perforin in NK cells
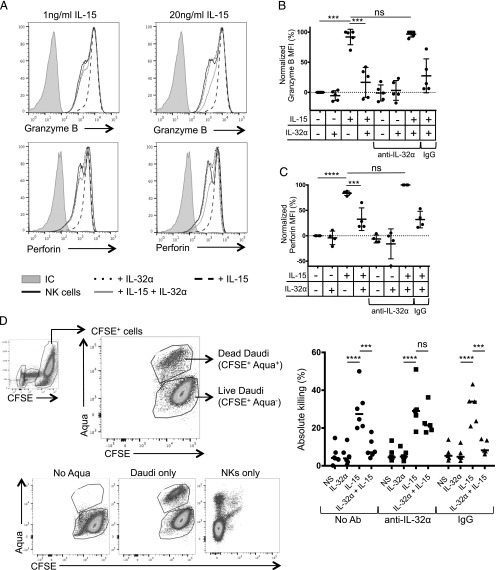
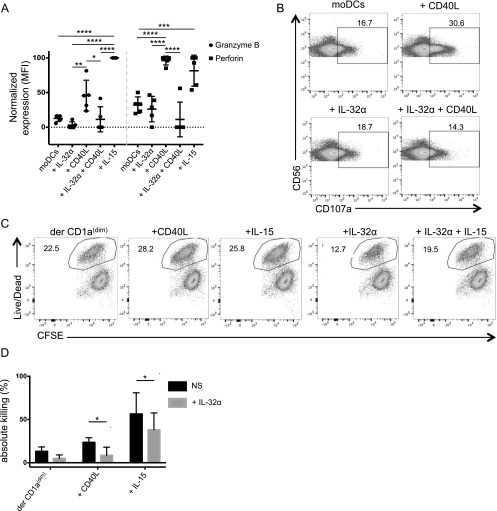
DCs were shown to license NK cells by presenting IL-15 (14, 22, 35). To measure whether IL-32α would synergize or inhibit this property of IL-15, we stimulated NK cells with different concentrations of one or both cytokines and measured the production of the effector molecules granzyme B and perforin. As expected, both 1 and 20 ng/ml IL-15 induced an increase in granzyme B and perforin (Fig. 3A; thick dashed line). This was consistent with the mean fluorescence intensity values that were measured for granzyme B (Fig. 3B) and perforin (Fig. 3C). Addition of IL-32α by itself did not induce granzyme B and perforin expression by NK cells (Fig. 3B, 3C). However, the addition of IL-32α to IL-15–conditioned NK cells resulted in a significant decrease in granzyme B (by 16.6% ± 20) and perforin (by 32.6% ± 22.3) expression, compared with NK cells stimulated with IL-15 alone (Fig. 3B, 3C). To confirm the antagonistic effect of IL-32α, we used a neutralizing Ab to IL-32α. We found that the ability of IL-15 to induce the effector molecules granzyme B and perforin could be entirely rescued (from 27.5% ± 28.2 to 96.7% ± 4.9 for granzyme B; and from 31.9% ± 16.3 to 100% ± 0.3 for perforin) in the presence of the neutralizing Ab compared with the control Ab (Fig. 3B, 3C). Other IL-32 isoforms, IL-32β and IL-32γ, did not induce any significant changes to granzyme B and perforin expression in NK cells in the absence or the presence of IL-15 (Supplemental Fig. 1). Overall, these results demonstrate that IL-32α acts directly on NK cells to regulate their effector molecule expression following IL-15 priming.
FIGURE 3.
IL-32α controls NK cell effector molecule expression. (A) NK cells were isolated from human PBMCs and incubated overnight with IL-32α (100 ng/ml), IL-15 (1 or 20 ng/ml), or both cytokines. The production of granzyme B and perforin was assessed by flow cytometry. Histograms show the expression of granzyme B and perforin in NK cells upon exposure to the different cytokines. (B) Graph shows granzyme B expression in at least four experiments. A neutralizing anti-32α Ab or an isotype-matched control (rabbit polyclonal) was added where indicated. Results were normalized to nonstimulated cells and plotted as a normalized mean fluorescence intensity. The level of granzyme B and perforin that was induced following IL-15 stimulation was set at 100%, whereas the expression of granzyme B and perforin in unstimulated NK cells was set at 0%. IL-32α was used at 100 ng/ml, and IL-15 was used at 1 ng/ml; each dot represents one experiment. Values are shown as average ± SD. (C) Similar to (B), graph shows perforin expression in at least four experiments. (D) Isolated NK cells were stimulated with IL-32α (100 ng/ml), IL-15 (1 ng/ml), and IL-32α + IL-15, and cocultured with CFSE-labeled Daudi cells. Dot plots show the gating strategy to assess percentage of live and dead CFSE+ Daudi cells. Graph shows the absolute killing by NK cells stimulated with IL-32α, IL-15, and IL-32α + IL-15 in absence or presence of anti–IL-32α neutralizing Ab or an isotype-matched control (rabbit polyclonal). Values are calculated as average ± SD. ***p ≤ 0.005, ****p ≤ 0.001. NS, nonstimulated; ns, not significant.
IL-32α regulates NK cell cytotoxic activity
Because IL-32α reduced the production and release of cytotoxic effectors, we hypothesized that this would result in a reduced killing of targets by NK cells. Thus, we exposed NK cells to IL-32α, IL-15, or both cytokines and measured their ability to kill a target cell line (Daudi) that lacks MHC class I molecules. As shown in Fig. 3D, unstimulated and IL-32α–exposed NK cells did not kill this target cell line. IL-15 induced killing of the target cell by ∼30 ± 11% across multiple experiments. As predicted, when rIL-32α was added in addition to IL-15, we observed a reduction in NK cell killing compared with that induced by IL-15 alone (from 30 ± 11% to 9.1 ± 5%; Fig 3D). Under these conditions, blocking IL-32α using neutralizing Ab could rescue the IL-15–induced NK cell cytotoxicity (from 9.1 ± 5% to 23.3 ± 7.4%; Fig. 3D). These results demonstrate that IL-32α is an antagonist for IL-15–mediated NK cell cytotoxic activity.
IL-32α inhibits IL-15–mediated STAT5 phosphorylation in NK cells
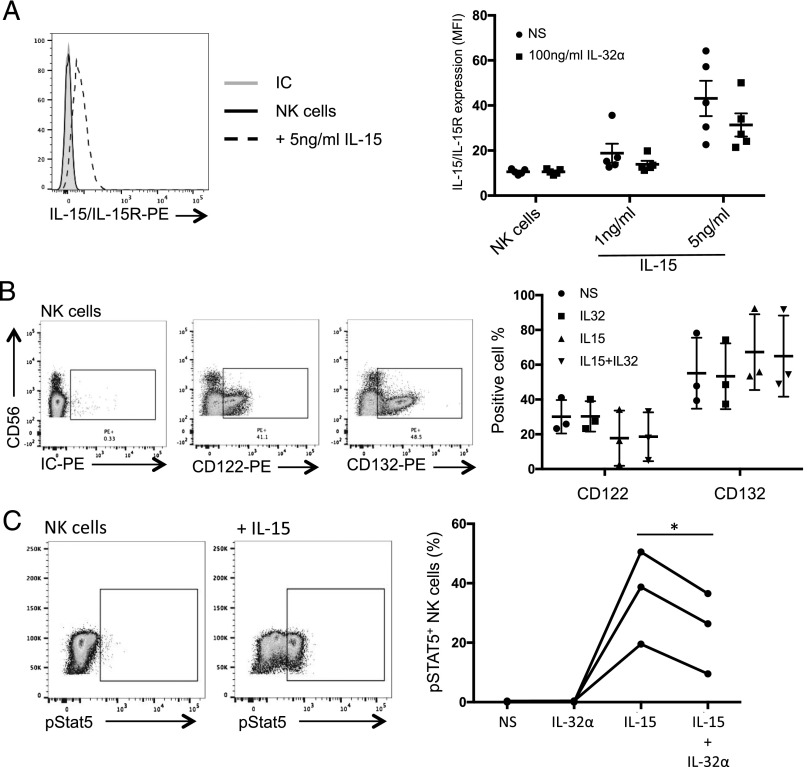
To investigate the mechanism by which IL-32α antagonized IL-15–mediated NK cell activation, we first assessed the potential inhibition of IL-15 binding to its receptor IL-15Rα by IL-32α. A unique mAb that recognizes the IL-15/IL-15Rα complex was used for staining NK cell surface after an exposure to IL-15 and IL-32α. As shown in Fig. 4A, left panel, addition of 1 ng/ml IL-15 to the cells was below the Ab detection limit. However, IL-15 was detected on the surface of NK cells when added at a concentration of 5 ng/ml. Although addition of 100 ng/ml IL-32α resulted in a reduction of IL-15 binding to its receptor (from 35% ± 20 to 19% ± 16.9), this was not significant (Fig. 4A, right panel). Furthermore, we assessed whether IL-32α would downregulate IL-15R to prevent IL-15 binding and consequently NK cell activation. The IL-15R shares the β- and γ-chains with IL-2R. Thus, we investigated the expression of CD122 (IL-2R β-chain) and CD132 (IL-2R γ-chain) on the surface of NK cells after exposure to IL-32α, IL-15, or both cytokines. As shown in Fig. 4B, IL-32α did not affect the expression of the IL-15R chains in the presence or absence of IL-15. Because IL-15R expression or IL-15 binding was not influenced by the presence of IL-32α, we investigated the pathway downstream of the receptor. Indeed, STAT5 has been shown to influence NK cell activation, inflammatory cytokine production, and cytotoxicity downstream of IL-15 (36, 37). Thus, NK cells were stimulated with IL-32α, IL-15, or both cytokines for 15 min, and the level of STAT5 phosphorylation was assessed by flow cytometry. As expected, IL-15 induced a significant phosphorylation of STAT5 (19.5–50.5%; Fig. 4C), which was not seen when the cells were exposed to IL-32α alone. Interestingly, we observed a reduction of STAT5 phosphorylation when the cells were stimulated with both IL-15 and IL-32α (12.3–16.5% reduction; Fig. 4C, right panel). This is consistent with the reduced NK cell cytotoxic activity that is observed in the presence of IL-32 (Fig. 3). Taken together, our data show that IL-32α regulates STAT5 phosphorylation in NK cells.
FIGURE 4.
IL-32α inhibits STAT5 phosphorylation in NK cells. (A) Left panel, PBMCs were cultured for 30 min on ice in the absence or presence of IL-15 (5 or 1 ng/ml) or IL-32α (100 ng/ml) and IL-15 (5 or 1 ng/ml). Then IL-15 binding to IL-15R was detected by flow cytometry using an Ab recognizing the IL-15/IL-15R complex. Histograms show the expression of IL-15/IL-15R complex on CD56+CD3− NK cells. One representative experiment of five is shown. Right, Plot shows the mean fluorescence intensity of IL-15/IL-15R complex expression on NK cells in five different donors. Geometric mean ± SEM is plotted. (B) NK cells were cultured in the absence or presence of IL-32α (100 ng/ml), IL-15 (1 ng/ml), or both cytokines overnight, and expression of CD122 and CD132 was assessed by flow cytometry. Left panel, Dot plots show a representative staining expression of CD122 and CD132 on NK cells. Right panel, The percentage of CD122+ and CD132+ NK cells measured in each condition is plotted. (C) NK cells were cultured in the absence or presence of IL-32α, IL-15, or both cytokines for 15 min, and STAT5 phosphorylation was assessed by flow cytometry. Left panel, Representative dot plots showing the expression of phosphorylated STAT5 without and following IL-15 activation. One of three experiments. Right panel, Graph shows the fraction of cells that express phosphorylated STAT5 following the different stimulations for all three donors (*p ≤ 0.05).
IL-32α acts on DCs to regulate NK cell effector molecule expression and killing capacity
We next wanted to assess the role of DC-derived IL-32 and IL-15 in NK cell activation. IFN-α–derived moDCs were generated and exposed to CD40L, IL-15, IL-32α, or CD40L together with IL-32α for 24 h. Cells were washed and cocultured with NK cells. Intracellular granzyme B and perforin expression was measured by flow cytometry (Fig. 5A). By staining NK cells, we found that CD40L- and IL-15–stimulated IFN-α moDCs induced higher levels of granzyme B compared with unstimulated DCs (45.654% ± 21.3 for CD40L; 100% ± 0.1 for IL-15 versus 12.8 ± 4.5) and perforin (96.2% ± 6.4 for CD40L and 81.5% ± 22.3 for IL-15 versus 31.9% ± 11.8) compared with the unstimulated moDCs or compared with IL-32α–conditioned moDCs (Fig. 5A). NK cells that were exposed to IL-32α– and CD40L-stimulated moDCs expressed lower amounts of granzyme B and perforin compared with NK cells that were exposed to CD40L-activated moDCs (11.4 ± 15.8 and 11.2 ± 25, respectively; Fig. 5A). To assess whether the reduced intracellular effector molecules seen following IL-32α exposure were due to an increased secretion or to a reduced production, we measured surface CD107a mobilization as a surrogate for NK cell degranulation upon conditioned DC activation. As shown in Fig. 5B, 16.7% of NK cells mobilized CD107a spontaneously upon exposure to unstimulated DCs. IL-32α–stimulated moDCs did not induce significant increase in NK CD107a mobilization compared with unstimulated DCs (18.7%). CD40L-activated moDCs induced 30.6% of the NK cells to mobilize CD107a (Fig. 5B). However, moDCs that were activated with both CD40L and IL-32α induced comparable levels of CD107a-mobilized NK cells (14.3%) as unstimulated moDCs (Fig. 5B). These results demonstrate that IL-32α regulates the production of effector molecules by activating NK cells, rather than inducing their increased secretion. This was further confirmed using ex vivo purified primary skin DCs and NK cells. Thus, dermal CD1adim DCs were stimulated with IL-32α, IL-15, CD40L, or IL-32α together with IL-15 for 24 h, washed, and then cocultured with NK cells for another 18 h. The primed NK cells were then assessed for their ability to kill target cells. As shown in Fig. 5C and 5D, NK cells that were primed by dermal CD1adim DCs were able to kill 13.2% ± 5 of the target cells. NKs that were activated by IL-15– or CD40L-stimulated dermal CD1adim DCs killed 56.5% ± 24.4 and 23.6% ± 5.4 of the target cells, respectively (Fig. 5C, 5D). Consistent with our previous results, NK cells that were primed by IL-32α–conditioned dermal CD1adim DCs killed only 5.07% ± 3.9 of the target cells. Remarkably, NK cells activated by dermal CD1adim DCs that were stimulated with IL-32α and IL-15 or IL-32α and CD40L killed 38.2% ± 19.4 (compared with 56.5% ± 24.4 with IL-15 alone) and 8.7 ± 8.3% (compared with 23.6% ± 5.4 with CD40L alone) of target cells, respectively (Fig. 5C, 5D). Thus, IL-32α is a potent inhibitor of IL-15 activity. Overall, our data demonstrate that, in addition to the direct effect of IL-32α on NK cells, it also acts indirectly, by manipulating DC function to suppress the priming of cytolytic NK cells.
FIGURE 5.
IL-32α acts on DCs to regulate NK cell effector molecule expression and killing capacity. (A) MoDCs were stimulated with IL-32α (100 ng/ml), CD40L (100 ng/ml), IL-32α + CD40L, or IL-15 (1 ng/ml) for 24 h and cocultured with NK cells for another 24 h. NK cells were assessed for intracellular granzyme B and perforin expression by flow cytometry. Plots show the normalized expression levels of granzyme B or perforin in multiple experiments (n = 5). Data were normalized by setting the highest value obtained after stimulation with IL-15 to 100%, and the values obtained in unstimulated cells to 0%. Values are calculated as average ± SD. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.005, ****p ≤ 0.001. (B) MoDCs were stimulated with CD40L (100 ng/ml), IL-32α (100 ng/ml), or IL-32α + CD40L for 24 h. They were then cocultured with isolated NK cells in the presence of anti-human CD107a-PE mAb or an isotype-matched control. Dot plots show the level of CD107a mobilization in response to different conditioned DCs. (C) Dermal CD1adim were sorted and stimulated with CD40L, IL-15, IL-32α, or IL-32α + IL-15 overnight. Stimulated or unstimulated (NS) DCs were cocultured with NK cells for 4 d. Cells were then cocultured with Daudi target cell line, and the percentage of dead target cells was assessed by flow cytometry. Dot plots show the percentage of dead target cells as indicated by the live/dead stain. (D) CD1adim DCs were stimulated and cocultured as in (C). Bar charts show normalized percentages of dead target cells in the absence (black) or presence of IL-32α (gray) (n = 5).
DC-derived IL-32α regulates NK cell–killing capacity
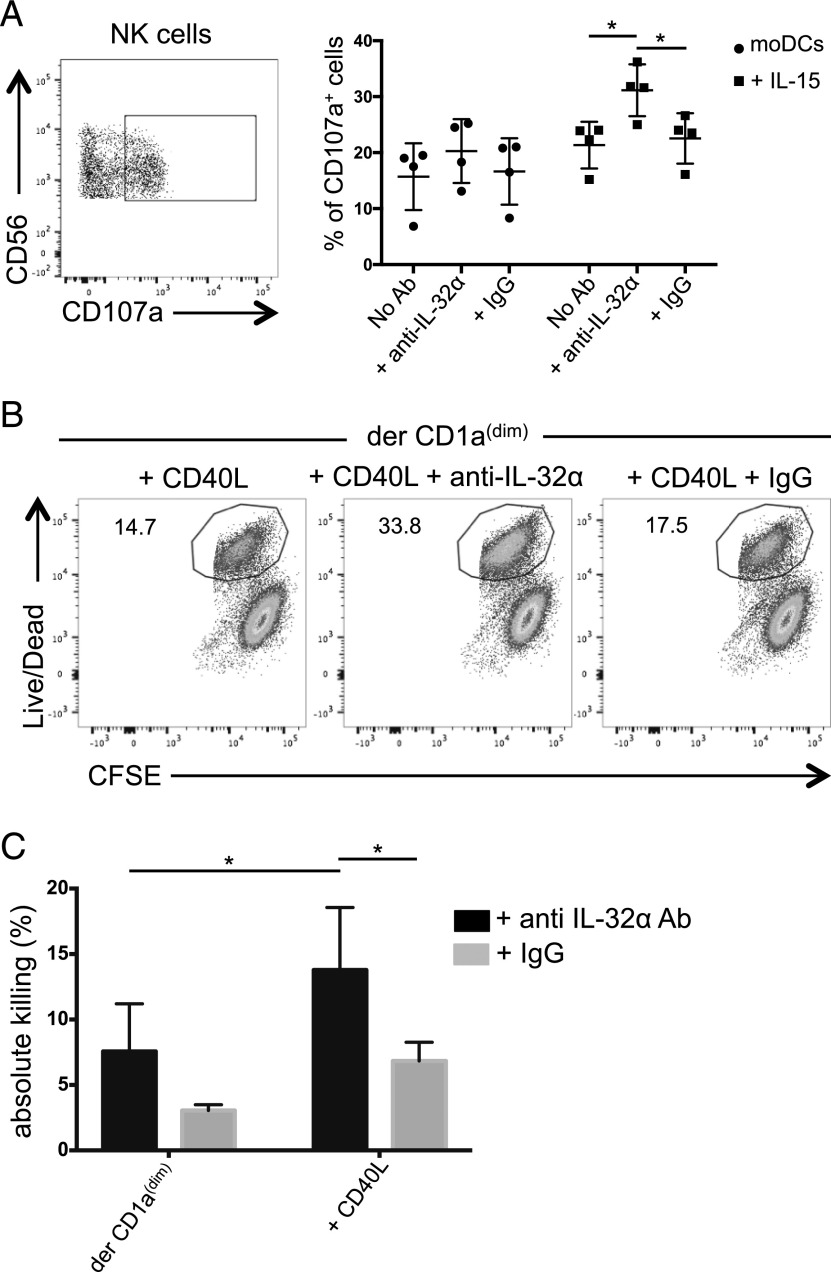
To test whether DC endogenous IL-32 may affect NK cell–killing capacity, we primed NK cells with moDCs that were prestimulated with IL-15 for 24 h to induce endogenous IL-32 expression or left unstimulated (Fig. 2B). Primed NK cells were then exposed to a target cell line, and CD107a mobilization was assessed. As shown in Fig. 6A, NK cells that were primed by IL-15–stimulated moDCs induced higher levels of surface CD107a expression than unstimulated DCs (21.3 ± 4.1% versus 15.7 ± 5.9%), a level that was further increased in the presence of anti–IL-32α Ab, but not with an isotype control or no Ab (31 ± 4.6% versus 22.3 ± 4.5%, respectively, and 21.3 ± 4.1%, respectively; Fig. 6A).
FIGURE 6.
DC-derived IL-32α regulates NK cell–killing capacity. (A) moDCs were stimulated with IL-15 for 24 h or left unstimulated and cocultured with NK cells for another 24 h in the presence of anti–IL-32α mAb or an isotype-matched control mAb. Left, Plot shows the gating used to assess the percentage of CD107a+ NK cells. Right, Graph shows the percentage of NK cells that mobilized CD107a in response to a target cell line, n = 4. Values are calculated as average ± SD. (B) Sorted dermal CD1adim were stimulated overnight with CD40L (100 ng/ml) and cocultured with NK cells in the presence of anti–IL-32α neutralizing Ab or an isotype-matched control for 3 d. NK cells were then exposed to a target cell line. Dot plots show the percentage of dead target cells as indicated by the live/dead stain. (C) As in (B), the graph shows the percentage of dead target cells in the presence of anti–IL-32α Ab (black) or an isotype-matched control (gray). Values were normalized for five donors. Maximal killing was defined using IL-15–stimulated NK cell. *p ≤ 0.05.
To assess the role of endogenous IL-32 in primary skin DCs, we stimulated dermal CD1adim DCs with CD40L for 48 h to induce IL-32 production and cocultured them with NK cells for additional 3 d in the presence or absence of anti–IL-32α neutralizing Ab or an isotype-matched control. NK cells were then assessed for their ability to kill target cells. As shown in Fig. 6B, NK cells that were primed by CD40L-activated dermal CD1adim DCs induced 14.7% killing. The presence of a control mAb during DC:NK cell coculture did not induce a significant change in the ability of the NK cells to kill (17.5%). However, blocking IL-32α during the coculture resulted in more dead target cells (33.8%) (Fig. 6B). As shown in Fig. 6C, blocking endogenous IL-32α in four donors significantly enhanced NK cell killing compared with control mAb only when the DCs were activated with CD40L. Overall, DC-derived IL-32α is induced directly or indirectly by IL-15 and suppresses the cytolytic capacity of NK cells.
IL-32α regulates IL-18 production in DCs
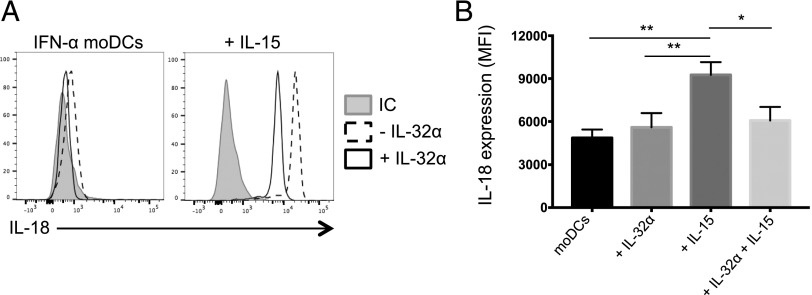
Our data, showing that IL-32α–conditioned DCs negatively regulate NK cell cytotoxic capacity (Fig. 5), led us to hypothesize that IL-32 can also act on DCs to reduce the expression of NK cell–activating cytokines. IL-15 is known to increase NK cell cytotoxic ability by inducing the production of granzyme B (38). IL-18 is a proinflammatory cytokine that is important for IFN-γ production and can act in synergy with IL-15 to enhance NK cell activation (39–41). We assessed the production of IL-18 in IFN-α moDCs that were stimulated overnight with IL-32α, IL-15, or a combination of IL-15 and IL-32α. As shown in Fig. 7, IL-15–induced IL-18 expression in moDCs and addition of IL-32α significantly reduced the production of IL-18 in these cells. Taken together, these results suggest that IL-32α can modulate IL-15 activity in NK cells as well as DCs.
FIGURE 7.
IL-32α inhibits IL-18 expression in DCs. IFN-α moDCs were stimulated with IL-32α, IL-15, or both cytokines overnight, and intracellular cytokine production was measured by flow cytometry. (A) Histograms show the expression of IL-18 by stimulated or unstimulated cells in the presence (solid) or absence of IL-32α (dashed). Gray filled histograms show an isotype-matched control (IC). (B) Graph shows IL-18 expression in stimulated DCs. Geometric mean of fluorescence intensity was plotted after subtracting the background expression of the isotypic control mAb (n = 5). *p ≤ 0.05, **p ≤ 0.01.
Discussion
The capacity of DCs to activate both innate and adaptive immune responses is largely dependent on the cytokines they produce. In this study, we assessed the role of DC-derived IL-15 and IL-32 in controlling NK cell response. We show that IL-15 and IL-32 are two cytokines that are expressed by skin LCs and dermal CD1adim DCs. IL-32 is induced in DCs upon IL-15 exposure. Functionally, IL-15 and IL-32α act sequentially to activate and dampen NK cell cytolytic capacity.
IL-32 is a newly described human cytokine that exists in several isoforms (23). We found that LCs express all four isoforms of IL-32, but predominantly the isoform α. Most studies focused on the IL-32γ, which is known to synergize with other cell activators such as LPS (TLR4 ligand) or muramyldipeptide (NOD2 ligand) (42, 43). IL-32γ can trigger TNF-α and IL-6 production, the maturation of DCs (24), and the activation of NK cell cytotoxicity through cell death receptor 3 (44). Furthermore, IL-32γ has been shown to be protective in tuberculosis (30, 45, 46). In contrast, little is known about the IL-32α isoform, except that it was indicated to have a regulatory function by inhibiting the production of TNF-α or IL-6 in PBMCs (25). Understanding the biology of the different IL-32 isoforms is important to understand why in some diseases, including cancer (47–49), HIV (50), tuberculosis (30), and leprosy (24, 51), IL-32 presence in patients was associated with either a positive or a negative outcome. In this work, we focus on the biology of the α isoform of IL-32, which we found to be expressed in two skin DC subsets.
We show that IL-15 is a potent inducer of IL-32 in DCs. IL-15 was indeed the most efficient inducer of a variety of activators that we examined, including flagellin, LPS, polyinosinic-polycytidylic acid, CLO75, muramyldipeptide, IL-2, IL-7, and CD40L (data not shown). Interestingly, both IL-15 and IL-32 are expressed and produced by LCs and CD1adimCD141− DCs. An indicator of the relationship between IL-15 and IL-32 was recently reported by Montoya et al. (30), in which IL-32 constituted part of an IL-15–induced set of genes during latent M. tuberculosis infection in patients’ blood. Nevertheless, the functional relationship between these cytokines has not been demonstrated. DCs serve as an important source for IL-15, which is presented to NK cells and enhances their cytolytic function (38, 39). We identified a mechanism in human NK cells that allows the regulation of the powerful effect of IL-15 and that might exist to prevent autoimmunity (52). This mechanism seems to be dependent on IL-32α from DCs, as the latter is not induced beyond its basal levels in NK cells in response to IL-15 stimulation (Supplemental Fig. 2). Our findings complement another recent study showing that IL-15–induced NK cell proliferation is negatively regulated by inhibitory receptors (53). Indeed, the control of NK cell activation and inhibition by and following IL-15 is critical. The absence of IL-15 or overexpression of IL-32α during infection or cancer might lead to progression or persistence of the disease due to the lack of NK cell killing (54–56). In contrast, an overexpression of IL-15 without proper regulation could cause autoimmunity (57) or even induce leukemia through DNA hypermethylation and chromosome instability (52).
Our study revealed a role for IL-32α in regulating IL-15–mediated NK cell activity. Our extensive analysis on a possible mechanism for this effect did not reveal a role for IL-32α in downregulating IL-2R β- and γ-chain expression (shared between IL-2R and IL-15R) or a significant inhibition in IL-15 binding to its receptor. However, we found that IL-32α acts by partially inhibiting STAT5 phosphorylation. Indeed, STAT5 has been described to promote NK cell cytolytic activity (36, 37). Thus, a detailed mechanism by which IL-32α inhibits STAT5 phosphorylation by IL-15 is yet to be identified. Overall, the inhibition of STAT5 phosphorylation by IL-32α permits to explain in part the complex mechanism by which it regulates NK cell activity.
Although IL-32α could efficiently inhibit NK cell cytotoxicity, exogenous IL-32β and IL-32γ did not share this property. The role of endogenous DC-derived IL-32β and IL-32γ in this process remains unknown due to a lack of specific neutralizing reagents. Thus, IL-32α is added to the list of other cytokines that inhibit NK cell functions. For example, IL-6 has been shown to antagonize NK cytotoxicity by reducing granzyme B and perforin in the systemic juvenile idiopathic arthritis murine model, and its presence in patient sera was correlated with lower NK activity (58). TGF-β is known to reduce NK cell function by repressing the mTor pathway (59) and downregulating NKG2D or DAP10 expression, leading to an impaired cytotoxic activity of human NK cells (44, 60). Our findings show that IL-32α can act directly on NK cells and on DCs to reduce their capacity to prime cytolytic NK cells and their IL-18 production. IL-18, along with IL-12, is known to induce IFN-γ production by NK cells (41) or can synergize with IL-15 (39) and contributes to their cytotoxic capacity.
Overall, we show that IL-32α is the main isoform produced by human skin LCs and dermal CD1adimCD141− DCs in response to IL-15 and in turn regulates IL-15–induced NK cell effector function. Whether a similar cytokine that is induced by and regulates IL-15 activity exists in mice is not known. IL-32α is induced and can act on both DCs and NK cells to dampen their inflammatory and effector functions. This mechanism is of importance as it could potentially allow the control of NK cell overactivation upon infection and autoimmune diseases. Alternatively, altering the IL-15/IL-32α balance in favor of IL-32α might impair NK cytotoxicity, leading to cancer or persistent viral or bacterial infections.
Supplementary Material
Acknowledgments
We thank Dr. Marina Cella, Dr. Alexander Barrow, Dr. Erica Maria Lantelme, as well as Dorjan Brinja and Adiel Munk at the Department of Pathology and Immunology, Washington University School of Medicine, for help. We thank the surgeons, nurses, and staff at the Barnes Jewish Hospital and Department of Surgery, Washington University School of Medicine, for providing access to skin samples. We thank Dr. Gwendalyn Randolph, Dr. Robert Schreiber, and Dr. Andrey Shaw for generous help and discussions, and Lisa Wu, Emily Manin, and Dr. Jean-Pierre Gorvel for critical reading of the manuscript.
This work was supported by a grant from the Siteman Cancer Center and funding from the Department of Pathology and Immunology, Washington University School of Medicine (to E.K.).
The online version of this article contains supplemental material.
- Ct
- cycle threshold
- DC
- dendritic cell
- FC
- fold change
- LC
- Langerhans cell
- moDC
- monocyte-derived DC.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Banchereau J., Steinman R. M. 1998. Dendritic cells and the control of immunity. Nature 392: 245–252. [DOI] [PubMed] [Google Scholar]
- 2.Seneschal J., Clark R. A., Gehad A., Baecher-Allan C. M., Kupper T. S. 2012. Human epidermal Langerhans cells maintain immune homeostasis in skin by activating skin resident regulatory T cells. Immunity 36: 873–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu C. I., Becker C., Metang P., Marches F., Wang Y., Toshiyuki H., Banchereau J., Merad M., Palucka A. K. 2014. Human CD141+ dendritic cells induce CD4+ T cells to produce type 2 cytokines. J. Immunol. 193: 4335–4343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klechevsky E. 2015. Functional diversity of human dendritic cells. Adv. Exp. Med. Biol. 850: 43–54. [DOI] [PubMed] [Google Scholar]
- 5.Klechevsky E., Flamar A. L., Cao Y., Blanck J. P., Liu M., O’Bar A., Agouna-Deciat O., Klucar P., Thompson-Snipes L., Zurawski S., et al. 2010. Cross-priming CD8+ T cells by targeting antigens to human dendritic cells through DCIR. Blood 116: 1685–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Jong M. A., Geijtenbeek T. B. 2010. Langerhans cells in innate defense against pathogens. Trends Immunol. 31: 452–459. [DOI] [PubMed] [Google Scholar]
- 7.Penel-Sotirakis K., Simonazzi E., Péguet-Navarro J., Rozières A. 2012. Differential capacity of human skin dendritic cells to polarize CD4+ T cells into IL-17, IL-21 and IL-22 producing cells. PLoS One 7: e45680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chu C. C., Ali N., Karagiannis P., Di Meglio P., Skowera A., Napolitano L., Barinaga G., Grys K., Sharif-Paghaleh E., Karagiannis S. N., et al. 2012. Resident CD141 (BDCA3)+ dendritic cells in human skin produce IL-10 and induce regulatory T cells that suppress skin inflammation. J. Exp. Med. 209: 935–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Banchereau J., Thompson-Snipes L., Zurawski S., Blanck J. P., Cao Y., Clayton S., Gorvel J. P., Zurawski G., Klechevsky E. 2012. The differential production of cytokines by human Langerhans cells and dermal CD14(+) DCs controls CTL priming. Blood 119: 5742–5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caux C., Massacrier C., Vanbervliet B., Dubois B., Durand I., Cella M., Lanzavecchia A., Banchereau J. 1997. CD34+ hematopoietic progenitors from human cord blood differentiate along two independent dendritic cell pathways in response to granulocyte-macrophage colony-stimulating factor plus tumor necrosis factor alpha. II. Functional analysis. Blood 90: 1458–1470. [PubMed] [Google Scholar]
- 11.Schmitt N., Bustamante J., Bourdery L., Bentebibel S. E., Boisson-Dupuis S., Hamlin F., Tran M. V., Blankenship D., Pascual V., Savino D. A., et al. 2013. IL-12 receptor β1 deficiency alters in vivo T follicular helper cell response in humans. Blood 121: 3375–3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zitvogel L. 2002. Dendritic and natural killer cells cooperate in the control/switch of innate immunity. J. Exp. Med. 195: F9–F14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Langers I., Renoux V., Reschner A., Touzé A., Coursaget P., Boniver J., Koch J., Delvenne P., Jacobs N. 2014. Natural killer and dendritic cells collaborate in the immune response induced by the vaccine against uterine cervical cancer. Eur. J. Immunol. 44: 3585–3595. [DOI] [PubMed] [Google Scholar]
- 14.Jonsson A. H., Yokoyama W. M. 2009. Natural killer cell tolerance licensing and other mechanisms. Adv. Immunol. 101: 27–79. [DOI] [PubMed] [Google Scholar]
- 15.Gerosa F., Baldani-Guerra B., Nisii C., Marchesini V., Carra G., Trinchieri G. 2002. Reciprocal activating interaction between natural killer cells and dendritic cells. J. Exp. Med. 195: 327–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sallusto F. 2013. DCs: a dual bridge to protective immunity. Nat. Immunol. 14: 890–891. [DOI] [PubMed] [Google Scholar]
- 17.Pallandre J. R., Krzewski K., Bedel R., Ryffel B., Caignard A., Rohrlich P. S., Pivot X., Tiberghien P., Zitvogel L., Strominger J. L., Borg C. 2008. Dendritic cell and natural killer cell cross-talk: a pivotal role of CX3CL1 in NK cytoskeleton organization and activation. Blood 112: 4420–4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brilot F., Strowig T., Roberts S. M., Arrey F., Münz C. 2007. NK cell survival mediated through the regulatory synapse with human DCs requires IL-15Ralpha. J. Clin. Invest. 117: 3316–3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lucas M., Schachterle W., Oberle K., Aichele P., Diefenbach A. 2007. Dendritic cells prime natural killer cells by trans-presenting interleukin 15. Immunity 26: 503–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lim D. S., Yawata N., Selva K. J., Li N., Tsai C. Y., Yeong L. H., Liong K. H., Ooi E. E., Chong M. K., Ng M. L., et al. 2014. The combination of type I IFN, TNF-α, and cell surface receptor engagement with dendritic cells enables NK cells to overcome immune evasion by dengue virus. J. Immunol. 193: 5065–5075. [DOI] [PubMed] [Google Scholar]
- 21.Seeger P., Bosisio D., Parolini S., Badolato R., Gismondi A., Santoni A., Sozzani S. 2014. Activin A as a mediator of NK-dendritic cell functional interactions. J. Immunol. 192: 1241–1248. [DOI] [PubMed] [Google Scholar]
- 22.Vivier E., Tomasello E., Baratin M., Walzer T., Ugolini S. 2008. Functions of natural killer cells. Nat. Immunol. 9: 503–510. [DOI] [PubMed] [Google Scholar]
- 23.Kim S. H., Han S. Y., Azam T., Yoon D. Y., Dinarello C. A. 2005. Interleukin-32: a cytokine and inducer of TNFalpha. Immunity 22: 131–142. [DOI] [PubMed] [Google Scholar]
- 24.Schenk M., Krutzik S. R., Sieling P. A., Lee D. J., Teles R. M., Ochoa M. T., Komisopoulou E., Sarno E. N., Rea T. H., Graeber T. G., et al. 2012. NOD2 triggers an interleukin-32-dependent human dendritic cell program in leprosy. Nat. Med. 18: 555–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choi J. D., Bae S. Y., Hong J. W., Azam T., Dinarello C. A., Her E., Choi W. S., Kim B. K., Lee C. K., Yoon D. Y., et al. 2009. Identification of the most active interleukin-32 isoform. Immunology 126: 535–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meyer N., Zimmermann M., Burgler S., Bassin C., Woehrl S., Moritz K., Rhyner C., Indermitte P., Schmid-Grendelmeier P., Akdis M., Menz G., et al. 2010. IL-32 is expressed by human primary keratinocytes and modulates keratinocyte apoptosis in atopic dermatitis. J. Allergy Clin. Immunol. 125: 858–865.e10. [DOI] [PubMed] [Google Scholar]
- 27.Sakitani K., Hirata Y., Hayakawa Y., Serizawa T., Nakata W., Takahashi R., Kinoshita H., Sakamoto K., Nakagawa H., Akanuma M., et al. 2012. Role of interleukin-32 in Helicobacter pylori-induced gastric inflammation. Infect. Immun. 80: 3795–3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Monteleone K., Di Maio P., Cacciotti G., Falasca F., Fraulo M., Falciano M., Mezzaroma I., D’Ettorre G., Turriziani O., Scagnolari C. 2014. Interleukin-32 isoforms: expression, interaction with interferon-regulated genes and clinical significance in chronically HIV-1-infected patients. Med. Microbiol. Immunol. 203: 207–216. [DOI] [PubMed] [Google Scholar]
- 29.Yousif N. G., Al-Amran F. G., Hadi N., Lee J., Adrienne J. 2013. Expression of IL-32 modulates NF-κB and p38 MAP kinase pathways in human esophageal cancer. Cytokine 61: 223–227. [DOI] [PubMed] [Google Scholar]
- 30.Montoya D., Inkeles M. S., Liu P. T., Realegeno S., Teles R. M., Vaidya P., Munoz M. A., Schenk M., Swindell W. R., Chun R., et al. 2014. IL-32 is a molecular marker of a host defense network in human tuberculosis. Sci. Transl. Med. 6: 250ra114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Artyomov M. N., Munk A., Gorvel L., Korenfeld D., Cella M., Tung T., Klechevsky E. 2015. Modular expression analysis reveals functional conservation between human Langerhans cells and mouse cross-priming dendritic cells. J. Exp. Med. 212: 743–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dubsky P., Saito H., Leogier M., Dantin C., Connolly J. E., Banchereau J., Palucka A. K. 2007. IL-15-induced human DC efficiently prime melanoma-specific naive CD8+ T cells to differentiate into CTL. Eur. J. Immunol. 37: 1678–1690. [DOI] [PubMed] [Google Scholar]
- 33.Ben Amara A., Gorvel L., Baulan K., Derain-Court J., Buffat C., Vérollet C., Textoris J., Ghigo E., Bretelle F., Maridonneau-Parini I., Mege J. L. 2013. Placental macrophages are impaired in chorioamnionitis, an infectious pathology of the placenta. J. Immunol. 191: 5501–5514. [DOI] [PubMed] [Google Scholar]
- 34.Klechevsky E., Banchereau J. 2013. Human dendritic cells subsets as targets and vectors for therapy. Ann. N. Y. Acad. Sci. 1284: 24–30. [DOI] [PubMed] [Google Scholar]
- 35.Amakata Y., Fujiyama Y., Andoh A., Hodohara K., Bamba T. 2001. Mechanism of NK cell activation induced by coculture with dendritic cells derived from peripheral blood monocytes. Clin. Exp. Immunol. 124: 214–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gotthardt D., Sexl V. 2017. STATs in NK-cells: the good, the bad, and the ugly. Front. Immunol. 7: 694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ni J., Cerwenka A. 2016. STAT5 loss awakens the dark force in natural killer cells. Cancer Discov. 6: 347–349. [DOI] [PubMed] [Google Scholar]
- 38.Koka R., Burkett P., Chien M., Chai S., Boone D. L., Ma A. 2004. Cutting edge: murine dendritic cells require IL-15R alpha to prime NK cells. J. Immunol. 173: 3594–3598. [DOI] [PubMed] [Google Scholar]
- 39.French A. R., Holroyd E. B., Yang L., Kim S., Yokoyama W. M. 2006. IL-18 acts synergistically with IL-15 in stimulating natural killer cell proliferation. Cytokine 35: 229–234. [DOI] [PubMed] [Google Scholar]
- 40.Chaix J., Tessmer M. S., Hoebe K., Fuséri N., Ryffel B., Dalod M., Alexopoulou L., Beutler B., Brossay L., Vivier E., Walzer T. 2008. Cutting edge: priming of NK cells by IL-18. J. Immunol. 181: 1627–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Biron C. A., Nguyen K. B., Pien G. C., Cousens L. P., Salazar-Mather T. P. 1999. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu. Rev. Immunol. 17: 189–220. [DOI] [PubMed] [Google Scholar]
- 42.Nakayama M., Niki Y., Kawasaki T., Takeda Y., Ikegami H., Toyama Y., Miyamoto T. 2013. IL-32-PAR2 axis is an innate immunity sensor providing alternative signaling for LPS-TRIF axis. Sci. Rep. 3: 2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Netea M. G., Azam T., Ferwerda G., Girardin S. E., Walsh M., Park J. S., Abraham E., Kim J. M., Yoon D. Y., Dinarello C. A., Kim S. H. 2005. IL-32 synergizes with nucleotide oligomerization domain (NOD) 1 and NOD2 ligands for IL-1beta and IL-6 production through a caspase 1-dependent mechanism. Proc. Natl. Acad. Sci. USA 102: 16309–16314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Park Y. P., Choi S. C., Kiesler P., Gil-Krzewska A., Borrego F., Weck J., Krzewski K., Coligan J. E. 2011. Complex regulation of human NKG2D-DAP10 cell surface expression: opposing roles of the γc cytokines and TGF-β1. Blood 118: 3019–3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bai X., Kim S. H., Azam T., McGibney M. T., Huang H., Dinarello C. A., Chan E. D. 2010. IL-32 is a host protective cytokine against Mycobacterium tuberculosis in differentiated THP-1 human macrophages. J. Immunol. 184: 3830–3840. [DOI] [PubMed] [Google Scholar]
- 46.Bai X., Shang S., Henao-Tamayo M., Basaraba R. J., Ovrutsky A. R., Matsuda J. L., Takeda K., Chan M. M., Dakhama A., Kinney W. H., et al. 2015. Human IL-32 expression protects mice against a hypervirulent strain of Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 112: 5111–5116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nold-Petry C. A., Rudloff I., Baumer Y., Ruvo M., Marasco D., Botti P., Farkas L., Cho S. X., Zepp J. A., Azam T., et al. 2014. IL-32 promotes angiogenesis. J. Immunol. 192: 589–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peng L. S., Zhuang Y., Li W. H., Zhou Y. Y., Wang T. T., Chen N., Cheng P., Li B. S., Guo H., Yang S. M., et al. 2014. Elevated interleukin-32 expression is associated with Helicobacter pylori-related gastritis. PLoS One 9: e88270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hong J. T., Son D. J., Lee C. K., Yoon D. Y., Lee D. H., Park M. H. 2017. Interleukin 32, inflammation and cancer. Pharmacol. Ther. 174: 127–137. [DOI] [PubMed] [Google Scholar]
- 50.Smith A. J., Toledo C. M., Wietgrefe S. W., Duan L., Schacker T. W., Reilly C. S., Haase A. T. 2011. The immunosuppressive role of IL-32 in lymphatic tissue during HIV-1 infection. J. Immunol. 186: 6576–6584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ribeiro-Dias F., Saar Gomes R., de Lima Silva L. L., Dos Santos J. C., Joosten L. A. 2017. Interleukin 32: a novel player in the control of infectious diseases. J. Leukoc. Biol. 101: 39–52. [DOI] [PubMed] [Google Scholar]
- 52.Mishra A., Liu S., Sams G. H., Curphey D. P., Santhanam R., Rush L. J., Schaefer D., Falkenberg L. G., Sullivan L., Jaroncyk L., et al. 2012. Aberrant overexpression of IL-15 initiates large granular lymphocyte leukemia through chromosomal instability and DNA hypermethylation. Cancer Cell 22: 645–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Anton O. M., Vielkind S., Peterson M. E., Tagaya Y., Long E. O. 2015. NK cell proliferation induced by IL-15 transpresentation is negatively regulated by inhibitory receptors. J. Immunol. 195: 4810–4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marcondes A. M., Mhyre A. J., Stirewalt D. L., Kim S. H., Dinarello C. A., Deeg H. J. 2008. Dysregulation of IL-32 in myelodysplastic syndrome and chronic myelomonocytic leukemia modulates apoptosis and impairs NK function. Proc. Natl. Acad. Sci. USA 105: 2865–2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gillgrass A., Gill N., Babian A., Ashkar A. A. 2014. The absence or overexpression of IL-15 drastically alters breast cancer metastasis via effects on NK cells, CD4 T cells, and macrophages. J. Immunol. 193: 6184–6191. [DOI] [PubMed] [Google Scholar]
- 56.Morvan M. G., Lanier L. L. 2016. NK cells and cancer: you can teach innate cells new tricks. Nat. Rev. Cancer 16: 7–19. [DOI] [PubMed] [Google Scholar]
- 57.Jabri B., Abadie V. 2015. IL-15 functions as a danger signal to regulate tissue-resident T cells and tissue destruction. Nat. Rev. Immunol. 15: 771–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cifaldi L., Prencipe G., Caiello I., Bracaglia C., Locatelli F., De Benedetti F., Strippoli R. 2015. Inhibition of natural killer cell cytotoxicity by interleukin-6: implications for the pathogenesis of macrophage activation syndrome. Arthritis Rheumatol. 67: 3037–3046. [DOI] [PubMed] [Google Scholar]
- 59.Viel S., Marçais A., Guimaraes F. S., Loftus R., Rabilloud J., Grau M., Degouve S., Djebali S., Sanlaville A., Charrier E., et al. 2016. TGF-β inhibits the activation and functions of NK cells by repressing the mTOR pathway. Sci. Signal. 9: ra19. [DOI] [PubMed] [Google Scholar]
- 60.Crane C. A., Han S. J., Barry J. J., Ahn B. J., Lanier L. L., Parsa A. T. 2010. TGF-beta downregulates the activating receptor NKG2D on NK cells and CD8+ T cells in glioma patients. Neuro-oncol. 12: 7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.