Abstract
Background
Understanding the dynamics of malaria transmission in diverse endemic settings is key for designing and implementing locally adapted and sustainable control and elimination strategies. A parasitological and epidemiological survey was conducted in September–October 2012, as a baseline underlying a 3-year population-based longitudinal cohort study. The aim was to characterize malaria transmission patterns in two contrasting ecological rural sites in the Peruvian Amazon, Lupuna (LUP), a riverine environment, and Cahuide (CAH), associated with road-linked deforestation.
Methods
After a full population census, 1941 individuals 3 years and older (829 in LUP, 1112 in CAH) were interviewed, clinically examined and had a blood sample taken for the detection of malaria parasites by microscopy and PCR. Species-specific parasite prevalence was estimated overall and by site. Multivariate logistic regression models assessed risk factors for parasite infection by PCR, while SaTScan detected spatial clusters of PCR-positive individuals within each site. In addition, data from routine malaria surveillance in the period 2009–2012 were obtained.
Results
Parasite prevalence by PCR was higher in CAH than in LUP for Plasmodium vivax (6.2% vs. 3.9%) and for Plasmodium falciparum (2.6% vs. 1.2%). Among PCR-confirmed infections, asymptomatic (Asy) parasite carriers were always more common than symptomatic (Sy) infections for P. vivax (Asy/Sy ratio: 2/1 in LUP and 3.7/1 in CAH) and for P. falciparum (Asy/Sy ratio: 1.3/1 in LUP and 4/1 in CAH). Sub-patent (Spat) infections also predominated over patent (Pat) infections for both species: P. vivax (Spat/Pat ratio: 2.8/1 in LUP and 3.7/1 in CAH) and P. falciparum malaria (Spat/Pat ratio: 1.9/1 in LUP and 26/0 in CAH). For CAH, age, gender and living in a household without electricity were significantly associated with P. vivax infection, while only age and living in a household with electricity was associated with P. falciparum infection. For LUP, only household overcrowding was associated with P. falciparum infection. The spatial analysis only identified well-defined clusters of P. vivax and P. falciparum infected individuals in CAH. Reported malaria incidence indicated that malaria transmission has long occurred in LUP with primarily seasonal patterns, and confirmed a malaria outbreak in CAH since May 2012.
Conclusions
This parasitological and epidemiological baseline assessment demonstrates that malaria transmission and parasite prevalence is heterogeneous in the Peruvian Amazon, and influenced by local socio-demographics and ecological contexts. Riverine and road construction/deforestation contexts must be taken into account in order to carry out effective anti-malaria control and elimination efforts.
Electronic supplementary material
The online version of this article (doi:10.1186/s12936-017-1957-y) contains supplementary material, which is available to authorized users.
Keywords: Malaria, Transmission, PCR, Heterogeneity, Hotspot, Peruvian Amazon
Background
Malaria remains an important public health problem in Peru, especially in the Amazonian department of Loreto where most malaria occurs [1–4]. Following the resurgence of malaria in the 1990s [5–8], which reached an historic peak of 158,115 cases in 1997 [9], the Loreto Regional Directorate of Health (LRDH) and national Peruvian Ministry of Health (MoH) organized control efforts that were intensified with the support of international agencies, particularly the Global Fund for AIDS, Tuberculosis and Malaria (GFATM) and the US Agency for International Development (USAID) [10]. Comprehensive community-based interventions were initiated and intensified that included: delivery of long-lasting insecticidal mosquito nets (LLINs) [11], environmental management with community participation, training of community health workers and microscopists [12], and “test and treat” interventions incorporating artemisinin-based combination therapy (ACT) for Plasmodium falciparum. It is thought that these interventions contributed to a reduction of microscopically-confirmed malaria cases in Loreto, from 54,291 cases in 2005 to 10,504 and 11,793 cases in 2010 and 2011, respectively [2, 13], and to an increasingly low-level, residual transmission in the region [2, 14].
Routine malaria control activities in the Peruvian Amazon are based on passive case detection (PCD) using light microscopy (LM) [2, 15] on patients presenting with fever or malaria-compatible illness at local health facilities. A positive LM result leads to government-provided, species-specific anti-malarial treatment. In areas with low and residual transmission, active, household- or field-based malaria surveillance is challenging because most infected individuals have very low-level parasitaemia, often exceeding the limit of detection of LM [16–18]. Indeed, several cross-sectional surveys in rural villages surrounding Iquitos city (capital of Loreto) have reproducibly demonstrated that the prevalence of asymptomatic parasite carriers ranges from 4 to 15%, and that molecular diagnostic tools such as polymerase chain reaction (PCR) is far more sensitive than LM [14, 19–21] for identifying the presence of parasitaemia. Villages in those studies were located along the road connecting Iquitos to the Nauta district, thus presenting varying degrees of development-driven deforestation associated with increased human-biting activity of Anopheles darlingi (the main malaria vector in the Peruvian Amazon) [8]. Collectively these observations suggest that asymptomatic carriers are a hidden human infectious reservoir that, combined with favourable ecological conditions for mosquito breeding and resting sites around places where people live or work, maintain local malaria transmission in Amazonia, hence hypoendemicity and residual malaria [22–25].
The hypothesis that asymptomatic Plasmodium vivax and P. falciparum parasitaemias contribute to the maintenance of malaria hypoendemicity in the Amazon region drives the work of our Amazonia International Center of Excellence in Malaria Research (Amazonia ICEMR), which was established in 2010 as one of ten such centers supported by the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH) [26]. The Amazonia ICEMR is conducting population-based longitudinal cohort studies with the aim of assessing how sub-microscopic, asymptomatic infections contribute to maintaining transmission in epidemiological contrasting settings of the Peruvian Amazon [27]. This paper focuses on the socio-demographics and epidemiological characteristics of two contrasting exemplars of complex malaria transmission in the Peruvian Amazon, the village of Cahuide, a rural site with road-associated deforestation, and Lupuna rural site with riverine environment. Population-based estimates of P. vivax and P. falciparum prevalence by LM and PCR at the baseline enrollment surveys in September–October 2012 are also discussed and those estimates were related to data from routine malaria surveillance and implemented control efforts to better understand the dynamics of malaria transmission in both sites.
Methods
Ethics statement
Ethics clearance for the study was obtained from the Ethics Review Board of the Universidad Peruana Cayetano Heredia, Lima, Peru (SIDISI code # 5739) and from the University of California San Diego Human Subjects Protection Program (Project # 100765). Permissions were received from health and local authorities after explaining the purpose and procedures of the study. Signed informed consent was obtained prior the study enrollment to participation and blood sampling by all adults and the parents of all participating children <18 years. In addition to parental/guardian consent, children older than 7 years provided a signed informed assent. All the methods were carried out in accordance with approved guidelines.
Study design
A census and an epidemiological survey were conducted as baseline activities of a 3-year population-based, longitudinal cohort study in the Lupuna (LUP) and Cahuide (CAH) sites in the northeastern Peruvian Amazon Department of Loreto. The study population included individuals aged 3 years and older who resided in study sites and agreed to participate in the cohort providing a written signed consent.
Study area
LUP site is located on the outskirts of Iquitos district, 10 km from downtown (latitude 03°44.591′S longitude 73°19.615′W) (Fig. 1). It is a forested area only accessible by crossing the Nanay River by boat. LUP is comprised of three contiguous villages, Santa Rita (SR), San Jose de Lupuna town (LT), and San Pedro (SP) situated about 500–1000 m from the riverbanks near the administrative border between San Juan and Iquitos districts. Villagers mainly work in agricultural activities, such as cassava cultivation and charcoal production. CAH includes three rural villages in the southern part of San Juan district, La Habana (LH), Doce de Abril (DA) and Cahuide town (CT), whose houses are located on both sites of the Iquitos-Nauta road between the 54th and 63th km. These three villages were established in the 1980s after the intensification of deforestation and extension of the road connecting Iquitos city to the Nauta district. CT, the most populated CAH village, is a centre of palm roof production, and has a port close to the intersection between the road and the Itaya River (around 58th km, latitude 04°13.785′S longitude 73°276′W). The climate of study area is tropical, warm and humid with a rainy season from November to May and a dry season from June to October [14]. Annual average temperature is around 27 °C, the relative humidity above 80%, with average annual rainfall of 4 m. Anopheles darlingi is the primary malaria vector in the area [28].
Fig. 1.

Study area. Sites: Lupuna (LUP), Cahuide (CAH). Villages: Santa Rita (SR), San Juan de Lupuna town (LT), San Pedro (SP), La Habana (LH), Doce de Abril (DA), Cahuide town (CT)
Plasmodium vivax and P. falciparum cases are reported to the Ministry of Health annually for both sites throughout the entire year [28]. As in most Peruvian endemic areas, malaria surveillance in study sites relies on passive case detection (PCD). Patients presenting with fever or illness compatible with malaria are systematically tested by LM at Ministry of Health-supported LUP and CAH health posts located in LT and CT, respectively. Microscopic diagnosis is available during weekdays between 8 a.m. and 2 p.m., but not during the weekend. Plasmodium vivax malaria was treated with chloroquine (CQ) for 3 days (10 mg/g on days 1 and 2, and 5 mg/kg on day 3), plus primaquine (PQ) for 7 days (0.5 mg/kg/day); P. falciparum malaria was treated with mefloquine (MQ) (12.5 mg/kg/day for 2 days) plus artesunate (AS) (4 mg/kg/day for 3 days), according to Peruvian national policy.
Census and geo-referencing
A complete census of the population in each village was conducted in July–August 2012, collecting individual data on socio-demographics (e.g. age, gender, education, occupation, socio-economic status, time living in the village), and on previous malaria episodes. Each house was identified with a unique number and geo-referenced using a handheld Global Positioning System (GPS) device (Garmin’s GPSMAP 60CSx, Garmin International Inc., USA), and household characteristics collected (e.g. household size, predominant construction materials in house, ownership of animals, and availability of essential services such as potable water, sewage system and electricity). Each individual subject was given a seven-digit unique code number combining the village, household and individual code.
Cohort enrollment and baseline survey
Individuals aged 3 years and older available at the time of the baseline survey (September–October 2012) were enrolled in the study area after consenting. Whenever selected individuals were absent, the household was revisited up to four times in a period of 30 days to maximize subject participation. Each participant had the axillary temperature taken, history of fever or any other malaria symptoms were recorded, and a finger-prick blood sample collected for immediate microscopy (thick and thin blood smears) and on filter paper (Whatman grade 3, Whatman, Springfield Mill, USA) for later molecular tests. Filter paper dried blood samples were individually stored at 4 °C with desiccant until processed. Subjects with microscopically-confirmed infections were referred to health posts for treatment according to Peruvian national guidelines.
Retrospective routine surveillance data
Additionally, routine surveillance data, namely reported malaria episodes by species in Iquitos and San Juan district from 2009 to 2012 were obtained from the Regional Directorate of Health, Loreto-Ministry of Health in Iquitos. Peruvian policy mandates weekly notification of confirmed malaria cases (by standard LM) at health facilities and data aggregation at district and department level. Since malaria surveillance data could be reliably disaggregated only to the district level but not to the village level, a retrospective review of all registered individuals detected by PCD between January 2009 and December 2012 was carried out in all health facilities near the study sites. Any other surveillance in addition to routine PCD conducted in CAH and LUP in the 12 months before the survey was recorded.
Laboratory procedures
Microscopy
Thick and thin smears were stained for 10 min with a 10% Giemsa solution, and parasite density was calculated by counting the number of asexual parasites for 200 white blood cells (WBC) in the thick smear, assuming a concentration of 8000 WBCs/μl. Slides were read on site, then a day later by an expert at microscopy at the reference laboratory in Iquitos. A slide was declared negative if no malaria parasite was found after examining 100 fields [29]. Quality control was done blindly on all positive slides and 10% of randomly chosen negative slides by a senior technician at Universidad Peruana Cayetano Heredia, Instituto de Medicina Tropical-“Alexander von Humboldt” in Lima, Peru.
Real time PCR
Filter paper blood spots were cut into ~6 mm2 pieces for DNA extraction using QIAamp DNA Microkit of QIAGEN following the manufacturer instructions. A quantitative real-time polymerase chain reaction (qPCR) method targeting the 18s rRNA gene region was used for molecular diagnosis, following the protocol reported by Mangold et al. [30] Primers were 5-TAACGAACGAGATCTTAA-3 and 5-GTTCCTCTAAGAAGCTTT-3. Cycling conditions were initial denaturation at 95 °C for 2 min, followed by amplification for 45 cycles of 20 s at 95 °C, 20 s at 52 °C, and 30 s at 68 °C. To confirm amplicon identify, amplification was immediately followed by a melt program consisting of 5 s at 65 °C and a stepwise temperature increase of 0.5 °C/s to 95 °C. Melting curves accurately differentiated P. vivax from P. falciparum.
Data analysis
Census and survey data were doubly entered and cross-checked in Access (Microsoft Corp, USA). Data analysis was performed using R v.2.15 software (R Development Core Team, R Foundation for Statistical Computing, Austria). Baseline characteristics between sites were compared using the Chi squared test. Species-specific parasite prevalence by LM and by PCR were estimated overall and by site, and their two-sided 95% confidence intervals (CI) were calculated using the Wilson score method.
The case definition for malaria parasite (Plasmodium spp.) infection was an individual with a positive PCR result regardless of symptoms. Cases were further classified as patent (detectable by LM) or sub-patent (not detectable by LM). A symptomatic infection was defined as an individual with confirmation of parasites in the blood sample at any level who also had fever or history of fever, headache, chills or general discomfort in the previous 7 days. Uni- and multivariate mixed-effects logistic regression models, with fixed effects and a random intercept that accounted for individual clustering at household, were used to determine risk factors for species-specific malaria infection in each site The following potential risk factors were assessed: age, gender, time living in the village, education level, forest-related job as main economical activity (including the intensity of these activities during the week), trip in the past month to another endemic area, the history of malaria in the past year, household overcrowding (more than three individuals sleeping per room as average in a household), low household bed net coverage (<80% of beds covered with a net in a household), housing structure (predominant material in roof and floor), and the availability of electricity. Factors with p values <0.1 for the Wald test in the univariate analysis were considered for inclusion in the multivariate model. Using manual backward, final models retained all factors that were significantly associated with malaria infection (Wald p values <0.05). Interactions were systematically checked for up to order two. Likelihood ratio tests (LRTs) were used to assess statistical differences between nested models.
The QGIS software QGIS v.2.16 (QGIS developer team, Open Source Geospatial Foundation) was used to map all surveyed households and to classify them according to the number of household members with malaria infections identified by PCR. The SaTScan software v.9.3 (M Kulldorff and Information Management Services Inc, USA) was used to identify spatial clustering of households with malaria infections in each site, using the following characteristics: pure spatial analysis, Poisson probability model [31], latitude/longitude coordinates, report of most likely clusters with no geographical overlap of secondary clusters, maximum spatial cluster size equal to 50% of total population. The analysis was first done without adjustment for covariates, and then done including the variables identified as fixed-effect risk factors by the mixed-effects logistic regression analyses. To adjust for covariates, the number of malaria confirmed infections and the total number of screened individuals had to be specified for each particular household location and combination of covariates in the case and population files, respectively. SaTScan applied multiple circular windows across the study area, each circle representing a possible cluster. Clusters were assessed based on 999 Monte Carlo simulations to determine the probability of observed frequency of infected individuals being due to chance relative to expected frequency under the null hypothesis of no clustering. The null hypothesis was rejected if any resulting p value of assessed clusters was <0.05 and the window with the maximum log likelihood ratio (LLR) was identified as the most likely cluster. The relative risk (RR) reported for each identified cluster was the estimated risk within the cluster divided by the estimated risk outside the cluster [31].
Results
A total of 487 households (HHs) and 2447 individuals were identified during the census in July–August 2012, distributed over the two sites as follows: LUP (211 HHs; 1007 individuals) and CAH (276; 1440). In addition, the distribution by village was: LT (95 HHs; 433 individuals), SR (66; 348) and SP (50; 226) for LUP, and CT (173; 922), LH (46; 224) and DA (57; 294) for CAH (Table 1). The overall ratio of female to male was 0.9; more than half of the population in both sites was younger than 25 years.
Table 1.
Household and participants in census and baseline survey
| Census | Survey | |||||
|---|---|---|---|---|---|---|
| HH | Individuals | HH | Individuals | |||
| N | N | n | % | n | % | |
| Lupuna (LUP) | ||||||
| Lupuna town (LT) | 95 | 433 | 88 | 92.6 | 352 | 81.3 |
| Santa Rita (SR) | 66 | 348 | 63 | 95.5 | 292 | 83.9 |
| San Pedro (SP) | 50 | 226 | 45 | 90.0 | 185 | 81.9 |
| Total | 211 | 1007 | 196 | 92.9 | 829 | 82.3 |
| Cahuide (CAH) | ||||||
| Cahuide town (CT) | 173 | 922 | 163 | 94.2 | 734 | 79.6 |
| La Habana (LA) | 46 | 224 | 37 | 80.4 | 147 | 65.6 |
| Doce de Abril (DA) | 57 | 294 | 52 | 91.2 | 231 | 78.6 |
| Total | 276 | 1440 | 252 | 91.3 | 1112 | 77.2 |
The cohort study enrolled 1941 of the 2447 censused inhabitants (79.3%) in 448 HH (92.0%) available at the time of the baseline survey. Of these 1941 individuals, 829 (42.7%) lived in LUP and 1117 (57.3%) in CAH. Socio-demographic characteristics and history of past malaria episodes by site and by village are presented in Table 2 and Additional file 1: Table S1, respectively. Individuals under 25 years represented 49.3 and 54.5% of the participants respectively in LUP and CAH (p = 0.008). Males slightly outnumbered females (ratio female/male = 0.89) without differences between sites. The proportion of participants aged >10 years old who have resided in the site ten or more years was significantly higher in LUP (85.0%) than in CAH (41.3%) (p < 0.001). Among adults 18 years and older, the proportion of individuals who had completed secondary education level was higher in LUP (47.0%) than in CAH (32.8%) (p < 0.001). The most commonly reported occupation among adults was farmers, followed by housewives, indoor laborers/employees and traders with similar proportions in both sites; security guards (p = 0.002) and loggers (p < 0.001) were more frequent in CAH than in LUP. Participants reporting having experienced malaria in their lifetime were more common in LUP (73.4%) than in CAH (67.0%) (p = 0.003). However, this was not the case when only recent episodes were considered, with more participants reporting have had malaria episodes within the past 12 months in CAH (48.9%) than in LUP (14.1%) (p < 0.001).
Table 2.
Baseline socio-demographic characteristics of study participants
| Lupuna (LUP) | Cahuide (CAH) | Total | ||||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Gender | ||||||
| Female | 406 | 49.0 | 510 | 45.9 | 916 | 47.2 |
| Male | 423 | 51.0 | 602 | 54.1 | 1025 | 52.8 |
| Age (years)* | ||||||
| <5 | 55 | 6.6 | 88 | 7.9 | 143 | 7.4 |
| 5–14.9 | 196 | 23.6 | 336 | 30.3 | 532 | 27.5 |
| 15–24.9 | 158 | 19.1 | 191 | 17.2 | 349 | 18.0 |
| 25–39.9 | 159 | 19.2 | 215 | 19.4 | 374 | 19.3 |
| 40–54.9 | 132 | 15.9 | 164 | 14.8 | 296 | 15.3 |
| >55 | 129 | 15.6 | 115 | 10.4 | 244 | 12.6 |
| Missing | 0 | 3 | 3 | |||
| Time in village (age ≥ 10 years)* | ||||||
| <2 | 26 | 3.9 | 207 | 24.6 | 233 | 15.4 |
| 2–9.9 | 74 | 11.1 | 288 | 34.2 | 362 | 24.0 |
| ≥10 | 567 | 85.0 | 348 | 41.3 | 915 | 60.6 |
| Missing | 3 | 3 | 6 | |||
| Education (age ≥ 18 years)* | ||||||
| None | 21 | 4.0 | 25 | 4.1 | 46 | 4.0 |
| Incomplete primary | 130 | 24.9 | 200 | 32.6 | 330 | 29.0 |
| Complete primary | 126 | 24.1 | 187 | 30.5 | 313 | 27.6 |
| Secondary | 226 | 43.2 | 185 | 30.2 | 411 | 36.2 |
| Superior | 20 | 3.8 | 16 | 2.6 | 36 | 3.2 |
| Missing | 3 | 3 | 6 | |||
| Main occupation (age ≥ 18 years)* | ||||||
| None | 29 | 5.6 | 21 | 3.4 | 50 | 4.4 |
| Student | 24 | 4.6 | 9 | 1.5 | 33 | 2.9 |
| Housewife | 124 | 23.9 | 145 | 23.7 | 269 | 23.8 |
| Trader | 47 | 9.1 | 65 | 10.6 | 112 | 9.9 |
| Labourer, employee | 72 | 13.9 | 88 | 14.4 | 160 | 14.2 |
| Guardian | 2 | 0.4 | 43 | 7.0 | 45 | 4.0 |
| Farmer | 178 | 34.4 | 180 | 29.4 | 358 | 31.7 |
| Logger | 22 | 4.2 | 55 | 9.0 | 77 | 6.8 |
| Fisher, hunter | 13 | 2.5 | 5 | 0.8 | 18 | 1.6 |
| Boat driver | 7 | 1.4 | 1 | 0.2 | 8 | 0.7 |
| Missing | 8 | 4 | 12 | |||
| Lifetime malaria episodes* | ||||||
| 0 | 215 | 26.6 | 365 | 33.0 | 580 | 30.3 |
| 1 | 133 | 16.5 | 314 | 28.4 | 447 | 23.3 |
| 2–3 | 175 | 21.7 | 271 | 24.5 | 446 | 23.3 |
| ≥4 | 285 | 35.3 | 157 | 14.2 | 442 | 23.1 |
| Missing | 21 | 5 | 26 | |||
| Malaria episodes (previous 12 months)* | ||||||
| 0 | 704 | 85.9 | 566 | 51.1 | 1270 | 65.9 |
| 1 | 93 | 11.3 | 429 | 38.7 | 522 | 27.1 |
| 2–3 | 22 | 2.7 | 100 | 9.0 | 122 | 6.3 |
| ≥4 | 1 | 0.1 | 13 | 1.2 | 14 | 0.7 |
| Missing | 9 | |||||
* Significant difference between sites (p < 0.05)
Table 3 and Additional file 1: Table S2 present the household characteristics of enrolled participants by site and by village, respectively. Household crowding was higher in CAH (57.6%) than in LUP (41.9%) (p < 0.001). In both sites, most individuals lived in houses with wooden walls and roofs composed of palm leaf; concrete (brick and cement) and tin were also common building materials, especially in the LT village of LUP (p < 0.001) (Additional file 1: Table S2). Wooden floors built on stilts predominated in houses of CAH, while dirt floors were more frequent in LUP, especially in SR (p < 0.001). Most participants had electricity at home in CAH (55.7%), primarily those living along the road and in the DA village (p < 0.001); however this electrical service mainly exclusively benefited participants living in LT village in LUP (p < 0.001). The use of potable water for drinking and food preparation was reported by only a small proportion of individuals in LUP (15.6%) and CAH (30.7%), while firewood was reported as the main fuel used for cooking by most participants (>80%) in both sites. Moreover, most participants in both sites reported having precarious or lack of sanitation facilities, as well as lack of trash disposal systems. The majority (97.0%) of participants in CAH reported that their houses were sprayed indoors with insecticide in the past 3 months, while only 12.4% reported this in LUP (p < 0.001). Household bed net coverage was high in both sites; the predominant material of bed nets reported by participants varied across sites. In all villages of LUP, most bed nets (about 90%) were LLINs. In CAH, about half of subjects reported having LLINs and half reported using non-impregnated insecticide nets made of tocuyo (non-processed cotton) or nylon.
Table 3.
Baseline household characteristics of study participants
| Lupuna (LUP) | Cahuide (CAH) | Total | ||||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Overcrowding (>3 persons/bedroom)* | ||||||
| No | 482 | 58.1 | 471 | 42.4 | 953 | 49.1 |
| Yes | 347 | 41.9 | 639 | 57.6 | 986 | 50.9 |
| Missing | 0 | 2 | 2 | |||
| Wall material* | ||||||
| Brick, cement | 130 | 15.7 | 51 | 4.6 | 181 | 9.3 |
| Wood | 629 | 75.9 | 919 | 82.8 | 1548 | 79.8 |
| Palm | 47 | 5.7 | 61 | 5.5 | 108 | 5.6 |
| Tin, other | 23 | 2.8 | 79 | 7.1 | 102 | 5.3 |
| Missing | 0 | 2 | 2 | |||
| Roof material* | ||||||
| Tin | 271 | 32.7 | 157 | 14.2 | 428 | 22.1 |
| Palm | 558 | 67.3 | 947 | 85.8 | 1505 | 77.9 |
| Missing | 0 | 8 | 8 | |||
| Floor material* | ||||||
| Cement | 203 | 24.5 | 141 | 12.7 | 344 | 17.7 |
| Wood | 98 | 11.8 | 640 | 57.7 | 738 | 38.1 |
| Dirt | 528 | 63.7 | 329 | 29.6 | 857 | 44.2 |
| Missing | 0 | 2 | 2 | |||
| Electricity* | ||||||
| Yes | 298 | 35.9 | 618 | 55.7 | 916 | 47.2 |
| No | 531 | 64.1 | 492 | 44.3 | 1023 | 52.8 |
| Missing | 0 | 2 | 2 | |||
| Potable water for drinking* | ||||||
| Yes | 129 | 15.6 | 337 | 30.7 | 466 | 24.2 |
| No | 700 | 84.4 | 760 | 69.3 | 1460 | 75.8 |
| Missing | 0 | 15 | 15 | |||
| Source of water* | ||||||
| Piped into dwelling | 6 | 0.7 | 44 | 4.1 | 50 | 2.6 |
| Public tap | 272 | 32.8 | 292 | 27.5 | 564 | 29.8 |
| Open well | 309 | 37.3 | 358 | 33.7 | 667 | 35.3 |
| River, rain | 242 | 29.2 | 368 | 34.7 | 610 | 32.3 |
| Missing | 0 | 50 | 50 | |||
| Sanitation facility* | ||||||
| Flush toilet | 4 | 0.5 | 3 | 0.3 | 7 | 0.4 |
| Pit latrine | 168 | 20.4 | 306 | 27.6 | 474 | 24.5 |
| Ground hole, cesspool | 279 | 33.9 | 518 | 46.7 | 797 | 41.2 |
| No facility, field | 373 | 45.3 | 283 | 25.5 | 656 | 33.9 |
| Missing | 5 | 2 | 7 | |||
| Trash disposal* | ||||||
| Burning trash | 240 | 29.0 | 467 | 42.4 | 707 | 36.6 |
| Bury trash | 56 | 6.8 | 163 | 14.8 | 219 | 11.3 |
| Field, river | 510 | 61.5 | 466 | 42.3 | 976 | 50.6 |
| Other | 23 | 2.8 | 5 | 0.5 | 28 | 1.5 |
| Missing | 0 | 11 | 11 | |||
| Cooking fuel* | ||||||
| Gas | 22 | 2.7 | 40 | 3.6 | 62 | 3.2 |
| Kerosene, charcoal | 13 | 1.6 | 137 | 12.4 | 150 | 7.7 |
| Firewood | 793 | 95.8 | 932 | 84.0 | 1725 | 89.1 |
| Missing | 1 | 3 | 4 | |||
| Insecticide sprayed (previous 3 months)* | ||||||
| Yes | 93 | 12.4 | 988 | 97.0 | 1081 | 61.1 |
| No | 656 | 87.6 | 31 | 3.0 | 687 | 38.9 |
| Missing | 80 | 93 | 173 | |||
| Bednet coverage (bednets/beds), % | ||||||
| <80 | 28 | 3.4 | 32 | 2.9 | 60 | 3.1 |
| ≥80 | 801 | 96.6 | 1078 | 97.1 | 1879 | 96.9 |
| Missing | 0 | 2 | 2 | |||
| Bednet material* | ||||||
| None | 0 | 0.0 | 2 | 0.2 | 2 | 0.1 |
| Tocuyo | 41 | 4.9 | 400 | 36.1 | 441 | 22.8 |
| Nylon | 33 | 4.0 | 124 | 11.2 | 157 | 8.1 |
| LLINs | 755 | 91.1 | 557 | 50.2 | 1312 | 67.7 |
| Other | 0 | 0.0 | 26 | 2.3 | 26 | 1.3 |
| Missing | 0 | 3 | 3 | |||
* Significant difference between sites (p < 0.05)
All 1941 individuals enrolled in the cohort had samples obtained for malaria diagnosis by LM at the time of the baseline survey; most of these (1770 individuals, 92.2%) had available sample for PCR analysis. By LM, the overall parasite prevalence was 2.1% (38 P. vivax, 2 P. falciparum infections), and by PCR 7.2% (93 P. vivax, 35 P. falciparum) (Table 4), with the highest figures in CAH site (p < 0.05). Of the total 38 P. vivax infections by LM, 30 were found in CAH (16 with parasitaemia <100 parasites/μl), and only 8 in LUP (one with parasitaemia <100 parasites/μl); while the two microscopically confirmed P. falciparum infections were found in LUP (one with parasitaemia <100 parasites/μl). The presence of gametocytes was recorded in 15 (50.0%) and 3 (37.5%) of the total microscopically confirmed P. vivax infections in CAH and LUP, respectively. Gametocytes were not observed in microscopically confirmed P. falciparum infections. Parasite prevalence by PCR was higher in CAH than in LUP for P. vivax (6.2% vs. 3.9%, p = 0.03) and for P. falciparum (2.6% vs. 1.2%, p = 0.03). Considering PCR-confirmed infections, asymptomatic (Asy) parasite carriers were always more common than symptomatic (Sy) infections for P. vivax (Asy/Sy ratio: 2/1 in LUP and 3.7/1 in CAH) and for P. falciparum (Asy/Sy ratio: 1.3/1 in LUP and 4/1 in CAH). Similarly, sub-patent (Spat) infections also predominated over patent (Pat) infections for both species: P. vivax (Spat/Pat ratio: 2.8/1 in LUP and 3.7/1 in CAH) and P. falciparum malaria (Spat/Pat ratio: 1.9/1 in LUP and 26/0 in CAH).
Table 4.
Malaria prevalence (by LM and PCR) by study site
| Lupuna (LUP) | Cahuide (CAH) | Total | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | % [95% CI] | n | % | % [95% CI] | n | % | % [95% CI] | ||||
| Microscopy | ||||||||||||
| Total analysed samples (N) | 829 | 1112 | 1941 | |||||||||
| P. vivax* | 8 | 1.0 | 0.4 | 2.0 | 30 | 2.7 | 1.9 | 3.9 | 38 | 2.0 | 1.4 | 2.7 |
| P. falciparum | 2 | 0.2 | 0.0 | 1.0 | 0 | 0.0 | 2 | 0.1 | 0.0 | 0.4 | ||
| Overall* | 10 | 1.2 | 0.6 | 2.3 | 30 | 2.7 | 1.9 | 3.9 | 40 | 2.1 | 1.5 | 2.8 |
| Asymptomatic P. vivax | 3 | 0.4 | 0.1 | 1.1 | 24 | 2.2 | 1.4 | 3.2 | 27 | 1.4 | 0.9 | 2.0 |
| Symptomatic P. vivax | 5 | 0.6 | 0.2 | 1.5 | 6 | 0.5 | 0.2 | 1.2 | 11 | 0.6 | 0.3 | 1.0 |
| Asymptomatic P. falciparum | 1 | 0.1 | 0.0 | 0.8 | 0 | 0.0 | 1 | 0.1 | 0.0 | 0.3 | ||
| Symptomatic P. falciparum | 1 | 0.1 | 0.0 | 0.8 | 0 | 0.0 | 1 | 0.1 | 0.0 | 0.3 | ||
| PCR | ||||||||||||
| Total analysed samples (N) | 777 | 1013 | 1790 | |||||||||
| P. vivax* | 30 | 3.9 | 2.7 | 5.5 | 63 | 6.2 | 4.8 | 7.9 | 93 | 5.2 | 4.2 | 6.4 |
| P. falciparum* | 9 | 1.2 | 0.6 | 2.3 | 26 | 2.6 | 1.7 | 3.8 | 35 | 2.0 | 1.4 | 2.7 |
| Overall* | 39 | 5.0 | 3.6 | 6.9 | 89 | 8.8 | 7.2 | 10.7 | 128 | 7.2 | 6.0 | 8.5 |
| Asymptomatic P. vivax | 20 | 2.6 | 1.6 | 4.0 | 53 | 5.2 | 4.0 | 6.8 | 73 | 4.1 | 3.2 | 5.1 |
| Symptomatic P. vivax | 10 | 1.3 | 0.7 | 2.4 | 10 | 1.0 | 0.5 | 1.9 | 20 | 1.1 | 0.7 | 1.8 |
| Asymptomatic P. falciparum | 5 | 0.6 | 0.2 | 1.6 | 23 | 2.3 | 1.5 | 3.4 | 28 | 1.6 | 1.1 | 2.3 |
| Symptomatic P. falciparum | 4 | 0.5 | 0.2 | 1.4 | 3 | 0.3 | 0.1 | 0.9 | 7 | 0.4 | 0.2 | 0.8 |
| Sub-patent P. vivax | 22 | 2.8 | 1.8 | 4.3 | 41 | 4.0 | 3.0 | 5.5 | 63 | 3.5 | 2.7 | 4.5 |
| Patent P. vivax | 8 | 1.0 | 0.5 | 2.1 | 22 | 2.2 | 1.4 | 3.3 | 30 | 1.7 | 1.2 | 2.4 |
| Sub-patent P. falciparum | 8 | 1.0 | 0.5 | 2.1 | 26 | 2.6 | 1.7 | 3.8 | 34 | 1.9 | 1.3 | 2.7 |
| Patent P. falciparum | 1 | 0.1 | 0.0 | 0.8 | 0 | 0.0 | 1 | 0.1 | 0.0 | 0.4 | ||
* Significant difference between sites (p < 0.05)
Given the relatively small number of infections detected by LM, uni- and multivariate mixed-effects logistic regression analyses were carried out only for confirmed infections by PCR for each site. In final models the estimates of the random-effects coefficients confirmed the influence of clustered sampled data at household level. In LUP, household overcrowding (i.e., more than three individuals sleeping per room as average in a household) was the only factor significantly associated with P. falciparum infection (OR 4.9, 95% CI [1.0–24.6]). No significant factors were found for P. vivax infection (Table 5). In CAH, age, gender and electricity availability (a proxy for income) remained independently associated with P. vivax malaria infection in the multivariate model (Table 6). While individuals between 15 and 39 years (AOR 2.8, 95% CI [1.4–5.6]) and those older than 40 years (AOR 2.4, 95% CI [1.2–5.1]) had higher odds of P. vivax infection than those <15 years, male participants were two times more likely to have a P. vivax infection than female participants (AOR 2.0, 95% CI [1.1–3.6]). Moreover, the lack of electricity increased the odds of infection in household members by a factor of 1.9 (AOR 1.9, 95% CI [1.1–3.4]) compared with individuals living in households that had available this public service. Considering P. falciparum, the odds of infection confirmed by PCR significantly increased at younger age (test for trend p < 0.001), with individuals <15 years being four times more likely to have P. falciparum infections than adults >40 years (AOR 5.5, 95% CI [1.3–24.4]). In this case, the availability of electricity was associated with highest risk of P. falciparum in household members (AOR 2.8, 95% CI [1.1–7.4]) compared with individuals living in households without electricity. No association was found between P. vivax and P. falciparum infection with regard to time of residence in the village, education level, occupation, trip in the past month to another endemic area, the history of malaria in the past year, and housing characteristics of study participants.
Table 5.
Univariate risk factor analysis for P. vivax and P. falciparum infection in Lupuna (LUP)
| P. vivax by PCR | P. falciparum by PCR | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | N | % | OR | OR [95% CI] | n | N | % | OR | OR [95% CI] | |||
| Gender | ||||||||||||
| Female | 13 | 382 | 3.4 | Ref | 3 | 382 | 0.8 | Ref | ||||
| Male | 17 | 395 | 4.3 | 1.3 | 0.6 | 2.7 | 6 | 395 | 1.5 | 1.9 | 0.5 | 7.7 |
| Age (years) | ||||||||||||
| <15 | 9 | 234 | 3.8 | Ref | 5 | 234 | 2.1 | 6.3 | 0.7 | 58.7 | ||
| 15–39 | 15 | 291 | 5.2 | 1.4 | 0.6 | 3.2 | 3 | 291 | 1.0 | 2.7 | 0.3 | 27.6 |
| ≥40 | 6 | 252 | 2.4 | 0.6 | 0.2 | 1.7 | 1 | 252 | 0.4 | Ref | ||
| Time in village (years) | ||||||||||||
| <10 | 7 | 242 | 2.9 | Ref | 3 | 242 | 1.2 | Ref | ||||
| ≥10 | 23 | 535 | 4.3 | 1.5 | 0.6 | 3.6 | 6 | 535 | 1.1 | 0.9 | 0.2 | 3.7 |
| Education | ||||||||||||
| None | 3 | 89 | 3.4 | Ref | 1 | 89 | 1.1 | Ref | ||||
| Incomplete primary | 6 | 245 | 2.4 | 0.7 | 0.2 | 2.9 | 2 | 245 | 0.8 | 0.7 | 0.1 | 8.6 |
| Complete primary | 6 | 153 | 3.9 | 1.2 | 0.3 | 4.8 | 2 | 153 | 1.3 | 1.1 | 0.1 | 13.3 |
| Secondary | 15 | 290 | 5.2 | 1.6 | 0.4 | 5.5 | 4 | 290 | 1.4 | 1.2 | 0.1 | 11.8 |
| Forest-related job | ||||||||||||
| No | 21 | 563 | 3.7 | Ref | 7 | 563 | 1.2 | Ref | ||||
| Yes (≤5 day/week) | 8 | 153 | 5.2 | 1.4 | 0.6 | 3.3 | 2 | 153 | 1.3 | 0.9 | 0.2 | 4.9 |
| Yes (>5 day/week) | 0 | 56 | 0.0 | NC | 0 | 56 | 0.0 | NC | ||||
| Trip previous month | ||||||||||||
| No | 30 | 771 | 3.9 | Ref | 9 | 771 | 1.2 | Ref | ||||
| Yes | 0 | 6 | 0.0 | NC | 0 | 6 | 0.0 | NC | ||||
| Malaria episodes (previous 12 months) | ||||||||||||
| 0 | 25 | 662 | 3.8 | Ref | 9 | 662 | 1.4 | Ref | ||||
| ≥1 episodes | 5 | 107 | 4.7 | 1.2 | 0.5 | 3.3 | 0 | 107 | 0.0 | NC | ||
| Overcrowding (>3 persons/bedroom) | ||||||||||||
| No | 17 | 452 | 3.8 | Ref | 2 | 452 | 0.4 | Ref | ||||
| Yes | 13 | 325 | 4.0 | 1.1 | 0.5 | 2.2 | 7 | 325 | 2.2 | 4.9* | 1.0 | 24.6 |
| Bednet coverage, % | ||||||||||||
| ≥80 | 30 | 751 | 4.0 | Ref | 9 | 751 | 1.2 | Ref | ||||
| <80 | 0 | 26 | 0.0 | NC | 0 | 26 | 0.0 | NC | ||||
| Roof material | ||||||||||||
| Calamine | 9 | 245 | 3.7 | Ref | 2 | 245 | 0.8 | Ref | ||||
| Palm | 21 | 532 | 3.9 | 1.1 | 0.5 | 2.4 | 7 | 532 | 1.3 | 1.6 | 0.3 | 8.3 |
| Floor material | ||||||||||||
| Cement | 5 | 184 | 2.7 | Ref | 1 | 184 | 0.5 | Ref | ||||
| Wood | 6 | 93 | 6.5 | 2.5 | 0.7 | 8.3 | 0 | 93 | 0.0 | NC | ||
| Dirt | 19 | 500 | 3.8 | 1.4 | 0.5 | 3.8 | 8 | 500 | 1.6 | 2.9 | 0.2 | 38.1 |
| Electricity | ||||||||||||
| Yes | 13 | 271 | 4.8 | Ref | 1 | 271 | 0.4 | Ref | ||||
| No | 17 | 506 | 3.4 | 0.7 | 0.3 | 1.4 | 8 | 506 | 1.6 | 4.3 | 0.5 | 36.0 |
Random-effects variance for P. falciparum model = 0.62 (including only overcrowding)
Ref reference, NC non calculated
* p < 0.05
Table 6.
Univariate and multivariate risk factor analysis for P. vivax and P. falciparum infection in Cahuide (CAH)
| P. vivax by PCR | P. falciparum by PCR | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | N | % | OR | OR [95% CI] | AOR | AOR [95% CI] | n | N | % | OR | OR [95% CI] | AOR | AOR [95% CI] | |||||
| Gender | ||||||||||||||||||
| Female | 19 | 471 | 4.0 | Ref | Ref | 12 | 471 | 2.5 | Ref | |||||||||
| Male | 44 | 542 | 8.1 | 2.1 | 1.2 | 3.8 | 2.0* | 1.1 | 3.6 | 14 | 542 | 2.6 | 1.0 | 0.5 | 2.3 | |||
| Age (years) | ||||||||||||||||||
| <15 | 13 | 401 | 3.2 | Ref | Ref | 17 | 401 | 4.2 | 5.6* | 1.3 | 24.8 | 5.5* | 1.3 | 24.4 | ||||
| 15–39 | 30 | 362 | 8.3 | 2.8* | 1.4 | 5.5 | 2.8* | 1.4 | 5.6 | 7 | 362 | 1.9 | 2.4 | 0.5 | 12.0 | 2.3 | 0.5 | 11.3 |
| ≥40 | 20 | 249 | 8.0 | 2.6* | 1.2 | 5.5 | 2.4* | 1.2 | 5.1 | 2 | 249 | 0.8 | Ref | Ref | ||||
| Time in village (years) | ||||||||||||||||||
| <2 | 17 | 237 | 7.2 | Ref | 5 | 237 | 2.1 | Ref | ||||||||||
| 2–9 | 26 | 448 | 5.8 | 0.8 | 0.4 | 1.7 | 15 | 448 | 3.3 | 1.6 | 0.5 | 4.6 | ||||||
| ≥10 | 20 | 328 | 6.1 | 0.9 | 0.4 | 1.9 | 6 | 328 | 1.8 | 0.8 | 0.2 | 2.8 | ||||||
| Education | ||||||||||||||||||
| None | 7 | 135 | 5.2 | Ref | 5 | 135 | 3.7 | Ref | ||||||||||
| Incomplete primary | 23 | 397 | 5.8 | 1.2 | 0.5 | 3.0 | 10 | 397 | 2.5 | 0.7 | 0.2 | 2.0 | ||||||
| Complete primary | 17 | 242 | 7.0 | 1.6 | 0.6 | 4.1 | 5 | 242 | 2.1 | 0.5 | 0.1 | 1.9 | ||||||
| Secondary | 16 | 237 | 6.8 | 1.5 | 0.6 | 3.9 | 6 | 237 | 2.5 | 0.7 | 0.2 | 2.2 | ||||||
| Forest-related job | ||||||||||||||||||
| No | 37 | 750 | 4.9 | Ref | Ref | 25 | 750 | 3.3 | Ref | |||||||||
| Yes (≤5 day/week) | 4 | 38 | 10.5 | 1.6 | 0.8 | 3.1 | 1.1 | 0.3 | 3.3 | 0 | 183 | 0.0 | NC | |||||
| Yes (>5 day/week) | 16 | 168 | 9.5 | 3.4* | 1.6 | 7.2 | 1.1 | 0.6 | 2.3 | 1 | 79 | 1.3 | 0.4 | 0.1 | 3.4 | |||
| Trip previous month | ||||||||||||||||||
| No | 61 | 969 | 6.3 | Ref | 26 | 969 | 2.7 | Ref | ||||||||||
| Yes | 2 | 44 | 4.5 | 0.7 | 0.2 | 3.2 | 0 | 44 | 0.0 | NC | ||||||||
| Malaria episodes (previous 12 months) | ||||||||||||||||||
| 0 | 31 | 499 | 6.2 | Ref | 16 | 499 | 3.2 | Ref | ||||||||||
| ≥1 episodes | 32 | 512 | 6.3 | 1.0 | 0.6 | 1.7 | 10 | 512 | 2.0 | 0.6 | 0.3 | 1.3 | ||||||
| Overcrowding (>3 persons/bedroom) | ||||||||||||||||||
| No | 23 | 425 | 5.4 | Ref | 14 | 425 | 3.3 | Ref | ||||||||||
| Yes | 40 | 588 | 6.8 | 1.3 | 0.7 | 2.3 | 12 | 588 | 2.0 | 0.6 | 0.3 | 1.4 | ||||||
| Bednet coverage, % | ||||||||||||||||||
| ≥80 | 60 | 985 | 6.1 | Ref | 26 | 985 | 2.6 | Ref | ||||||||||
| <80 | 3 | 28 | 10.7 | 2.1 | 0.5 | 8.8 | 0 | 28 | 0.0 | NC | ||||||||
| Roof material | ||||||||||||||||||
| Calamine | 14 | 144 | 9.7 | Ref | 7 | 144 | 4.9 | Ref | ||||||||||
| Palm | 49 | 865 | 5.7 | 0.6 | 0.3 | 1.1 | 19 | 865 | 2.2 | 0.4 | 0.2 | 1.1 | ||||||
| Floor material | ||||||||||||||||||
| Cement | 9 | 122 | 7.4 | Ref | 6 | 122 | 4.9 | Ref | ||||||||||
| Wood | 32 | 590 | 5.4 | 0.7 | 0.3 | 1.6 | 14 | 590 | 2.4 | 0.5 | 0.2 | 1.3 | ||||||
| Dirt | 22 | 301 | 7.3 | 1.0 | 0.4 | 2.5 | 6 | 301 | 2.0 | 0.4 | 0.1 | 1.3 | ||||||
| Electricity | ||||||||||||||||||
| Yes | 26 | 560 | 4.6 | Ref | Ref | 20 | 560 | 3.6 | 2.7 | 1.1 | 7.1 | 2.8 | 1.1 | 7.4 | ||||
| No | 37 | 453 | 8.2 | 1.9* | 1.0 | 3.3 | 1.9 | 1.1 | 3.4 | 6 | 453 | 1.3 | Ref | Ref | ||||
Random-effects variance for adjusted P. vivax model = 0.48
Random-effects variance for adjusted P. falciparum model = 0.36
Ref reference, NC non calculated
* p < 0.05
Figures 2 and 3 show the spatial distribution of positive PCR individuals by species and for each site. Despite some aggregation of cases within adjacent houses in LUP, no significant spatial clustering of P. vivax and P. falciparum infections were identified using SaTScan (Fig. 2), mainly because of the low number of infections. However, spatial analysis demonstrated that species-specific malaria infection was non-randomly distributed in CAH. Indeed, the most likely spatial cluster of P. vivax infections (RR = 4.2, p = 0.028) was in the southern part of CT village (Fig. 3a), including 59 surveyed individuals with available PCR results (5.8% of total in CAH) in 16 households (6.6% of total). The cluster represented 20.6% (13/63) of all P. vivax confirmed infections by PCR. However, this cluster disappeared when the spatial analysis was adjusted for gender, age and electricity availability (factors associated with the infection according to risk factor analysis). For P. falciparum, the most likely cluster adjusted for age and electricity availability was the closest area to the fluvial port in CT (RR = 6.6, p = 0.04). With a radius of 99 meters, the cluster included 61 individuals (6.0% of the total) in 14 households (5.7% of the total) (Fig. 3b). This cluster represented 30.8% (8/26) of all P. falciparum confirmed infections by PCR. Notably, most individuals and households of the adjusted cluster were included within a previously identified bigger cluster (radius = 180 m) without adjustment for covariates.
Fig. 2.
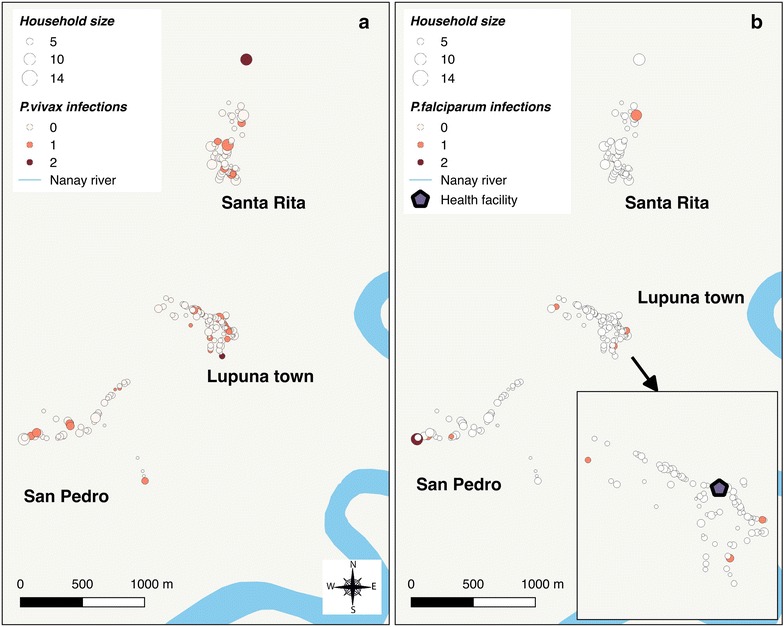
Distribution of P. vivax- (a) and P. falciparum- (b) infected individuals in Lupuna
Fig. 3.

Distribution of P. vivax- (a) and P. falciparum- (b) infected individuals in Cahuide (CAH) with location of significant spatial clusters
The retrospective analysis of reported malaria cases between 2009 and 2012 showed that the malaria incidence in LUP varied across years, with some periods (mainly in year 2009) not following the same trends of malaria incidence compared to the Iquitos district (Fig. 4a). Despite this temporal heterogeneity in the malaria incidence in LUP, a seasonal increase of malaria cases was annually identified between April and July from 2010 and 2012. In 2012, malaria cases from LUP villages (124 P. vivax, 7 P. falciparum) represented about 15% of the total 880 reported cases in Iquitos district. Regarding malaria intervention efforts, deliveries of LLINs by GFATM’s PAMAFRO malaria project in 2008 and 2010 were the main malaria control intervention in LUP during the evaluated period. On the other hand, malaria cases in CAH between 2009 and 2011 occurred with stochastic patterns (with no more than 70 P. vivax and 10 P. falciparum reported cases per year), and did not follow the same trends of the malaria incidence than San Juan district during that period (Fig. 4b). The rapid increase of malaria cases in study villages of CAH since May 2012 following intensive and prolonged rains triggered comprehensive control efforts leaded by RHDL-MoH, such as successive mass-screening interventions with LM and treatment of confirmed infections (four rounds between May and August 2012), indoor spraying with residual insecticide (more than 90% of household coverage according to RHDL-MoH in May 2012), and the distribution of LLINs (one per household in July 2012) (Additional file 2: Figure S1). Households in CAH had previously received LLINs from PAMAFRO project only in 2007 [11], reaching high coverage levels. Although the outbreak was partially controlled by August 2012, malaria cases increased again in November–December 2012. At the end of the year, malaria cases (1221 P. vivax cases and 28 P. falciparum cases) from CAH villages accounted for about 35% of the total 3617 reported cases in San Juan district in 2012.
Fig. 4.

Weekly reported malaria cases in Lupuna (a) and Cahuide (b), and in their respective districts, Iquitos and San Juan (2009–2012)
Discussion
The baseline analysis presented here of a population-based, longitudinal malaria cohort study in two contrasting ecological villages in the Peruvian Amazon indicates substantial prevalence of malaria parasitaemia in the region and is an important benchmark for ongoing longitudinal studies. The riverine rural site, Lupuna (LUP), is home to a fairly stable population (85% of individuals >10 years having lived in the village 10 years or more). Here, the combined analysis of survey findings and routine reported malaria incidence suggests that malaria transmission has long occurred with primarily seasonal patterns. In contrast, Cahuide (CAH), a rural site with road-driven deforestation and a more transient population (about 60%), has experienced a notable and concerning increase of cases since May 2012 that compelled the regional government to carry out intensive outbreak control measures until August 2012 (1 month before the survey). Low parasite rates were observed in both sites during the survey, with high predominance of asymptomatic and sub-patent infections over symptomatic and patent infections. Moreover, the site-specific analysis of malaria prevalence and reported incidence suggested a high degree of heterogeneity at the micro-geographic level and over time for both P. falciparum and P. vivax.
Community surveys for determining malaria parasite carriage are commonly used to estimate malaria transmission in endemic areas [32, 33]. Because cross-sectional designs only include collecting data at one time point related to a specific outcome of interest (malaria), they are unable to capture the variations in malaria transmission over time (i.e., seasonal, epidemic or sporadic patterns in malaria transmission) [32]. Therefore, a better interpretation of the obtained prevalence rates in LUP and CAH requires understanding the appropriate context (riverine, road-associated) in Amazonia, and analysis of past trends in malaria incidence in relation with important events and factors influencing malaria transmission. Such analysis will be deepened by the longitudinally acquired data over the course of our ICEMR project (e.g., epidemiological, entomological, and ecological data), and will underlie the implementation of future control and elimination strategies.
The richness of these datasets is important to consider with regard to how future analyses will guide anti-malaria interventions. In riverine, rural LUP, malaria prevalence by PCR may indicate the seasonal-time dependent parasite carriage level in an endemic area (i.e., low seasonal transmission during the baseline survey) with no major recent changes in the population socio-demographics and in local control measures. In contrast, in road/deforestation and riverine rural CAH, prevalence of parasite carriers (with confirmed gametocytaemia by LM in some of them) reflects the parasite reservoir that enables persistence of malaria transmission by both P. vivax and P. falciparum. This was observed here to have occurred despite the intensive response from RHDL-MoH to a severe malaria outbreak from May to August 2012. These interpretations are further supported by a recently published entomological study of our Amazonian ICEMR that found that the highest monthly entomological inoculation rates (EIRs) are associated with seasonal transmission in both sites, with an important factor being seasonally high river levels that enable mosquito breeding [28]. Indeed, the entomological evaluation confirmed the presence of infected An. darlingi (by both P. vivax and P. falciparum) and higher human biting rates (HBRs) in LUP between February and June (maximum EIR in April) in successive years 2011 and 2012; while for CAH, the highest monthly EIR (2.52) with also confirmed P. vivax- and P. falciparum-infected An. darlingi occurred during the 2012 malaria outbreak. Interestingly, despite decreased HBR levels leading to a much lower estimated EIR in CAH in October 2012 (period of baseline survey), the entomological study found a high infection rate of collected mosquitoes (1.47%) which indicated onward malaria transmission in the area [28], and the potential new increase of cases if ecological conditions were favourable. This is exactly what happened in November–December 2012 [13] following the early onset of heavy rains generating an abrupt height increase of level rivers since October 2012, and consequently damaging and flooding to villages located along the Amazonas River and its tributaries [9].
As previously described elsewhere in the Peruvian Amazon [16–21], the findings presented here from both LUP and CAH consistently demonstrated that a large majority of malaria infections—by both P. vivax and P. falciparum—were asymptomatic (64–85%) and sub-microscopic (75–77%), despite differences in malaria prevalence and variations in malaria transmission over time. A limitation of such cross-sectional surveys is that they might not correctly estimate the proportion of infections that later might become symptomatic or have higher parasite density levels (as detectable by LM) [24, 34]. Few longitudinal studies in Amazonia, in particular Peru and Brazil, have provided data regarding such evolution [20, 22]. A 6-month cohort study in peri-Iquitos villages combining PCD, ACD and weekly monitoring of symptoms found that an important proportion of malaria infections which were asymptomatic at the time of detection (either by LM or PCR), developed malaria symptoms (reported and/or measured fever) after 1 week of follow-up, resulting in only 35–40% of asymptomatic infections among all confirmed cases in study villages [20, 34]. Another study conducted in western Brazilian Amazonia in 2004–2005 [22], including cross-sectional surveys, PCD and ACD over a 14-month period, found that among 93 asymptomatic infections confirmed by PCR during surveys, only 10 (10.7%) developed symptoms consistent with malarial disease over the subsequent 2 months of follow-up. Large prospective population cohorts with long and rigorous follow-up, as those recently implemented by the Amazonian ICEMR [26, 27], offer the opportunity to better delineate clinical and parasitological evolution of extant and/or newly acquired infections that were only detected by PCR. Population-based longitudinal study design is required to measure accurately the natural history of true and persistent asymptomatic and sub-microscopic carriers, and assess their contribution in maintaining malaria transmission in the Amazon region.
Considering the site-specific socio-demographics, household characteristics and ecological conditions in LUP and CAH, risk factor and spatial analyses of malaria infections were performed separately for each site. The low number of infections in LUP detected during the survey in low transmission season would primarily explain the difficulty in identifying malaria risk factors and spatial clusters of high transmission in the site for both P. vivax and P. falciparum. Household overcrowding, the only factor found to be significantly associated with P. falciparum infection in LUP, has also been reported in several studies conducted in areas where transmission is exclusively due to P. falciparum, or co-endemic with P. vivax [35, 36]. The effect of household overcrowding on malaria risk may be explained by the increase in human-released chemo-attractants for mosquitoes in reduced spaces [37], leading to higher human–vector contact rates. Despite the lack of association between P. falciparum infection and other proxy variables for socio-economic status in LUP, it is important to note that overcrowding can also reflect poor economic household conditions. The complex association between poverty and malaria is well known, which likely operates in both directions [38, 39]: poor households are less able to afford prevention and control measures and the higher burden of malaria may push these same individuals deeper into poverty.
Regardless of how the relationship of species-specific malaria with potential risk factors in CAH might be explained, it must be considered that our parasitological baseline survey probably captured the incipient onset of a second local outbreak of P. vivax and P. falciparum malaria. This outbreak likely included both: parasite carriers that remained despite the response to a first severe outbreak occurred between May and August 2012, and new infections by either similar or different parasite populations than those of the first outbreak. The increased risk for P. vivax in males and individuals aged >15 years together with the confirmed infection of mosquitoes in the site [28] suggests that transmission during the second outbreak may have been primarily determined by new infections (mostly asymptomatic infections with low parasitaemia) rather than by hypnozoite-triggered relapses. This explanation is further supported by entomological findings in the same site indicating that An. darlingi mosquitoes bite mainly outdoors [28], and by other studies in the Peruvian Amazon suggesting that the higher malaria risk of adult males is associated with increased human–vector contact rates following outdoor- and forest-related occupational activities (even though the latter activities were not resulted directly associated with the infection in the present study) [14, 40].
The differences in malaria risk between species with respect to the availability of electricity at household can be explained by the different connotations that this public service can have. While the lack of electricity may reflect poor socio-economic status associated with increased P. vivax infections and consequently suggest that transmission is not entirely outdoors; the electricity availability as risk factor for P. falciparum may be related with an increase of evening outdoor activities (e.g., watching television, chatting, resting, etc.) of household members [41]. On the other hand, the development of host immunity with the intensity of exposure to P. falciparum species in CAH (site that reports P. falciparum cases every year) may explain the significant relationship of P. falciparum infections with younger age groups [42–44], as well as, the finding that all P. falciparum infections had low parasite densities non-detectable by LM [16]. The results presented here from CAH differ from previous reports showing that prevalence and/or incidence of P. vivax decreased significantly faster with age than that of P. falciparum, suggesting that acquiring clinical immunity is different species-specific, as has been seen in other co-endemic countries such as Papua New Guinea [45], Thailand [46], Vanuatu [47], Ethiopia [48], and Brazil [42, 49]. The data presented here regarding LM−, PCR+ malaria infection do need to be interpreted with caution, considering the limitation of cross-sectional malaria epidemiology studies because risk for transmission is highly dynamic and multifactorial. The longitudinal cohort data from both sites, once available, will allow for more precise understanding of the complex risk factors for incident malaria infection. The rich data sets will allow for more sophisticated analysis, for example, using complex mixed-effects regression models to account for repeated measures, clustering within household/village, and hierarchical levels of risk factors. Further, the identification and characterization of parasite populations will provide insights into complex malaria transmission dynamics in diverse scenarios, for example those characterized by changing seasonal endemic and/or epidemic transmission due to climate change, or those influenced by human mobility patterns due to social policies.
The high heterogeneity in malaria transmission in the Peruvian Amazon creates opportunities for targeted interventions [14]. However the identification of hotspots of malaria transmission and their characterization are not always straightforward. In our study, SaTScan analysis detected clusters with the highest malaria prevalence for both P. vivax and P. falciparum only in the CAH site. Since the cluster located on the south of CAH for P. vivax was found to be associated with an increased presence of male individuals, age groups older than 15 years and the lack of electricity at households, an adjusted spatial analysis for these covariates was performed resulting in the disappearance of the cluster. The observation indicating that the aggregation of P. vivax infections was mainly determined by the aggregation of groups with high-risk than by specific ecological conditions within the site has also been reported in other villages of the Peruvian Amazon [14]. Local mosquito and human mobility patterns may explain the absence of clustering of specific environmental factors along the Iquitos-Nauta road. Indeed, the presence of several breeding and resting sites near households along the road which enlarge after rains or floods from Itaya River could result into a wider dispersal of An. darlingi, and the high mobility of infected individuals through the unique and highly accessible road could further increase the dispersal of parasites. Conversely, the cluster for P. falciparum remained in the closest area to the river even after being adjusted for age and electricity availability (variables associated with P. falciparum infection). Its highest prevalence may be explained by the unprecedented floods from Itaya River in 2012 resulting in large and multiple breeding sites in the area, but also by the presence of a fluvial port to which individuals (resident and non-residents) arrive after being exposed to malaria due to travel-related economical activities in other endemic riverine villages.
Conclusions
Parasitological measures (by LM and PCR), and individual and household characteristics collected during the baseline survey of a 3-year population-based malaria cohort study in the Amazonian Lupuna and Cahuide villages, together with contextual data obtained from malaria surveillance systems and health facilities, allowed for a good characterization of their population and malaria transmission patterns. On a population basis in both sites, low parasite rates were observed, with findings that asymptomatic and sub-patent infections predominated, compared to symptomatic and patent infections. However, the site-specific analysis of malaria prevalence and reported incidence showed substantial heterogeneity in malaria transmission at the micro-geographical level and over time, likely influenced by the site-specific socio-demographics, household characteristics, and ecological conditions in the villages. Riverine and road construction/deforestation contexts must be taken into account in order to carry out effective anti-malaria control and elimination efforts.
Additional files
Additional file 1: Table S1. Baseline socio-demographic characteristics of study participants by villages. Table S2. Baseline household characteristics of study participants by villages.
Additional file 2: Figure S1. Weekly reported malaria cases in Cahuide (B) in 2012, and main control interventions implemented. (1) Mass screening and treatment (MS&T) and indoor spraying with residual insecticide with >90% of household coverage; (2, 3, 5, 6, 7) MS&T; (4) distribution of LLINs.
Authors’ contributions
JV, ALC and DG conceived and designed the study. MG and RC supervised the fieldwork. DG, RR and PM supervised the laboratory assays. ARA, MG, GCE and CP contributed to the data management and the consolidation of the fieldwork and laboratory data. ARA conducted the analysis, prepared figures and tables, interpreted the results, and wrote the first draft of the paper. JV, ALC and DG contributed to the result interpretation and writing of the paper. All authors read and approved the final manuscript.
Acknowledgements
We thank all residents and local authorities from the Loreto villages of Cahuide and Lupuna for their enthusiastic participation in the study, as well as all field workers for their dedication during the fieldwork. This study was funded by cooperative agreement U19AI089681 from the United States Public Health Service, NIH/NIAID, funded as the Amazonian International Center of Excellence in Malaria Research.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1186/s12936-017-1957-y) contains supplementary material, which is available to authorized users.
Contributor Information
Angel Rosas-Aguirre, Email: angelrosasa@gmail.com.
Mitchel Guzman-Guzman, Email: mitch_guzman@hotmail.com.
Dionicia Gamboa, Email: dionigamboa@yahoo.com.
Raul Chuquiyauri, Email: raulharo@yahoo.com.
Roberson Ramirez, Email: roberson_leo@hotmail.com.
Paulo Manrique, Email: paulo_nvnv@yahoo.com.
Gabriel Carrasco-Escobar, Email: gabriel.carrasco@upch.pe.
Carmen Puemape, Email: cpoema9@gmail.com.
Alejandro Llanos-Cuentas, Email: alejandro.llanos.c@upch.pe.
Joseph M. Vinetz, Email: jvinetz@ucsd.edu
References
- 1.Nájera JA, González-Silva M, Alonso PL. Some lessons for the future from the Global Malaria Eradication Programme (1955–1969) PLoS Med. 2011;8:e1000412. doi: 10.1371/journal.pmed.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosas-Aguirre A, Gamboa D, Manrique P, Conn JE, Moreno M, Lescano AG, et al. Epidemiology of Plasmodium vivax malaria in Peru. Am J Trop Med Hyg. 2016;95:133–144. doi: 10.4269/ajtmh.16-0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Griffing SM, Gamboa D, Udhayakumar V. The history of 20th century malaria control in Peru. Malar J. 2013;12:303. doi: 10.1186/1475-2875-12-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cueto M. El regreso de las epidemias: salud y sociedad en el Perú del siglo XX. IEP; 1997.
- 5.Williams HA, Vincent-Mark A, Herrera Y, Chang OJ. A retrospective analysis of the change in anti-malarial treatment policy: Peru. Malar J. 2009;8:85. doi: 10.1186/1475-2875-8-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruebush TK, Neyra D, Cabezas C. Modifying national malaria treatment policies in Peru. J Public Health Policy. 2004;25:328–345. doi: 10.1057/palgrave.jphp.3190032. [DOI] [PubMed] [Google Scholar]
- 7.Guarda JA, Asayag CR, Witzig R. Malaria reemergence in the Peruvian Amazon region. Emerg Infect Dis. 1999;5:209–215. doi: 10.3201/eid0502.990204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vittor AY, Gilman RH, Tielsch J, Glass G, Shields T, Lozano WS, et al. The effect of deforestation on the human-biting rate of Anopheles darlingi, the primary vector of falciparum malaria in the Peruvian Amazon. Am J Trop Med Hyg. 2006;74:3–11. [PubMed] [Google Scholar]
- 9.Soto-Calle V, Rosas-Aguirre A, Llanos-Cuentas A, Abatih E, DeDeken R, Rodriguez H, et al. Spatio-temporal analysis of malaria incidence in the Peruvian Amazon Region between 2002 and 2013. Sci Rep. 2017;7:40350. doi: 10.1038/srep40350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flores W, Chang J, Barillas E. Rapid assessment of the performance of malaria control strategies implemented by countries in the Amazon subregion using adequacy criteria: case study. Malar J. 2011;10:379. doi: 10.1186/1475-2875-10-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosas-Aguirre A, Guzmán-Guzmán M, Moreno-Gutierrez D, Rodriguez-Ferrucci H, Vargas-Pacherrez D, Acuña-González Y. Long-lasting insecticide-treated bednet ownership, retention and usage one year after their distribution in Loreto, Peru. Rev Peru Med Exp Salud Pública. 2011;28:228–236. doi: 10.1590/S1726-46342011000200009. [DOI] [PubMed] [Google Scholar]
- 12.Rosas-Aguirre Á, Gamboa D, Rodriguez H, Llanos-Zavalaga F, Aguirre K, Llanos-Cuentas A. Use of standardized blood smear slide sets for competency assessment in the malaria microscopic diagnosis in the Peruvian Amazon. Rev Peru Med Exp Salud Pública. 2010;27:540–547. doi: 10.1590/S1726-46342010000400008. [DOI] [PubMed] [Google Scholar]
- 13.Ministerio de Salud del Perú Tendencia y situación de las enfermedades sujetas a vigilancia epidemiológica: malaria. Bol Epidemiol. 2015;24:975–986. [Google Scholar]
- 14.Rosas-Aguirre A, Speybroeck N, Llanos-Cuentas A, Rosanas-Urgell A, Carrasco-Escobar G, Rodriguez H, et al. Hotspots of malaria transmission in the Peruvian Amazon: rapid assessment through a parasitological and serological survey. PLoS ONE. 2015;10:e0137458. doi: 10.1371/journal.pone.0137458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ministerio de Salud del Perú. Norma técnica para la atención de la malaria y malaria severa en el Perú. NTS Nro. 054-MINSA/DGSP-V.01, modificada en Febrero 2015. MINSA; 2015.
- 16.Okell LC, Ghani AC, Lyons E, Drakeley CJ. Submicroscopic infection in Plasmodium falciparum-endemic populations: a systematic review and meta-analysis. J Infect Dis. 2009;200:1509–1517. doi: 10.1086/644781. [DOI] [PubMed] [Google Scholar]
- 17.Bousema T, Okell L, Felger I, Drakeley C. Asymptomatic malaria infections: detectability, transmissibility and public health relevance. Nat Rev Microbiol. 2014;12:833–840. doi: 10.1038/nrmicro3364. [DOI] [PubMed] [Google Scholar]
- 18.Lindblade KA, Steinhardt L, Samuels A, Kachur SP, Slutsker L. The silent threat: asymptomatic parasitaemia and malaria transmission. Expert Rev Anti Infect Ther. 2013;11:623–639. doi: 10.1586/eri.13.45. [DOI] [PubMed] [Google Scholar]
- 19.Parekh FK, Hernandez JN, Krogstad DJ, Casapia WM, Branch OH. Prevalence and risk of Plasmodium falciparum and P. vivax malaria among pregnant women living in the hypoendemic communities of the Peruvian Amazon. Am J Trop Med Hyg. 2007;77:451–457. [PMC free article] [PubMed] [Google Scholar]
- 20.Branch O, Casapia WM, Gamboa DV, Hernandez JN, Alava FF, Roncal N, et al. Clustered local transmission and asymptomatic Plasmodium falciparum and Plasmodium vivax malaria infections in a recently emerged, hypoendemic Peruvian Amazon community. Malar J. 2005;4:27. doi: 10.1186/1475-2875-4-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roshanravan B, Kari E, Gilman RH, Cabrera L, Lee E, Metcalfe J, et al. Endemic malaria in the Peruvian Amazon Region of Iquitos. Am J Trop Med Hyg. 2003;69:45–52. [PubMed] [Google Scholar]
- 22.da Silva-Nunes M, Moreno M, Conn JE, Gamboa D, Abeles S, Vinetz JM, et al. Amazonian malaria: asymptomatic human reservoirs, diagnostic challenges, environmentally-driven changes in mosquito vector populations, and the mandate for sustainable control strategies. Acta Trop. 2012;121:281–291. doi: 10.1016/j.actatropica.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vinetz JM, Gilman RH. Asymptomatic Plasmodium parasitemia and the ecology of malaria transmission. Am J Trop Med Hyg. 2002;66:639–640. doi: 10.4269/ajtmh.2002.66.639. [DOI] [PubMed] [Google Scholar]
- 24.Coura JR, Suárez-Mutis M, Ladeia-Andrade S. A new challenge for malaria control in Brazil: asymptomatic Plasmodium infection—a review. Mem Inst Oswaldo Cruz. 2006;101:229–237. doi: 10.1590/S0074-02762006000300001. [DOI] [PubMed] [Google Scholar]
- 25.Barbosa S, Gozze AB, Lima NF, Batista CL, da Silva Bastos M, Nicolete VC, et al. Epidemiology of disappearing Plasmodium vivax malaria: a case study in rural Amazonia. PLoS Negl Trop Dis. 2014;8:e3109. doi: 10.1371/journal.pntd.0003109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rao MR. International centers of excellence for malaria research. Am J Trop Med Hyg. 2015;93:1–4. doi: 10.4269/ajtmh.15-0407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moss WJ, Dorsey G, Mueller I, Laufer MK, Krogstad DJ, Vinetz JM, et al. Malaria epidemiology and control within the international centers of excellence for malaria research. Am J Trop Med Hyg. 2015;93:5–15. doi: 10.4269/ajtmh.15-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moreno M, Saavedra MP, Bickersmith SA, Lainhart W, Tong C, Alava F, et al. Implications for changes in Anopheles darlingi biting behaviour in three communities in the peri-Iquitos region of Amazonian Peru. Malar J. 2015;14:290. doi: 10.1186/s12936-015-0804-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ministerio de Salud del Perú . Norma técnica de salud para el control de calidad del diagnóstico microscópico de malaria. Lima: MINSA; 2010. [Google Scholar]
- 30.Mangold KA, Manson RU, Koay ESC, Stephens L, Regner M, Thomson RB, et al. Real-time PCR for detection and identification of Plasmodium spp. J Clin Microbiol. 2005;43:2435–2440. doi: 10.1128/JCM.43.5.2435-2440.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kulldorff M. SaTScan—software for the spatial, temporal, and space-time scan statistics. Boston: Harvard Medical School and Harvard Pilgrim Health Care; 2010. [Google Scholar]
- 32.Tusting LS, Bousema T, Smith DL, Drakeley C. Measuring changes in Plasmodium falciparum transmission: precision, accuracy and costs of metrics. Adv Parasitol. 2014;84:151–208. doi: 10.1016/B978-0-12-800099-1.00003-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elissa N, Migot-Nabias F, Luty A, Renaut A, Touré F, Vaillant M, et al. Relationship between entomological inoculation rate, Plasmodium falciparum prevalence rate, and incidence of malaria attack in rural Gabon. Acta Trop. 2003;85:355–361. doi: 10.1016/S0001-706X(02)00266-8. [DOI] [PubMed] [Google Scholar]
- 34.Rosas-Aguirre A, Erhart A, Llanos-Cuentas A, Branch O, Berkvens D, Abatih E, et al. Modelling the potential of focal screening and treatment as elimination strategy for Plasmodium falciparum malaria in the Peruvian Amazon Region. Parasites Vectors. 2015;8:261. doi: 10.1186/s13071-015-0868-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ernst KC, Adoka SO, Kowuor DO, Wilson ML, John CC. Malaria hotspot areas in a highland Kenya site are consistent in epidemic and non-epidemic years and are associated with ecological factors. Malar J. 2006;5:78. doi: 10.1186/1475-2875-5-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ricci F. Social implications of malaria and their relationships with poverty. Mediterr J Hematol Infect Dis. 2012;4:2012048. doi: 10.4084/mjhid.2012.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zwiebel LJ, Takken W. Olfactory regulation of mosquito–host interactions. Insect Biochem Mol Biol. 2004;34:645–652. doi: 10.1016/j.ibmb.2004.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosas-Aguirre A, Ponce OJ, Carrasco-Escobar G, Speybroeck N, Contreras-Mancilla J, Gamboa D, et al. Plasmodium vivax malaria at households: spatial clustering and risk factors in a low endemicity urban area of the northwestern Peruvian coast. Malar J. 2015;14:176. doi: 10.1186/s12936-015-0670-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Teklehaimanot A, Mejia P. Malaria and poverty. Ann N Y Acad Sci. 2008;1136:32–37. doi: 10.1196/annals.1425.037. [DOI] [PubMed] [Google Scholar]
- 40.Chuquiyauri R, Paredes M, Peñataro P, Torres S, Marin S, Tenorio A, et al. Socio-demographics and the development of malaria elimination strategies in the low transmission setting. Acta Trop. 2012;121:292–302. doi: 10.1016/j.actatropica.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gryseels C, Durnez L, Gerrets R, Uk S, Suon S, Set S, et al. Re-imagining malaria: heterogeneity of human and mosquito behaviour in relation to residual malaria transmission in Cambodia. Malar J. 2015;14:165. doi: 10.1186/s12936-015-0689-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ladeia-Andrade S, Ferreira MU, de Carvalho ME, Curado I, Coura JR. Age-dependent acquisition of protective immunity to malaria in riverine populations of the Amazon Basin of Brazil. Am J Trop Med Hyg. 2009;80:452–459. [PubMed] [Google Scholar]
- 43.Clark EH, Silva CJ, Weiss GE, Li S, Padilla C, Crompton PD, et al. Plasmodium falciparum malaria in the Peruvian Amazon, a region of low transmission, is associated with immunologic memory. Infect Immun. 2012;80:1583–1592. doi: 10.1128/IAI.05961-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Doolan DL, Dobaño C, Baird JK. Acquired immunity to malaria. Clin Microbiol Rev. 2009;22:13–36. doi: 10.1128/CMR.00025-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lin E, Kiniboro B, Gray L, Dobbie S, Robinson L, Laumaea A, et al. Differential patterns of infection and disease with P. falciparum and P. vivax in young Papua New Guinean children. PLoS ONE. 2010;5:e9047. doi: 10.1371/journal.pone.0009047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Phimpraphi W, Paul RE, Yimsamran S, Puangsa-art S, Thanyavanich N, Maneeboonyang W, et al. Longitudinal study of Plasmodium falciparum and Plasmodium vivax in a Karen population in Thailand. Malar J. 2008;7:99. doi: 10.1186/1475-2875-7-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maitland K, Williams TN, Bennett S, Newbold CI, Peto TE, Viji J, et al. The interaction between Plasmodium falciparum and P. vivax in children on Espiritu Santo island, Vanuatu. Trans R Soc Trop Med Hyg. 1996;90:614–620. doi: 10.1016/S0035-9203(96)90406-X. [DOI] [PubMed] [Google Scholar]
- 48.Seyoum D, Kifle YG, Rondeau V, Yewhalaw D, Duchateau L, Rosas-Aguirre A, et al. Identification of different malaria patterns due to Plasmodium falciparum and Plasmodium vivax in Ethiopian children: a prospective cohort study. Malar J. 2016;15:208. doi: 10.1186/s12936-016-1253-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.da Silva-Nunes M, Codeço CT, Malafronte RS, da Silva NS, Juncansen C, Muniz PT, et al. Malaria on the Amazonian frontier: transmission dynamics, risk factors, spatial distribution, and prospects for control. Am J Trop Med Hyg. 2008;79:624–635. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Baseline socio-demographic characteristics of study participants by villages. Table S2. Baseline household characteristics of study participants by villages.
Additional file 2: Figure S1. Weekly reported malaria cases in Cahuide (B) in 2012, and main control interventions implemented. (1) Mass screening and treatment (MS&T) and indoor spraying with residual insecticide with >90% of household coverage; (2, 3, 5, 6, 7) MS&T; (4) distribution of LLINs.


