Abstract
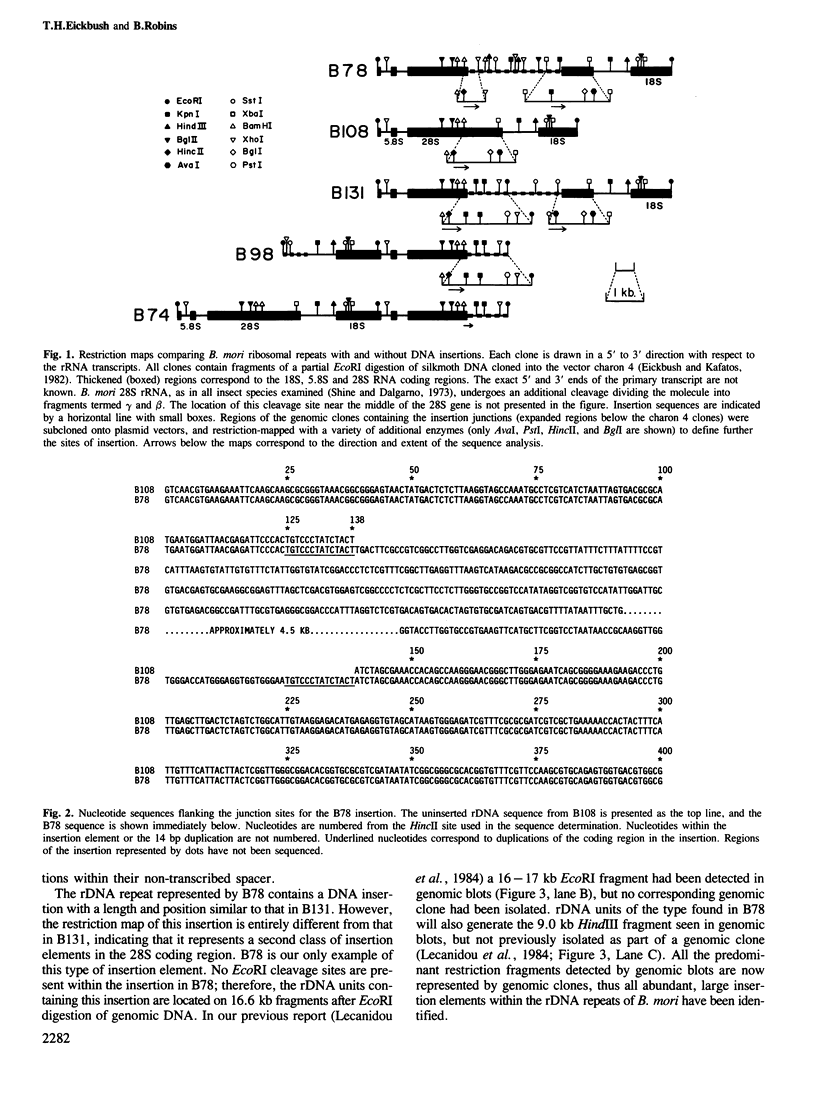
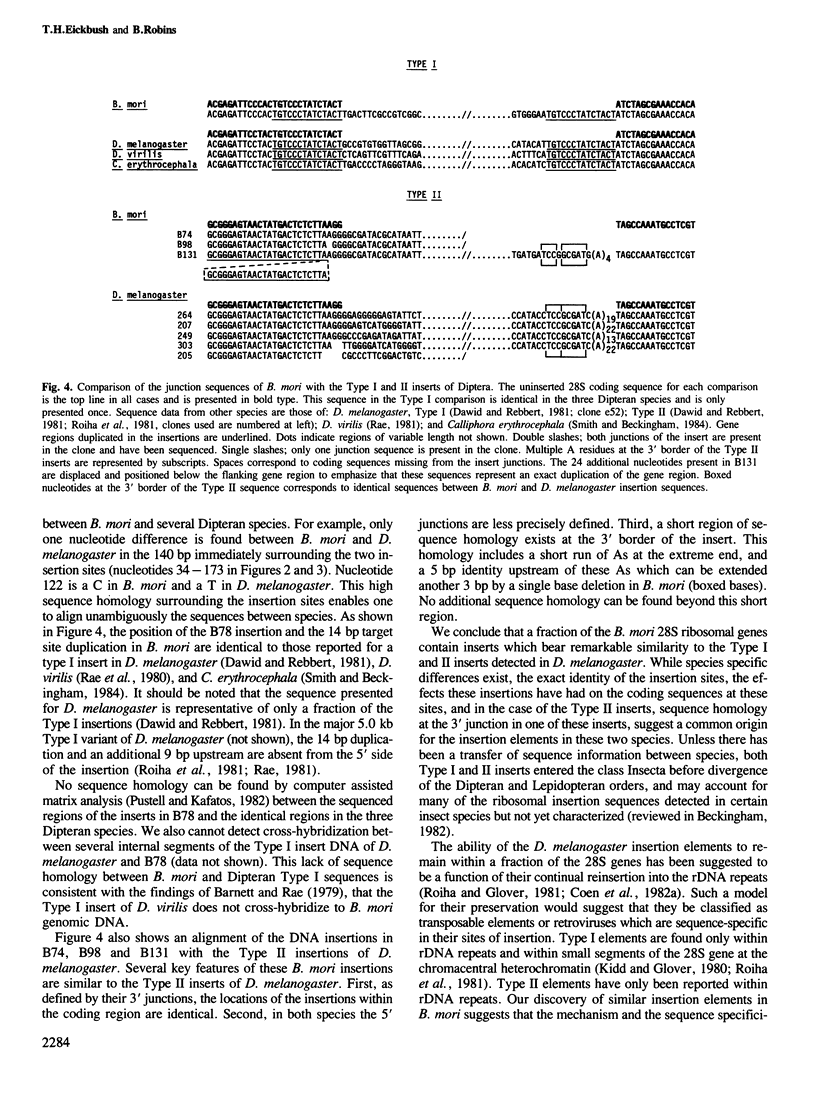
We have examined the 28S ribosomal genes of the silkmoth, Bombyx mori, for the presence of insertion sequences. Two types of insertion sequences were found, each approximately 5 kb in length, which do not share sequence homology. Comparison of the nucleotide sequences of the junction regions with the uninserted gene reveals that one type of insertion has resulted in a 14 bp duplication of the 28S coding region at the insertion site. The location of this insertion and the 14 bp duplication are identical to that found in the Type I ribosomal insertion element of Drosophila melanogaster. The second type of insertion element is located at a site corresponding to approximately 75 bp upstream of the first type. The location of this insertion, the variability detected at its 5' junction, and a short region of sequence homology at its 3' junction suggest that it is related to the Type II element of D. melanogaster. This is the first example of a Type II-like rDNA insertion outside of sibling species of D. melanogaster, and the first example of a Type I-like rDNA insertion outside of the higher Diptera.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnett T., Rae P. M. A 9.6 kb intervening sequence in D. virilis rDNA, and sequence homology in rDNA interruptions of diverse species of Drosophila and other diptera. Cell. 1979 Apr;16(4):763–775. doi: 10.1016/0092-8674(79)90092-8. [DOI] [PubMed] [Google Scholar]
- Beckingham K., White R. The ribosomal DNA of Calliphora erythrocephala; and analysis of hybrid plasmids containing ribosomal DNA. J Mol Biol. 1980 Mar 15;137(4):349–373. doi: 10.1016/0022-2836(80)90162-x. [DOI] [PubMed] [Google Scholar]
- Coen E. S., Thoday J. M., Dover G. Rate of turnover of structural variants in the rDNA gene family of Drosophila melanogaster. Nature. 1982 Feb 18;295(5850):564–568. doi: 10.1038/295564a0. [DOI] [PubMed] [Google Scholar]
- Coen E., Strachan T., Dover G. Dynamics of concerted evolution of ribosomal DNA and histone gene families in the melanogaster species subgroup of Drosophila. J Mol Biol. 1982 Jun 15;158(1):17–35. doi: 10.1016/0022-2836(82)90448-x. [DOI] [PubMed] [Google Scholar]
- Dawid I. B., Rebbert M. L. Nucleotide sequences at the boundaries between gene and insertion regions in the rDNA of Drosophilia melanogaster. Nucleic Acids Res. 1981 Oct 10;9(19):5011–5020. doi: 10.1093/nar/9.19.5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawid I. B., Wellauer P. K., Long E. O. Ribosomal DNA in Drosophila melanogaster. I. Isolation and characterization of cloned fragments. J Mol Biol. 1978 Dec 25;126(4):749–768. doi: 10.1016/0022-2836(78)90018-9. [DOI] [PubMed] [Google Scholar]
- Eickbush T. H., Kafatos F. C. A walk in the chorion locus of Bombyx mori. Cell. 1982 Jun;29(2):633–643. doi: 10.1016/0092-8674(82)90179-9. [DOI] [PubMed] [Google Scholar]
- Kidd S. J., Glover D. M. A DNA segment from D. melanogaster which contains five tandemly repeating units homologous to the major rDNA insertion. Cell. 1980 Jan;19(1):103–119. doi: 10.1016/0092-8674(80)90392-x. [DOI] [PubMed] [Google Scholar]
- Lecanidou R., Eickbush T. H., Kafatos F. C. Ribosomal DNA genes of Bombyx mori: a minor fraction of the repeating units contain insertions. Nucleic Acids Res. 1984 Jun 11;12(11):4703–4713. doi: 10.1093/nar/12.11.4703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long E. O., Dawid I. B. Expression of ribosomal DNA insertions in Drosophila melanogaster. Cell. 1979 Dec;18(4):1185–1196. doi: 10.1016/0092-8674(79)90231-9. [DOI] [PubMed] [Google Scholar]
- Manning R. F., Samols D. R., Gage L. P. The genes for 18S, 5.8S and 28S ribosomal RNA of Bombyx mori are organized into tandem repeats of uniform length. Gene. 1978 Oct;4(2):153–166. doi: 10.1016/0378-1119(78)90027-6. [DOI] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Pustell J., Kafatos F. C. A high speed, high capacity homology matrix: zooming through SV40 and polyoma. Nucleic Acids Res. 1982 Aug 11;10(15):4765–4782. doi: 10.1093/nar/10.15.4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae P. M. Coding region deletions associated with the major form of rDNA interruption in Drosophila. Nucleic Acids Res. 1981 Oct 10;9(19):4997–5010. doi: 10.1093/nar/9.19.4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae P. M., Kohorn B. D., Wade R. P. The 10 kb Drosophila virilis 28S rDNA intervening sequence is flanked by a direct repeat of 14 base pairs of coding sequence. Nucleic Acids Res. 1980 Aug 25;8(16):3491–3504. doi: 10.1093/nar/8.16.3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritossa F. M. Unstable redundancy of genes for ribosomal RNA. Proc Natl Acad Sci U S A. 1968 Jun;60(2):509–516. doi: 10.1073/pnas.60.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roiha H., Glover D. M. Chracterisation of complete type II insertions in cloned segments of ribosomal DNA from Drosophila melanogaster. J Mol Biol. 1980 Jun 25;140(2):341–355. doi: 10.1016/0022-2836(80)90110-2. [DOI] [PubMed] [Google Scholar]
- Roiha H., Glover D. M. Duplicated rDNA sequences of variable lengths flanking the short type I insertions in the rDNA of Drosophila melanogaster. Nucleic Acids Res. 1981 Nov 11;9(21):5521–5532. doi: 10.1093/nar/9.21.5521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roiha H., Miller J. R., Woods L. C., Glover D. M. Arrangements and rearrangements of sequences flanking the two types of rDNA insertion in D. melanogaster. Nature. 1981 Apr 30;290(5809):749–753. doi: 10.1038/290749a0. [DOI] [PubMed] [Google Scholar]
- Roiha H., Read C. A., Browne M. J., Glover D. M. Widely differing degrees of sequence conservation of the two types of rDNA insertion within the melanogaster species sub-group of Drosophila. EMBO J. 1983;2(5):721–726. doi: 10.1002/j.1460-2075.1983.tb01491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. Occurrence of heat-dissociable ribosomal RNA in insects: the presence of three polynucleotide chains in 26 S RNA from cultured Aedes aegypti cells. J Mol Biol. 1973 Mar 25;75(1):57–72. doi: 10.1016/0022-2836(73)90528-7. [DOI] [PubMed] [Google Scholar]
- Smith V. L., Beckingham K. The intron boundaries and flanking rRNA coding sequences of Calliphora erythrocephala rDNA. Nucleic Acids Res. 1984 Feb 10;12(3):1707–1724. doi: 10.1093/nar/12.3.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartof K. D., Dawid I. G. Similarities and differences in the structure of X and Y chromosome rRNA genes of Drosophila. Nature. 1976 Sep 2;263(5572):27–30. doi: 10.1038/263027a0. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Wellauer P. K., Dawid I. B. Ribosomal DNA in Drosophila melanogaster. II. Heteroduplex mapping of cloned and uncloned rDNA. J Mol Biol. 1978 Dec 25;126(4):769–782. doi: 10.1016/0022-2836(78)90019-0. [DOI] [PubMed] [Google Scholar]
- Wellauer P. K., Dawid I. B., Tartof K. D. X and Y chromosomal ribosomal DNA of Drosophila: comparison of spacers and insertions. Cell. 1978 Jun;14(2):269–278. doi: 10.1016/0092-8674(78)90113-7. [DOI] [PubMed] [Google Scholar]
- de Cicco D. V., Glover D. M. Amplification of rDNA and type I sequences in Drosophila males deficient in rDNA. Cell. 1983 Apr;32(4):1217–1225. doi: 10.1016/0092-8674(83)90304-5. [DOI] [PubMed] [Google Scholar]


