Abstract
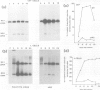
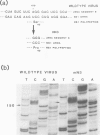
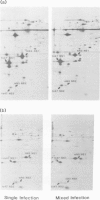
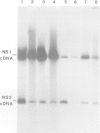
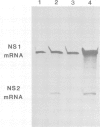
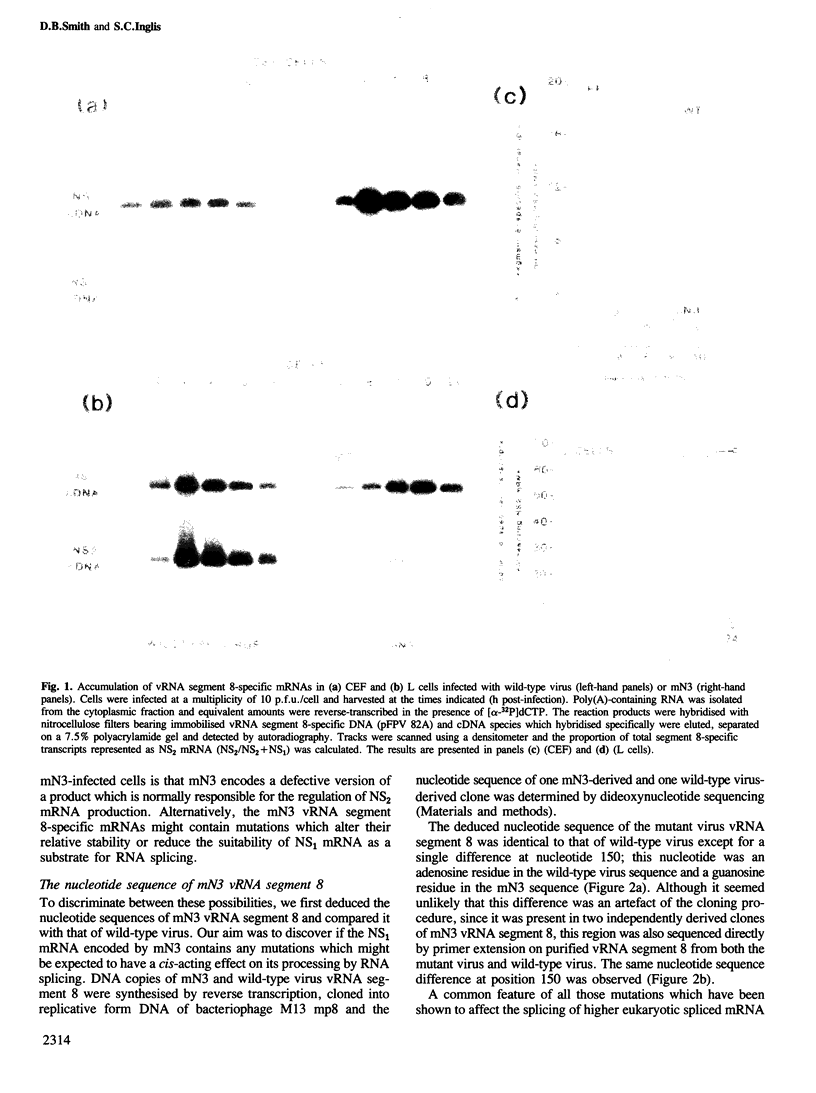
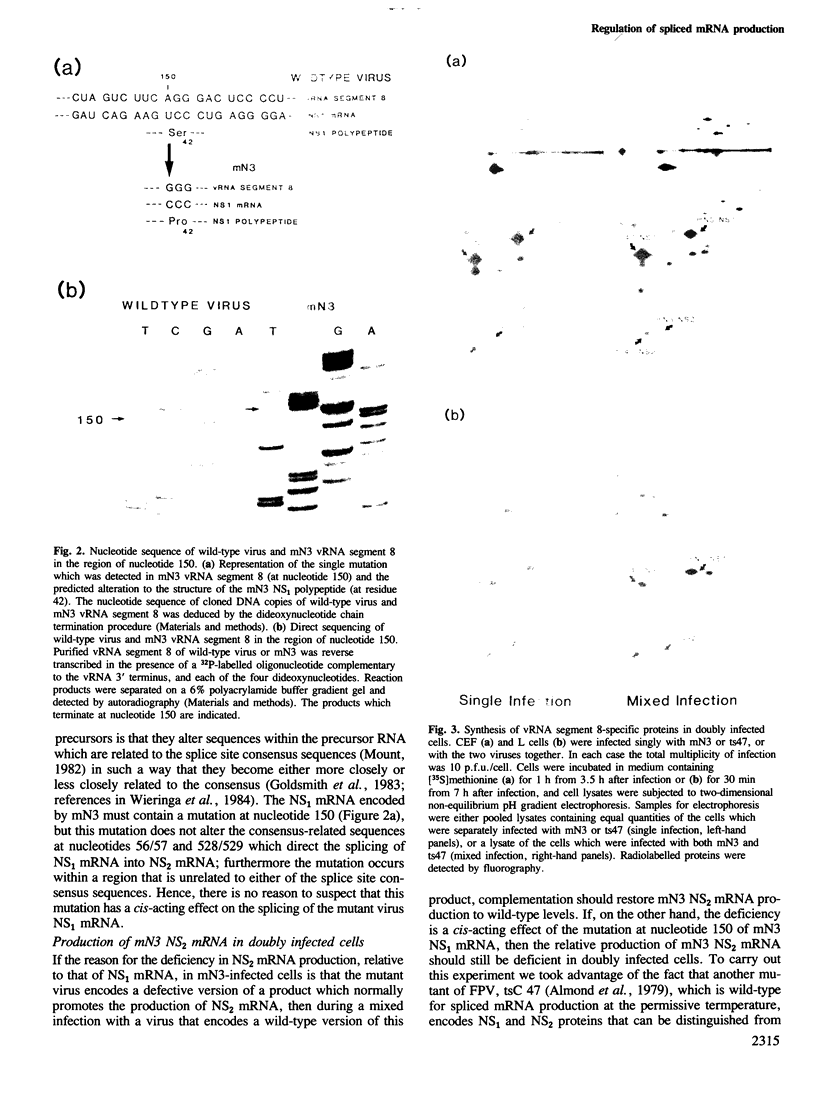
The influenza virus NS2 mRNA is generated through processing by cellular enzymes of a transcript (the NS1 mRNA) of virion RNA segment 8. Production of this mRNA is altered in cells infected with a mutant of influenza A (fowl plague) virus. The proportion of segment 8 transcripts which accumulated in a spliced form was found to be considerably lower in mutant virus-infected cells than in cells infected with wild-type virus, and the amplification in production of NS2 mRNA relative to that of the NS1 mRNA, which normally occurs during infection with wild-type virus, was not observed with the mutant. The NS1 mRNA specified by the mutant virus has unaltered splice recognition sites and was apparently processed normally during a mixed infection with a strain of virus which is wild-type for production of NS2 mRNA. These results suggest that the production of NS2 mRNA is regulated by virus-specific products; these products may act by increasing the efficiency of splicing of NS1 mRNA.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almond J. W., McGeoch D., Barry R. D. Temperature-sensitive mutants of fowl plague virus: isolation and genetic characterization. Virology. 1979 Jan 30;92(2):416–427. doi: 10.1016/0042-6822(79)90146-6. [DOI] [PubMed] [Google Scholar]
- BREITENFELD P. M., SCHAFER W. The formation of fowl plague virus antigens in infected cells, as studied with fluorescent antibodies. Virology. 1957 Oct;4(2):328–345. doi: 10.1016/0042-6822(57)90067-3. [DOI] [PubMed] [Google Scholar]
- Biggin M. D., Gibson T. J., Hong G. F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borland R., Mahy B. W. Deoxyribonucleic acid-dependent ribonucleic acid polymerase activity in cells infected with influenza virus. J Virol. 1968 Jan;2(1):33–39. doi: 10.1128/jvi.2.1.33-39.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKLIN R. M., BREITENFELD P. M. The abortive infection of Earle's L-cells by fowl plague virus. Virology. 1959 Jul;8(3):293–307. doi: 10.1016/0042-6822(59)90031-5. [DOI] [PubMed] [Google Scholar]
- Goldsmith M. E., Humphries R. K., Ley T., Cline A., Kantor J. A., Nienhuis A. W. "Silent" nucleotide substitution in a beta+-thalassemia globin gene activates splice site in coding sequence RNA. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2318–2322. doi: 10.1073/pnas.80.8.2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Hay A. J., Lomniczi B., Bellamy A. R., Skehel J. J. Transcription of the influenza virus genome. Virology. 1977 Dec;83(2):337–355. doi: 10.1016/0042-6822(77)90179-9. [DOI] [PubMed] [Google Scholar]
- Inglis S. C., Brown C. M. Differences in the control of virus mRNA splicing during permissive or abortive infection with influenza A (fowl plague) virus. J Gen Virol. 1984 Jan;65(Pt 1):153–164. doi: 10.1099/0022-1317-65-1-153. [DOI] [PubMed] [Google Scholar]
- Inglis S. C., Brown C. M. Spliced and unspliced RNAs encoded by virion RNA segment 7 of influenza virus. Nucleic Acids Res. 1981 Jun 25;9(12):2727–2740. doi: 10.1093/nar/9.12.2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglis S. C., Carroll A. R., Lamb R. A., Mahy B. W. Polypeptides specified by the influenza virus genome I. Evidence for eight distinct gene products specified by fowl plague virus. Virology. 1976 Oct 15;74(2):489–503. doi: 10.1016/0042-6822(76)90355-x. [DOI] [PubMed] [Google Scholar]
- Inglis S. C., Gething M. J., Brown C. M. Relationship between the messenger RNAs transcribed from two overlapping genes of influenza virus. Nucleic Acids Res. 1980 Aug 25;8(16):3575–3589. doi: 10.1093/nar/8.16.3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglis S. C., Mahy B. W. Polypeptides specified by the influenza virus genome. 3. Control of synthesis in infected cells. Virology. 1979 May;95(1):154–164. doi: 10.1016/0042-6822(79)90410-0. [DOI] [PubMed] [Google Scholar]
- Inglis S. C., McGeoch D. J., Mahy B. W. Polypeptides specified by the influenza virus genoma. 2. Assignement of protein coding functions to individual genome segments by in vitro translation. Virology. 1977 May 15;78(2):522–536. doi: 10.1016/0042-6822(77)90128-3. [DOI] [PubMed] [Google Scholar]
- Lamb R. A., Etkind P. R., Choppin P. W. Evidence for a ninth influenza viral polypeptide. Virology. 1978 Nov;91(1):60–78. doi: 10.1016/0042-6822(78)90355-0. [DOI] [PubMed] [Google Scholar]
- Lamb R. A., Lai C. J., Choppin P. W. Sequences of mRNAs derived from genome RNA segment 7 of influenza virus: colinear and interrupted mRNAs code for overlapping proteins. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4170–4174. doi: 10.1073/pnas.78.7.4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb R. A., Lai C. J. Expression of unspliced NS1 mRNA, spliced NS2 mRNA, and a spliced chimera mRNA from cloned influenza virus NS DNA in an SV40 vector. Virology. 1984 May;135(1):139–147. doi: 10.1016/0042-6822(84)90124-7. [DOI] [PubMed] [Google Scholar]
- Lamb R. A., Lai C. J. Sequence of interrupted and uninterrupted mRNAs and cloned DNA coding for the two overlapping nonstructural proteins of influenza virus. Cell. 1980 Sep;21(2):475–485. doi: 10.1016/0092-8674(80)90484-5. [DOI] [PubMed] [Google Scholar]
- Lamb R. A., Lai C. J. Spliced and unspliced messenger RNAs synthesized from cloned influenza virus M DNA in an SV40 vector: expression of the influenza virus membrane protein (M1). Virology. 1982 Dec;123(2):237–256. doi: 10.1016/0042-6822(82)90258-6. [DOI] [PubMed] [Google Scholar]
- Mount S. M. A catalogue of splice junction sequences. Nucleic Acids Res. 1982 Jan 22;10(2):459–472. doi: 10.1093/nar/10.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevins J. R., Wilson M. C. Regulation of adenovirus-2 gene expression at the level of transcriptional termination and RNA processing. Nature. 1981 Mar 12;290(5802):113–118. doi: 10.1038/290113a0. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Porter A. G., Smith J. C., Emtage J. S. Nucleotide sequence of influenza virus RNA segment 8 indicates that coding regions for NS1 and NS2 proteins overlap. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5074–5078. doi: 10.1073/pnas.77.9.5074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers G. N., Paulson J. C., Daniels R. S., Skehel J. J., Wilson I. A., Wiley D. C. Single amino acid substitutions in influenza haemagglutinin change receptor binding specificity. Nature. 1983 Jul 7;304(5921):76–78. doi: 10.1038/304076a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. F., Searle P. F., Williams J. G. Characterisation of bacterial clones containing DNA sequences derived from Xenopus laevis vitellogenin mRNA. Nucleic Acids Res. 1979 Feb;6(2):487–506. doi: 10.1093/nar/6.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark G. R., Williams J. G. Quantitative analysis of specific labelled RNA'S using DNA covalently linked to diazobenzyloxymethyl-paper. Nucleic Acids Res. 1979 Jan;6(1):195–203. doi: 10.1093/nar/6.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieringa B., Hofer E., Weissmann C. A minimal intron length but no specific internal sequence is required for splicing the large rabbit beta-globin intron. Cell. 1984 Jul;37(3):915–925. doi: 10.1016/0092-8674(84)90426-4. [DOI] [PubMed] [Google Scholar]
- Wilson M. C., Darnell J. E., Jr Control of messenger RNA concentration by differential cytoplasmic half-life. Adenovirus messenger RNAs from transcription units 1A and 1B. J Mol Biol. 1981 May 25;148(3):231–251. doi: 10.1016/0022-2836(81)90537-4. [DOI] [PubMed] [Google Scholar]
- Wolstenholme A. J., Barrett T., Nichol S. T., Mahy B. W. Influenza virus-specific RNA and protein syntheses in cells infected with temperature-sensitive mutants defective in the genome segment encoding nonstructural proteins. J Virol. 1980 Jul;35(1):1–7. doi: 10.1128/jvi.35.1.1-7.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]