Abstract
Cardiac allograft vasculopathy (CAV) is a progressive process involving both the epicardial and microvascular coronary systems. Timing of the development of abnormalities in these 2 compartments and correlation between changes in physiology and anatomy are undefined. Invasive evaluation of coronary artery anatomy and physiology with intravascular ultrasound (IVUS), fractional flow reserve (FFR), coronary flow reserve (CFR) and the index of microcirculatory resistance (IMR) was performed in the left anterior descending coronary artery during 151 angiographic evaluations of asymptomatic heart transplant (HT) recipients between 0 and >5 years after HT. There was no angiographic evidence of significant CAV, but during the first year after HT, FFR decreased significantly (0.89±0.06 vs. 0.85±0.07, p=0.001) and IVUS-derived % plaque volume increased significantly (15.6±7.7 to 22.5±12.3%, p=0.0002) resulting in a significant inverse correlation between epicardial physiology and anatomy (r=- 0.58, p<0.0001). IMR was lower in these patients, compared to those ≥ 2 years after HT (24.1±14.3 vs. 29.4±18.8 units, p=0.05), suggesting later spread of CAV to the microvasculature. As IMR increased, FFR increased (0.86±0.06 to 0.90±0.06, p=0.0035 comparing recipients with IMR ≤20 to those with IMR ≥40), despite no difference in % plaque volume (21.0±11.2 vs. 20.5±10.5%, p=NS). In conclusion, early after HT, both anatomic and physiologic evidence of epicardial CAV were found. Later after HT, the physiologic effect of epicardial CAV may be less, due to increased microvascular dysfunction.
It is now possible to independently assess epicardial coronary artery physiology by measuring myocardial fractional flow reserve (FFR) and microvascular physiology by measuring the index of microcirculatory resistance (IMR) relatively easily and simultaneously with a single coronary guidewire.1-3 The purpose of this study is to investigate changes over time in epicardial coronary artery anatomy and physiology after heart transplantation, as determined by intravascular (ultrasound) IVUS and FFR. By also measuring IMR, we hope to better understand the timing of the development of cardiac allograft vasculopathy (CAV) in the microvasculature and its relationship to the anatomic and physiologic changes in the epicardial arteries of these patients.
Methods
Stable heart transplant recipients undergoing coronary angiography were eligible for this study. The protocol was approved by Stanford University’s Administrative Panel on Human Subjects and each patient provided informed written consent. After routine right heart catheterization and right ventricular endomyocardial biopsy, coronary angiography was performed in the usual fashion.
Each patient then received 3,000 to 5,000 units of intravenous heparin and 100 to 200 micrograms of intracoronary nitroglycerin via a 6 French left coronary guiding catheter. A 0.014 inch coronary pressure wire was calibrated outside of the body and then advanced into the left anterior descending artery left anterior descending so that the pressure sensor, which is 3 centimeters from the tip of the wire, was located at the ostium of the guiding catheter, where equal pressure readings from the catheter and wire were confirmed. The wire was then advanced to the mid to distal segment of the left anterior descending.
With commercially available software, the shaft of the pressure wire can act as a proximal thermistor and the pressure sensor can act as a distal thermistor; the transit time of an injectate can be determined using a validated thermodilution technique.4,5 The resting mean transit time of saline down the left anterior descending was calculated by rapidly injecting 3 milliliters of room temperature saline into the left main coronary artery 3 times and averaging the results. Either intravenous adenosine (140 micrograms/kilogram/minute) or intracoronary papaverine (15 milligrams) was then administered to induce maximal hyperemia. The hyperemic mean transit time was then calculated in a similar fashion to the resting mean transit time. Distal coronary and aortic phasic and mean pressures were recorded simultaneously.
Coronary flow reserve was defined as the resting mean transit time divided by the hyperemic mean transit time.4,5 FFR was measured by dividing the mean distal coronary pressure (measured with the wire) by the mean aortic pressure (measured with the catheter), during peak hyperemia. IMR was calculated by dividing the mean distal pressure by the inverse of the hyperemic mean transit time, or more simply, by multiplying the mean distal pressure by the hyperemic mean transit time.1 An example of simultaneous FFR, CFR and IMR measurements is shown in Figure 1.
Figure 1.
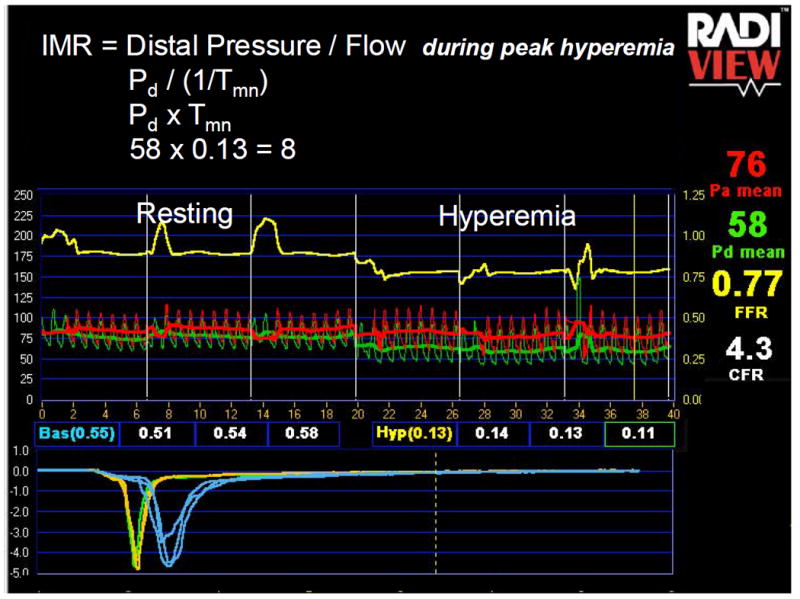
Example of FFR, CFR and IMR Measurement
In this example of a patient one year out from HT, the FFR in the left anterior descending artery is very abnormal at 0.77 (distal pressure, 58 mmHg divided by aortic pressure, 76mmHg), despite an angiographically normal appearing vessel. IVUS revealed moderate diffuse intimal thickening with a % plaque volume of 33%. CFR is calculated by dividing the resting mean transit time (0.55 seconds) by the hyperemic mean transit time (0.13 seconds), both of which are derived from averaging 3 transit times calculated from the thermodilution curves created by saline injection. The CFR is 4.3 which would usually be considered normal, but the low FFR suggests that the normal CFR in this patient was likely once higher. IMR is calculated as described in the figure by multiplying the hyperemic distal pressure (58 mmHg) by the hyperemic mean transit time (0.13 seconds) and was very low at 8 units, suggesting normal microvascular function.
A 40 megaherz IVUS catheter was then advanced over the pressure wire to the mid to distal left anterior descending. An automated pullback of the catheter at 0.5 millimeters per second was performed and the IVUS images were recorded. Offline, the IVUS images were digitized and two-dimensional analysis of cross-sections taken every 0.5 mm was performed. Measurements included maximum and minimum vessel and lumen diameters and areas, maximum percent cross-sectional narrowing, plaque area, and maximal intimal thickness. Three-dimensional volumetric analysis was performed using Simpson’s method (Echo Plaque, Indec Systems, Inc.). Measurements included the average vessel, lumen and plaque area of the entire imaged left anterior descending segment. In order to standardize for varying degrees of pullback length and for vessel size, the percent plaque volume, defined as the plaque volume divided by the vessel volume, was calculated. Plaque morphology was graded as soft, fibrofatty, mixed or calcific as previously described.6
The Stanford Quantitative Coronary Angiography Core Laboratory, blinded to the physiologic and IVUS results, performed quantitative coronary angiography on the proximal, mid and distal left anterior descending coronary artery from an angiogram with cranial angulation and minimal panning of the table. Using the guiding catheter for calibration and an edge detection system (Sanders Data Systems), the reference diameters and minimum lumen diameter for the 3 sites were calculated and the greatest % diameter stenosis recorded.
Data are expressed as means ±standard deviation. The mean FFR, IMR, CFR and IVUS parameters at various time periods after HT were compared with unpaired Student’s t test. Linear regression was applied to determine correlations between physiologic and IVUS parameters. Cases were grouped based on the time after HT, from baseline (within 2 months of HT), to1 year, 2 year, 3-5 year, and >5 years after HT. ANOVA was used to compare changes in physiologic and IVUS parameters over time. A p value <0.05 was considered statistically significant. Statistical analysis was performed using Stat View 5.0 (SAS Institute).
Results
Patient characteristics are summarized in Table 1. A total of 151 cases (92 patients) were included in the study. Quantitative coronary angiography demonstrated no stenosis in the left anterior descending > 40% in any patient. Data from 46 of these patients have been reported in a previous publication.7
Table 1.
Clinical Characteristics
| Characteristic | (n=92) |
|---|---|
| Donor Age (years) | 34 ± 13 |
| Recipient Age (years) | 53 ± 11 |
| Recipient Gender Male | 79% |
| Pre-Transplant Hypertension | 14% |
| Pre-Transplant Diabetes | 13% |
| Pre-Transplant Smoking | 19% |
| Ischemic Time (minutes) | 208 ± 51 |
| Ischemic Cardiomyopathy | 57% |
| Recipient Cytomegalovirus Positive | 65% |
| Cytomegalovirus Mismatch (D+/R-) | 12% |
FFR decreased significantly in cases at baseline compared to 1 year after HT (0.89±0.06 to 0.85±0.07, p=0.001, Figure 2). Beyond the first year, FFR gradually increased in a nonsignificant fashion (0.85±0.07 at year 1 to 0.89±0.06 at >5 years post HT, P=0.29). IMR did not change significantly during the 5 time periods (24.7±13.5 vs. 23.6±15.1 vs. 28.2±21.1 vs. 31.0±18.3 vs. 28.9±16.7, p=0.35). However, when comparing cases < 2 years to those ≥2 years after HT, IMR increased (24.1±14.3 vs. 29.4±18.8 units, p=0.05). There was a nonsignificant increase in the hyperemic mean transit time (0.36 ± 0.20 vs. 0.40 ± 0.22 seconds, p=0.32) and a significant increase in distal coronary pressure (67±12.5 vs. 72.7 ±14.8, p=0.01) in those cases < 2 years compared to those ≥2 years after HT. CFR did not change significantly during the 5 time periods after HT (3.0±1.5 vs. 3.4±1.9 vs. 3.2±1.8 vs. 2.9±1.1 vs. 3.3±1.4, p=0.72), nor was there a significant difference in CFR when comparing cases < 2 years to those ≥ 2 years after HT (3.19 vs. 3.09, p=0.70).
Figure 2.
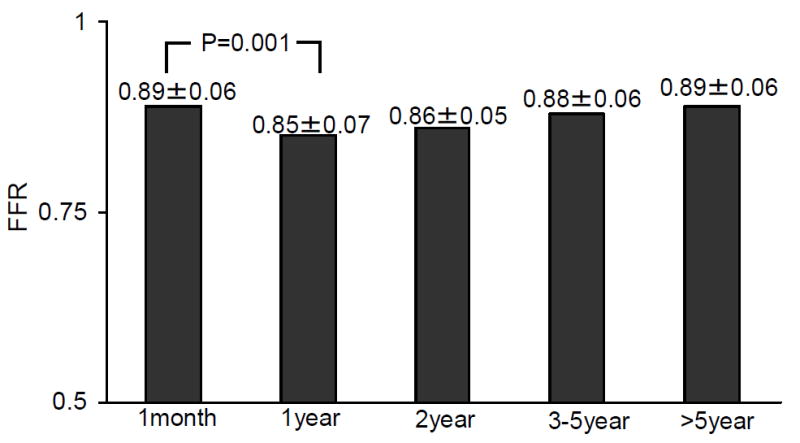
Change in FFR based on Time from Transplantation
On average, the IVUS pullback began 66.5±14.3mm distal to the left anterior descending ostium. Volumetric and cross-sectional IVUS findings are shown in Table 2. Average lumen area decreased significantly over time (11.1± 2.7 vs. 9.3±2.7 vs. 9.4±1.8 vs. 9.0±2.1 vs. 7.0±1.2, p<0.0001) and % plaque volume increased significantly over time (15.6 ± 7.7 vs. 22.5 ± 12.3 vs. 22.7 ± 11.4 vs. 24.1±10.6 vs. 26.1±10.7, p=0.002).
Table 2.
Intravascular Ultrasound Findings
| Time After Transplantation | 1 month (n=46) | 1 year (n=48) | 2 years (n=22) | 3-5 years (n=27) | >5 years (n=10) |
|---|---|---|---|---|---|
| Volumetric | |||||
| Average Vessel Area (mm3/mm) | 13.3±3 | 12.5±3 | 12.5±3 | 12.2±4§ | 9.7±2§ |
| Average Plaque Area (mm3/mm) | 2.1±1* | 3.0±2* | 3.1±2 | 3.2±2 | 2.7±2 |
| Cross-sectional | |||||
| Minimum Lumen Area (mm) | 4.9±2 | 4.6±2 | 4.2±1 | 4.2±2‡ | 2.9±1‡ |
| Maximum % Cross-Sectional Narrowing | 27±14† | 39±20† | 41±20 | 46±16 | 46±18 |
| Maximum Intimal Thickness (mm) | 0.7±0.5‡ | 0.9±0.4‡ | 1.1±0.6 | 1.0±0.6 | 0.9±0.5 |
| Plaque Morphology | |||||
| Soft | 90% | 73% | 67% | 65% | 52% |
| Fibrofatty | 4% | 13% | 14% | 12% | 10% |
| Mixed | 2% | 4% | 9% | 1% | 6% |
| Calcific | 4% | 9% | 9% | 15% | 32% |
p=0.02,
p=0.003,
p=0.03,
p=0.05
FFR correlated inversely with % plaque volume and maximum % cross-sectional narrowing in cases < 2 years post transplant (r=-0.58, p<0.0001 and r=-0.57, p<0.0001, respectively), cases in which on average IMR was lower. FFR did not correlate with these IVUS parameters in cases ≥ 2 years after transplantation (r=-0.14, p=0.27 and r=-0.22, p=0.08, respectively), cases in which IMR on average was higher. In cases in which IMR was ≤ 20, FFR was significantly lower compared to cases in which IMR was ≥ 40, despite equivalent IVUS findings (Table 3). CFR did not correlate with % plaque volume (r=0.13, p=0.11), maximum % cross-sectional narrowing (r=0.11, p=0.18) or FFR (r=0.06, p=0.49), but did correlate significantly with IMR (r=0.51, p<0.0001).
Table 3.
Relationship Between IMR, FFR and IVUS Results
| Variable | IMR ≤ 20 (n=67) | 20 < IMR < 40 (n=50) | IMR ≥40 (n=34) |
|---|---|---|---|
| % Plaque Volume | 21.0±11% | 20.8±11% | 20.5±11% |
| Maximum % Cross Sectional | 38.9±18% | 39.4±18% | 40.4±19% |
| Narrowing FFR | 0.86±0.06* | 0.87±0.07 | 0.90±0.06* |
P=0.0035
Discussion
The major finding in this study is the interplay between the physiologic changes in the coronary microvasculature and the epicardial coronary artery over time in HT recipients, and the effect this interplay has on the correlation between epicardial artery physiology and anatomy. Early after HT, anatomic changes in the epicardial artery as assessed by IVUS, correlate with significant changes in epicardial artery physiology, as assessed by FFR. On average, microvascular resistance (IMR) is lower during the first 2 years after HT compared to later after HT, presumably due to late involvement of the microvasculature by CAV. Late after HT, the increase in microvascular resistance results in a decrease in the maximum achievable flow down the epicardial vessel and lessens the physiologic impact of structural abnormalities in the epicardial artery. For this reason, FFR may not provide a good representation of epicardial artery plaque burden late after HT, unless microvascular resistance (IMR) is found to be low.
In patients who have not had HT, but have microvascular dysfunction from another cause (e.g., myocardial infarction) a similar disconnect between the anatomic findings and physiologic implications may be present. It does not imply that FFR is inaccurate in these patients. The FFR result continues to inform one about the percentage of maximum achievable myocardial flow in the presence of an epicardial stenosis. In the setting of microvascular dysfunction, the maximum achievable flow is blunted, the impact of an epicardial stenosis lessened, and the expected physiologic benefit from stenting lessened.
CAV is a progressive process involving the epicardial as well as microvascular systems. The changes in physiology and anatomy over time in these two compartments are poorly defined and changes in one may significantly impact assessment of the other. The standard technique for detecting epicardial CAV is IVUS, which has been shown to be more sensitive than angiography and to be predictive of long-term adverse outcome.8-10 IVUS is limited however by the fact that it provides anatomic information, only, and does not interrogate the microvasculature.
The standard technique for evaluating coronary physiology in HT recipients has been to use a Doppler wire to measure CFR.11 CFR interrogates the entire coronary circulation and is unable to distinguish abnormal epicardial physiology from microvascular physiology.12 Additionally, CFR is limited by poor reproducibility. 13,14 These factors may explain why some previous studies have shown a significant decrease in CFR over time after HT15, and others a significant increase.16
FFR is an index which provides information about the degree to which epicardial artery disease is affecting myocardial perfusion.17 It is unique compared to CFR in that it is specific for the epicardial artery and is extremely reproducible.13,14,18 FFR has been well-validated in the non-HT population, but not well-studied in HT recipients. We have previously published a study evaluating FFR at a single time point in HT recipients, predominantly early after HT, and found a strong correlation between FFR and the IVUS-determined epicardial % plaque volume.7
IMR is a new index that we recently proposed as a method for interrogating the status of the coronary microcirculation, independent of the epicardial artery.1-3 We have found that it correlates well with an accepted experimental technique for determining microvascular resistance1 and that it is more reproducible than CFR.14 The ability to assess easily and accurately both the epicardial artery and the microvasculature simultaneously by measuring FFR and IMR with a single wire is a potential advantage over the standard methods of using IVUS and a Doppler velocity wire.
In this study we found that early after HT (<2 years) there was a significant inverse correlation between epicardial artery physiology as measured with FFR and epicardial artery anatomy as measured with IVUS. During the first year after HT, FFR decreased significantly while plaque volume increased significantly. On average, the minimum achievable microvascular resistance, as measured with IMR, was low early (during the first 2 years) after HT compared to later. Because of the low microvascular resistance, the peak epicardial flow, as estimated by the hyperemic mean transit time, was higher (shorter hyperemic mean transit time). The higher flow results in a greater pressure gradient (lower distal pressure and FFR) across an epicardial stenosis and likely explains the strong correlation between FFR and IVUS findings.
Later after HT (≥2 years) IMR increased, suggesting that CAV involves the microvasculature later than it does the epicardial artery. Changes in myogenic tone that occur in the microvasculature after HT and may initially lower resistance could result in microvascular edema which eventually leads to increased resistance.19 During the later time periods (2 years post HT to >5 years post HT), there was little change in plaque volume and a more significant late decrease in vessel volume. FFR gradually increased during this time, coinciding with the increase in IMR (Table 3).
A major limitation of this study is the lack of serial analyses meaning that late patients may not be well matched with early patients. For example, in a subset of patients in this study in which IMR was measured at the time of HT and then 1 year later, we have previously reported that IMR was significantly higher at baseline, presumably due to the effects of donor heart ischemia and/or early recipient immunologic effects.20 We also found in a group with IVUS evaluation at baseline and 1 year after HT that negative remodeling played a more prominent role in lumen loss early after HT than seen in this study.21 Likely the changes in coronary physiology are not universal, but recipients with certain clinical characteristics may be more or less susceptible. Because of the lack of long-term serial data, we could not do subgroup analyses. Long-term follow-up of matched patients will be crucial to confirm the findings in this study. In addition, this study did not include consecutive patients and was a retrospective analysis; some patients were excluded because of medical conditions, such as elevated creatinine, and others for logistic reasons. Finally, determining the prognostic importance of these physiologic measurements and volumetric IVUS findings will be critical.
Acknowledgments
This work was supported in part by grants 1 K23 HL072808-01A1 (WFF) and 1 PO1-AI50153 (HAV) from the National Institutes of Health, Heart Lung and Blood Institute, Bethesda, MD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fearon WF, Balsam LB, Farouque HM, Caffarelli AD, Robbins RC, Fitzgerald PJ, Yock PG, Yeung AC. Novel index for invasively assessing the coronary microcirculation. Circulation. 2003;107:3129–3132. doi: 10.1161/01.CIR.0000080700.98607.D1. [DOI] [PubMed] [Google Scholar]
- 2.Fearon WF, Aarnoudse W, Pijls NH, De Bruyne B, Balsam LB, Cooke DT, Robbins RC, Fitzgerald PJ, Yeung AC, Yock PG. Microvascular resistance is not influenced by epicardial coronary artery stenosis severity: experimental validation. Circulation. 2004;109:2269–2272. doi: 10.1161/01.CIR.0000128669.99355.CB. [DOI] [PubMed] [Google Scholar]
- 3.Aarnoudse W, Fearon WF, Manoharan G, Geven M, van de Vosse F, Rutten M, De Bruyne B, Pijls NH. Epicardial stenosis severity does not affect minimal microcirculatory resistance. Circulation. 2004;110:2137–2142. doi: 10.1161/01.CIR.0000143893.18451.0E. [DOI] [PubMed] [Google Scholar]
- 4.De Bruyne B, Pijls NHJ, Smith L, Wievegg M, Heyndrickx GR. Coronary thermodilution to assess flow reserve: experimental validation. Circulation. 2001;104:2003–2006. doi: 10.1161/hc4201.099223. [DOI] [PubMed] [Google Scholar]
- 5.Pijls NH, De Bruyne B, Smith L, Aarnoudse W, Barbato E, Bartunek J, Bech GJ, Van De Vosse F. Coronary thermodilution to asses flow reserve: validation in humans. Circulation. 2002;105:2480–2484. doi: 10.1161/01.cir.0000017199.09457.3d. [DOI] [PubMed] [Google Scholar]
- 6.Mintz GS, Nissen SE, Anderson WD, Bailey SR, Erbel R, Fitzgerald PJ, Pinto FJ, Rosenfield K, Siegel RJ, Tuzcu EM, Yock PG. American College of Cardiology Clinical Expert Consensus Document on Standards for Acquisition, Measurement and Reporting of Intravascular Ultrasound Studies (IVUS). A report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2001;37:1478–92. doi: 10.1016/s0735-1097(01)01175-5. [DOI] [PubMed] [Google Scholar]
- 7.Fearon WF, Nakamura M, Lee DP, Rezaee M, Vagelos RH, Hunt SA, Fitzgerald PJ, Yock PG, Yeung AC. Simultaneous assessment of fractional and coronary flow reserves in cardiac transplant recipients: Physiologic Investigation for Transplant Arteriopathy (PITA Study) Circulation. 2003;108:1605–10. doi: 10.1161/01.CIR.0000091116.84926.6F. [DOI] [PubMed] [Google Scholar]
- 8.St Goar FG, Pinto FJ, Alderman EL, Valantine HA, Schroeder JS, Gao SZ, Stinson EB, Popp RL. Intracoronary ultrasound in cardiac transplant recipients In vivo evidence of “angiographically silent” intimal thickening. Circulation. 1992;85:979–987. doi: 10.1161/01.cir.85.3.979. [DOI] [PubMed] [Google Scholar]
- 9.Kobashigawa JA, Tobis JM, Starling RC, Tuzcu EM, Smith AL, Valantine HA, Yeung AC, Mehra MR, Anzai H, Oeser BT, Abeywickrama KH, Murphy J, Cretin N. Multicenter intravascular ultrasound validation study among heart transplant recipients: outcomes after five years. J Am Coll Cardiol. 2005;45:1532–1537. doi: 10.1016/j.jacc.2005.02.035. [DOI] [PubMed] [Google Scholar]
- 10.Tuzcu EM, Kapadia SR, Sachar R, Ziada KM, Crowe TD, Feng J, Magyar WA, Hobbs RE, Starling RC, Young JB, McCarthy P, Nissen SE. Intravascular ultrasound evidence of angiographically silent progression in coronary atherosclerosis predicts long-term morbidity and mortality after cardiac transplantation. J Am Coll Cardiol. 2005;45:1538–1542. doi: 10.1016/j.jacc.2004.12.076. [DOI] [PubMed] [Google Scholar]
- 11.Caracciolo EA, Wolford TL, Underwood RD, Donohue TJ, Bach RG, Miller LW, Kern MJ. Influence of intimal thickening on coronary blood flow responses in orthotopic heart transplant recipients. A combined intravascular Doppler and ultrasound imaging study. Circulation. 1995;92:II182–90. doi: 10.1161/01.cir.92.9.182. [DOI] [PubMed] [Google Scholar]
- 12.Kern MJ. Coronary physiology revisited : practical insights from the cardiac catheterization laboratory. Circulation. 2000;101:1344–51. doi: 10.1161/01.cir.101.11.1344. [DOI] [PubMed] [Google Scholar]
- 13.De Bruyne B, Bartunek J, Sys SU, Pijls NH, Heyndrickx GR, Wijns W. Simultaneous coronary pressure and flow velocity measurements in humans. Feasibility, reproducibility, and hemodynamic dependence of coronary flow velocity reserve, hyperemic flow versus pressure slope index, and fractional flow reserve. Circulation. 1996;94:1842–9. doi: 10.1161/01.cir.94.8.1842. [DOI] [PubMed] [Google Scholar]
- 14.Ng M, Yeung AC, Fearon WF. Invasive Assessment of the Coronary Microcirculation: Superior Reproducibility and Less Hemodynamic Dependence of Index of Microcirculatory Resistance as Compared to Coronary Flow Reserve. Circulation. 2006;113:2054–2061. doi: 10.1161/CIRCULATIONAHA.105.603522. [DOI] [PubMed] [Google Scholar]
- 15.Mazur W, Bitar JN, Young JB, Khalil AA, Vardan S, Short BC, Rivera JM, Raizner AE, Farmer JA, Zoghbi WA, Kleiman NS. Progressive deterioration of coronary flow reserve after heart transplantation. Am Heart J. 1998;136:504–509. doi: 10.1016/s0002-8703(98)70228-5. [DOI] [PubMed] [Google Scholar]
- 16.Konig A, Spes CH, Schiele TM, Rieber J, Stempfle HU, Meiser B, Theisen K, Mudra H, Reichart B, Klauss V. Coronary Doppler measurements do not predict progression of cardiac allograft vasculopathy: analysis by serial intracoronary Doppler, dobutamine stress echocardiography, and intracoronary ultrasound. J Heart Lung Transplant. 2002;21:902–905. doi: 10.1016/s1053-2498(01)00416-8. [DOI] [PubMed] [Google Scholar]
- 17.Pijls NH, De Bruyne B, Peels K, Van Der Voort PH, Bonnier HJ, Bartunek J, Koolen JJ. Measurement of fractional flow reserve to assess the functional severity of coronary-artery stenoses. N Engl J Med. 1996;334:1703–1708. doi: 10.1056/NEJM199606273342604. [DOI] [PubMed] [Google Scholar]
- 18.Pijls NH, Van Gelder B, Van der Voort P, Peels K, Bracke FA, Bonnier HJ, el Gamal MI. Fractional flow reserve. A useful index to evaluate the influence of an epicardial coronary stenosis on myocardial blood flow. Circulation. 1995;92:3183–3193. doi: 10.1161/01.cir.92.11.3183. [DOI] [PubMed] [Google Scholar]
- 19.Moien-Afshari F, Skarsgard PL, McManus BM, Laher I. Cardiac transplantation and resistance artery myogenic tone. Can J Physiol Pharmacol. 2004;82:840–8. doi: 10.1139/y04-100. [DOI] [PubMed] [Google Scholar]
- 20.Fearon WF, Hirohata A, Nakamura M, Luikart H, Lee DP, Vagelos R, Hunt SA, Valantine HA, Fitzgerald PJ, Yock PG, Yeung AC. Discordant Changes in Epicardial and Microvascular Coronary Physiology After Cardiac Transplantation: Physiologic Investigation for Transplant Arteriopathy II (P.I.T.A. II) Study. J Heart Lung Transplant. 2006;25:765–771. doi: 10.1016/j.healun.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 21.Fearon WF, Potena L, Hirohata A, Sakurai R, Yamasaki M, Luikart H, Lee J, Vana ML, Cooke JP, Mocarski ES, Yeung AC, Valantine HA. Changes in coronary arterial dimmensions early after cardiac transplantation. Transplantation. 2006 doi: 10.1097/01.tp.0000256335.84363.9b. in press. [DOI] [PubMed] [Google Scholar]


