Abstract
N-Arachidonoyl dopamine (NADA) is an endogenous lipid that potently activates the transient receptor potential vanilloid 1 (TRPV1), which mediates pain and thermosensation. NADA is also an agonist of cannabinoid receptors 1 and 2. We have reported that NADA reduces the activation of cultured human endothelial cells by LPS and TNF-α. Thus far, in vivo studies using NADA have focused on its neurologic and behavioral roles. In this article, we show that NADA potently decreases in vivo systemic inflammatory responses and levels of the coagulation intermediary plasminogen activator inhibitor 1 in three mouse models of inflammation: LPS, bacterial lipopeptide, and polymicrobial intra-abdominal sepsis. We also found that the administration of NADA increases survival in endotoxemic mice. Additionally, NADA reduces blood levels of the neuropeptide calcitonin gene-related peptide but increases the neuropeptide substance P in LPS-treated mice. We demonstrate that the anti-inflammatory effects of NADA are mediated by TRPV1 expressed by nonhematopoietic cells and provide data suggesting that neuronal TRPV1 may mediate NADA’s anti-inflammatory effects. These results indicate that NADA has novel TRPV1-dependent anti-inflammatory properties and suggest that the endovanilloid system might be targeted therapeutically in acute inflammation.
Introduction
The endogenous lipid N-arachidonoyl dopamine (NADA) is composed of an arachidonic acid backbone conjugated to a dopamine moiety (1). NADA is a putative endocannabinoid and possesses activity via the G protein–coupled cannabinoid receptors (2, 3). NADA has also been classified as an endovanilloid, which is a group of endogenous activators of the transient receptor potential vanilloid 1 (TRPV1) and include the endocannabinoid anandamide (AEA) and a variety of lipoxygenase products (4–6). TRPV1, a nonselective cation channel, is primarily known for its role in sensing pain and temperature. Of the known endovanilloids, NADA is the most potent activator of TRPV1 and is considered to be a principal endogenous TRPV1 ligand (1, 5, 7, 8). TRPV1 is also activated by a variety of noxious insults, including heat > 42°C, low pH, and capsaicin, the active ingredient in chili peppers (5).
TRPV1 is highly expressed in the peripheral nervous system, specifically in a subset of small-diameter primary sensory neurons in trigeminal nerve and dorsal root ganglia that detect noxious stimuli and in the inferior (nodose) ganglion of the vagus nerve (9–13). The peripheral terminals of TRPV1-expressing nociceptors innervate most organs and tissues. TRPV1 expressed in the peripheral nervous system functions to integrate multiple noxious stimuli, ultimately leading to the release of neuropeptides, such as calcitonin gene–related peptide (CGRP) and substance P. These neuropeptides elicit pain and neurogenic inflammatory responses (14–18). TRPV1 is also expressed in the CNS, including the spinal cord, striatum, hippocampus, cerebellum, and amygdala (19–23). Finally, TRPV1 is found in nonneuronal cells, including leukocytes, smooth muscle cells, and endothelial cells (11, 17, 24–27). In this regard, TRPV1 activation has been reported to regulate the activation and proinflammatory properties of CD4+ T cells (28)
NADA and TRPV1 have overlapping distributions, because NADA has been detected in the striatum, cerebellum, hippocampus, thalamus, brainstem, and dorsal root ganglia (7, 19, 20, 23, 29). Moreover, NADA has been proposed to modulate neuronal homeostasis by reducing or inducing cation influx via activation of cannabinoid receptors and TRPV1, respectively (1, 30). In addition to its neurologic and behavioral effects, limited studies suggest that NADA has anti-inflammatory effects on nonneuronal cells, such as astrocytes, leukocytes, and endothelial cells (2, 24, 31–34).
We previously found that treatment with NADA reduces the activation of cultured endothelial cells by TNF-α, as well as by bacterial lipopeptides (TLR2 agonists) and LPS (endotoxin, TLR4 agonist) (24). Furthermore, our data using pharmacological inhibitors suggested that the activities of NADA may be mediated through cannabinoid (CB) receptors and TRP channels. Thus, we proposed that NADA may fine-tune the inflammatory activation of endothelial cells through TRPs, which are known to have a similar function in neurons (24). These studies led us to hypothesize that NADA may moderate sepsis-induced inflammation. The majority of the in vivo work on NADA has focused on its neurologic and behavioral effects, and NADA’s effects on inflammation have not been characterized (2, 35–38). Although there are mixed studies suggesting that TRPV1 activation affects inflammation in models of sepsis, to our knowledge, no prior study has investigated in vivo the role of NADA or the NADA–TRPV1 axis in sepsis (17, 27, 39, 40). Therefore, we tested the hypothesis that the administration of NADA would reduce acute systemic inflammation in sepsis via activation of TRPV1. We find that treatment with NADA reduces systemic inflammation in toxicity and infection models of sepsis and improves survival of endotoxemic mice. We determine that NADA’s anti-inflammatory effects depend upon TRPV1 expressed outside of the hematopoietic compartment. We also find that the ability of NADA to regulate systemic inflammation does not require leukocyte or endothelial TRPV1, pointing to a potential role for neuronal TRPV1 expression in the anti-inflammatory effects of NADA.
Materials and Methods
Mice
C57BL/6J wild-type (WT), B6.129 × 1-Trpv1tm1Jul/J (Trpv1−/−), and congenic B6.SJL-Ptprca (CD45.1+) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Experiments used 8-wk-old male or female mice. The University of California, San Francisco Institutional Animal Care and Use Committee approved all animal studies, and experiments were performed in accordance with the Public Health Service Policy on the Humane Care and Use of Laboratory Animals. Unless otherwise noted, adult mice were euthanized using CO2 asphyxiation, followed by bilateral thoracotomies and exsanguination by direct cardiac puncture.
Treatment of mice with LPS, Pam3Cys, NADA, AEA, 2-arachidonoyl glycerol, and capsaicin
In studies focused on the effects of NADA and TRPV1 on acute systemic inflammation, WT and Trpv1−/− mice were injected i.v. (challenged) with Pam3Cys (10 mg/kg; EMC Microcollections, Tubingen, Germany), LPS (5 mg/kg; Sigma-Aldrich, St. Louis, MO), or 0.9% saline (carrier for LPS and Pam3Cys). Five minutes later, they were treated i.v. with vehicle alone (5% Tween-20 in PBS) or with NADA (1–10 mg/kg), AEA (10–40 mg/kg), 2-arachidonoyl glycerol (2-AG; 10 mg/kg), or capsaicin (0.2 mg/kg) (Tocris, Minneapolis, MN) dissolved in vehicle. Unless otherwise noted, whole blood was collected via cardiac puncture for cytokine analysis 2 h postinjection using citrate as the anticoagulant. In each experiment, at least four mice were used per condition, and experiments were performed at least twice in different weeks. The doses of Pam3Cys and LPS used were established in pilot dose-response studies.
In separate experiments, we assessed the effects of NADA administration on survival of endotoxemic mice. Eight-week-old WT male mice were challenged with LPS (7 mg/kg, i.p.) and then received two i.v. doses of NADA (5 mg/kg) or carrier. The first dose of NADA was given 5 min after LPS challenge, and the second was given at t = 2 h. Mice were monitored at 4 and 8 h and then every 8 h through 48 h. Moribund mice were euthanized and counted as nonsurvivors, as required by our Institutional Animal Care and Use Committee. A moribund state was defined by inanition, the lack of response to gentle shaking of the cage, and the inability of the mice to right themselves when placed on their sides. Individuals who were blinded to the treatment groups monitored mice and made decisions about whether they were moribund and required euthanasia.
Immunoassays and MTT assay
IL-6, CCL-2, IL-10, and plasminogen activator inhibitor (PAI)-1 levels were quantified in mouse plasma samples by ELISA (R&D Systems, Minneapolis, MN and Innovative Research, Novi, MI). Substance P and CGRPα/β levels were quantified in mouse plasma samples by enzyme immunoassay (Enzo Life Sciences, Farmingdale, NY and Cayman Chemicals, Ann Arbor, MI, respectively), and absorbance was read using a FLUOstar OPTIMA fluorescent plate reader (BMG Labtech, Cary, NC). Plasma samples were also analyzed for cytokines with a Bio-Plex Pro Mouse 23-Plex Group 1 magnetic bead–based multiplex assay (Bio-Rad, Hercules, CA) using a MAGPIX instrument and xPONENT analysis software (Luminex, Austin, TX), according to the manufacturers’ specifications. The lower limit of detection and intra-assay percentage coefficient of variability (CV) for this panel of analytes were IL-1β = 47.68 pg/ml (4.8% CV), TNF-α = 13.62 pg/ml (4.7% CV), IL-10 = 4.99 pg/ml (3.4% CV), CCL2 = 35.82 pg/ml (4.7% CV), CCL3 = 1.53 pg/ml (4.3% CV), CCL4 = 2.46 pg/ml (3.9% CV), CCL5 = 1.69 pg/ml (3.4% CV), IL-12p40 = 5.74 pg/ml (3.2% CV), and CXCL1 = 3.54 pg/ml (1.9% CV). MTT assays were performed to assess cell viability, as described (24).
Liquid chromatography–tandem mass spectrometry
An analytical liquid chromatography–tandem mass spectrometry (LC-MS/MS) method to detect endocannabinoids in plasma was developed at Cayman Chemical. Plasmas were collected 0, 5, 10, 30, or 60 min after i.v. administration of NADA to WT mice. Plasma samples were analyzed by LC-MS/MS using a NADA standard prepared at Cayman Chemical, as described (24).
Cecal ligation and puncture
Cecal ligation and puncture (CLP) was performed on 8-wk-old WT female mice, as described (41). Briefly, mice were anesthetized with isoflurane and then were administered a 1-ml bolus of saline s.c. to account for fluid loss. The abdominal cavity was opened, and the cecum was exteriorized and ligated midway between the tip and the ileocecal junction. The cecum was punctured once using a 20-gauge needle, and light pressure was applied to extrude a small amount of stool from the puncture site. The cecum was replaced into the abdomen, the incision was closed using a silk suture, and the skin was closed using a clip stapler. Sham mice underwent the identical procedure, but without the cecal ligation or puncture. NADA (10 mg/kg, i.v.; Cayman Chemicals) or vehicle (5% Tween-20 in PBS) was administered to mice immediately after surgery and again 2 h postoperatively. For analgesia, bupivacaine was applied locally to the incision site during closure, and buprenorphine was administered s.c. immediately prior to incision and again at 4 h. Mice were placed on a warming pad throughout surgery and postoperatively until they were fully recovered.
Preparation of primary murine cells
PBMCs were isolated from murine whole blood collected in citrate by gradient centrifugation using Lymphoprep (Axis-Shield, Oslo, Norway), as per the manufacturer’s instructions. Primary peritoneal cells were isolated from adult mice as described (42). Primary lung endothelial cells and microglia were prepared from 6–7-d-old mouse pups immediately after decapitation, as described (43–45). The purity of the cell populations was verified by flow cytometry, as described below. Endothelial cells from WT and Trpv1−/− mice cells were 90.3 and 90.9% pure, respectively. Bone marrow was isolated from 8-wk-old WT and Trpv1−/− male donor mice as described, and erythrocytes were lysed using RBC Lysis Buffer (Sigma-Aldrich) (46).
Bone marrow chimera studies
For the generation of chimeric mice, bone marrow hematopoietic cells were isolated and suspended in PBS to a final concentration of 1 × 107 cells per milliliter (46). Bone marrow chimeras were produced by lethally irradiating recipient congenic CD45.1 mice with a split dose of 1100 rad, followed by i.v. administration of 2 × 106 WT or Trpv1−/− donor bone marrow cells. To allow sufficient recovery time from the bone marrow transplantation, experiments were performed 8–9 wk after irradiation. Recipient mice were fed antibiotic pellets containing sulfamethoxazole and trimethoprim 1 wk prior to and 3 wk after radiation. Bone marrow engraftment was verified by flow cytometry, as described below. Chimeras were challenged with LPS and treated with NADA, as described above.
Ex vivo NADA and inflammatory agonist treatments
Endothelial cells were grown to confluence in 48-well plates before agonist treatment. Microglia were plated at 8 × 104 cells per well in 48-well plates and used 2 d after plating. In all experiments, cells were preincubated with NADA or vehicle (ethanol) at the indicated concentrations for 1 h prior to and continuously during the inflammatory agonist treatment. Pam3Cys (10 μg/ml) or LPS (10 ng/ml) was added for 6 h. Preparations of Pam3Cys contained <0.002 EU/ml endotoxin based on the kinetic turbidimetric Limulus amebocyte lysate assay.
Flow cytometry
Mice were euthanized, whole blood was collected by cardiac puncture, and erythrocytes were lysed using RBC Lysis Buffer. Cultured primary murine cells were detached with Accutase (Innovative Cell Technologies, San Diego, CA). Cells were passed through a 70-μm cell strainer, counted, and aliquoted at 1 × 106 cells per sample. Cells were washed using PBS without Ca2+ and Mg2+ and incubated with LIVE/DEAD violet fluorescent reactive dye (Thermo Fisher Scientific, Waltham, MA) in PBS for 30 min at 4°C to assess cell viability. Cells were then washed using Flow Cytometry Staining Buffer (FCSB; R&D Systems) and incubated with rat anti-mouse IgG (1 μg; Thermo Fisher Scientific) in FCSB for 15 min at 4°C. Next, cells isolated from whole blood were incubated for 30 min at 4°C with specific Ab (1 μg) or isotype control (1 μg). Samples were washed twice with FCSB and then fixed using BD Cytofix (250 μl; BD Biosciences, San Jose, CA) for 30 min at 4°C. Samples were washed once more with FCSB and analyzed by flow cytometry (BD LSR II Flow Cytometer; BD Biosciences). Abs used were purchased from BD Biosciences unless otherwise noted: FITC-mouse anti-mouse CD45.1, allophycocyanin-mouse anti-mouse CD45.2, FITC-mouse IgG2a κ, allophycocyanin-mouse IgG2a κ, FITC-rat anti-mouse CD102, PE-rat anti-mouse CD31, FITC-rat anti-mouse IgG2a κ, PE-rat anti-mouse IgG2a κ, and Alexa Fluor 750–rat anti-mouse αM/CD11b (R&D Systems).
Statistics
Survival curves were compared using a log-rank (Mantel–Cox) test. All other data were analyzed using a two-tailed Mann–Whitney U tests. A p value <0.05 was considered statistically significant for all data. With the exception of the survival studies, the data in graphs are presented as mean ± SD. All experiments were repeated at least twice. Group sizes are indicated in the figure legends.
Results
NADA reduces systemic inflammation and PAI-1 levels in mice challenged with LPS or Pam3Cys
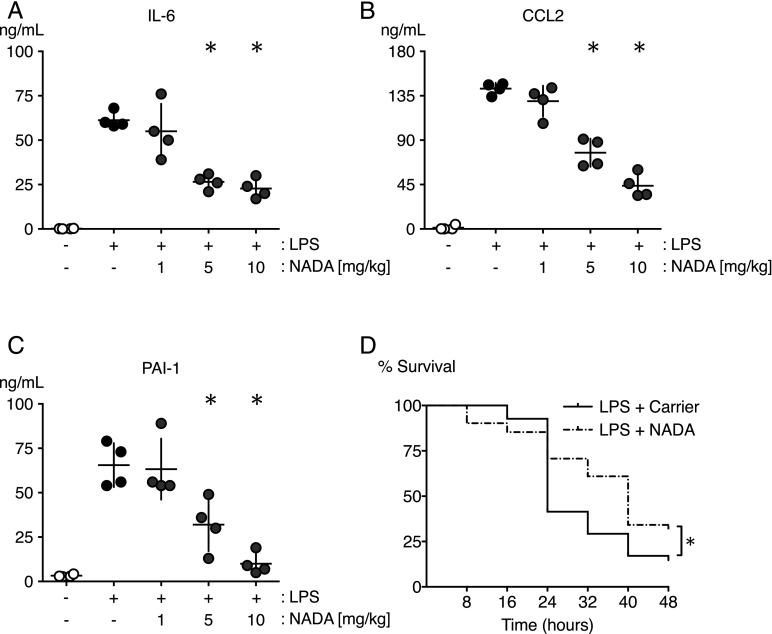
To investigate the contribution of NADA to systemic inflammation and coagulopathy, we challenged mice with LPS (5 mg/kg, i.v.) immediately prior to treatment with NADA (1, 5, and 10 mg/kg, i.v.). NADA doses of 5 and 10 mg/kg significantly reduced plasma concentrations of IL-6 and CCL2 at t = 2 h after LPS challenge (Fig. 1A, 1B). In comparison with vehicle-treated control mice, NADA also reduced the LPS-induced upregulation of PAI-1 (Fig. 1C), an antifibrinolytic factor that is associated with an increased incidence of organ failure and death in sepsis (47). NADA alone did not affect baseline levels of inflammatory mediators or PAI-1 (data not shown). These results are consistent with data that we obtained using a 23-plex immunoassay, in which NADA decreased LPS-induced production of the proinflammatory cytokines IL-1β, TNF-α, CCL2, CCL3, CCL4, CCL5, and IL-12p40 while significantly increasing the levels of the anti-inflammatory cytokine IL-10 (data not shown).
FIGURE 1.
NADA reduces circulating IL-6, CCL2, and PAI-1 levels and increases survival in endotoxemic mice. (A–C) WT mice were challenged i.v. with LPS or carrier and immediately thereafter were treated i.v. with NADA (1, 5, or 10 mg/kg) or vehicle. Levels of IL-6 (A), CCL2 (B), and PAI-1 (C) were quantified in plasma after 2 h (n = 4 mice per group). *p < 0.05 LPS-treated mice in the presence or absence of NADA, Mann–Whitney U test. (D) Survival was followed over a 48-h period in WT mice challenged i.p. with LPS (7 mg/kg) and treated with two i.v. doses of NADA (5 mg/kg) or carrier at t = 5 min and 2 h (n = 41 mice per group). *p < 0.0287, log-rank test.
Although LPS is found exclusively on Gram-negative bacteria, TLR2-activating lipoproteins are expressed by Gram-positive and Gram-negative bacteria (48). To test whether NADA reduces TLR2-mediated inflammation, we challenged mice with Pam3Cys (10 mg/kg, i.v.), a TLR2 ligand, and immediately thereafter administered NADA (10 mg/kg, i.v.). Similar to LPS, administration of NADA reduced levels of IL-6, CCL2, and PAI-1, but increased IL-10 levels in plasma 2 h after Pam3Cys challenge (Supplemental Fig. 1).
NADA administration increases survival in endotoxemic mice
To test the hypothesis that the administration of NADA would have a functional effect on outcomes of acute systemic inflammation, we assessed the effects of NADA on 48-h survival of mice challenged with LPS (7 mg/kg, i.p.). Survival was significantly higher in NADA-treated mice versus carrier-treated mice (Fig. 1D). Overall survival was higher and median survival times were longer in NADA-treated mice (48-h survival 31.7%, median survival 40 h) versus carrier-treated mice (48-h survival 14.6%, median survival 24 h).
NADA has a short half-life in the circulation
We measured plasma NADA levels at intervals through 60 min after treatment of mice with NADA (10 mg/kg, i.v.) using LC-MS/MS. Although the half-maximal concentration of NADA was achieved at 2.7 min, suggesting rapid clearance from the blood, NADA levels remained at 100 ng/ml at the final time point of 60 min (Supplemental Fig. 2). It is unclear whether NADA is catabolized or distributed into tissues.
NADA promotes an anti-inflammatory cytokine profile and reduces PAI-1 in the CLP model of sepsis in mice
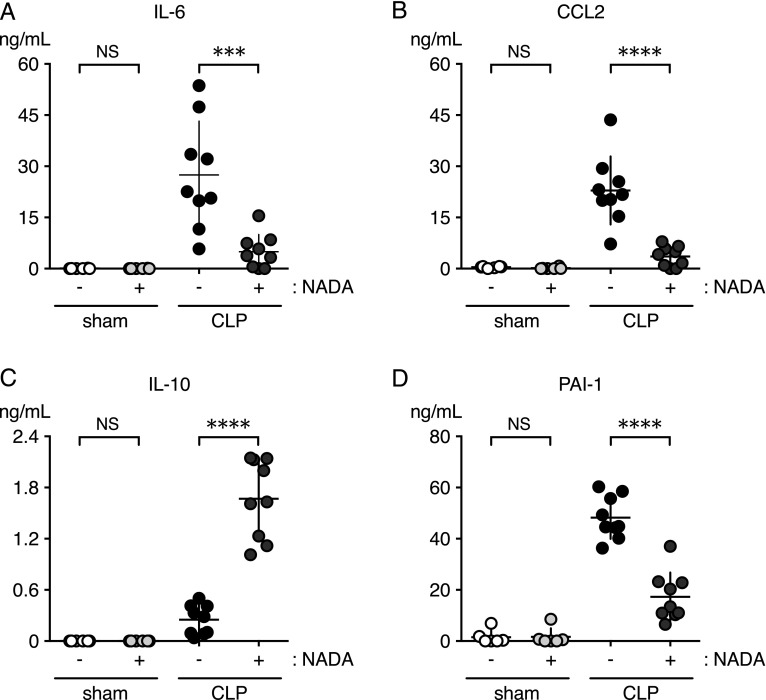
We assessed the effects of NADA in a CLP model of polymicrobial intra-abdominal sepsis to determine its effects during active infection. We administered NADA immediately after CLP surgery and again 2 h later, and then quantified plasma cytokines and PAI-1 at 6 h. NADA treatment significantly reduced IL-6, CCL2, and PAI-1 levels and increased IL-10 levels (Fig. 2). Mice that underwent sham surgery had background levels of cytokines in the presence or absence of NADA. Thus, NADA reduces the systemic proinflammatory response during an active infection.
FIGURE 2.
NADA modulates cytokines and PAI-1 in a CLP model of sepsis. WT mice underwent CLP or sham surgeries and were given NADA or vehicle i.v. immediately after surgery and again 2 h later. Levels of IL-6 (A), CCL2 (B), IL-10 (C), and PAI-1 (D) were quantified in plasmas 6 h after surgery (n = 4–10 mice per group). ***p < 0.001, ****p < 0.0001, CLP mice in the presence or absence of NADA, Mann–Whitney U test.
NADA’s anti-inflammatory activity in vivo requires TRPV1
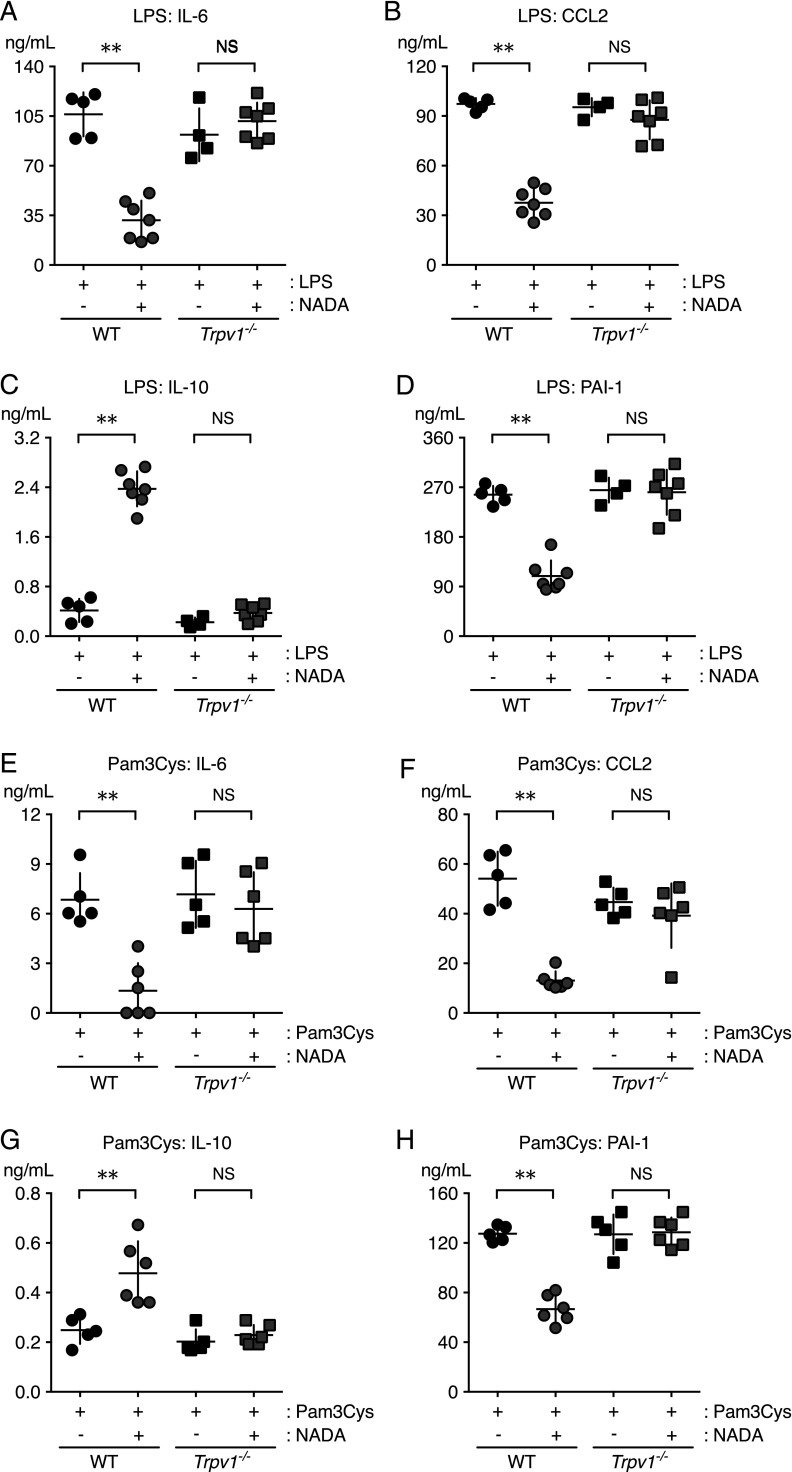
To determine the role of TRPV1 expression in the anti-inflammatory effects of NADA, we compared the responses of WT and Trpv1−/− mice challenged with LPS (5 mg/kg, i.v.) or Pam3Cys (10 mg/kg, i.v.), immediately followed by NADA (10 mg/kg, i.v.) or vehicle. In contrast to WT mice, the administration of NADA had no effect on plasma levels of IL-6, CCL2, IL-10, or PAI-1 in Trpv1−/− mice challenged with LPS or Pam3Cys (Fig. 3). NADA alone did not affect baseline levels of inflammatory mediators or PAI-1 in WT or Trpv1−/− mice (data not shown). These data indicate that TRPV1 mediates the anti-inflammatory effects of NADA in LPS- and Pam3Cys-treated mice.
FIGURE 3.
TRPV1 mediates NADA anti-inflammatory activity in mice challenged with LPS or Pam3Cys. WT and Trpv1−/− mice were challenged i.v. with LPS (A–D) or Pam3Cys (E–H) and immediately thereafter were treated i.v. with NADA or vehicle (n = 4 mice per group). Plasma concentrations of IL-6 (A and E), CCL2 (B and F), IL-10 (C and G), and PAI-1 (D and H) were quantified at 2 h. There were no significant differences between WT and Trpv1−/− mice challenged with LPS or Pam3Cys in the absence of NADA or in WT and Trpv1−/− mice treated with NADA alone (data not shown). **p < 0.01, LPS-challenged (A–D) or Pam3Cys-challenged (E–H) WT mice treated with NADA versus carrier, Mann–Whitney U test. NS, no significant difference between LPS-challenged or Pam3Cys-challenged Trpv1−/− mice treated with NADA versus carrier.
Capsaicin and AEA reduce inflammation and PAI-1 in endotoxemic mice
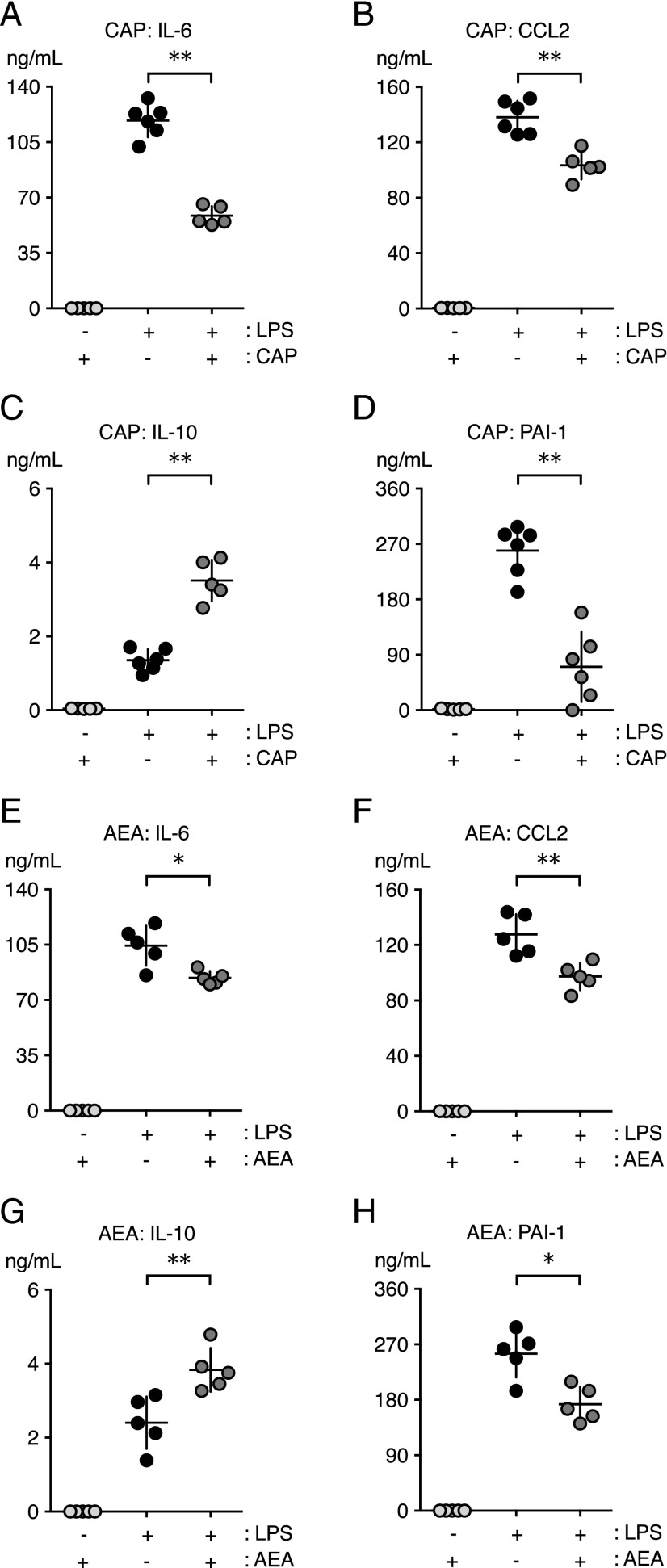
NADA activates CB receptors (CBRs) in addition to TRPV1, and our prior study supported a dominant role for the CBRs in the anti-inflammatory effects of NADA in human endothelial cells (24). To determine whether TRPV1 activation might affect LPS-induced systemic inflammation independently of CBR activation, we studied the effects of capsaicin, a TRPV1-specific agonist that is devoid of effects on CBR 1 (CB1R) or CBR 2 (CB2R). Similar to NADA, the administration of capsaicin (0.2 mg/kg, i.v.) reduced IL-6, CCL2, and PAI-1 levels and increased IL-10 levels in LPS-treated mice (Fig. 4A–D). Capsaicin doses of 0.01 or 0.05 mg/kg had no effect on LPS-induced cytokines or PAI-1 (data not shown), and capsaicin alone did not affect baseline expression of these mediators.
FIGURE 4.
Capsaicin (CAP) and AEA reduce LPS-induced acute inflammation in mice. WT mice were challenged i.v. with LPS or carrier and immediately thereafter were administered CAP [(A–D) 0.2 mg/kg, i.v.], AEA [(E–H) 40 mg/kg, i.v.], or carrier (i.v.; data not shown) (n = 5–6 mice per group). Levels of IL-6 (A and E), CCL2 (B and F), IL-10 (C and G), and PAI-1 (D and H) were quantified in plasmas at 2 h. (A–H) Levels of inflammatory mediators and PAI-1 were at baseline in mice treated with CAP or AEA in the absence of LPS. *p < 0.05, **p < 0.01, LPS-challenged mice in the presence or absence of CAP or AEA, Mann–Whitney U test.
We also tested the effects of the endocannabinoid AEA, which, like NADA, activates CBRs and TRPV1 (6, 49). However, AEA is a less potent TRPV1 agonist than NADA (36). A high dose of AEA (40 mg/kg) abrogated LPS-induced upregulation of IL-6, CCL2, and PAI-1 and increased IL-10 (Fig. 4E–H). The effects of AEA on cytokines and PAI-1 were less pronounced than those of NADA or capsaicin, and these effects were not observed with lower doses of AEA (10 or 20 mg/kg; data not shown). Treatment with the endocannabinoid 2-AG, which activates CBRs but not TRPV1, did not affect LPS-induced inflammation (Supplemental Fig. 3). Our data showing anti-inflammatory effects of capsaicin and AEA support the hypothesis that TRPV1 activation has anti-inflammatory effects in sepsis.
NADA exerts its anti-inflammatory effects in vivo via nonhematopoietic TRPV1
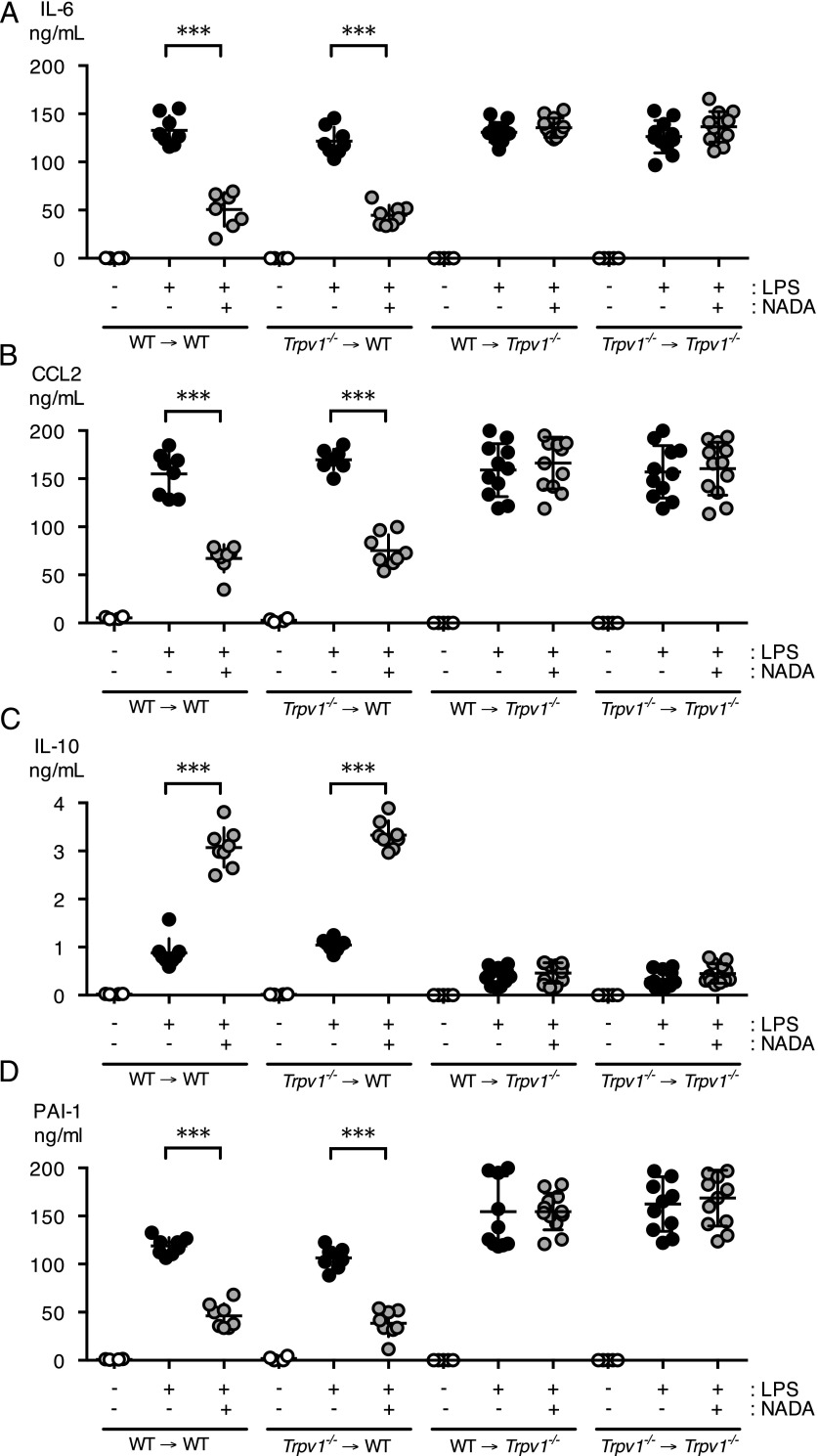
TRPV1 is expressed in multiple cell lineages, including hematopoietic and nonhematopoietic cells. Therefore, we used bone marrow chimeras to determine the role of hematopoietic versus nonhematopoietic TRPV1 expression in NADA’s anti-inflammatory properties. We transplanted Trpv1−/− (CD45.2+) bone marrow cells into irradiated WT (CD45.1+) recipient mice to generate mice that lack TRPV1 expression in hematopoietic cells (Trpv1−/− → WT). We also generated reverse chimeras that exclusively express TRPV1 in hematopoietic cells by transplanting WT (CD45.1+) bone marrow into irradiated Trpv1−/− (CD45.2+) recipients (WT → Trpv1−/−). As controls, WT (CD45.1+) bone marrow was transplanted into irradiated WT (CD45.2+) recipients (WT → WT), and Trpv1−/− bone marrow cells were transplanted into Trpv1−/− recipients (Trpv1−/− → Trpv1−/−). We verified bone marrow engraftment by flow cytometry, with >95% of leukocytes staining as donor cells (data not shown). We then challenged mice with LPS (5 mg/kg, i.v.) and treated them with NADA (10 mg/kg, i.v.). In contrast to our results in global Trpv1−/− mice, NADA reduced levels of IL-6, CCL2, and PAI-1, and increased IL-10 levels, in mice lacking TRPV1 expression in their hematopoietic cells (Trpv1−/− → WT) (Fig. 5). Furthermore, in mice lacking TRPV1 expression outside of the bone marrow (WT → Trpv1−/−), NADA had no effect on levels of IL-6, CCL-2, PAI-1, or IL-10 (Fig. 5). These data indicate that NADA does not require hematopoietic TRPV1 to exert its anti-inflammatory effects in vivo in endotoxemic mice.
FIGURE 5.
Nonhematopoietic TRPV1 mediates the anti-inflammatory action of NADA in endotoxemic mice. (A–D) Irradiated CD45.1+ WT recipient mice were reconstituted with bone marrow from CD45.2+ Trpv1−/− mice (Trpv1−/− → WT). Irradiated CD45.2+ Trpv1−/− recipient mice were reconstituted with bone marrow from CD45.1+ WT mice (WT → Trpv1−/−) or Trpv1−/− mice (Trpv1−/− → Trpv1−/−). CD45.2+ WT mice were reconstituted with CD45.1+ donor bone marrow (WT → WT). Following engraftment bone marrow chimeras were challenged i.v. with LPS or vehicle and 5 min later were treated i.v. with NADA (10 mg/kg) or vehicle (n = 6–12 mice per group). Plasma levels of IL-6 (A), CCL2 (B), IL-10 (C), and PAI-1 (D) were quantified at 2 h. (A–D) NADA alone did not affect baseline levels of inflammatory mediators or PAI-1 in any of the bone marrow chimeras. The Mann–Whitney U test was used to calculate statistical significance. ***p < 0.001, LPS-treated mice in the presence or absence of NADA.
NADA exerts its anti-inflammatory effects independently of TRPV1 in cultured primary murine hematopoietic cells and endothelial cells
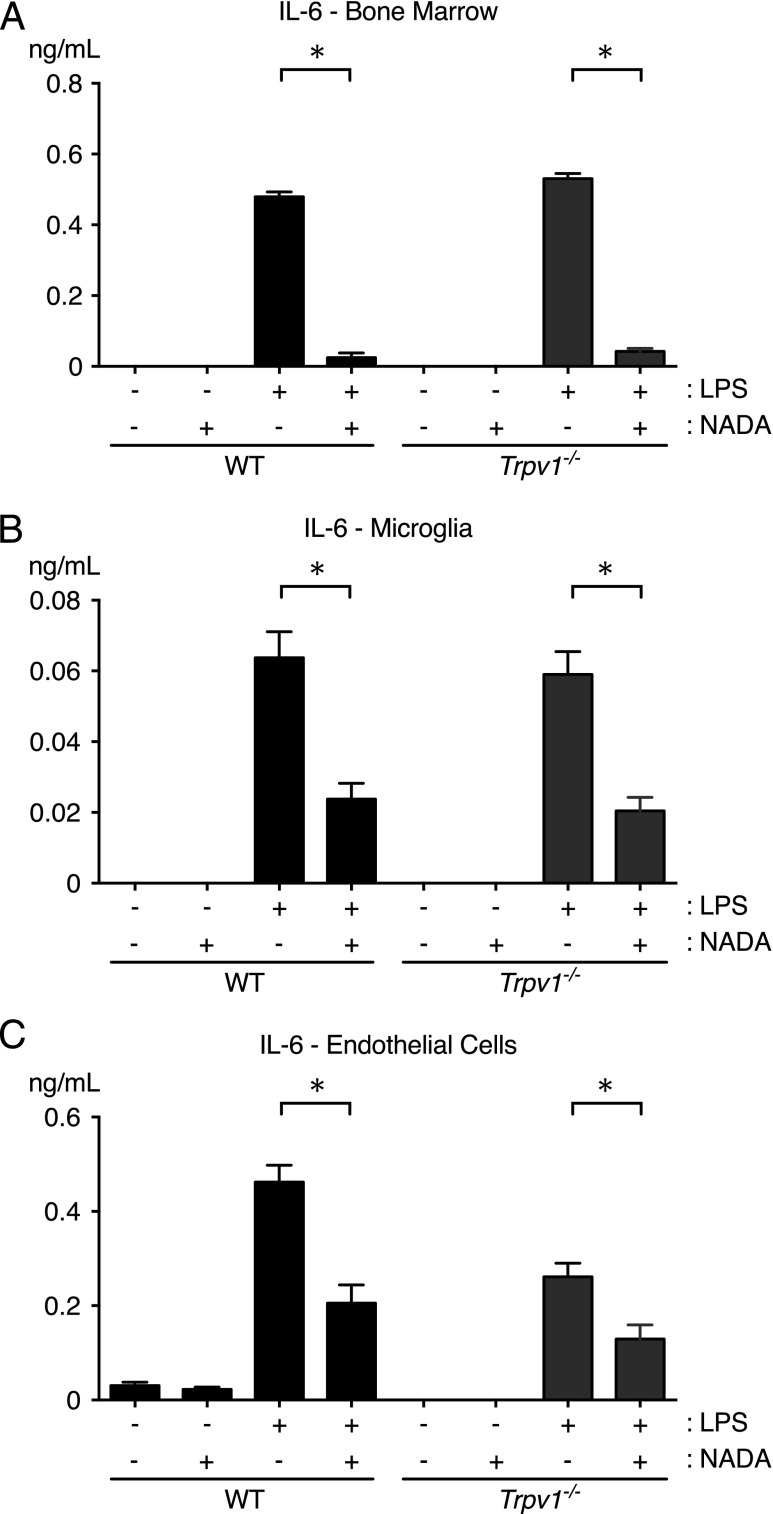
Because our bone marrow chimera studies suggested that NADA mediates its anti-inflammatory effects via nonhematopoietic TRPV1, we performed ex vivo studies using cultured myeloid cells and endothelial cells prepared from WT and Trpv1−/− mice. For each of these studies, cells were incubated for 1 h with NADA and then stimulated with LPS or Pam3Cys. NADA treatment reduced IL-6 production induced by LPS or Pam3Cys in WT and Trpv1−/− bone marrow cells (Fig. 6A, shown for LPS). Similar results were obtained in PBMCs and thioglycollate-elicited peritoneal macrophages from WT and Trpv1−/− mice (data not shown). Because irradiation may not fully deplete brain-resident microglia, we were concerned that the results of our bone marrow chimera studies could have been due to the residual TRPV1-expressing microglia in irradiated WT mice transplanted with Trpv1−/− marrow (50). Similar to bone marrow cells, PBMCs, and peritoneal macrophages, NADA treatment reduced the LPS-induced upregulation of IL-6 in microglia from WT and Trpv1−/− mice (Fig. 6B). Finally, we tested the effects of NADA on LPS-induced activation of primary lung endothelial cells isolated from WT and Trpv1−/− mice. NADA induced a comparable decrease in LPS-induced IL-6 secretion by endothelial cells from Trpv1−/− and WT mice (Fig. 6C). Collectively, these ex vivo data suggest that the effects of NADA on acute inflammation are not mediated through TRPV1 expressed by endothelial cells, cells derived from the hematopoietic lineage, or microglia. Furthermore, they suggest that NADA reduces inflammatory activation of these endothelial cells, monocytes, macrophages, and microglia through TRPV1-independent mechanisms. This is consistent with our prior work showing that CB1R and CB2R antagonists block the anti-inflammatory effects of NADA on primary human endothelial cells (24).
FIGURE 6.
TRPV1 does not mediate the anti-inflammatory activity of NADA in LPS-treated hematopoietic cells or endothelial cells ex vivo. Cultured primary murine bone marrow cells (A), microglia (B), and lung endothelial cells (C) were treated for 1 h with NADA (1 μM) and then stimulated with LPS (10 ng/ml) for an additional 6 h in the continued presence of NADA (n = 4 wells per group). IL-6 levels were quantified in culture supernatants. Identical results were observed with PBMCs and thioglycollate-elicited peritoneal macrophages (data not shown). *p < 0.05, LPS-treated cells in the presence or absence of NADA, Mann–Whitney U test.
NADA modulates neuropeptide expression via TRPV1
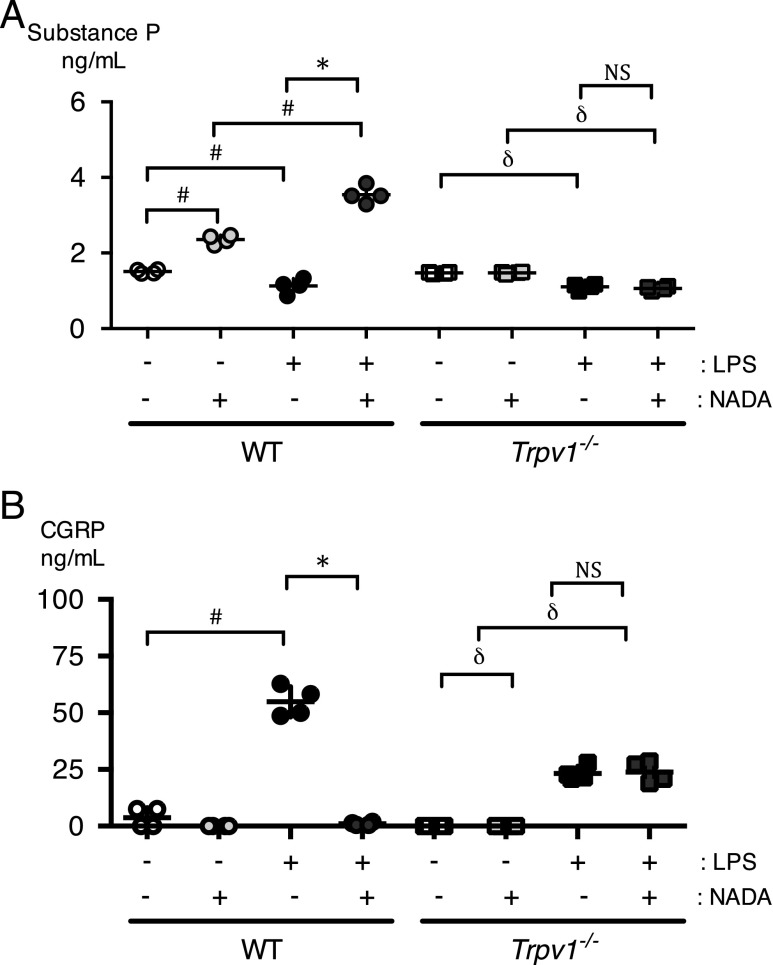
We hypothesized that NADA may exert its anti-inflammatory effects via the release of the neuropeptides CGRP and substance P, both of which are released by TRPV1-expressing neurons in sensory ganglia. We quantified plasma levels of substance P (Fig. 7A) and CGRP (Fig. 7B) in mice treated with NADA and LPS. NADA augmented substance P levels in carrier-treated and LPS-treated WT mice. The augmentation of substance P by NADA was greater in LPS-treated mice compared with carrier-treated mice. In contrast to WT mice, NADA had no effects on substance P levels in LPS-treated or carrier-treated Trpv1−/− mice. LPS alone slightly, but significantly, reduced substance P levels in WT and Trpv1−/− mice. In contrast, LPS alone induced CGRP in WT and Trpv1−/− mice. The upregulation of CGRP by LPS alone was more pronounced in WT mice compared with Trpv1−/− mice. Finally, NADA dramatically decreased CGRP levels in LPS-treated WT mice but had no effect on CGRP levels in LPS-treated Trpv1−/− mice. Capsaicin had similar effects as NADA on neuropeptide release (data not shown).
FIGURE 7.
NADA regulates neuropeptide release via TRPV1 in endotoxemic mice. WT and Trpv1−/− mice were challenged i.v. with LPS or carrier for LPS (normal saline) and then immediately treated i.v. with NADA (10 mg/kg) or vehicle for NADA. Substance P (A) and CGRP (B) levels were quantified in plasma at 2 h (n = 4–6 mice per group). The Mann–Whitney U test was used to calculate statistical significance. *p < 0.05, LPS-treated WT mice in the presence or absence of NADA. #p < 0.05, WT mice treated with carrier alone versus WT mice treated with LPS alone or NADA alone, or mice treated with NADA versus mice treated with LPS and NADA. δp < 0.05, Trpv1−/− mice treated with carrier alone versus mice treated with LPS alone, or Trpv1−/− mice treated with NADA alone versus mice treated with LPS + NADA. NS, no significant between LPS-treated Trpv1−/− mice in the presence and absence of NADA.
Discussion
We have provided the first evidence, to our knowledge, that the endocannabinoid/endovanilloid NADA abates acute systemic inflammation in vivo, in a TRPV1-dependent manner. Treatment with NADA reduces plasma levels of proinflammatory cytokines and PAI-1 and increases levels of the anti-inflammatory cytokine IL-10 in mice with intra-abdominal sepsis induced by CLP and following challenge with microbial TLR2 and TLR4 agonists. Furthermore, the administration of NADA increases survival in endotoxemic mice. NADA has mixed effects on circulating levels of the neuropeptides: it reduces CGRP but augments substance P in endotoxemic mice. Our data in Trpv1−/− bone marrow chimeras suggest that TRPV1 expressed in nonhematopoietic cells mediates the anti-inflammatory effects of NADA. These data, in conjunction with our ex vivo data using microglia, PBMCs, peritoneal macrophages, bone marrow cells, and endothelial cells, raise the intriguing possibility that NADA exerts its anti-inflammatory effects in vivo via neuronal TRPV1. We provide further evidence of an anti-inflammatory effect of TRPV1 activation by showing that capsaicin, a specific TRPV1 agonist, and AEA, a weak TRPV1 agonist, also reduce LPS-induced systemic inflammation. During septic shock, the excessive production of proinflammatory mediators and the failure to appropriately resolve inflammation, in conjunction with dysregulation of coagulation pathways, lead to organ failure, the most common cause of demise in this high-mortality syndrome (51–53). We speculate that the NADA–TRPV1 axis may protect against sepsis-induced complications, such as multiple organ failure and death, by abrogating the positive-feedback loop that propagates pathological inflammation by reducing proinflammatory cytokines and PAI-1, which is an inhibitor of fibrinolysis that facilitates clot persistence. Collectively, our results suggest that the endovanilloid system, and particularly TRPV1, represents a novel potential therapeutic target for the treatment of disorders characterized by dysregulated acute inflammation, such as sepsis.
Because NADA is putatively the principal endogenous TRPV1 agonist, we sought to determine the contribution of TRPV1 to the immunomodulatory effects of NADA in mice. The effects of NADA on circulating levels of cytokines, PAI-1, and neuropeptides induced by challenge with TLR agonists were absent in Trpv1−/− mice, indicating that TRPV1 is required to elicit these early parameters of systemic and neuroinflammation. Functional TRPV1 expressed by a number of nonneuronal cell populations has been reported to regulate immune responses (24–28, 39, 40, 54). However, our data with bone marrow chimeras suggest that TRPV1 expressed outside of the hematopoietic compartment mediates NADA’s anti-inflammatory effects in endotoxemic mice. This is further supported by our ex vivo data that bone marrow, peritoneal cells, PBMCs, and microglia from Trpv1−/− mice all retain equivalent responsiveness to the anti-inflammatory properties of NADA compared with cells from WT mice. Similarly, our ex vivo data using primary endothelial cells suggest that endothelial TRPV1 is not responsible for NADA’s in vivo anti-inflammatory properties in mice. We previously reported that CBRs may mediate the anti-inflammatory effects of NADA in human endothelial cells, suggesting that CB1R and/or CB2R may be responsible for the anti-inflammatory effects of NADA on leukocyte and endothelial cell populations (24). These results support the notion that TRPV1 engagement in a nonhematopoietic-derived cell population primarily mediates the anti-inflammatory effects of NADA in early sepsis. However, our studies do not rule out the possibility that the TRPV1-independent anti-inflammatory effects of NADA on immune and endothelial cells via other receptors, such as CBRs, may alter outcomes of sepsis, including later complications, such as multiple organ failure.
Consistent with the cumulative evidence that points to a protective role for TRPV1 in murine sepsis models, we observe that TRPV1 activation displays a protective phenotype of reduced proinflammation, increased anti-inflammation, and downregulation of PAI-1 in toxicity and infection models of sepsis (17, 27, 39, 40). However, in contrast to other reports on TRPV1 in sepsis, we did not detect differences in the cytokine responses of Trpv1−/− and WT mice treated with LPS or Pam3Cys in the absence of NADA. Because we used early time points (2–6 h), our study does not define the role of TRPV1 at later stages of sepsis, which may account for such differences. Our study suggests the novel possibility that NADA may regulate sepsis-induced systemic inflammation through the activation of neuronal TRPV1. The nervous system is increasingly being recognized as playing key roles in inflammation, and strong links have been established between the immune and nervous systems. Neurogenic inflammation is believed to be a critical component of inflammatory initiation and the pain response (55, 56). There is also strong evidence that the links between the nervous and immune systems are bidirectional, with each able to modulate the activity of the other. In the area of sepsis, current evidence points to a neuroimmune response mediated through the vagus nerve and spleen (57, 58). Notably, TRPV1 is expressed in the vagus nerve, raising the possibility that the vagus nerve might be involved in the neuroimmune response (59).
Neurogenic inflammation is regulated by neuropeptides, such as CGRP and substance P, which are released from the peripheral terminals of sensory neurons (16). Neuropeptides act in an autocrine or paracrine manner on various target cells, such as vascular endothelial and smooth muscle cells, to regulate vasodilation and capillary permeability, and on innate and adaptive immune cells to regulate chemotaxis and activation (16, 56). There is limited information regarding the importance of neuropeptides in sepsis. CGRP has been reported to mediate aspects of septic shock in endotoxemic rats (60). Additionally, elevated levels of plasma substance P and CGRP have been reported to be associated with worse outcomes in humans with sepsis (61, 62).
Activation of TRPV1 on pain-sensing neuronal receptors (nociceptors) by a variety of stimuli promotes the secretion of CGRP and substance P, which, by inducing inflammation and vasodilation, facilitate the arrival of circulating leukocytes and inflammatory mediators to sites of tissue distress (7, 9, 17, 63–68). However, substance P has also been reported to promote an anti-inflammatory wound-healing phenotype by inducing IL-10 and a switch to M2 macrophages, as well as by inhibiting NO synthase and TNF-α production (69–71). We observed that the administration of NADA increases plasma levels of substance P in a TRPV1-dependent manner and that LPS augments NADA’s induction of substance P. In contrast, we found that NADA abrogates LPS-induced upregulation of CGRP. We postulate that NADA may exert its anti-inflammatory effects by simultaneously decreasing CGRP and augmenting substance P in the systemic circulation, although the effects of NADA on neuropeptide release and on acute systemic inflammation may be independent of one another. It is possible that NADA treatment reduced CGRP secretion via the acute desensitization of TRPV1, but this seems unlikely because, similar to other reports, we observed that NADA TRPV1-dependently increases levels of substance P in the circulation. Finally, the neuropeptides in the plasma may have been derived from nonneuronal cells, such as keratinocytes, which have been shown to express CGRPβ, or subsets of leukocytes that express substance P (69, 72).
In conclusion, we have observed that the administration of NADA attenuates acute systemic inflammation and coagulopathy in murine models of acute inflammation and sepsis, and it increases survival and modulates the levels of circulating neuropeptides in endotoxemic mice. These in vivo immunomodulatory effects of NADA appear to be dependent upon TRPV1 expressed in nonhematopoietic cells. In contrast, NADA modulates inflammatory activation of primary leukocytes and endothelial cells ex vivo through receptors and pathways other than TRPV1. These results suggest that an endogenous NADA–TRPV1 neuronal axis may dampen the proinflammatory response in the early phases of sepsis. Further studies will be needed to thoroughly elucidate the mechanisms by which NADA exerts its effects in vivo, as well as to determine the role of the NADA–TRPV1 axis in the later phases of sepsis and in sepsis-induced organ injury. Nonetheless, our observations may have important implications in sepsis and suggest that the endovanilloid system may represent a unique neuroimmunological therapeutic target for the treatment of acute systemic inflammation and sepsis.
Supplementary Material
Acknowledgments
We thank Dr. Audrey Gerard for guidance with generating bone marrow chimera mice.
J.H. and K.W. are cosenior authors.
This work was supported by grants from the Department of Anesthesia and Perioperative Care, University of California, San Francisco (to J.H.), the International Anesthesia Research Society (to J.H.), National Science Foundation Graduate Research Fellowship Program Grant 1144247 (to S.K.L.), and the University of California, San Francisco Research Evaluation and Allocation Committee/Research Allocation Program (Huntington Fund; to J.H.).
The online version of this article contains supplemental material.
- AEA
- anandamide
- 2-AG
- 2-arachidonoyl glycerol
- CB
- cannabinoid
- CBR
- CB receptor
- CB1R
- CBR 1
- CB2R
- CBR 2
- CD45.1+
- B6.SJL-Ptprca mice
- CGRP
- calcitonin gene–related peptide
- CLP
- cecal ligation and puncture
- CV
- coefficient of variability
- FCSB
- Flow Cytometry Staining Buffer
- LC-MS/MS
- liquid chromatography–tandem mass spectrometry
- NADA
- N-arachidonoyl dopamine
- PAI
- plasminogen activator inhibitor
- TRPV1
- transient receptor potential vanilloid 1
- Trpv1−/−
- B6.129 × 1-Trpv1tm1Jul/J
- WT
- wild-type.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Hu S. S., Bradshaw H. B., Benton V. M., Chen J. S., Huang S. M., Minassi A., Bisogno T., Masuda K., Tan B., Roskoski R., Jr., et al. 2009. The biosynthesis of N-arachidonoyl dopamine (NADA), a putative endocannabinoid and endovanilloid, via conjugation of arachidonic acid with dopamine. Prostaglandins Leukot. Essent. Fatty Acids 81: 291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bisogno T., Melck D., Bobrov M. Y., Gretskaya N. M., Bezuglov V. V., De Petrocellis L., Di Marzo V. 2000. N-acyl-dopamines: novel synthetic CB(1) cannabinoid-receptor ligands and inhibitors of anandamide inactivation with cannabimimetic activity in vitro and in vivo. Biochem. J. 351: 817–824. [PMC free article] [PubMed] [Google Scholar]
- 3.Felder C. C., Joyce K. E., Briley E. M., Mansouri J., Mackie K., Blond O., Lai Y., Ma A. L., Mitchell R. L. 1995. Comparison of the pharmacology and signal transduction of the human cannabinoid CB1 and CB2 receptors. Mol. Pharmacol. 48: 443–450. [PubMed] [Google Scholar]
- 4.Caterina M. J., Schumacher M. A., Tominaga M., Rosen T. A., Levine J. D., Julius D. 1997. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389: 816–824. [DOI] [PubMed] [Google Scholar]
- 5.Van Der Stelt M., Di Marzo V. 2004. Endovanilloids. Putative endogenous ligands of transient receptor potential vanilloid 1 channels. Eur. J. Biochem. 271: 1827–1834. [DOI] [PubMed] [Google Scholar]
- 6.Tóth A., Blumberg P. M., Boczán J. 2009. Anandamide and the vanilloid receptor (TRPV1). Vitam. Horm. 81: 389–419. [DOI] [PubMed] [Google Scholar]
- 7.Huang S. M., Bisogno T., Trevisani M., Al-Hayani A., De Petrocellis L., Fezza F., Tognetto M., Petros T. J., Krey J. F., Chu C. J., et al. 2002. An endogenous capsaicin-like substance with high potency at recombinant and native vanilloid VR1 receptors. Proc. Natl. Acad. Sci. USA 99: 8400–8405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mogg A. J., Mill C. E., Folly E. A., Beattie R. E., Blanco M. J., Beck J. P., Broad L. M. 2013. Altered pharmacology of native rodent spinal cord TRPV1 after phosphorylation. Br. J. Pharmacol. 168: 1015–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piomelli D., Sasso O. 2014. Peripheral gating of pain signals by endogenous lipid mediators. Nat. Neurosci. 17: 164–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Helliwell R. J., McLatchie L. M., Clarke M., Winter J., Bevan S., McIntyre P. 1998. Capsaicin sensitivity is associated with the expression of the vanilloid (capsaicin) receptor (VR1) mRNA in adult rat sensory ganglia. Neurosci. Lett. 250: 177–180. [DOI] [PubMed] [Google Scholar]
- 11.Cavanaugh D. J., Chesler A. T., Jackson A. C., Sigal Y. M., Yamanaka H., Grant R., O’Donnell D., Nicoll R. A., Shah N. M., Julius D., Basbaum A. I. 2011. Trpv1 reporter mice reveal highly restricted brain distribution and functional expression in arteriolar smooth muscle cells. J. Neurosci. 31: 5067–5077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cavanaugh D. J., Chesler A. T., Bráz J. M., Shah N. M., Julius D., Basbaum A. I. 2011. Restriction of transient receptor potential vanilloid-1 to the peptidergic subset of primary afferent neurons follows its developmental downregulation in nonpeptidergic neurons. J. Neurosci. 31: 10119–10127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tominaga M., Caterina M. J., Malmberg A. B., Rosen T. A., Gilbert H., Skinner K., Raumann B. E., Basbaum A. I., Julius D. 1998. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron 21: 531–543. [DOI] [PubMed] [Google Scholar]
- 14.Schumacher M. A. 2010. Transient receptor potential channels in pain and inflammation: therapeutic opportunities. Pain Pract. 10: 185–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laird J. M., Olivar T., Roza C., De Felipe C., Hunt S. P., Cervero F. 2000. Deficits in visceral pain and hyperalgesia of mice with a disruption of the tachykinin NK1 receptor gene. Neuroscience 98: 345–352. [DOI] [PubMed] [Google Scholar]
- 16.Holzer P. 1988. Local effector functions of capsaicin-sensitive sensory nerve endings: involvement of tachykinins, calcitonin gene-related peptide and other neuropeptides. Neuroscience 24: 739–768. [DOI] [PubMed] [Google Scholar]
- 17.Devesa I., Planells-Cases R., Fernández-Ballester G., González-Ros J. M., Ferrer-Montiel A., Fernández-Carvajal A. 2011. Role of the transient receptor potential vanilloid 1 in inflammation and sepsis. J. Inflamm. Res. 4: 67–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thán M., Németh J., Szilvássy Z., Pintér E., Helyes Z., Szolcsányi J. 2000. Systemic anti-inflammatory effect of somatostatin released from capsaicin-sensitive vagal and sciatic sensory fibres of the rat and guinea-pig. Eur. J. Pharmacol. 399: 251–258. [DOI] [PubMed] [Google Scholar]
- 19.Cristino L., de Petrocellis L., Pryce G., Baker D., Guglielmotti V., Di Marzo V. 2006. Immunohistochemical localization of cannabinoid type 1 and vanilloid transient receptor potential vanilloid type 1 receptors in the mouse brain. Neuroscience 139: 1405–1415. [DOI] [PubMed] [Google Scholar]
- 20.Mezey E., Tóth Z. E., Cortright D. N., Arzubi M. K., Krause J. E., Elde R., Guo A., Blumberg P. M., Szallasi A. 2000. Distribution of mRNA for vanilloid receptor subtype 1 (VR1), and VR1-like immunoreactivity, in the central nervous system of the rat and human. Proc. Natl. Acad. Sci. USA 97: 3655–3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steenland H. W., Ko S. W., Wu L. J., Zhuo M. 2006. Hot receptors in the brain. Mol. Pain 2: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Szolcsányi J., Joó F., Jancsó-Gábor A. 1971. Mitochondrial changes in preoptic neurons after capsaicin desensitization of the hypothalamic thermodetectors in rats. Nature 229: 116–117. [DOI] [PubMed] [Google Scholar]
- 23.Roberts J. C., Davis J. B., Benham C. D. 2004. [3H]Resiniferatoxin autoradiography in the CNS of wild-type and TRPV1 null mice defines TRPV1 (VR-1) protein distribution. Brain Res. 995: 176–183. [DOI] [PubMed] [Google Scholar]
- 24.Wilhelmsen K., Khakpour S., Tran A., Sheehan K., Schumacher M., Xu F., Hellman J. 2014. The endocannabinoid/endovanilloid N-arachidonoyl dopamine (NADA) and synthetic cannabinoid WIN55,212-2 abate the inflammatory activation of human endothelial cells. J. Biol. Chem. 289: 13079–13100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spinsanti G., Zannolli R., Panti C., Ceccarelli I., Marsili L., Bachiocco V., Frati F., Aloisi A. M. 2008. Quantitative Real-Time PCR detection of TRPV1-4 gene expression in human leukocytes from healthy and hyposensitive subjects. Mol. Pain 4: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Himi N., Hamaguchi A., Hashimoto K., Koga T., Narita K., Miyamoto O. 2012. Calcium influx through the TRPV1 channel of endothelial cells (ECs) correlates with a stronger adhesion between monocytes and ECs. Adv. Med. Sci. 57: 224–229. [DOI] [PubMed] [Google Scholar]
- 27.Fernandes E. S., Fernandes M. A., Keeble J. E. 2012. The functions of TRPA1 and TRPV1: moving away from sensory nerves. Br. J. Pharmacol. 166: 510–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bertin S., Aoki-Nonaka Y., de Jong P. R., Nohara L. L., Xu H., Stanwood S. R., Srikanth S., Lee J., To K., Abramson L., et al. 2014. The ion channel TRPV1 regulates the activation and proinflammatory properties of CD4+ T cells. Nat. Immunol. 15: 1055–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ji D., Jang C. G., Lee S. 2014. A sensitive and accurate quantitative method to determine N-arachidonoyldopamine and N-oleoyldopamine in the mouse striatum using column-switching LC-MS-MS: use of a surrogate matrix to quantify endogenous compounds. Anal. Bioanal. Chem. 406: 4491–4499. [DOI] [PubMed] [Google Scholar]
- 30.De Petrocellis L., Di Marzo V. 2009. Role of endocannabinoids and endovanilloids in Ca2+ signalling. Cell Calcium 45: 611–624. [DOI] [PubMed] [Google Scholar]
- 31.Navarrete C. M., Pérez M., de Vinuesa A. G., Collado J. A., Fiebich B. L., Calzado M. A., Muñoz E. 2010. Endogenous N-acyl-dopamines induce COX-2 expression in brain endothelial cells by stabilizing mRNA through a p38 dependent pathway. Biochem. Pharmacol. 79: 1805–1814. [DOI] [PubMed] [Google Scholar]
- 32.Navarrete C. M., Fiebich B. L., de Vinuesa A. G., Hess S., de Oliveira A. C., Candelario-Jalil E., Caballero F. J., Calzado M. A., Muñoz E. 2009. Opposite effects of anandamide and N-arachidonoyl dopamine in the regulation of prostaglandin E and 8-iso-PGF formation in primary glial cells. J. Neurochem. 109: 452–464. [DOI] [PubMed] [Google Scholar]
- 33.Yoo J. M., Park E. S., Kim M. R., Sok D. E. 2013. Inhibitory effect of N-Acyl dopamines on IgE-mediated allergic response in RBL-2H3 cells. Lipids 48: 383–393. [DOI] [PubMed] [Google Scholar]
- 34.Sancho R., Macho A., de La Vega L., Calzado M. A., Fiebich B. L., Appendino G., Muñoz E. 2004. Immunosuppressive activity of endovanilloids: N-arachidonoyl-dopamine inhibits activation of the NF-kappa B, NFAT, and activator protein 1 signaling pathways. J. Immunol. 172: 2341–2351. [DOI] [PubMed] [Google Scholar]
- 35.Sagar D. R., Smith P. A., Millns P. J., Smart D., Kendall D. A., Chapman V. 2004. TRPV1 and CB(1) receptor-mediated effects of the endovanilloid/endocannabinoid N-arachidonoyl-dopamine on primary afferent fibre and spinal cord neuronal responses in the rat. Eur. J. Neurosci. 20: 175–184. [DOI] [PubMed] [Google Scholar]
- 36.Price T. J., Patwardhan A., Akopian A. N., Hargreaves K. M., Flores C. M. 2004. Modulation of trigeminal sensory neuron activity by the dual cannabinoid-vanilloid agonists anandamide, N-arachidonoyl-dopamine and arachidonyl-2-chloroethylamide. Br. J. Pharmacol. 141: 1118–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Little P. J., Compton D. R., Johnson M. R., Melvin L. S., Martin B. R. 1988. Pharmacology and stereoselectivity of structurally novel cannabinoids in mice. J. Pharmacol. Exp. Ther. 247: 1046–1051. [PubMed] [Google Scholar]
- 38.Bezuglov V., Bobrov M., Gretskaya N., Gonchar A., Zinchenko G., Melck D., Bisogno T., Di Marzo V., Kuklev D., Rossi J. C., et al. 2001. Synthesis and biological evaluation of novel amides of polyunsaturated fatty acids with dopamine. Bioorg. Med. Chem. Lett. 11: 447–449. [DOI] [PubMed] [Google Scholar]
- 39.Clark N., Keeble J., Fernandes E. S., Starr A., Liang L., Sugden D., de Winter P., Brain S. D. 2007. The transient receptor potential vanilloid 1 (TRPV1) receptor protects against the onset of sepsis after endotoxin. FASEB J. 21: 3747–3755. [DOI] [PubMed] [Google Scholar]
- 40.Guptill V., Cui X., Khaibullina A., Keller J. M., Spornick N., Mannes A., Iadarola M., Quezado Z. M. 2011. Disruption of the transient receptor potential vanilloid 1 can affect survival, bacterial clearance, and cytokine gene expression during murine sepsis. Anesthesiology 114: 1190–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Toscano M. G., Ganea D., Gamero A. M. 2011. Cecal ligation puncture procedure. J. Vis. Exp. (51): 2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang X., Goncalves R., Mosser D. M. 2008. The isolation and characterization of murine macrophages. Curr. Protoc. Immunol. Chapter 14: Unit 14.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lim Y. C., Luscinskas F. W. 2006. Isolation and culture of murine heart and lung endothelial cells for in vitro model systems. Methods Mol. Biol. 341: 141–154. [DOI] [PubMed] [Google Scholar]
- 44.Harms A. S., Tansey M. G. 2013. Isolation of murine postnatal brain microglia for phenotypic characterization using magnetic cell separation technology. Methods Mol. Biol. 1041: 33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shin H. S., Xu F., Bagchi A., Herrup E., Prakash A., Valentine C., Kulkarni H., Wilhelmsen K., Warren S., Hellman J. 2011. Bacterial lipoprotein TLR2 agonists broadly modulate endothelial function and coagulation pathways in vitro and in vivo. J. Immunol. 186: 1119–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Swamydas M., Lionakis M. S. 2013. Isolation, purification and labeling of mouse bone marrow neutrophils for functional studies and adoptive transfer experiments. J. Vis. Exp. (77): e50586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mesters R. M., Flörke N., Ostermann H., Kienast J. 1996. Increase of plasminogen activator inhibitor levels predicts outcome of leukocytopenic patients with sepsis. Thromb. Haemost. 75: 902–907. [PubMed] [Google Scholar]
- 48.Kawai T., Akira S. 2010. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat. Immunol. 11: 373–384. [DOI] [PubMed] [Google Scholar]
- 49.Starowicz K., Nigam S., Di Marzo V. 2007. Biochemistry and pharmacology of endovanilloids. Pharmacol. Ther. 114: 13–33. [DOI] [PubMed] [Google Scholar]
- 50.Ajami B., Bennett J. L., Krieger C., Tetzlaff W., Rossi F. M. 2007. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat. Neurosci. 10: 1538–1543. [DOI] [PubMed] [Google Scholar]
- 51.Levi M., de Jonge E., van der Poll T. 2003. Sepsis and disseminated intravascular coagulation. J. Thromb. Thrombolysis 16: 43–47. [DOI] [PubMed] [Google Scholar]
- 52.Rittirsch D., Flierl M. A., Ward P. A. 2008. Harmful molecular mechanisms in sepsis. Nat. Rev. Immunol. 8: 776–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schouten M., Wiersinga W. J., Levi M., van der Poll T. 2008. Inflammation, endothelium, and coagulation in sepsis. J. Leukoc. Biol. 83: 536–545. [DOI] [PubMed] [Google Scholar]
- 54.Fernandes E. S., Liang L., Smillie S. J., Kaiser F., Purcell R., Rivett D. W., Alam S., Howat S., Collins H., Thompson S. J., et al. 2012. TRPV1 deletion enhances local inflammation and accelerates the onset of systemic inflammatory response syndrome. J. Immunol. 188: 5741–5751. [DOI] [PubMed] [Google Scholar]
- 55.Cao Y. Q., Mantyh P. W., Carlson E. J., Gillespie A. M., Epstein C. J., Basbaum A. I. 1998. Primary afferent tachykinins are required to experience moderate to intense pain. Nature 392: 390–394. [DOI] [PubMed] [Google Scholar]
- 56.Chiu I. M., von Hehn C. A., Woolf C. J. 2012. Neurogenic inflammation and the peripheral nervous system in host defense and immunopathology. Nat. Neurosci. 15: 1063–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Borovikova L. V., Ivanova S., Zhang M., Yang H., Botchkina G. I., Watkins L. R., Wang H., Abumrad N., Eaton J. W., Tracey K. J. 2000. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 405: 458–462. [DOI] [PubMed] [Google Scholar]
- 58.Rosas-Ballina M., Tracey K. J. 2009. The neurology of the immune system: neural reflexes regulate immunity. Neuron 64: 28–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ni D., Gu Q., Hu H. Z., Gao N., Zhu M. X., Lee L. Y. 2006. Thermal sensitivity of isolated vagal pulmonary sensory neurons: role of transient receptor potential vanilloid receptors. Am. J. Physiol. Regul. Integr. Comp. Physiol. 291: R541–R550. [DOI] [PubMed] [Google Scholar]
- 60.Hüttemeier P. C., Ritter E. F., Benveniste H. 1993. Calcitonin gene-related peptide mediates hypotension and tachycardia in endotoxic rats. Am. J. Physiol. 265: H767–H769. [DOI] [PubMed] [Google Scholar]
- 61.Joyce C. D., Fiscus R. R., Wang X., Dries D. J., Morris R. C., Prinz R. A. 1990. Calcitonin gene-related peptide levels are elevated in patients with sepsis. Surgery 108: 1097–1101. [PubMed] [Google Scholar]
- 62.Beer S., Weighardt H., Emmanuilidis K., Harzenetter M. D., Matevossian E., Heidecke C. D., Bartels H., Siewert J. R., Holzmann B. 2002. Systemic neuropeptide levels as predictive indicators for lethal outcome in patients with postoperative sepsis. Crit. Care Med. 30: 1794–1798. [DOI] [PubMed] [Google Scholar]
- 63.Kawasaki H., Takasaki K., Saito A., Goto K. 1988. Calcitonin gene-related peptide acts as a novel vasodilator neurotransmitter in mesenteric resistance vessels of the rat. Nature 335: 164–167. [DOI] [PubMed] [Google Scholar]
- 64.Zygmunt P. M., Petersson J., Andersson D. A., Chuang H., Sørgård M., Di Marzo V., Julius D., Högestätt E. D. 1999. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature 400: 452–457. [DOI] [PubMed] [Google Scholar]
- 65.Murata Y., Masuko S. 2006. Peripheral and central distribution of TRPV1, substance P and CGRP of rat corneal neurons. Brain Res. 1085: 87–94. [DOI] [PubMed] [Google Scholar]
- 66.Yaraee R., Ebtekar M., Ahmadiani A., Sabahi F. 2003. Neuropeptides (SP and CGRP) augment pro-inflammatory cytokine production in HSV-infected macrophages. Int. Immunopharmacol. 3: 1883–1887. [DOI] [PubMed] [Google Scholar]
- 67.Cuesta M. C., Quintero L., Pons H., Suarez-Roca H. 2002. Substance P and calcitonin gene-related peptide increase IL-1 beta, IL-6 and TNF alpha secretion from human peripheral blood mononuclear cells. Neurochem. Int. 40: 301–306. [DOI] [PubMed] [Google Scholar]
- 68.Tang Y., Feng Y., Wang X. 1998. Calcitonin gene-related peptide potentiates LPS-induced IL-6 release from mouse peritoneal macrophages. J. Neuroimmunol. 84: 207–212. [DOI] [PubMed] [Google Scholar]
- 69.Weinstock J. V. 2015. Substance P and the regulation of inflammation in infections and inflammatory bowel disease. Acta Physiol. (Oxf.) 213: 453–461. [DOI] [PubMed] [Google Scholar]
- 70.Ho W. Z., Kaufman D., Uvaydova M., Douglas S. D. 1996. Substance P augments interleukin-10 and tumor necrosis factor-alpha release by human cord blood monocytes and macrophages. J. Neuroimmunol. 71: 73–80. [DOI] [PubMed] [Google Scholar]
- 71.Jiang M. H., Chung E., Chi G. F., Ahn W., Lim J. E., Hong H. S., Kim D. W., Choi H., Kim J., Son Y. 2012. Substance P induces M2-type macrophages after spinal cord injury. Neuroreport 23: 786–792. [DOI] [PubMed] [Google Scholar]
- 72.Hou Q., Barr T., Gee L., Vickers J., Wymer J., Borsani E., Rodella L., Getsios S., Burdo T., Eisenberg E., et al. 2011. Keratinocyte expression of calcitonin gene-related peptide β: implications for neuropathic and inflammatory pain mechanisms. Pain 152: 2036–2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.