Abstract
Self-monitoring of lung function, vital signs, and symptoms is crucial for lung transplant recipients (LTRs) to ensure early detection of complications and prompt intervention. This study sought to identify patterns and correlates of adherence to self-monitoring among LTRs over the first 12-months post-discharge from transplant. This study analyzed existing data from the usual care arm participants of a randomized clinical trial who tracked self-monitoring activities using paper-and-pencil logs. Adherence was calculated as the percent of days LTRs recorded any self-monitoring data per interval: hospital discharge-2 months, 3–6 months, and 7–12 months. The sample (N=91) was mostly white (87.9%), male (61.5%), with a mean age of 57.2±13.8 years. Group-based trajectory analyses revealed 2 groups: 1) moderately adherent with slow decline (n=29, 31.9%) and 2) persistently nonadherent (n=62, 68.1%). Multivariate binary logistic regression revealed the following baseline factors increased the risk in the persistently nonadherent group: female (p=.035), higher anxiety (p=.008), and weaker sense of personal control over health (p=.005). Poorer physical health over 12-months were associated with increased risk in the persistently nonadherent group (p=.004). This study highlighted several modifiable factors for future interventions to target, including reducing post-transplant anxiety, and strengthening sense of personal control over health in LTRs.
Keywords: lung transplant, adherence, self-monitoring, self-management, group-based trajectory modeling
INTRODUCTION
To date, more than 55,000 adults have undergone lung transplantation worldwide, a number largely limited by the scarcity of organ donors.1 Despite improved physical functioning and quality of life, survival after lung transplantation is often compromised by the high susceptibility to infections and graft rejection with approximately 75% of lung transplant recipients (LTRs) developing infection and 50% developing acute rejection during the first year post-transplantation.2–4 Thus, there is a pressing need to detect complications early and intervene promptly to maximize outcomes in LTRs. Frequent self-monitoring of health conditions at home has been shown to be a reliable and valid approach for promoting early detection and prompt treatment of complications to reduce morbidity and mortality after lung transplantation.5,6
Prior to discharge, LTRs are instructed to perform self-monitoring activities including daily assessment of lung function using a home spirometer, vital signs, and common symptoms of post-transplant complications.7–9 One study showed that 100% adherence to home self-monitoring reduced total post-transplant medical costs by more than 50%.10 Despite the well-recognized importance of self-monitoring, adherence to self-monitoring among LTRs is less than ideal8,11 with nonadherence rates for performing spirometry reportedly as high as 54.6% and 65.9% at 12 and 24 months post-transplant, respectively.8 Without a clear understanding of the patterns and correlates of adherence to self-monitoring, it is difficult to design, implement, and evaluate interventions to address this problem.
According to the World Health Organization (WHO), there are five major dimensions affecting adherence behaviors: 1) social/economic factors; 2) patient-related factors; 3) condition-related factors; 4) therapy-related factors; and 5) health system-related factors.12 Factors in each of the five dimensions have been examined in the context of adherence to self-monitoring after lung transplantation.8,11,13,14 Yet, the published evidence regarding the correlates of adherence to self-monitoring has been limited in several ways. First, the evidence is limited in quantity. Our recent literature search15 of five large medical and nursing databases yielded only four published studies8,11,13,14 that examined the correlates of adherence to self-monitoring among LTRs. Second, results from these four studies were inconsistent, limiting the conclusions that can be drawn about the correlates of adherence to self-monitoring. For example, age was not a significant correlate of adherence to self-monitoring of lung function in Dew et al8 and Teichman et al14, while Kugler and colleagues11 found that younger age (<40 years old) was significantly correlated with poorer adherence to home spirometry. Third, current studies have focused on describing adherence for the entire sample at each assessment time-point, yet there is heterogeneity in the patterns of adherence over time. For example, some individuals might have good adherence behaviors at the beginning and slowly become nonadherent over time while other individuals might be consistently nonadherent over time. To address this gap and identify distinct groups of individuals who demonstrate similar adherence patterns over time, in this study we used an advanced statistical technique, group-based trajectory modeling.16,17 The aims of this study were to: 1) describe distinct patterns of adherence to self-monitoring and 2) identify correlates (representing each dimension of the WHO model) associated with patterns of adherence to self-monitoring in LTRs over the first year after discharge from transplantation.
METHODS
Study Design
This study was a secondary analysis of prospectively collected data from a completed randomized controlled trial, Phase III Trial of Pocket PATH®: A Computerized Intervention to Promote Self-Care (R01NR107011, PI: DeVito Dabbs).18 The primary aim of the parent study was to test the efficacy of a mobile health intervention, relative to usual care, to promote self-care behaviors (including adherence to daily self-monitoring) during the first year following discharge from the lung transplant hospitalization.18
Sample and Setting
We examined only the usual care group of the parent study for this investigation since our aim was to describe underlying patterns of adherence to self-monitoring in a naturalistic setting but the Pocket PATH intervention might influence adherence to self-monitoring. Eligibility criteria for the current study were: 1) lung transplant recipient; 2) > 18 years of age; 3) stable enough to be transferred from the cardiothoracic intensive care unit (ICU) to the acute care unit; 4) able to speak and read English, 5) no previous organ transplantation, 6) expected to be involved in post-transplant care, and 7) randomized to the usual care condition. All participants were recruited from the Cardiothoracic Transplant Program of the University of Pittsburgh Medical Center prior to hospital discharge. IRB approval was obtained for the parent and current study.
Measures
Outcome Variable: Adherence to Self-Monitoring
Prior to discharge, participants in the usual care group received a 30-minute scripted oral set of instructions from their transplant coordinator and an instruction binder which emphasized the importance of adhering to medical regimen, performing daily self-monitoring, and reporting abnormal values to their transplant team. More specifically, for the self-monitoring component, participants were instructed to self-monitor and record the date and values for a variety of health indicators daily on paper-and-pencil logs, including lung function using home spirometry, vital signs and symptoms. At 2-, 6-, and 12-months follow-up assessments, they were asked to bring paper logs for data collectors to make photocopies. Adherence was calculated as the percent of days that LTRs recorded data for any health indicator on the paper logs and this was calculated for each of the three intervals (hospital discharge to 2 months, 2 to 6 months, and 6 to 12 months). These intervals were selected to be consistent with the measurement time points in the parent study. Total days per interval were adjusted for any days that LTRs were re-hospitalized and therefore not expected to perform self-monitoring at home.
Potential Correlates of Adherence to Self-Monitoring
The choice of the potential correlates of adherence to examine in our analyses was informed by prior studies8,11,13,14 and based on the five dimensions of the WHO adherence model: social/economic, patient-related, condition-related, therapy-related, and healthcare system factors.12
Social/Economic factors
Socio-demographic characteristics were collected at baseline and included age, education, gender, race, employment status, marital status, and whether the respondents felt that their income met their household needs. Given that there were limited numbers of non-white LTRs, currently employed LTRs, or LTRs who felt their income did not meet needs in this study, we examined these variables descriptively but omitted them from multivariate model building processes. The quality of the patient’s relationship with his/her family caregiver was measured at baseline using the 15-item Dyadic Adjustment Scale (DAS).19 Possible scores range from 15–75 with higher scores indicating better relationship quality. Cronbach’s alpha was 0.80 for the current sample.
Patient-related factors
Self-care agency, defined as ones’ willingness and ability to perform self-care, was assessed using a 53-item scale, Perception of Self-Care Agency20, at baseline, 2-, 6-, and 12-months post-discharge. Higher scores indicate higher levels of perceived self-care agency with possible scores ranging from 53–265. Cronbach’s alpha was 0.94 for our current sample. The 18-item Multi-Dimensional Health Locus of Control Scale 21,22 (MHLC) was administered at baseline to measure the extent to which LTRs believed that health outcomes were 1) their own responsibility (internality) (Cronbach’s alpha = 0.78 for the current sample), 2) their health professionals’ responsibility (externality) (Cronbach’s alpha = 0.43 for the current sample), or 3) determined by chance alone (chance) (Cronbach’s alpha = 0.77 for the current sample). Each subscale includes 6 items using a Likert scale of 1–6, which were summed for a possible range of 6–36. Higher scores reflect stronger beliefs in each of the three domains. Given that Cronbach’s alpha was relatively low (0.43) for the externality subscale, we omitted it from our modeling processes.
Condition-related factors
The underlying lung disease (obstructive vs. non-obstructive) was collected from the medical record. The anxiety and depression subscales of the Symptom Checklist-90 (SCL-90)23,24 were administered at baseline, and longitudinally at 2-, 6-, and 12-months post-hospital discharge to assess symptoms of psychological distress during the past two weeks. Items were formatted using a 5-point Likert scale (0 “not at all” to 4 “extremely distressed”). The subscale scores were calculated by averaging the score for each item23,24 and the possible scores range from 0–4. Higher scores indicate greater levels of psychological distress (anxiety or depression). Cronbach’s alpha was 0.86 and 0.82 for anxiety and depression subscales, respectively, in our current sample. The physical component and mental component summary scores, were calculated from the Medical Outcomes Study Short Form-36 (MOS SF-36)25,26, which assessed LTRs’ health-related quality of life at 2-, 6-, and 12-month post-discharge. Higher scores reflect better health-related quality of life with possible scores ranging from 0–100.
Therapy-related factors included the type of transplant (single or double lung transplantation), whether the LTR was re-intubated or not post-transplant, days requiring chest drainage, and whether the post-operation need for ventilator support exceeded 48 hours. Data for these variables were abstracted from the medical record review.
Health system-related factors included length of hospital stay (days), number of days in ICU, and discharge destination (home or facilities other than home). Data for these variables were abstracted from the medical record review.
Statistical Analysis
Descriptive analyses were conducted using IBM® SPSS® Statistics (Version 23, IBM Corp., Armonk, NY). The level of statistical significance was set at 0.05 for two-tailed hypothesis testing. We calculated means and standard deviations for continuous variables without outliers and frequencies and percentages for categorical variables. For continuous variables with outliers, we reported median and interquartile ranges.
We used PROC TRAJ in SAS (Version 9.4, SAS Institute Inc., Cary, NC) to perform the group-based trajectory modeling16,17,27 to identify distinct patterns of adherence to self-monitoring in LTRs during the first 12 months after discharge from transplant hospitalization. Based on Bayesian Information Criteria (BIC) values for competing models, the model with smallest BIC based on Bayes factor was chosen as having the best fit.
For baseline correlates, we applied univariate logistic regression analyses to examine the association between each correlate and the predicted group membership. Baseline correlates with p-values less than 0.30 in the univariate models were considered as candidates for the multivariate logistic regression models. We chose this liberal p-value cutoff as this approach is more likely to retain important factors in the final model given a small sample size.28,29 A backward elimination approach using likelihood ratio Chi-squared test statistic28,29 was used to identify the final most parsimonious multivariate model retaining candidate factors significant at p<0.05. We calculated crude and adjusted odds ratios (ORs) with 95% confidence intervals (CIs).
For longitudinally measured correlates (self-care agency, SF-36 physical component summary (PCS), SF-36 mental component summary (MCS), and SCL-90 Anxiety and Depression subscales), we conducted linear mixed modeling to examine their associations with predicted group membership. In the linear mixed models, predicted trajectory group was treated as the grouping factor and the longitudinal correlates were modeled as outcomes. We reported group effect, time effect and group by time interaction effect for each longitudinal correlate.
RESULTS
Sample Characteristics
Individuals who did not provide any adherence data (n=3) and those who died during the study (n=8) were excluded from the analysis, which yielded a final sample of 91 LTRs for analysis. Baseline characteristics of the total sample are displayed in Table 1. The total sample was predominantly white (87.9%), male (61.5%), and currently married (70.3%), with a mean age of 57.19 (SD=13.76) years. Most participants had above a high school education (68.1%) and reported having incomes that met their needs (80.0%).
Table 1.
Sample Characteristics and Baseline Correlates of Membership in the Persistently Nonadherent to Self-Monitoring Group Based on Univariate Logistic Regression Analyses
| Adherence to Self-Monitoring
|
|||||
|---|---|---|---|---|---|
| Correlates | Total Sample (N=91) M±SD or n (%) | Moderately adherent with slow decline group (n=29) M±SD or n(%) |
Persistently nonadherent group (n=62) M±SD or n (%) |
Unadjusted Odd Ratio (OR) | 95% CI for OR |
| Social/Economic | |||||
|
| |||||
| Age (years) | 57.19±13.76 | 58.62±15.47 | 56.52±12.97 | 0.99 | 0.96, 1.02 |
| > High school education | 62 (68.1%) | 20 (69.0%) | 42 (67.7%) | 0.95 | 0.37, 2.44 |
| Currently employed | 7 (7.7%) | 3 (10.3%) | 4 (6.5%) | n/a | n/a |
| Non-white | 11 (12.1%) | 1 (3.4%) | 10 (16.1%) | n/a | n/a |
| Female | 35 (38.5%) | 6 (20.7%) | 29 (46.8%) | 3.37 | 1.21, 9.41 |
| Income met needs: No | 18 (20%) | 2 (6.9%) | 16 (26.2%) | n/a | n/a |
| Currently or living as married | 64 (70.3%) | 23 (79.3%) | 41 (66.1%) | 0.51 | 0.18, 1.44 |
| Quality of dyadic relationship* (possible score range: 15–75) | 68.00±7.00 | 68.00±9.75 | 67.00±5.50 | 0.98 | 0.92, 1.06 |
|
| |||||
| Patient-related | |||||
|
| |||||
| MHLC internal subscale (possible score range: 6–36) | 23.96±6.48 | 26.72±6.27 | 22.66±6.21 | 0.90 | 0.83, 0.97 |
| MHLC chance subscale (possible score range: 6–36) | 18.29±7.02 | 17.80±6.43 | 18.52±7.32 | 1.02 | 0.95, 1.08 |
| Self-care agency (possible score range: 53–265) | 222.24±23.90 | 224.14±20.78 | 221.35±25.3 | 1.00 | 0.98, 1.01 |
|
| |||||
| Condition-related | |||||
|
| |||||
| Obstructive lung disease | 37 (40.7%) | 10 (34.5%) | 27 (43.5%) | 1.47 | 0.59, 3.66 |
| SCL-90 anxiety score* (possible score range: 0–4) | 0.40±0.73 | 0.30±0.30 | 0.50±0.95 | 4.26 | 1.37, 13.28 |
| SCL-90 depression score (possible score range: 0–4) | 0.62±0.55 | 0.44±0.34 | 0.70±0.61 | 3.15 | 1.05, 9.42 |
|
| |||||
| Treatment-related | |||||
|
| |||||
| Single lung transplant | 16 (17.6%) | 7 (24.1%) | 9 (14.5%) | 0.53 | 0.18, 1.61 |
| Re-intubated | 26 (28.6%) | 6 (20.7%) | 20 (32.3%) | 1.83 | 0.64, 5.19 |
| Post-op ventilator needs ≥48 hours | 35 (38.5%) | 7 (24.1%) | 28 (45.2%) | 2.59 | 0.97, 6.94 |
| Days with chest drain* | 12.00±8.75 | 12.50±10.25 | 12.00±8.00 | 1.00 | 0.95, 1.06 |
|
| |||||
| Healthcare system-related | |||||
|
| |||||
| Length of hospital stay (days)* | 31.00±27.00 | 27.00±25.00 | 33.00±28.25 | 1.01 | 0.99, 1.03 |
| Days in ICU* | 6.00±10.00 | 4.00±5.00 | 7.00±11.50 | 1.01 | 0.97, 1.05 |
| Discharge to home setting | 78 (85.7%) | 24 (82.8%) | 54 (87.1%) | 0.71 | 0.21, 2.40 |
Note: n/a indicated that these variables were only examined descriptively but omitted from model building due to the limited variability in these variables.
Medians and inter-quartile ranges were reported for those variables with outliers.
M: Mean; SD: Standard deviation; CI: Confidence Interval; MHLC: Multidimensional Health Locus of Control; ICU: Intensive Care Unit; SCL-90: Symptom Checklist 90.
Patterns of Adherence to Self-Monitoring
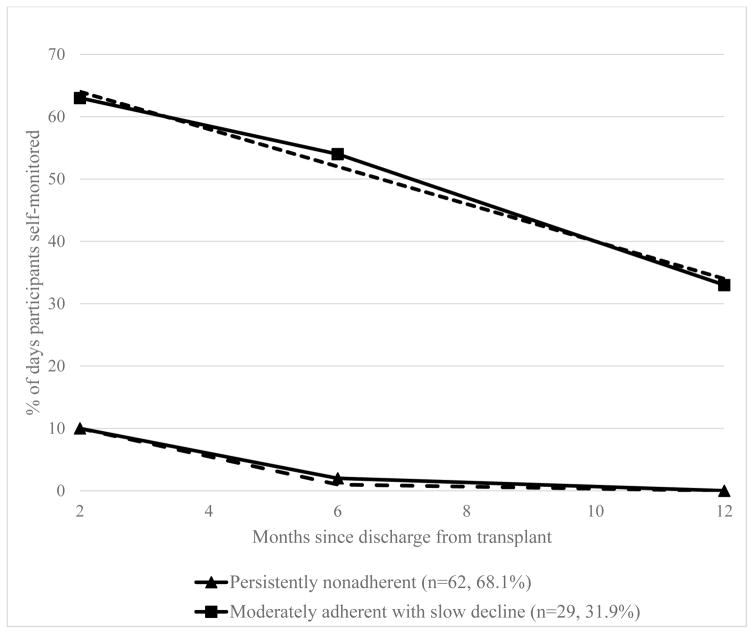
Based on the significance of estimated model parameters and BIC values from group-based trajectory modeling, a 2-group trajectory model provided the best fit:1) moderately adherent with slow decline (n=29, 31.9%) and 2) persistently nonadherent (n=62, 68.1%). As shown in Figure 1, the moderately adherent with slow decline group began with 63.84% adherence to self-monitoring and linearly declined at a small slope (b1=−4.03, p=0.002) over time. The persistently nonadherent group started at a low percent of adherence (10.02%) at 2 months and declined to 1% adherence at 6-months and 0% at 12 months, meaning they performed minimal self-monitoring over 12 months.
Figure 1. Estimated Trajectory Groups of Adherence to Self-Monitoring.
Note: Solid lines are mean percent adherence based on raw data and dashed lines are predicted mean percent adherence based on trajectory modeling.
Baseline Correlates of Adherence to Self-Monitoring
Based on the univariate analyses, 8 candidate correlates met the threshold of p<0.30: gender, marital status, internal health locus of control, anxiety, depression, type of transplant, whether re-intubated post-transplant, and post-operation ventilator needs. Using a backward elimination approach, we obtained the most parsimonious model with only 3 correlates retained, including gender, internal health locus of control, and anxiety. More specifically, the adjusted odds of being in the persistently nonadherent group (as opposed to the moderately adherent with slow decline group) was 3.39 (95% CI, 1.09–10.53) for females, 1.14 (95% CI: 1.04–1.26) for every one unit decrease in internal health locus of control, and 6.08 (95% CI, 1.60–23.07) for every one unit increase in anxiety (Table 2).
Table 2.
Adjusted Odds Ratios for Membership in the Persistently Nonadherent Group Based on Final Parsimonious Multivariate Logistic Regression
| Baseline Correlates | Adjusted OR (95% CI) | P value |
|---|---|---|
| Social/Economic | ||
| Female | 3.39 (1.09, 10.53) | .035 |
| Patient-related | ||
| Lower MHLC Internal subscale score | 1.14 (1.04, 1.26) | .008 |
| Condition-related | ||
| Higher SCL-90 anxiety score | 6.08 (1.60, 23.07) | .005 |
OR: Odds Ratio; CI: Confidence Interval; MHLC: Multidimensional Health Locus of Control; SCL-90: Symptom Checklist 90.
Longitudinal Correlates of Adherence to Self-Monitoring
Table 3 summarizes the descriptive statistics (means and standard deviations) and the linear mixed modeling results for longitudinal correlates of adherence to self-monitoring including, self-care agency, physical component summary score, mental component summary score, anxiety and depression subscales. There was a significant group effect for the physical component summary score (p=0.004), suggesting that lower physical component summary scores of the quality of life measure were associated with membership in the persistently nonadherent group over the 12 months of observation. None of the other longitudinal correlates were significant.
Table 3.
Descriptive Statistics and Linear Mixed Modeling Results for Longitudinal Correlates by Predicted Trajectory Group Membership
| Correlates | Moderately adherent with slow decline group (n=29) | Persistently nonadherent group (n=62) | p-values
|
||
|---|---|---|---|---|---|
| Group | Time | Group*Time Interaction | |||
| Self-Care Agency | |||||
| 2 months | 227.21 (26.94) | 226.03 (22.50) | 0.13 | 0.52 | 0.05 |
| 6 months | 235.28 (21.06) | 223.25 (23.18) | |||
| 12 months | 232.71 (24.17) | 225.29 (23.85) | |||
| SF-36 PCS | |||||
| 2 months | 41.58 (7.85) | 36.99 (9.30) | 0.004 | <0.001 | 0.47 |
| 6 months | 45.59 (9.33) | 38.91 (10.93) | |||
| 12 months | 46.11 (8.80) | 41.52 (10.51) | |||
| SF-36 MCS | |||||
| 2 months | 51.65 (11.99) | 51.44 (10.78) | 0.58 | 0.13 | 0.56 |
| 6 months | 52.17 (10.21) | 52.46 (9.54) | |||
| 12 months | 55.63 (5.89) | 53.06 (8.74) | |||
| SCL-90 Anxiety | |||||
| 2 months | 0.38 (0.34) | 0.57 (0.62) | 0.11 | 0.06 | 0.51 |
| 6 months | 0.40 (0.37) | 0.49 (0.49) | |||
| 12 months | 0.31 (0.34) | 0.39 (0.39) | |||
| SCL-90 Depression | |||||
| 2 months | 0.55 (0.51) | 0.67 (0.65) | 0.24 | 0.23 | 0.85 |
| 6 months | 0.58 (0.60) | 0.66 (0.48) | |||
| 12 months | 0.45 (0.30) | 0.59 (0.42) | |||
Note: Descriptive statistics in the table were shown as mean (standard deviation).
SF-36: Short Form-36; PCS: Physical Component Summary; MCS: Mental Component Summary; SCL-90: Symptom Checklist-90.
DISCUSSION
We applied group-based trajectory modeling to examine patterns and correlates of adherence to self-monitoring over the first year after discharge from transplant hospitalization. Findings revealed two distinct groups that showed different patterns of adherence to self-monitoring, namely a group of persistently nonadherent LTRs and a group that was moderately adherent with slow decline. We also identified several baseline and longitudinal correlates associated with trajectory group membership.
Although the moderately adherent with slow decline group demonstrated better adherence to self-monitoring than the persistently nonadherent group, the adherence level declined over time in both groups. This decline pattern in adherence behavior has been repeatedly observed in prior studies.8,18,30–32 In addition, our results showed that even with the better adherence group, they only started with moderate level of 63.84% adherence to self-monitoring at 2-months post-discharge. Furthermore, majority of our participants were classified as persistently nonadherent. This suggests that adherence to self-monitoring is a problematic issue in LTRs. It is possible that LTRs performed the self-monitoring activities but did not record the readings on the paper logs. However, results from a prior study11 which used Bluetooth-enabled spirometer showed that the nonadherence to self-monitoring was as high as 59.4% over 3 months observation. The study by Dew and colleagues8 in which a collateral report from the family members of LTRs was used also found the nonadherence to self-monitoring was as high as 54.6% by the end of 12 months observation. These data together suggest nonadherence to self-monitoring is prevalent in LTRs.15 A previous report8 revealed that LTRs stopped self-monitoring because they felt that their clinicians did not review the paper logs during office visits. This suggests that clinicians may need to reinforce the importance of self-monitoring and regularly check LTRs’ paper logs and their adherence to self-monitoring during clinical follow up visits. The study team led by DeVito Dabbs and colleagues18 showed that it is possible to promote adherence to self-monitoring using a mobile-based intervention. Future study may need to explore how to implement and sustain such effective strategies in clinical care practice and also test other possible cost-effective user friendly strategies to promote self-monitoring behaviors in LTRs.
Having lower internal health locus of control beliefs at baseline was a significant correlate of membership in the persistently nonadherent group, which is consistent with findings of Dew et al8 that having a weaker belief that one’s own actions influenced health outcomes increased the odds of being persistently nonadherent to performing spirometry. While the mean score of internal health locus of control for our overall sample is comparable to that in previous reports13,33, our results showed that the persistently nonadherent group possessed a significantly lower internal health locus of control than the moderately adherent group. This is not surprising because possessing a lower internal health locus of control indicates weaker belief that one has control over one’s own health. This perceived lack of personal control over one’s health may be due to insufficient post-transplant education or patient activation; thus these LTRs did not realize they could play an active role by self-monitoring and reporting any potential complications to their transplant team in a timely manner. This finding suggests that clinicians may need to activate and empower LTRs to engage in self-monitoring and reinforce the importance of taking personal responsibility for post-transplant care.
Our results demonstrated that poorer ratings for the physical health component of quality of life were associated with membership in the persistently nonadherent group over the 12-months post-discharge. This is consistent with findings of previous qualitative evidence that reported LTRs’ views that their perceived poor physical health is a major barrier for adherence to self-monitoring of lung function11,34 It has been repeatedly reported that during the posttransplant honeymoon period, LTRs are often overly optimistic and anticipate steady improvement and a new life free of transplant-related complications.35 When they then perceive their physical health to be poorer than expected, they may feel disappointed with the transplantation surgery and do not want to be reminded of their deteriorating status by having to record and view self-monitoring results34; thus they may be less likely to engage in the instructed self-monitoring activities. We also found that reporting a higher baseline level of anxiety was associated with being persistently nonadherent. The negative associations between psychological distress and 1) self-care agency, 2) adherence behaviors and 3) transplant-related health outcomes among LTRs and other solid organ recipients are well-established.36–38 Anxiety disorders are common in LTRs.37–39 It is plausible that those LTRs with a high anxiety level may suffer excess or irrational fears and do not want to know daily results of self-monitoring. Thus they may intentionally avoid self-monitoring.39 Clinicians may need to identify LTRs with higher physical and psychological burden and intervene to promote adherence to self-monitoring and better outcomes.
We found that female LTRs were at increased risk of being in the persistently nonadherent group. Being female has also been found to be a significant predictor of nonadherence to home spirometry by Dew and colleagues8. While the reason for this finding has not been well examined, it is possible that female LTRs may have more competing priorities with self-management40, which lead to limited attention to their own health issues, and thus, lower adherence. These findings suggest that clinicians may need to identify unique barriers to adherence among female LTRs and monitor their adherence more closely after discharge.
Limitations
This study has several limitations. Because our aim was to describe the natural course of adherence to self-monitoring over the first year post-transplant, we only studied LTRs randomized to the usual care arm of the parent study, which provided a relatively small sample size for this analysis (N=91). However, we did employ several approaches (e.g., we screened univariate relationships first to identify candidate correlates (p<0.30) in the multivariate models, and used backward elimination to reach a most parsimonious model) which permitted us to meet the recommended case-to-variable ratio of 10:1.41 Also, we relied on paper logs as a proxy measure of self-monitoring, which may have underestimated adherence because participants may have performed self-monitoring but did not necessarily document these activities on the paper logs. Yet, it is worth mentioning that most of the transplant programs rely on paper logs for LTRs to record self-monitoring activities. Thus, the findings from this study could be informative to a broad audience. This study was conducted at only one transplant center which may have limited the generalizability; however, the characteristics of our sample were representative of the United States LTRs samples.42
Implications for clinical care and future research
Based on our discussion above and evidence in the literature, several factors may explain low adherence rate to self-monitoring in LTRs, including 1) inherent burden in self-monitoring with regards to performing the measurement, recording on paper logs, tracking, and interpreting trends over time, 2) failure to see benefits of self-monitoring due to lack of clinicians’ check-in and reinforcement during follow-up clinical visits; and 3) poor physical health or psychological distress led to intentionally avoid self-monitoring. To address these barriers, LTRs and clinicians need to work together. For example, clinicians may start to educate and empower patients and family members with the knowledge and skills needed to perform self-monitoring earlier during the hospitalization after the transplant, instead of shortly before discharge. Furthermore, it is equally important for clinicians to regularly review patients’ self-monitoring logs and identify potential barriers to their completion during follow-up visits with LTRs, which may help further reinforce the importance and benefits of self-monitoring in LTRs. Nonetheless, the process of self-monitoring at home should be made user-friendly. Paper logs are the standard of usual care in many transplant programs, and they can be time-consuming and difficult to interpret for LTRs. Researchers have shown that mHealth-based intervention is effective in promoting adherence to self-monitoring in LTRs.18 Future studies need to explore how to broadly implement mHealth tools, such as blue-tooth enabled home spirometers with decisional-support aids showing the trends and alerting LTRs to contact transplant team in case of abnormal values. These types of strategies may reduce burden and empower patients and families in post-transplant care.43
CONCLUSIONS
Promoting adherence to self-monitoring may improve early detection and treatment of complications and thus optimize health outcomes for LTRs. Our findings suggested two distinct patterns of adherence to self-monitoring and pointed to several modifiable targets for interventions to promote adherence to self-monitoring among LTRs, such as reducing post-transplant anxiety, and strengthening the sense of personal control over health. Findings also suggest clinicians should target LTRs who are female or have poorer physical health as high-risk populations for poor adherence to self-monitoring.
Acknowledgments
This study was supported in part by Sigma Theta Tau Eta Chapter Research Award (PI: Hu) and Margaret E. Wilkes Scholarship from University of Pittsburgh School of Nursing (PI: Hu). The study was a secondary analysis of data collected during the parent project funded by NIH (NINR R01NR010711; PI: DeVito Dabbs). Portions of this research were presented at the 37th Annual Meeting of the Society of Behavioral Medicine (SBM) in April 2016, Washington DC.
Footnotes
Author Contributions: LH: conceptualization and design, analyzed the data, interpreted the results, wrote manuscript and reviewed/edited manuscript. ADD, MAD, JHL: conceptualization and design, interpreted the results, and reviewed/edited manuscript; SMS: provided consultation on data analysis and interpretations, reviewed/edited manuscript. We have complied with the author guidelines.
Conflict of Interest: None
Contributor Information
Lu Hu, New York University School of Medicine, Department of Population Health, Center for Healthful Behavior Change.
Annette DeVito Dabbs, University of Pittsburgh School of Nursing, Department of Acute and Tertiary Care.
Mary Amanda Dew, University of Pittsburgh School of Medicine, Department of Psychiatry.
Susan M. Sereika, University of Pittsburgh School of Nursing, Department of Health and Community Systems.
Jennifer H. Lingler, University of Pittsburgh School of Nursing, Department of Health and Community Systems.
References
- 1.Yusen RD, Edwards LB, Dipchand AI, et al. The registry of the International Society for Heart and Lung Transplantation: Thirty-third adult lung and heart-lung transplant report-2016; focus theme: Primary diagnostic indications for transplant. J Hear Lung Transplant. 2016;35(10):1170–1184. doi: 10.1016/j.healun.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 2.DeVito Dabbs A, Hoffman LA, Iacono AT, et al. Pattern and predictors of early rejection after lung transplantation. Am J Crit Care. 2003;12(6):497–505. [PubMed] [Google Scholar]
- 3.Burguete SR, Maselli DJ, Fernandez JF, Levine SM. Lung transplant infection. Respirology. 2013;18(1):22–38. doi: 10.1111/j.1440-1843.2012.02196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alrawashdeh M, Zomak R, Dew MA, et al. Pattern and predictors of hospital readmission during the first year after lung transplantation. Am J Transplant. 2016 doi: 10.1111/ajt.14064.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finkelstein SM, Lindgren B, Prasad B, et al. Reliability and validity of spirometry measurements in a paperless home monitoring diary program for lung transplantation. [Accessed January 5, 2015];Heart Lung. 1993 22(6):523–533. http://www.ncbi.nlm.nih.gov/pubmed/8288456. [PubMed] [Google Scholar]
- 6.Kugler C, Fuehner T, Dierich M, et al. Effect of adherence to home spirometry on bronchiolitis obliterans and graft survival after lung transplantation. Transplantation. 2009;88(1):129–134. doi: 10.1097/TP.0b013e3181aad129. [DOI] [PubMed] [Google Scholar]
- 7.Chhajed PN, Tamm M, Malouf MA, Glanville AR. Lung Transplantation: Management and Complications. Indian J Chest Dis Allied Sci. 2002;44:31–43. [PubMed] [Google Scholar]
- 8.Dew MA, DiMartini AF, DeVito Dabbs A, et al. Adherence to the medical regimen during the first two years after lung transplantation. Transplantation. 2008;85(2):193–202. doi: 10.1097/TP.0b013e318160135f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeVito Dabbs A, Song M-K, Myers B, et al. Clinical trials of health information technology interventions intended for patient use: unique issues and considerations. Clin Trials. 2013;10(6):896–906. doi: 10.1177/1740774513493149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adam TJ, Finkelstein SM, Parente ST, Hertz MI. Cost analysis of home monitoring in lung transplant recipients. Int J Technol Assess Health Care. 2007;23(2):216–222. doi: 10.1017/S0266462307070080. [DOI] [PubMed] [Google Scholar]
- 11.Kugler C, Gottlieb J, Dierich M, et al. Significance of patient self-monitoring for long-term outcomes after lung transplantation. Clin Transplant. 2010;24(5):709–716. doi: 10.1111/j.1399-0012.2009.01197.x. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization. Adherence to Long-Term Therapies: Evidence for Action. Switerland: 2003. [Google Scholar]
- 13.Lindgren BR, Snyder M, Sabati N, Adam T, Finkelstein M. Health locus of control and adherence with home spirometry use in lung transplant recipients. Prog Transplant. 2002;12(1):24–29. doi: 10.1177/152692480201200105. [DOI] [PubMed] [Google Scholar]
- 14.Teichman BJ, Burker EJ, Weiner M, Egan TM. Factors associated with adherence to treatment regimens after lung transplantation. [Accessed June 24, 2013];Prog Transplant. 2000 10(2):113–121. doi: 10.1177/152692480001000208. http://www.ncbi.nlm.nih.gov/pubmed/10933765. [DOI] [PubMed] [Google Scholar]
- 15.Hu L, Lingler JH, Sereika SM, et al. Nonadherence to the medical regimen after lung transplantation: A systematic review. Hear Lung J Acute Crit Care. 2017 doi: 10.1016/j.hrtlng.2017.01.006.. [DOI] [PubMed] [Google Scholar]
- 16.Jones BL, Nagin DS. Advances in Group-Based Trajectory Modeling and an SAS Procedure for Estimating Them. Sociol Methods Res. 2007;35(4):542–571. doi: 10.1177/0049124106292364. [DOI] [Google Scholar]
- 17.Jones BL, Nagin DS, Roeder K. A SAS Procedure Based on Mixture Models for Estimating Developmental Trajectories. Sociol Methods Res. 2001;29(3):374–393. doi: 10.1177/0049124101029003005. [DOI] [Google Scholar]
- 18.DeVito Dabbs A, Song MK, Myers BA, et al. A randomized controlled trial of a mobile health intervention to promote self-management after lung transplantation. Am J Transplant. 2016;16(7):2172–2180. doi: 10.1111/ajt.13701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spanier GB. Measuring Dyadic Adjustment: New Scales for Assessing the Quality of Marriage and Similar Dyads. J Marriage Fam. 1976;38(1):15–28. [Google Scholar]
- 20.Hanson B, Bickel L. Development and testing on perception of self-care agency. In: Riehl-Sisca J, editor. The Science and Art of Self-Care. Norwalk, Connecticut: Appleton-Century-Crofts; 1985. pp. 271–278. [Google Scholar]
- 21.Wallston BS, Wallston KA. Locus of control and health: a review of the literature. [Accessed October 3, 2013];Health Educ Monogr. 1978 6(2):107–117. doi: 10.1177/109019817800600102. http://www.ncbi.nlm.nih.gov/pubmed/357347. [DOI] [PubMed] [Google Scholar]
- 22.Wallston KA, Wallston BS, DeVellis R. Development of the Multidimensional Health Locus of Control (MHLC) Scales. Heal Educ Behav. 1978;6(1):160–170. doi: 10.1177/109019817800600107. [DOI] [PubMed] [Google Scholar]
- 23.Derogatis LR, Unger R. SYMPTOM CHECKLIST-90-REVISED. Corsini Encycl Psychol. 2000;2009:18–19. [Google Scholar]
- 24.Rodin G, Voshart K. Depression in the medically ill: an overview. [Accessed October 3, 2013];Am J Psychiatry. 1986 143(6):696–705. doi: 10.1176/ajp.143.6.696. http://www.ncbi.nlm.nih.gov/pubmed/3521339. [DOI] [PubMed] [Google Scholar]
- 25.Ware J, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. [Accessed July 17, 2014];Med Care. 1992 30(6):473–483. http://www.ncbi.nlm.nih.gov/pubmed/1593914. [PubMed] [Google Scholar]
- 26.Ware J, Gandek B. Overview of the SF-36 Health Survey and the International Quality of Life Assessment (IQOLA) Project. J Clin Epidemiol. 1998;51(11):903–912. doi: 10.1016/S0895-4356(98)00081-X. [DOI] [PubMed] [Google Scholar]
- 27.Nagin DS. Group-Based Modeling of Development. Cambridge, MA: Harvard University Press; 2005. [Google Scholar]
- 28.Babyak Ma. What you see may not be what you get: a brief, nontechnical introduction to overfitting in regression-type models. Psychosom Med. 2004;66(3):411–421. doi: 10.1097/01.psy.0000127692.23278.a9.. [DOI] [PubMed] [Google Scholar]
- 29.Steyerberg EW, Eijkemans MJ, Harrell FE, Habbema JD. Prognostic modeling with logistic regression analysis: in search of a sensible strategy in small data sets. Med Decis Mak. 2001;21:45–56. doi: 10.1177/0272989X0102100106. [DOI] [PubMed] [Google Scholar]
- 30.Massey EK, Tielen M, Laging M, et al. The role of goal cognitions, illness perceptions and treatment beliefs in self-reported adherence after kidney transplantation: a cohort study. J Psychosom Res. 2013;75(3):229–234. doi: 10.1016/j.jpsychores.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 31.Moreso F, Torres IB, Costa G, Seron D. Nonadherence to immunosuppression: challenges and solutions. Transpl Res Risk Manag. 2015;7:27. doi: 10.2147/TRRM.S50796.. [DOI] [Google Scholar]
- 32.Jiang Y, Sereika SM, De Vito Dabbs A, Handler SM, Schlenk EA. Acceptance and Use of Mobile Technology for Health Self-Monitoring in Lung Transplant Recipients during the First Year Post-Transplantation. Appl Clin Inform. 2016;7(2):430–445. doi: 10.4338/ACI-2015-12-RA-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DeVito Dabbs A, Kim Y, Hamdan-Mansour A, Thibodeau A, McCurry K. Health locus of control after lung transplantation: Implications for managing health. J Clin Psychol Med Settings. 2006;13(4):381–392. doi: 10.1007/s10880-006-9038-3. [DOI] [Google Scholar]
- 34.Sabati N, Snyder M, Edin-Stibbe C, Lindgren B, Finkelstein S. Facilitators and barriers to adherence with home monitoring using electronic spirometry. [Accessed June 24, 2013];AACN Clin Issues. 2001 12(2):178–185. doi: 10.1097/00044067-200105000-00002. http://www.ncbi.nlm.nih.gov/pubmed/11759546. [DOI] [PubMed] [Google Scholar]
- 35.Dabbs ADV, Hoffman LA, Swigart V, et al. Striving for normalcy: Symptoms and the threat of rejection after lung transplantation. Soc Sci Med. 2004;59(7):1473–1484. doi: 10.1016/j.socscimed.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 36.DeVito Dabbs A, Terhorst L, Song M-K, et al. Quality of recipient-caregiver relationship and psychological distress are correlates of self-care agency after lung transplantation. Clin Transplant. 2013;27(1):113–120. doi: 10.1111/ctr.12017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dew MA, Rosenberger EM, Myaskovsky L, et al. Depression and Anxiety as Risk Factors for Morbidity and Mortality after Organ Transplantation: A Systematic Review and Meta-Analysis. Transplantation. 2015 doi: 10.1097/TP.0000000000000901.. Epub ahead:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosenberger EM, Dew MA, DiMartini AF, DeVito Dabbs AJ, Yusen RD. Psychosocial issues facing lung transplant candidates, recipients and family caregivers. Thorac Surg Clin. 2012;22(4):517–529. doi: 10.1016/j.thorsurg.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.DiMartini AF, Crone C, Fireman M, Dew MA. Psychiatric Aspects of Organ Transplantation in Clinical Care. Crit Care Clin. 2008;24(4):1–30. doi: 10.1016/j.ccc.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Manteuffel M, Williams S, Chen W, Verbrugge RR, Pittman DG, Steinkellner A. Influence of patient sex and gender on medication use, adherence, and prescribing alignment with guidelines. J Womens Health (Larchmt) 2014;23(2):112–119. doi: 10.1089/jwh.2012.3972. [DOI] [PubMed] [Google Scholar]
- 41.Tabachnick B, Fidell L. Using Multivariate Statistics. 5. Needham Heights, MA: Pearson/Allyn & Bacon; 2007. [Google Scholar]
- 42.Organ Procurement and Transplantation Network. [Accessed January 13, 2015];View Data Reports - OPTN. http://optn.transplant.hrsa.gov/converge/latestData/viewDataReports.asp. Published 2012.
- 43.Fleming JN, Taber DJ, McElligott J, McGillicuddy JW, Treiber F. Mobile health in solid organ transplant: The time is now. Am J Transplant. 2017:1–14. doi: 10.1111/ajt.14225.. [DOI] [PubMed] [Google Scholar]