Abstract
Rationale
Human metapneumovirus (HMPV) is a recently discovered respiratory pathogen of the family Paramyxoviridae, the same of Respiratory Syncytial Virus (RSV). Premature children are at high risk of severe RSV infections, but it is unclear whether HMPV infection is more severe in hospitalized children with history of severe prematurity.
Methods
We conducted a retrospective analysis of the clinical respiratory presentation of all PCR-confirmed HMPV infections in preschool age children (≤5 yrs.) with and without history of severe prematurity (<32 weeks gestation). Respiratory distress scores were developed to examine the clinical severity of HMPV infections. Demographic and clinical variables were obtained from reviewing electronic medical records (EMR).
Results
A total of 571 pre-school children were identified by PCR-confirmed viral respiratory tract infection during the study period. HMPV was identified as a causative organism in 63 cases (11%). Fifty–eight (n=58) preschool age children with HMPV infection were included in this study after excluding those with significant co-morbidities. Our data demonstrated that 32.7% of children admitted with HMPV had history of severe prematurity. Preschool children with history of prematurity had more severe HMPV disease as illustrated by longer hospitalizations, new or increased need for supplemental O2, and higher severity scores independently of age, ethnicity and history of asthma.
Conclusion
Our study suggests that HMPV infection causes significant disease burden among preschool children with history of prematurity leading to severe respiratory infections and increasing health care resource utilization due to prolonged hospitalizations.
Keywords: human metapneumovirus, prematurity, asthma, wheezing
INTRODUCTION
Respiratory tract infections are the second leading cause of death among children who are less than 5 years of age worldwide.1 Human Metapneumovirus (HMPV), a relatively new respiratory pathogen of the family Paramyxoviridae – the same of Respiratory Syncytial Virus (RSV) – was discovered only a decade ago,2 but it is now recognized as a frequent cause of acute lower respiratory tract infections in the pediatric population.1,4–6 Most children who are less than 5 years old have already been infected with HMPV,3 with the overall prevalence of acute HMPV infection in the pediatric population ranging from 5–25% with some variation across different regions and age groups.5, 7–12 It has been estimated that approximately 27,000 HMPV-related hospitalizations will occur per year in the future among preschool children in the US.13 This estimation is alarming and emphasizes the need to investigate the epidemiology and pathogenesis of HMPV in children.
HMPV shares the same clinical respiratory signs and symptoms as RSV, including cough, wheezing, rales, hypoxemia and respiratory distress in high-risk groups.5, 14–16 HMPV lower respiratory tract infections contribute to 5–15% of all hospitalizations in infants and young children.17–18 Despite the importance of this pathogen in the pediatric population, no treatment or prevention strategies have been developed,19,20 which may reflect the lack of understanding of the risk factors that increase the morbidity and mortality of HMPV infection in children.19,20 Interestingly, prematurity has recently been suggested to be an important risk factor for severe HMPV infection.20–22 Young children with a history of prematurity are at increased risk of hospitalization and frequent outpatient visits due to HMPV bronchiolitis and pneumonia,20–23 epidemiologies that resemble the phylogenetically and clinically related pathogen RSV.21 Other risk factors associated with severe HMPV infection include immunosuppression, younger age and existence of underlying co-morbidities, such as asthma, congenital heart diseases, neuromuscular disorders, and other chronic pulmonary conditions.23–25
Although the aforementioned risk factors have been reported, there are limited data about the clinical presentation of severe HMPV infection in hospitalized premature children. Moreover, the link between the severity of HMPV respiratory infections and the history of prematurity in hospitalized children still needs to be better defined. Accordingly, the aim of this cross-sectional study was to examine the clinical severity of HMPV infection in hospitalized children ≤ 5 years of age with and without history of severe prematurity (<32 weeks gestation), using respiratory parameters derived from standardized bronchiolitis scores validated by our group,26, 27 and health care utilization (i.e, length of admission).
MATERIALS AND METHODS
Study Subjects
We conducted a retrospective cross-sectional analysis of a cohort of pre-school children ≤5 years of age who were admitted with HMPV infection, which was confirmed by PCR analysis, at Children’s National Medical Center (CNMC) between January 2013 and February 2014. Viral PCR were performed on subjects who presented to the hospital with suspected viral respiratory tract infection at discretion of clinician. We only included children with positive PCR for HMPV and excluded individuals with mixed viral infections.
Patients with significant co-morbidities such as cardiorespiratory conditions (other than asthma and prematurity), genetic syndromes, and immunosuppression were excluded from the study. This study was approved by the Institutional Review Board at Children’s National Medical Center.
Clinical and demographic variables
Clinical and demographic variables were obtained by reviewing electronic medical records (EMR) at CNMC. Demographic variables comprised gestational age in weeks, age, gender, and ethnicity. Other clinical variables included tachypnea, retractions, abnormal breath sounds (wheezing), asthma diagnosis, oxyhemoglobin saturation values by pulse oximetry (SaO2), supplemental oxygen (O2) requirement relative to patient’s baseline, length of hospitalization, and the need for admission to pediatric intensive care unit (PICU). In our institution, PICU admission criteria include worsening hypoxemia or hypercapnia, worsening respiratory distress, continuing requirement for more than 50% oxygen, hemodynamic instability, and apnea. In addition, in the setting of viral respiratory tract infection PICU admission is also required for the following : 1) initiation of “non-invasive advanced respiratory support”, which is defined as the use of high-flow nasal cannula or positive airway pressure via mask in continuous (CPAP) or bi-level (BIPAP) modes; and 2) initiation of mechanical ventilator support via endotracheal intubation or tracheostomy. Patients with baseline mechanical ventilator support are not automatically admitted to PICU unless that there is a modification in baseline parameters. The variable “need for invasive mechanical support” during HMPV infection was defined as new onset of mechanical support or increased in baseline parameters from baseline.
For the purpose of the study, clinical parameters were characterized as binary outcome for: severe prematurity defined a priori by a gestational age of less than 32 weeks to include extremely preterm and very preterm subjects based on World Health Organization (WHO) definition of prematurity,28 asthma status and oxygen supplementation. Tachypnea was stratified and scored in groups (0–3) according to respiratory rate definitions used in bronchiolitis scores as follows: 0 for <30 breaths per minute (bpm); 1 for 30–45 bpm; 2 for 46–60 bpm; and 3 for >60.27,29 “Asthma” was defined in this pediatric population using a definition that required the presence of at least one of the following criteria: (1) ever being diagnosed with asthma by a physician on the basis of criteria recommended for children in the National Asthma Education and Prevention Program (NAEPP) Guidelines; and (2) use of asthma therapy and/or presence of asthma symptoms in the past 12 months and previously described.30
Clinical Evaluation of Viral Respiratory Tract infection Severity Based on Lower Airway obstruction and Respiratory Distress
To retrospectively assess overall clinical severity of HMPV infection, we recorded wheezing, retractions, supplemental O2 need (which we found retrospectively to be more consistently documented than the exact initial SaO2 saturation) and tachypnea at the initial presentation based on EMR and combined them in a respiratory distress score (0–10). For this score, we used a stratified value for tachypnea (0–3) and combined assigned values with the binary need of O2 (0–2), presence of wheezing (0–3) and retractions (0–2), yielding a total maximal value of 10 points (Supplemental Table E1). Of note, the four clinical variables included in this respiratory distress score were selected because they represented the main phenotypical features of viral bronchiolitis in children, lower airway obstruction and respiratory distress,30 and they are the parameters included in bronchiolitis scores validated by our group (Modified Wood’s Clinical Asthma score (M-WCAS) and Tal severity score).27,29
Statistical Analysis
Data were analyzed using the software SAS version 9.3 (SAS Institute Inc., Cary, NC, USA). Descriptive statistics were used to calculate the prevalence of HMPV. Collected demographic and clinical data were compared with the use of a Chi-square test (categorical variables) or T-test or Wilcoxon rank-sum test, as appropriate for continuous variables. Significance was taken at the P < 0.05 level.
RESULTS
Prevalence of prematurity in hospitalized preschool children with HMPV infection
We reviewed records of all preschool age children (0–5 years) admitted to our institution during the 2013 season with a PCR confirmed diagnosis of viral respiratory tract infection (n = 571). We identified HMPV as the causative organism in 63 cases (11%). Fifty - eight (n = 58) preschool age children with HMPV infection were included in this study after excluding those with significant co-morbidities such as cardiorespiratory conditions (other than asthma and prematurity). In terms of age distribution, a history of severe prematurity (born at < 32 weeks gestational age) was present in 42% (n = 8/19) in infants less than a year of age and 24% (n=9/39) of individuals 1–5 years old. The overall prevalence of history of severe prematurity was 32.7% in the entire population (n=19/58), indicating that prematurity was highly prevalent in preschool children hospitalized due to HMPV infection. Clinical characteristics and comorbidities of premature children with HMPV infection are shown in Table 1. Comparison of demographic and baseline study variables of children with and without history of severe prematurity revealed no significant differences (Table 1).
Table 1. Baseline characteristics for study subjects with HMPV.
Comparison of children with and without history of severe prematurity (<32 weeks gestation) and comorbidities of premature subjects. For quantitative variables, data are presented as mean ± standard deviation (SD). For categorical variables, data are presented as count number (column percentage). The p values are obtained by either two-sample t test, or chi -square test, depending on the type of variable
| Variable | Non-premature | Premature | P value |
|---|---|---|---|
| N | 39 | 19 | |
| Gestational age (weeks), mean(SD) | 39(1.8) | 26.2(2.5) | <0.001 |
| Gender (Male), n (%) | 23(58) | 9(53) | 0.676 |
| Age (y), mean (SD) | 2.1(1.6) | 1.9(1.6) | 0.549 |
| Race/Ethnicity | |||
| – Black, n(%) | 23(58) | 8(42) | 0.221 |
| – White, n(%) | 5(13) | 7(37) | 0.051 |
| – Hispanic, n(%) | 11(28) | 4(21) | 0.545 |
| Asthma, n (%) | 15(38) | 10(58) | 0.252 |
| Prematurity comorbidities, n (%) | |||
| – Necrotizing enterocolitis (NEC) | 7(37) | ||
| – Bronchopulmonary dysplasia (BPD) | 13(68) | ||
| – Tracheostomy/home mechanical support | 3(16) | ||
| – Intraventricular hemorrhage (IVH) | 7(37) |
HMPV in premature children is associated with higher clinical severity
To assess HMPV severity in premature children, we first evaluated the absolute degree of hypoxemia assessed with the value of SaO2 at presentation, which was significantly lower in children with history of severe prematurity (mean SaO2 89%; 95% CI = 85.5%–94.1%) relative to children born at term (mean SaO2 95%; 95% CI = 94.4%–96.7%). We also investigated the odds of needing supplemental O2 (new or increased from baseline) in severely premature children hospitalized with HMPV infection. After adjusting for age, gender and history of asthma (logistic regression), we identified that severely premature children were 6 times more likely to need supplemental O2 (adjusted OR 5.9; 95% CI = 1.25–26.; p = 0.02). We next examined overall clinical severity using a respiratory distress score (0–10; Supplemental Table E1), which includes the most characteristic phenotypical features of viral bronchiolitis: lower airway obstruction (wheezing) and respiratory distress (sub-costal retractions, supplemental O2 need and tachypnea).26, 27 Severely premature born children with HMPV had more severe respiratory distress scores relative to non-premature HMPV-infected children (score mean +/− SD in full term HMPV-infected 4.7 +/− 3.3 and in severely premature HMPV-infected 7.3+/−2.3, p<0.01; Table 2). Individual parameters of respiratory distress were also significantly increased except for wheezing that did not reach statistical significance (Table 2).
Table 2. Clinical severity parameters in children with HMPV infection.
Comparison of children with and without history of severe prematurity. For quantitative variables, data are presented as mean ± standard deviation (SD). For categorical variables, data are presented as count number (column percentage). The p values are obtained by either two-sample t test, or chi -square test, depending on the type of variables.
| Non-premature | Premature | P value | |
|---|---|---|---|
| N | 39 | 19 | |
| Initial O2 Saturation (%), mean +/− SD | 95+/−3.4 | 89+/−7.1 | <0.01 |
| Mean Total Respiratory Distress Score (0–10) | 4.7+/− 3.3 | 7.3 +/−2.3 | <0.01 |
| – Supplemental O2 (new or increased from baseline, n (%) | 15(38.4) | 16(84) | <0.01 |
| – Stratified tachypnea (0–3), mean +/− SD | 1.21 +/−0.9 | 1.73+/−0.7 | 0.04 |
| – Wheezing, n (%) | 22(56.4) | 15(79) | 0.09 |
| – Subcostal retractions, n (%) | 20(51.2) | 15(79) | 0.04 |
In terms of the clinical presentation of premature children infected with HMPV, we found that lung auscultation and chest radiographs (CXR) were abnormal in virtually all cases. Specifically, diffuse or localized crackles were present in 95% of the cases (n = 18/19) and focal infiltrates were identified in 74% (n = 14/19) of premature children infected with HMPV. Lung infiltrates in premature children infected with HMPV were typically poorly defined air occupying lesions affecting different segments of both lungs (Figure 1). These focal infiltrates were not associated with ground-glass pattern, pleural effusion or necrotizing pneumonia in any of the cases. Fever (>100.4 F°) was present in 68% of the cases (n = 13/19) but it was typically low degree and present only during the onset of the symptoms. In terms of laboratory abnormalities, mild-moderate leukocytosis was identified in 26% of the cases (n = 5/19) and mild thrombocytopenia was present in two of the premature children infected with HMPV.
Figure 1. Comparative Chest Radiographs (CXRs) of a 2–year–old premature girl with HMPV infection.
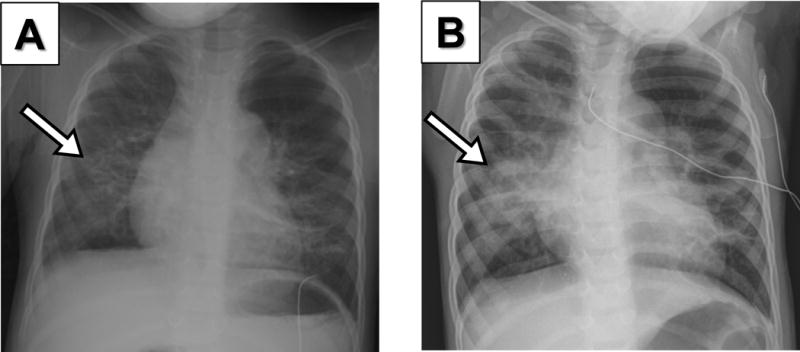
(A) Initial CXR during admission to the hospital due to HMPV infection showing a focal infiltrate in the right lower lobe (arrow). (B) 48h later CXR shows progression of right lower lung opacities (arrow) that correlated with worsening of crackles, poor air entry, respiratory distress and hypoxemia.
Severe prematurity is associated with longer hospitalization in children with HMPV infection
Preschool children with HMPV infection had a median duration of hospitalization of 4 days (95% CI 2.6–4.7 days, Figure 2). In contrast, preschool children with history of severe prematurity had a significantly longer hospitalization during HMPV infection with a median of 9 days (95% CI 6.8–12.7 days, Figure 2). Interestingly, the length of hospitalization during HMPV infection was non-significantly different in preschool children with the diagnosis of asthma relative to that in individuals without history of this condition (non-asthmatic children median 3.2 days, 95% CI 2.2–6.3 vs. asthmatic children median 4.7 days, 95% CI 4.1–6.4; p > 0.05). During the study period, 23 children with HMPV infection were admitted PICU (39%). Thirty–three percent of non-premature children with HMPV infection needed PICU admission (n = 13/39). In the group of severely premature children, 58% were admitted to PICU (n = 11/19). Premature children hospitalized with HMPV infection were more likely to require non-invasive respiratory support (n = 5/39 non-premature vs. n = 7/19 premature; p < 0.05). No significant differences were found in the need for invasive mechanical ventilation, (n = 5/39 non-premature vs. n = 5/19 premature; p = 0.07) and no mortality was present during the study period.
Figure 2. Mean duration of hospitalization in children with HMPV infection.
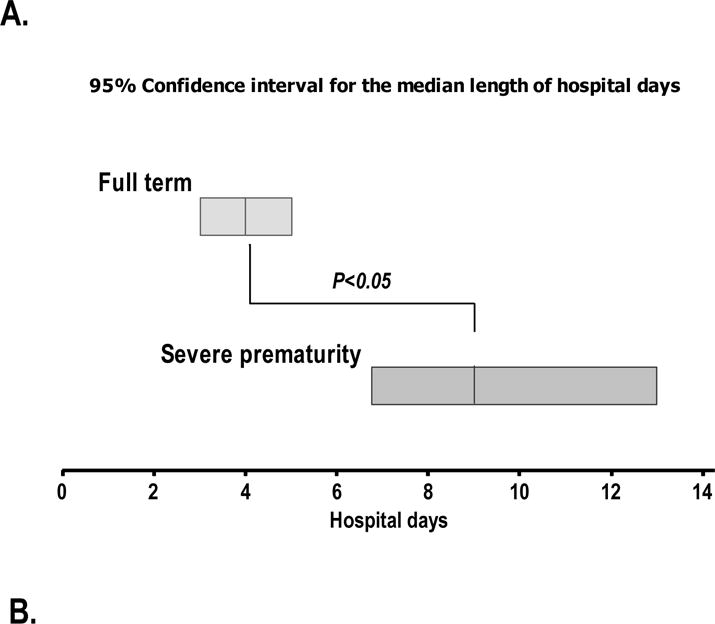
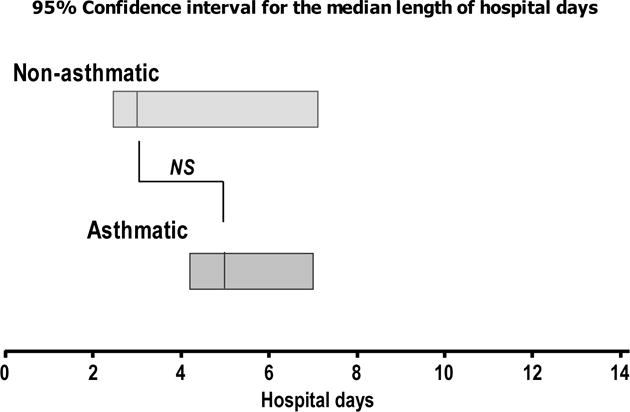
Bars represent the 95% confidence interval for the median length of admission (hospital days) in in children with and without history of severe prematurity (Panel A) or asthma (Panel B). p-values obtained by non-parametric Mann-Withney test contrasting median hospital days in the two groups presented.
DISCUSSION
Our data demonstrated that hospitalized preschool children (≤5 years old) with HMPV and history of severe prematurity (born ≤ 32 weeks gestational age) had severe HMPV disease, illustrated by high clinical severity scores (wheezing, retractions, supplemental O2 need and tachypnea) relative to children without history of prematurity. We also identified that severe prematurity was associated with a two-fold increase in the duration of HMPV hospitalization in preschool aged children. Accordingly, this study provides new evidence to support that severe prematurity is an important risk factor to be considered in the development of preventive strategies to reduce morbidity, mortality and high-costs generated by HMPV infection during the first years of life.
HMPV and RSV infection have similar clinical respiratory signs and symptoms, including cough, wheezing, rales, hypoxemia and respiratory distress in the same high-risk groups.5,14–16 Premature children are at increased risk of severe RSV infections that can lead to hospitalization, PICU admission, and death.25 The clinical features of lower respiratory tract infection and the disease spectrum caused by HMPV and RSV are quite similar, with similar possible risk factors. In this study, a focus on prematurity’s association with the severity of HMPV has been established. Our results revealed that premature children aged 0–5 acquired a more clinically severe HMPV infection than children who were born non-premature. In agreement with our observations, a study by Papenburg et al. identified that prematurity was more frequent among HMPV hospitalized children who were aged 0–5 months compared to non-premature children who presented to the pediatric clinic.21 Similar to our present results, their severity score was based on the inclusion of at least one of the following criteria: admission to PICU, hospitalization stay >5days, and requirement for supplemental oxygen.21 Moreover, prematurity was independently associated with increased severity of HMPV infection among hospitalized children of the same cohort (OR, 13.97; 95% CI, 1.5–130).21 Population-based studies have identified that HMPV can cause severe respiratory compromise in premature infants and children with or without chronic lung disease.32 In another investigation by Robinson et al. 34% of HMPV hospitalized children were born premature, suggesting that prematurity could be an important risk factor as it is for RSV,33 however, the severity of HMPV infection in premature children was not quantified. In our study we modified bronchiolitis severity scores validated in hospitalized children27,29 to allow the retrospective assessment of the most characteristic phenotypical features of viral bronchiolitis: lower airway obstruction (wheezing) and respiratory distress (sub-costal retractions, supplemental O2 need and tachypnea).26, 27 Given that EMR review did not allow us to assign mild-severe categories for wheezing and retractions accurately, we used binary variables with weighted scores and combined with hypoxemia and stratified tachypnea (0–3). Using this approach we were able to determine that children with history of severe prematurity develop more hypoxemia, stratified tachypnea and sub-costal retractions during HMPV infection. Wheezing alone was not more common in children with history of prematurity, suggesting that rather than triggering airway reactivity, HMPV primarily exacerbates abnormal alveolar gas/exchange in the lungs of young children with history of severe prematurity. This is further supported by the presence of crackles and alveolar infiltrates in most of the premature children infected with HMPV (Figure 1).
We also investigated whether the severe HMPV infection in premature children may impact health care resource utilization in preschool children hospitalized with HMPV infection. To this end, we contrasted the length of hospitalization (hospital-days) in subjects with and without a history of severe prematurity. Our results showed that the mean duration of hospitalization in infants with severe prematurity was two times higher (9 days vs 4 days; P < 0.05, see Figure 2). By comparison, asthmatic children with HMPV did not have longer hospitalizations than non-asthmatic children. This indicates that additional factors (other than airway hyperreactivity/asthma) are present in children with history of severe prematurity that make them more susceptible to respiratory viruses in early life. Based on our data reporting that hypoxemia is a common clinical presentation of HMPV in this population, it is possible that prematurity predisposes to more alveolar damage during HMPV infection. In parallel with longer hospitalization and frequent need for supplemental O2, we also identified that children with history of severe prematurity often required advanced respiratory support, which in our institution includes the use of high-flow nasal cannula or positive airway pressure via mask in continuous (CPAP) or bi-level (BIPAP) modes in the pediatric intensive care unit. Collectively, our data indicate that HMPV infection in children with history prematurity is associated with severe respiratory disease and consequent increase in health care utilization.
Our findings add more support to the prevalent notion that the length and costs of hospitalizations in severely preterm infants are very high,34,35 with the cost being inversely proportional to decreasing gestational age (GA) and birth weight.34,35 This is further aggravated by the fact that premature infants are at high-risk of recurrent hospitalization due to lower respiratory tract infections, as well as chronic pulmonary symptoms.36 Moreover, although severely premature children have a high incidence of hospitalizations in the first few years of life,37,38 there is also evidence than these children need more respiratory health care services later in childhood.37,38 This may in part be due to other co-morbidities associated with prematurity such as neurological impairment, artificial airways, and respiratory support that result in high care resource utilization at least during the first 5 years of life.31 Accordingly, our current data demonstrates that there is an association between prematurity and severe HMPV infection during the first 5 years of life and thus provide additional support for the potential use of preventive measures for HMPV infected children with history of prematurity to ameliorate morbidity and mortality in this vulnerable population through childhood. Some future preventive strategies against HMPV may include the use of passive prophylaxis as it has been implemented with a F protein-directed monoclonal antibody (mAb) -palivizumab against RSV. However, it is important to mention that mAb prophylaxis-based approaches have pros and cons. For instance, while palivizumab was initially shown to reduce RSV-associated hospitalization by 39–78% in selected high-risk premature infants,39 subsequent cost-economic analyses of palivizumab prophylaxis indicated that it did not represent good value for money when used unselectively in children.40 On the other hand, subgroup analysis suggests that prophylaxis with palivizumab may be cost-effective for some subgroups, particularly those with chronic lung disease of prematurity or congential heart disease (high – risk groups).40 In addition to risk stratification, we also believe that clinicians should consider local socioeconomic factors to determine whether mAb–based prophylaxis approaches are cost-effective in their clinical setting and geographic area.
The main limitations of the present study are its retrospective design, the small sample size, the limited number of predictor variables included in the multivariate analysis, and the fact that it was conducted in a specialized, tertiary referral hospital. With respect to the first point, because the data were taken from electronic medical records (EMR), and the majority of variables analyzed were hard variables, it is unlikely that its retrospective design significantly compromised the validity of data collected. Second, although our statistical analysis included a small number of subjects and limited predictor variables, we obtained an adequate signal (significant results) after including the majority of variables that have been reported in the literature as validated markers of bronchiolitis severity and potential confounders.26,27 However, as is the case in cross-sectional studies, residual confounding and type II error (due to small sample size) cannot be excluded, so interpretation of our results needs to be cautious. It is also important to mention that important variables could not be obtained in our EMR review, including insurance information, the duration between symptom onset and hospitalization as well as specific medical treatment(s) provided. Our study subjects underwent viral PCR analysis at the discretion of the clinician, which can result in selection bias. Lastly, it is important to emphasize the fact that the study was conducted in a specialized, tertiary referral hospital makes it likely that the patients included represent the extreme of the spectrum of severity of all patients with HMPV infection, which could limit the generalization of results to other contexts (external validity). However, the similarity of the results to previous studies linking prematurity with HMPV,20–22 as well as with the phylogenetically and clinically related pathogen RSV,2 suggests that the results of this study could be extrapolated to other contexts.
In summary, our data demonstarted that preschool children with a history of prematurity had the more severe HMPV disease, according to clinical scoring of wheezing, retractions, supplemental O2 need and tachypnea. Importantly, greater clinical severity also translated into increased health care resource utilization with a two-fold increase in the duration of hospitalization relative to individuals with HMPV infection and no history of prematurity.
Supplementary Material
Table E1 Clinical severity score in children with HMPV infection.
Clinical parameters of the respiratory distress severity score (0–10) that includes need for supplemental O2, stratified tachypnea, wheezing and retractions.
Acknowledgments
Work supported by Grants NHLBI/HL090020 (K12 Genomics of Lung), NICHC/HD001399 (K12 Child Health Research Career Development Award), UL1TR000075 KL2TR000076 Awards from the NIH National Center for Advancing Translational Sciences
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.World Health Organization. 2013 http://www.who.int/gho/publications/world_health_statistics/EN_WHS2013_Full.pdf.
- 2.van den Hoogen BG, de Jong JC, Groen J, Kuiken T, de Groot R, Fouchier RA, et al. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat Med. 2001;7:719–24. doi: 10.1038/89098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Freymuth F, Vabret A, Lebon P, Legrand L, Bach N, Brouard J, et al. Le Métapneumovirus humain. Virologie. 2004;8:413–23. [Google Scholar]
- 4.Edwards KM, Zhu Y, Griffin MR, Weinberg GA, Hall CB, Szilagyi PG, et al. New Vaccine Surveillance Network. Burden of human metapneumovirus infection in young children. N Engl J Med. 2013;368(7):633–43. doi: 10.1056/NEJMoa1204630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williams JV, Harris PA, Tollefson SJ, Halburnt-Rush LL, Pingsterhaus JM, Edwards KM, et al. Human metapneumovirus and lower respiratory tract disease in otherwise healthy infants and children. N Engl J Med. 2004;350(5):443–50. doi: 10.1056/NEJMoa025472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Widmer K, Zhu Y, Williams JV, Griffin MR, Edwards KM, Talbot HK. Rates of hospitalizations for respiratory syncytial virus, human metapneumovirus, and influenza virus in older adults. J Infect Dis. 2012;206(1):56–62. doi: 10.1093/infdis/jis309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mullins JA, Erdman DD, Weinberg GA, Edwards K, Hall CB, Walker FJ, et al. Human metapneumovirus infection among children hospitalized with acute respiratory illness. Emerg Infect Dis. 2004;10:700–5. doi: 10.3201/eid1004.030555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McAdam AJ, Hasenbein ME, Feldman HA, Cole SA, Offermann JT, Riley AM, et al. Human metapneumovirus in children tested at a tertiary-care hospital. J Infect Dis. 2004;190:20–6. doi: 10.1086/421120. [DOI] [PubMed] [Google Scholar]
- 9.Boivin G, De Serres G, Cote S, Gilca R, Abed Y, Rochette L, Bergeron MG, et al. Human metapneumovirus infections in hospitalized children. Emerg Infect Dis. 2003;9:634–40. doi: 10.3201/eid0906.030017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Esper F, Martinello RA, Boucher D, Weibel C, Ferguson D, Landry ML, et al. A 1-year experience with human metapneumovirus in children aged < 5 years. JInfect Dis. 2004;189:1388–96. doi: 10.1086/382482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sloots TP, Mackay IM, Bialasiewicz S, Jacob KC, McQueen E, Harnett GB, et al. Human metapneumovirus, Australia, 2001–2004. Emerg Infect Dis. 2006;12:1263–6. doi: 10.3201/eid1208.051239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van den Hoogen BG, van Doornum GJ, Fockens JC, Cornelissen JJ, Beyer WE, de Groot R, et al. Prevalence and clinical symptoms of human metapneumovirus infection in hospitalized patients. J Infect Dis. 2003;188:1571–7. doi: 10.1086/379200. [DOI] [PubMed] [Google Scholar]
- 13.Williams JV, Edwards KM, Weinberg GA, Griffin MR, Hall CB, Zhu Y, Szilagyi PG, et al. Population-based incidence of human metapneumovirus infection among hospitalized children. J Infect Dis. 2010;201:1890–8. doi: 10.1086/652782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jartti T, van den Hoogen B, Garofalo RP, Osterhaus AD, Ruuskanen O. Metapneumovirus and acute wheezing in children. Lancet. 2002;360(9343):1393–4. doi: 10.1016/S0140-6736(02)11391-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Madhi SA, Ludewick H, Kuwanda L, van Niekerk N, Cutland C, Little T, et al. Pneumococcal coinfection with human metapneumovirus. J Infect Dis. 2006;193(9):1236–43. doi: 10.1086/503053. [DOI] [PubMed] [Google Scholar]
- 16.Williams JV, Tollefson SJ, Heymann PW, Carper HT, Patrie J, Crowe JE. Human metapneumovirus infection in children hospitalized for wheezing. J Allergy Clin Immunol. 2005;115(6):1311–2. doi: 10.1016/j.jaci.2005.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tecu C, Mihai ME, Alexandrescu VI, Orăşeanu D, Zapucioiu C, Ivanciuc AE, et al. Single and multipathogen viral infections in hospitalized children with acute respiratory infections. Roum Arch Microbiol Immunol. 2013;72(4):242–9. [PubMed] [Google Scholar]
- 18.Papenburg J, Boivin G. The distinguishing features of human metapneumovirus and respiratory syncytial virus. Rev Med Virol. 2010;20:245–60. doi: 10.1002/rmv.651. [DOI] [PubMed] [Google Scholar]
- 19.Feuillet F, Lina B, Rosa-Calatrava M, Boivin G. Ten years of human metapneumovirus research. J Clin Virol. 2012;53(2):97–105. doi: 10.1016/j.jcv.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 20.Schuster JE, Williams JV. Human Metapneumovirus. Pediatrics in Review. 2013;12(34):558. doi: 10.1542/pir.34-12-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Papenburg J, Hamelin MÈ, Ouhoummane N, Carbonneau J, Ouakki M, Boivin G. Comparison of risk factors for human metapneumovirus and respiratory syncytial virus disease severity in young children. Journal of Infectious Diseases. 2012;206(2):178–189. doi: 10.1093/infdis/jis333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schildgen V, van den Hoogen B, Fouchier R, et al. Human Metapneumovirus: lessons learned over the first decade. Clin Microbiol Rev. 2011;24(4):734–54. doi: 10.1128/CMR.00015-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spaeder MC, Custer JW, Bembea MM, Aganga DO, Song X, Scafidi S. A multicenter outcomes analysis of children with severe viral respiratory infection due to human metapneumovirus. Pediatric Critical Care Medicine. 2013;14(3):268–272. doi: 10.1097/PCC.0b013e3182720fc7. [DOI] [PubMed] [Google Scholar]
- 24.Englund JA, Boeckh M, Kuypers J, Garrett NW, Hackman RC, Morrow RA, et al. Brief communication:fatal human metapneumovirus infection in stem-cell transplant recipients. Ann Intern Med. 2006;144(5):344–9. doi: 10.7326/0003-4819-144-5-200603070-00010. [DOI] [PubMed] [Google Scholar]
- 25.Pelletier G, Déry P, Abed Y, Boivin G. Respiratory tract reinfections by the new human metapneumovirus in an immunocompromised child. Emerg Infect Dis. 2002;8(9):976–8. doi: 10.3201/eid0809.020238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodríguez DA, Rodriguez-Martinez CE, Cárdenas AC, Quilaguy IE, Mayorga LY, Falla LM, et al. Predictors of severity and mortality in children hospitalized with respiratory syncytial virus infection in a tropical region. Pediatr Pulmonol. 2013 doi: 10.1002/ppul.22781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duarte-Dorado DM, Madero-Orostegui DS, Rodriguez-Martinez CE, Nino G. Validation of a scale to assess the severity of bronchiolitis in a population of hospitalized infants. J Asthma. 2013 Sep 19; doi: 10.3109/02770903.2013.834504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller A-B, Narwal R, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379:2162–72. doi: 10.1016/S0140-6736(12)60820-4. [DOI] [PubMed] [Google Scholar]
- 29.Tal A, Bavilski C, Yohai D, Bearman JE, Gorodischer R, Moses SW. Dexamethasone and salbutamol in the treatment of acute wheezing in infants. Pediatrics. 1983;71:13–8. [PubMed] [Google Scholar]
- 30.Gutierrez MJ, Zhu J, Rodriguez-Martinez CE, Nino CL, Nino G. Nocturnal phenotypical features of obstructive sleep apnea (OSA) in asthmatic children. Pediatric Pulmonology. 2013;48(6):592–600. doi: 10.1002/ppul.22713. [DOI] [PubMed] [Google Scholar]
- 31.Petrou S, Mehta Z, Hockley C, Cook-Mozaffari P, Henderson J, Goldacre M. The impact of preterm birth on hospital inpatient admissions and costs during the first 5 years of life. Pediatrics. 2003;112(6 Pt 1):1290–7. doi: 10.1542/peds.112.6.1290. [DOI] [PubMed] [Google Scholar]
- 32.Klein MI, Coviello S, Bauer G, Benitez A, Serra ME, Schiatti MP, et al. The impact of infection with human metapneumovirus and other respiratory viruses in young infants and children at high risk for severe pulmonary disease. J Infect Dis. 2006;193(11):1544–51. doi: 10.1086/503806. [DOI] [PubMed] [Google Scholar]
- 33.Robinson JL, Lee BE, Bastien N, Li Y. Seasonality and clinical features of human metapneumovirus infection in children in Northern Alberta. Journal of Medical Virology. 2005;76(1):98–105. doi: 10.1002/jmv.20329. [DOI] [PubMed] [Google Scholar]
- 34.Rogowski J. Measuring the cost of neonatal and perinatal care. Pediatrics. 1999;103(1 suppl E):329–35. [PubMed] [Google Scholar]
- 35.Marbella AM, Chetty VK, Layde PM. Neonatal hospital lengths of stay, readmissions, and charges. Pediatrics. 1998;101(1 pt 1):32–6. doi: 10.1542/peds.101.1.32. [DOI] [PubMed] [Google Scholar]
- 36.Renard ME, Truffert P, Groupe EPIPAGE Clinical respiratory outcome of very preterm newborn at 5 years. The EPIPAGE cohort Arch Pediatr. 2008;15:592–4. doi: 10.1016/S0929-693X(08)71844-X. [DOI] [PubMed] [Google Scholar]
- 37.Gray D, Woodward LJ, Spencer C, Inder TE, Austin NC. Health service utilisation of a regional cohort of very preterm infants over the first 2 years of life. J Paediatr Child Health. 2006;42(6):377–83. doi: 10.1111/j.1440-1754.2006.00876.x. [DOI] [PubMed] [Google Scholar]
- 38.Rautava L, Häkkinen U, Korvenranta E, Andersson S, Gissler M, Hallman M, et al. Health and the use of health care services in 5-year-old very-low-birth-weight infants. Acta Paediatr. 2010;99(7):1073–9. doi: 10.1111/j.1651-2227.2010.01737.x. [DOI] [PubMed] [Google Scholar]
- 39.The IMpact-RSV Study Group. Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics. 1998;102(3):531–7. [PubMed] [Google Scholar]
- 40.Wang D, 1, Bayliss S, Meads C. Palivizumab for immunoprophylaxis of respiratory syncytial virus (RSV) bronchiolitis in high-risk infants and young children: a systematic review and additional economic modelling of subgroup analyses. Health Technol Assess. 2011;15(5):iii–iv. 1–124. doi: 10.3310/hta15050. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table E1 Clinical severity score in children with HMPV infection.
Clinical parameters of the respiratory distress severity score (0–10) that includes need for supplemental O2, stratified tachypnea, wheezing and retractions.


