Abstract
Spontaneous rupture of the Achilles tendon is increasingly common in the middle aged population. However, the cause for the particularly high incidence of injury in this age group is not well understood. Therefore, the objective of this study was to identify age-specific differences in the Achilles tendon-muscle complex using an animal model. Functional measures were performed in vivo and tissues were harvested following euthanasia for mechanical, structural, and histological analysis from young, middle aged, and old rats. Numerous alterations in tendon properties were detected across age groups, including inferior material properties (maximum stress, modulus) with increasing age. Differences in function were also observed, as older animals exhibited increased ankle joint passive stiffness and decreased propulsion force during locomotion. Macroscale differences in tendon organization were not observed, although cell density and nuclear shape did vary between age groups. Muscle fiber size and type distribution were not notably affected by age, indicating that other factors may be more responsible for age-specific Achilles tendon rupture rates. This study improves our understanding of the role of aging in Achilles tendon biomechanics and ankle function, and helps provide a potential explanation for the disparate incidence of Achilles tendon ruptures in varying age groups.
Keywords: Biomechanics, Injury, Fatigue, Skeletal Muscle, Elasticity, Orthopaedics, Age
INTRODUCTION
Achilles tendon injuries are most common in middle-aged men, especially those involved in recreational sports. Various studies have each reported a consistent increased incidence rate since the 1950s that is greater than the rate of population increase alone (Lantto et al., 2015; Leppilahti et al., 1996; Suchak et al., 2005). Furthermore, there is evidence that the median age and overall incidence in older individuals specifically of Achilles tendon ruptures have both increased over the past decade (Huttunen et al., 2014). The mechanisms underlying this age-specific disparity are not well understood, but have been suggested to include increased participation in demanding sports by older individuals combined with tissue degeneration associated with aging (Ng et al., 2011; Peffers et al., 2015).
Specifically, age-dependent changes in Achilles tendon physiology and mechanics, such as decreased blood flow and increased tissue stiffness, have been implicated as potential causes for the high incidence of ruptures in this age group. However, although peritendinous blood flow is decreased while at rest in older individuals, blood flow in Achilles tendons during exercise is similar throughout ages, suggesting that vascular factors are not likely solely responsible for the increased incidence with age of Achilles tendon injuries (Langberg et al., 2001). Importantly, in vivo human studies evaluating biomechanical changes in aging Achilles tendon have yielded contradictory results as to whether Achilles tendon stiffness and strain in older individuals is decreased, increased, or similar to younger individuals(Karamanidis and Arampatzis, 2006; Kubo et al., 2007; Onambele et al., 2006). Additionally, there has been suggestion that regular physical activity as athletes age can help to avoid degeneration by increasing tendon size, strength, and nutrient delivery (Smith et al., 2002). Conversely, a recent study found no differences in Achilles tendon cross-sectional area, collagen content, or mechanical properties between adult long distance runners who were physically active or inactive in young age, suggesting that physical activity during youth did not improve Achilles tendon properties (Lenskjold et al., 2015). In summary, previous clinical studies have differing results which do not establish a clear relationship between age and Achilles tendon ruptures, likely due to an incomplete fundamental understanding of how aging affects Achilles tendon physiology and biomechanics. Given the practical limitations of human studies, there exists a clear need for a rigorous animal study that leverages the benefits of using a non-human model to better and more comprehensively define the role of aging in Achilles tendon structure and function.
Animal models offer a highly controlled system to study Achilles tendon biomechanics, and have demonstrated a potential explanation for the contrasting incidence in Achilles tendon rupture across sex (Pardes et al., 2016). However, it is unknown if aging also alters Achilles tendon mechanical behavior that could help explain the particularly high frequency of Achilles tendon ruptures in middle-aged men. There is preliminary support for this hypothesis, as changes in the viscoelastic response of the gastrocnemius-Achilles (GC-AT) muscle-tendon unit have been identified with increasing age (Plate et al., 2013). However, more comprehensive investigation is needed in order to better define the impact of aging on Achilles tendon-muscle properties and ankle function in vivo and ex vivo, ultimately helping to elucidate the mechanism underlying the age-specific incidence of ruptures observed clinically.
Therefore, the objective of this study was to identify functional, mechanical, and structural differences among Achilles tendon-muscle units from young, middle aged, and old male rats. We hypothesized that middle aged and old rats would exhibit increased joint stiffness and decreased Achilles tendon material quality compared to young rats, as well as decreased matrix organization.
METHODS
Design
All procedures were approved by the University of Pennsylvania’s Animal Care and Use Committee. Young (8 mo), Middle Aged (19 mo), and Old (28 mo) male F344XBN rats, approximating respective human ages of 18, 43, and 63 years (Quinn, 2005), were acquired from the National Institute of Aging (n=16/group) and euthanized following in vivo functional testing. This animal model shows musculoskeletal changes similar to those observed in human aging and has been previously used specifically for the study of the rotator cuff and Achilles tendon-muscle units aging response (Mannava et al., 2011; Plate et al., 2013).
Gait analysis
Animals (n=12–16/group) were acclimated to an instrumented walkway, and spatial, temporal, and kinetic parameters were quantified during autonomous locomotion as previously described (Fryhofer et al., 2016). Briefly, maximum ground reaction forces (medial/lateral, braking, propulsion, vertical) were calculated following isolation of the hindlimb on either one of two six degree-of-freedom force plates and reported as percent of animal body weight (%BW). Spatiotemporal parameters (stride length, stride width, speed, and stance time) were determined by semi-automated analysis of images captured by digital camera during locomotion using MATLAB (Mathworks; Natick, MA).
Passive joint function
Passive ankle range of motion (ROM) and stiffness were measured using a custom device while animals (n=16/group) were anesthetized as previously described (Fryhofer et al., 2016). Bilinear fits were applied to torque-angle data (within a consistent torque range across all animals) in order to calculate toe and linear stiffness for both dorsiflexion and plantarflexion. Range of motion was measured relative to the geometric zero position (ankle and tibia oriented perpendicularly).
Tendon sample preparation
Animals were euthanized (mass [mean±SD]: Young 393±25g, Middle Aged 521±27g, Old 504±44g) and Achilles tendon-foot units were harvested and either processed for histological assays or frozen until preparation for structural and mechanical analysis. Prior to all ex vivo testing and analyses, specimens were randomized and blinded to the study investigators. High frequency ultrasound/mechanical testing specimens (same specimens were used for both assays) were fine dissected to remove non-tendinous soft tissue and measured for tendon midsubstance cross sectional area (CSA) using a custom laser-based device (Freedman et al., 2016). Stain dots were applied for optical strain tracking, the calcaneus-foot complex was embedded in PMMA, and the proximal end of the tendon was attached between sandpaper using cyanoacrylate to leave a gauge length of 12 mm (Freedman et al., 2016).
High frequency ultrasound (HFUS)
Prior to mechanical testing, sagittal B-mode images of tendons loaded at 1N in a 1X PBS bath(n=11–12/group) were captured at 0.25mm increments using a 40MHz scanner (MS550D; VisualSonics, CA) as previously described (Riggin et al., 2014). A custom MATLAB program was used to analyze the 3–4 central-most images and determine tendon matrix alignment (reported as circular standard deviation) and density (reported as echogenicity).
Mechanical testing
Samples (n=11–12/group/protocol) were tested to evaluate quasistatic properties (ramp to failure with optical strain tracking) or viscoelastic, dynamic, and fatigue properties (stress relaxation, low-strain frequency sweep, load-controlled fatigue testing) using one of two previously described protocols (Freedman et al., 2016; Pardes et al., 2016). Briefly, the Achilles tendon was maintained perpendicular to the foot to mimic in vivo loading and submerged in a 1X PBS bath at 37°C. The Electropuls E3000 testing frame (Instron; Norwood, MA) was used with a 250 N load cell, while images were captured by a digital camera (Basler; Exton, PA) and 200 mm lens (Nikon; Melville, NY). Fatigue samples were tested until failure or completion of 20,000 cycles. Mechanical properties were evaluated at 50 cycles (early response) and 1500 cycles (latest possible cycle number to include data from >90% of all specimens). Load-displacement data for all tests were acquired by WaveMatrix (Instron; Norwood, MA) and imported into MATLAB for calculation of mechanical properties (Freedman et al., 2016).
Tendon histology
Achilles tendons (n=4–8/group) were fixed, decalcified, and embedded in paraffin using standard techniques and sectioned sagitally at 7μm (Fryhofer et al., 2016). Sections were then stained with Hematoxylin-Eosin (H&E) or Safranin-O/Fast Green and imaged at 100X. Images were evaluated for cell density and nuclear shape factor (0= circle; 1=line) using a commercial software (Bioquant Osteo II; Nashville, TN) (Rooney et al., 2016). Safranin-O positive staining was determined using a previously described thresholding method in ImageJ (Fryhofer et al., 2016).
Muscle histology
Gastrocnemius-soleus muscle mid-belly (n=7/group) was excised at the time of sacrifice, flash frozen, and stored at −80°C until axial cryosectioning at 10μm, as described previously (Pardes et al., 2016). Immunofluorescence imaging was performed on sections stained for laminin and myosin heavy chain (MyHC) types 1, 2a, and 2b. The anti-MyHC antibodies developed by Stefano Schiaffino were obtained from the Developmental Studies Hybridoma Bank, created by the NICHD of the NIH and maintained at The University of Iowa, Department of Biology, Iowa City, IA 52242. MyHC2x expression was presumed from unstained fibers. After staining, three images were taken from both the superficial and deep regions of each muscle. The SMASH application in MATLAB was used to calculate muscle fiber size (minimum Feret diameter) and type distribution for each region (Smith and Barton, 2014).
Statistics
One-way ANOVAs were used to compare groups for functional, structural, and mechanical Achilles tendon-muscle properties, and significant relationships (p<0.05) were further evaluated using post hoc Student’s t-tests with Bonferroni corrections (α = 0.05/3), except for cycles completed and tendon histological properties. For cycles completed, ranks were assigned (1, 0–5000 cycles; 2, 5000–10000 cycles, 3, 10000–15000 cycles; 4, 15000–20000 cycles) and the non-parametric Kruskal-Wallis test with Dunn’s post hoc tests was used. Non-parametric Kruskal–Wallis tests were also used to evaluate differences in tendon histological properties between groups (α = 0.05). All data are presented as mean ± standard deviation except for fatigue cycles completed and tendon histology, which are reported as median (interquartile range).
RESULTS
Gait analysis revealed that lateral force increased and propulsion force decreased with increasing age (Fig. 1A–B), while no significant differences in braking or vertical forces were detected (Fig. 1C–D). Animals also took slower, shorter, and wider steps as they aged (Fig. 1E–G).
Figure 1. Kinetic and spatiotemporal gait analysis.
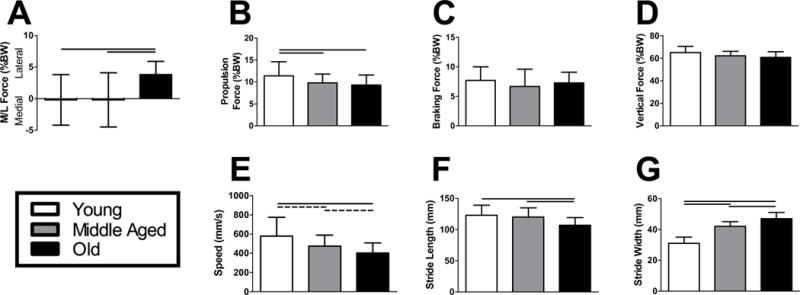
Old animals had increased (A) lateral force and decreased (B) propulsion force during locomotion. Peak (C) braking and (D) vertical forces did not exhibit an age-dependent response. As animals increased in age, their (E) walking speed and (F) stride length declined, while their (G) stride width increased. n = 12–16/group. Data represented as means and error bars indicating standard deviation. Solid lines indicate significant differences (p < 0.05/3) and dashed lines indicate trends (p < 0.10).
Evaluation of passive ankle joint function demonstrated no differences in plantarflexion range of motion (ROM) or toe stiffness across age groups, but plantarflexion linear stiffness was increased in Middle Aged animals compared to the other groups (Fig. 2A–B). Aging resulted in decreased dorsiflexion ROM and increased linear, but not toe, stiffness (Fig. 2C–D).
Figure 2. Passive ankle joint kinematics.
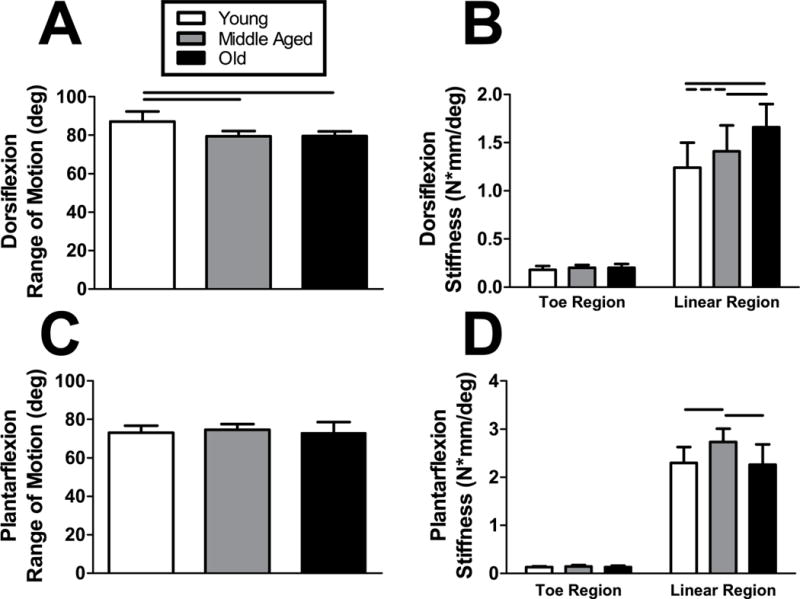
(A) Dorsiflexion range of motion was inferior in both Middle Aged and Old animals compared to the Young group. Conversely, the linear region of (B) plantarflexion stiffness increased as animals aged. (C) There were no differences in plantarflexion range of motion across all groups, despite an increase in the linear region of (D) plantarflexion stiffness in Middle Aged animals. No differences in dorsiflexion or plantarflexion toe region stiffness were detected. n = 16/group. Data represented as means and error bars indicating standard deviation. Solid lines indicate significant differences (p < 0.05/3) and dashed lines indicate trends (p < 0.10).
HFUS analysis revealed tendon matrix organization and density did not differ with increasing age (Fig. 3A–B). Tendon cross-sectional area, however, increased 13% and 10% from Young to Middle Aged and Old groups, respectively (Fig. 3C).
Figure 3. Achilles tendon structure.
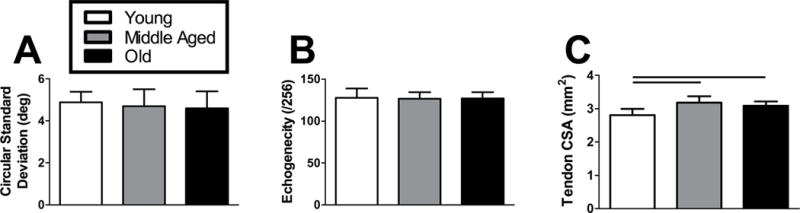
High frequency ultrasound (HFUS) assessment of tendon structure revealed no differences in (A) circular standard deviation (matrix organization) or (B) echogenicity (matrix density). A laser-based device was used to measure (C) tendon cross-sectional area, which was greater in Middle Aged and Old animals compared to the Young group. n = 11–12/group. Data represented as means and error bars indicating standard deviation. Solid lines indicate significant differences (p < 0.05/3).
While maximum force was not different between groups, maximum stress was superior in Young animals compared to the Middle Aged and Old groups (Fig. 4A–B). Stiffness was not significantly different between groups, but tendons from the Young animals demonstrated an average modulus 35% and 26% greater than tendons from the Middle Aged and Old animals, respectively (Fig. 4C–D). Age was not a significant factor on the maximum strain and toughness (Fig. 4E–F).
Figure 4. Achilles tendon quasi-static mechanical properties.
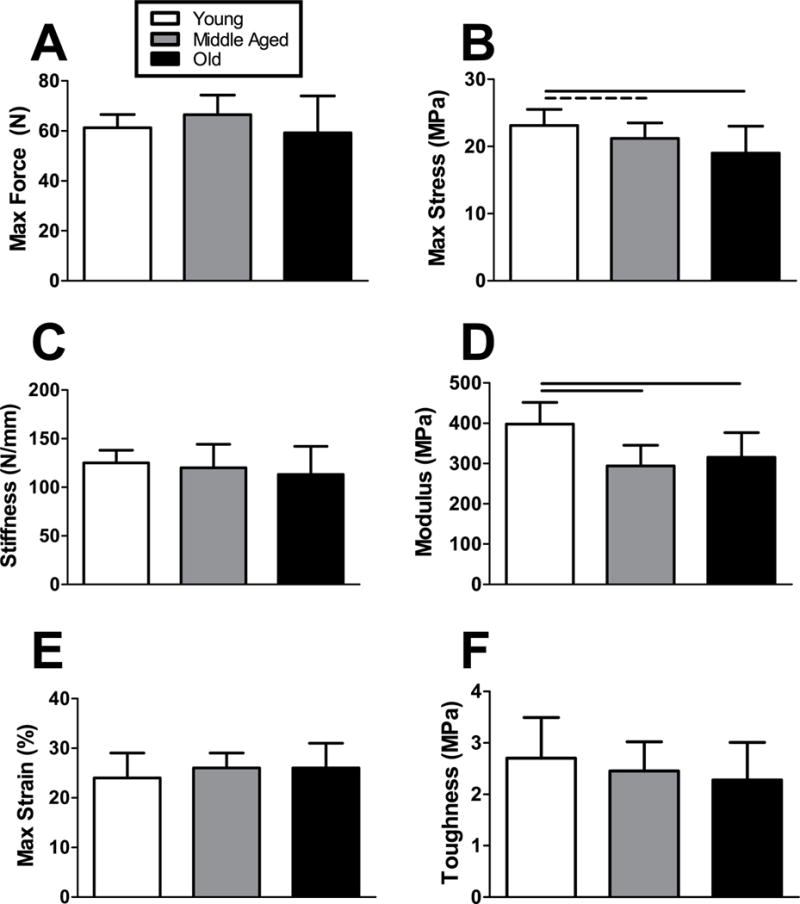
(A) Max force did not differ across age groups, but (B) max stress was superior in the Young animals during a ramp-to-failure protocol. Similarly, (B) stiffness did not vary consistently with age but (C) modulus was greatest in the Young group. (E) Max strain and (F) toughness were not significantly different across age groups, but were strongly correlated (R=0.80, p<0.0001). n = 11–12/group. Data represented as means and error bars indicating standard deviation. Solid lines indicate significant differences (p < 0.05/3) and dashed lines indicate trends (p < 0.10).
No differences in the tendon viscoelastic properties percent relaxation or tan(6) were detected between groups (Fig. 5A–B). Conversely, the dynamic modulus, |E*|, was 37% greater in the Young group compared to tendons from Middle aged and Old animals at all frequencies tested (Fig. 5C).
Figure 5. Achilles tendon viscoelastic and dynamic mechanical properties.
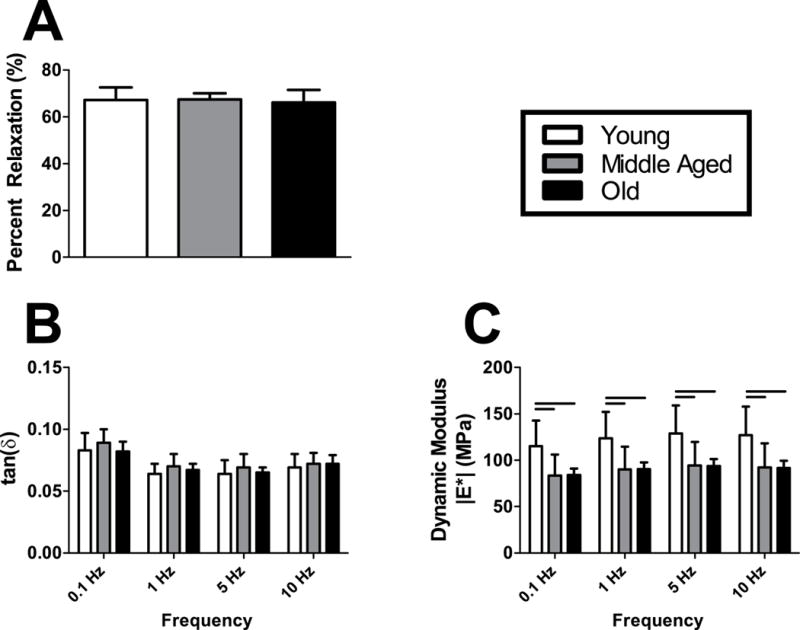
Stress relaxation testing revealed no differences in (A) percent relaxation across age groups. A low-strain frequency sweep detected no changes in (B) tan(6) in response to aging, but (C) dynamic modulus was superior in the Young group at all frequencies tested. n = 11–12/group. Data represented as means and error bars indicating standard deviation. Solid lines indicate significant differences (p < 0.05/3).
Tendons from Middle Aged and Old animals exhibited superior fatigue life compared to Young animals (Fig. 6A). Peak strain, laxity, and secant stiffness were not significantly different between groups after 50 or 1500 cycles of loading (Fig. 6B–D). Secant modulus was increased in Young animals, though this only reached statistical significance at 50 and not 1500 cycles (Fig. 6E). Additionally, there was a trend towards decreased hysteresis in Middle Aged animals after 1500 cycles (Fig. 6F).
Figure 6. Achilles tendon fatigue mechanical properties.
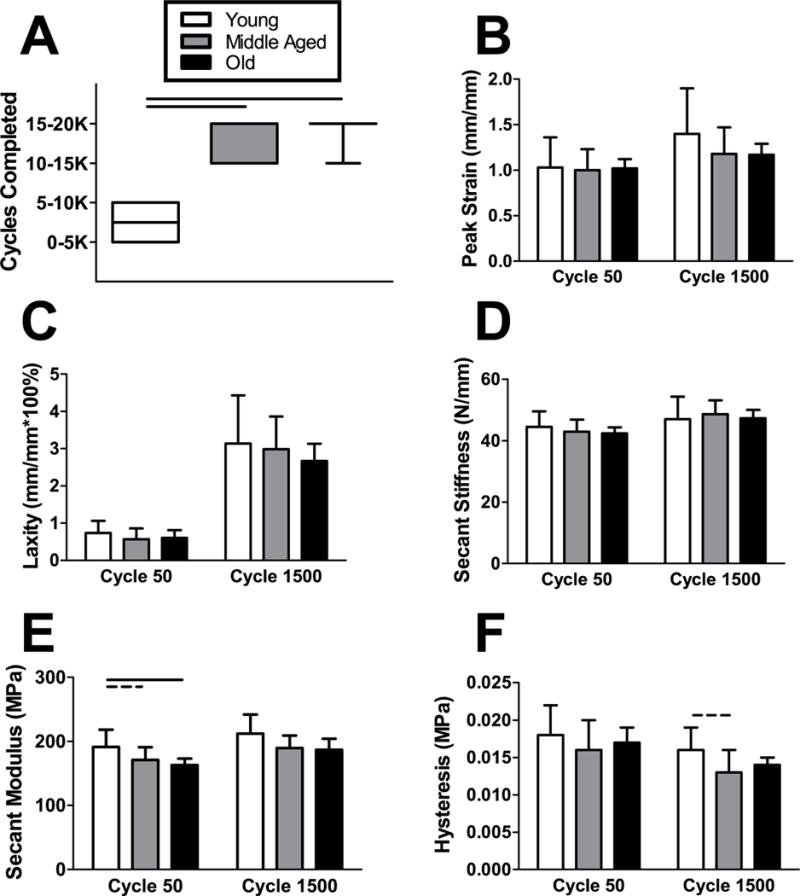
Tendons from Young animals had a shorter (A) fatigue life than the Middle Aged and Old groups. After 50 and 1500 cycles of fatigue loading, there were no differences in (B) peak strain, (C) laxity), or (D) secant stiffness between groups. (E) Secant modulus was increased in Young animals, though this only reached statistical significance at 50 and not 1500 cycles. There was a trend towards decreased (F) hysteresis in Middle Aged animals after 1500 cycles only. n = 11–12/group. Data represented as means and error bars indicating standard deviation, except fatigue cycles completed (five number summary box plots). Solid lines indicate significant differences (p < 0.05/3) and dashed lines indicate trends (p < 0.10).
Histological analysis revealed that Achilles tendon cell density decreased from Young and Middle Aged animals to Old animals (Fig. 7A). Nuclear shape also became more rounded in the Old animals compared to more spindle shaped in the Middle Aged and Young animals (Fig. 7B). Very little proteoglycan content was detected and no differences were observed across age groups (Fig. 7C). Representative images for tendon histology are shown for Young, Middle Aged, and Old for both H&E and Safranin-O/Fast Green (Saf-O) staining (Fig. 7D–I).
Figure 7. Achilles tendon histological properties.
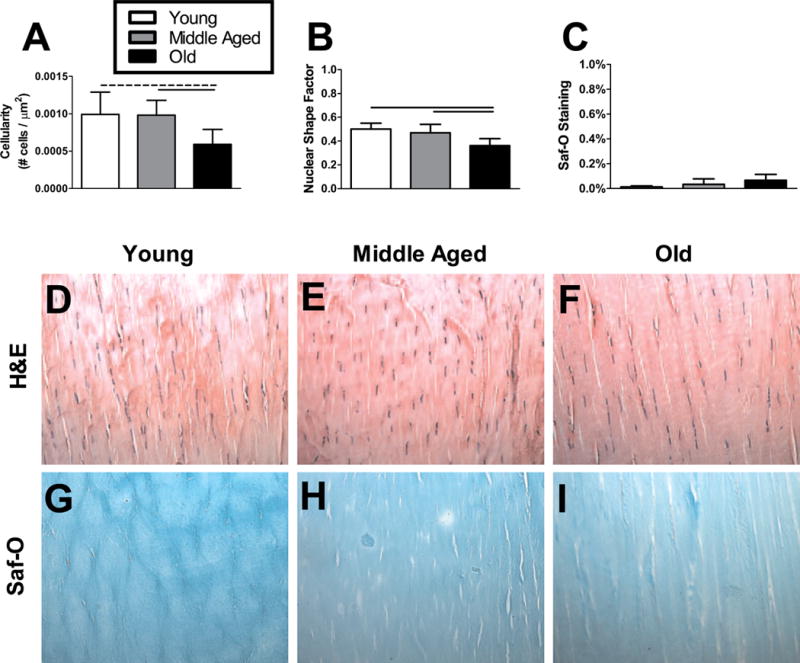
Young and Middle Aged animals had greater (A) cell density in their Achilles tendon midsubstance compared to Old animals. (B) Cell shape was more rounded in the tendons from Old animals than Middle Aged or Young animals. There was no significant change in (C) proteoglycan content across age groups. n = 4–8/group. Data represented as means and error bars indicating standard deviation. Solid lines indicate significant differences (p < 0.05/3) and dashed lines indicate trends (p < 0.10). Representative images are shown for each group (D–I).
Muscle fiber size and type distribution did not vary dramatically with age (Fig. 8A–C). However, there were trends toward decreased superficial fiber size and deep type 2a fiber content in the Old animals compared to Young animals.
Figure 8. Muscle fiber size and type distribution.
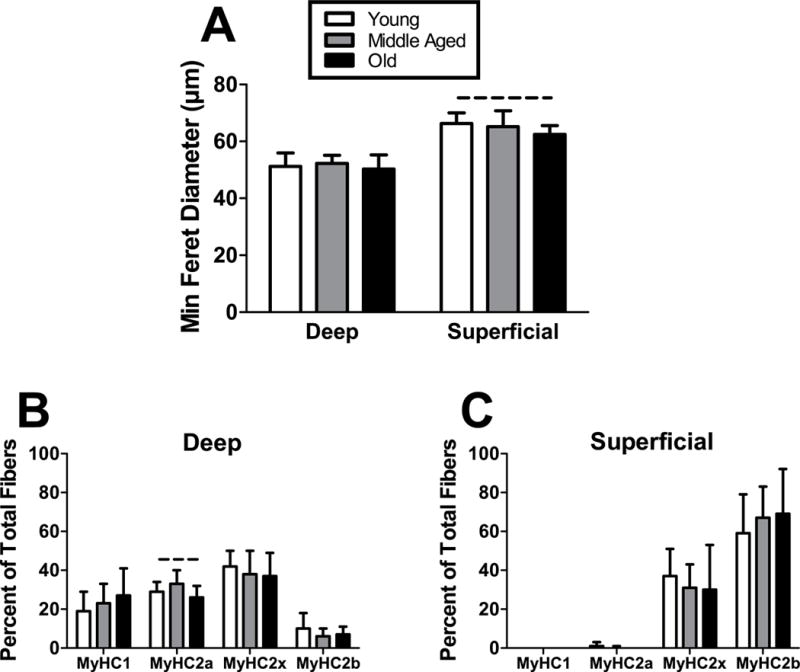
(A) Fiber size was not different across age groups in the deep region, but there was a trend towards inferior fiber size in Old animals in the superficial region. In the deep region, (B) fiber type distribution generally did not vary across age, except for a trend towards decreased type 2a in Old animals. Fiber type distribution in the (C) superficial region was not significantly altered by age. n = 7/group. Data represented as means and error bars indicating standard deviation. Solid lines indicate significant differences (p < 0.05/3) and dashed lines indicate trends (p < 0.10).
DISCUSSION
Aging is the single greatest risk factor for tendon disorders, yet the impact of aging on Achilles tendon-muscle properties remains incomplete (Cury et al., 2016; Stenroth et al., 2012). Previous studies have discovered microstructural changes in the tendon with increasing age, including decreased collagen fibril diameters, decreased crimp angle, and altered collagen type III and elastic content, but their link to macro-scale tendon and ankle mechanics is not intuitive or well understood (Slane et al., 2015; Slane and Thelen, 2015). Furthermore, human studies have reported conflicting results on the effects of aging on Achilles tendon mechanical function, which other investigators suggested may be in part due to low sample size, methodological differences, and spatial definitions of the tendon (Slane et al., 2016; Slane and Thelen, 2015; Stenroth et al., 2012). Therefore, the objective of this study was to leverage the advantages of a highly controlled animal model to comprehensively investigate functional, mechanical, and structural/compositional differences across Achilles tendon-muscle units from young, middle aged, and old male rats.
With regard to ankle joint function, Middle Aged and Old rats demonstrated similar deficits to those observed in humans as a consequence of aging. Specifically, increased passive stiffness and decreased range of motion are hallmark features of aging joints, including the ankle (Menz, 2015). It has been demonstrated that such joint-level changes are directly associated functional ability in human studies (Menz et al., 2005; Spink et al., 2011). This is in agreement with the results of our rodent gait analysis, which revealed that increasing age led to increased lateral force and decreased propulsion force, as well as shorter, slower, and wider strides during locomotion. Furthermore, our findings are highly consistent with human walking and running gait analysis studies (Devita et al., 2016; Franz, 2016; Ko et al., 2012). Given that greater calf muscle activation (both magnitude and variability) and metabolic cost of walking have been observed in older individuals or in cases of abnormal tendon mechanics, it is possible that these adaptations may represent a compensatory mechanism to overcome passive plantarflexion stiffness and achieve sufficient ankle range of motion during locomotion, at least in middle aged adults (Mian et al., 2007; Mian et al., 2006; Schmitz et al., 2009; Suydam et al., 2015). Further increases in muscle activation during demanding push-off movements, such as jumping, could then lead to overstraining and subsequent injury of an already mechanically inferior tendon, as evidenced by our ex vivo mechanical analysis (Slane and Thelen, 2015). However, this potential injury mechanism requires future investigation in a more clinically relevant age group (40–50 years old rather than 65+ years old) and during challenging physical activities typically associated with spontaneous Achilles tendon rupture.
Mechanical testing revealed superior tendon material quality in the Young group, consistent with our initial hypothesis. Our results agree with previous animal and clinical studies which reported an increase in tendon compliance with increasing age, which may lead to subsequent injury (Cury et al., 2016; Dudhia et al., 2007; Joseph et al., 2014; Mian et al., 2007; Slane and Thelen, 2015; Svensson et al., 2016). It is important to note that these effects may vary across different tendons, as others have reported age-specific increases in the stiffness and modulus of the tibialis anterior tendon, for example (Slane et al., 2015; Wood et al., 2011; Zaseck et al., 2016). We did not identify any age-related differences in tendon viscoelastic properties, although such differences appear to be present in the entire Achilles tendon-muscle unit in vivo (Plate et al., 2013). Interestingly, we also observed relatively few differences in Achilles tendon fatigue properties, indicating that the tendon may perform similarly during lower intensity activities involving cyclical loading, regardless of age. However, as Achilles tendon ruptures generally occur spontaneously due to a single rapid, high intensity loading event, this suggests that quasi-static failure properties may better explain the mechanical etiology of acute injury, whereas fatigue resilience is more indicative of repetitive performance of daily functions like walking (Freedman et al., 2016; Maffulli et al., 2000). This concept is supported by the inferior max stress and modulus detected in the older groups of the current study, which likely have a role in the mechanism underlying the disparate incidence of ruptures in the middle aged population reported previously.
Contrary to our initial hypothesis, we did not observe consistent alterations in tendon matrix organization or composition with increasing age, though some differences were observed. Previous studies have reported changes in mRNA levels and activity of proteins involved extracellular matrix organization and turnover that could compromise the structural integrity of aged tendon (Thorpe et al., 2016; Yu et al., 2013; Zaseck et al., 2016). However, results from our HFUS analysis suggest that these distinct molecular profiles do not necessarily translate into macroscale age-specific differences in tendon matrix alignment or density. Cell density was inferior in the Old group, in agreement with previous work which noted decreased cellularity in Achilles tendons from elderly rats (Cury et al., 2016). Tendon cell shape has been reported to change from round to spindle shaped during tissue maturation, yet our results suggest that this phenomena may be relevant to the aging process as well (Svensson et al., 2016). The lack of differences in GAG staining between age groups was expected, given that proteoglycan content is a minor component of uninjured Achilles tendons and has only been shown to decrease with age in some, but not all, tendons and ligaments (Kostrominova and Brooks, 2013; Pardes et al., 2016; Svensson et al., 2016; Thornton et al., 2015). This is also consistent with our mechanical testing results, specifically that Achilles tendon viscoelasticity did not vary with age. The increase in Achilles tendon cross-sectional area (CSA) with age is consistent with previous studies, which some have suggested may be due to increased water content or collagen-III content (Cury et al., 2016; Stenroth et al., 2012; Svensson et al., 2016). Yet other investigators have reported no difference or even decreased water content (perhaps corresponding to changes in GAG content) in tendons and ligaments with increasing age, indicating that collagen-III may be the predominant factor responsible for increased CSA (Svensson et al., 2016; Thornton et al., 2015). Given that collagen-III fibers are thinner and more compliant than collagen-I fibers, an age-related elevation of collagen-III content would also help to explain the decreased resistance to tensile force of older Achilles tendons observed in our study and should be further investigated in the future (Cury et al., 2016).
Aging is generally considered to result in deterioration of muscle mass, quality, and strength (sarcopenia) (Doherty, 2003; Larsson et al., 1979). While we did observe a trend towards decreased superficial fiber size with increasing age, widespread alterations of muscle composition were not detected. Interestingly, a recent study revealed that the decline in muscle size and strength is not solely responsible for the decreased propulsive power observed during gait in older adults, and that these individuals may actually underutilize their available muscular potential (Franz, 2016). Thus, the complex interplay between tendon material properties, ankle joint stiffness, and muscle sarcopenia is not fully understood, but has potentially significant impacts on mechanical performance and injury risk, warranting additional investigation in animal and human models (Danos et al., 2016; Slane and Thelen, 2015).
There are limitations to this study. First, only male animals were used to study the role of aging in Achilles tendon biomechanics, structure, and ankle function. It has been previously demonstrated by our group and others that sex differences exist in Achilles tendon homeostasis, incidence of injury, and healing response (Fryhofer et al., 2016; Pardes et al., 2016). Given that men are much more likely to rupture their Achilles tendon, however, we chose to isolate the effects of age by performing the current study in a uniform male cohort. Additionally, the mean age for Achilles tendon rupture is similar between males and females (Pardes et al., 2016). Nevertheless, it is still possible that some aspects of the tendon’s response to aging are sex-specific and this should be investigated in future studies. It is also important to note that the impacts of aging on tendon and ligaments appear to be tissue-specific, as described earlier and as evidenced by the distinct age groups most affected by specific injuries (e.g., ACL ruptures in younger adults, Achilles ruptures in middle-aged adults, and rotator cuff tears in the elderly).Thus, the results of the current aging study should be interpreted specifically for Achilles tendon physiology and may not be broadly generalizable as relevant to all tendons and ligaments Next, it is likely that the counterintuitive result of decreased fatigue life in the Young tendons is at least in part due to their decreased cross-sectional area, which resulted in greater peak cyclical stress experienced throughout load-controlled fatigue testing. All tendons were cycled between ~8–40% of their max force in order to ensure consistent loading regardless of age. It would be possible to instead perform fatigue loading based on age-specific max stress, but this could confound direct comparison of fatigue properties. Therefore, since both approaches have their respective benefits and shortcomings, we opted for the method that allowed for the most intuitive comparisons of mechanical properties between age groups. Finally, it should be noted that passive dorsiflexion of the ankle of uninjured rats at all ages resulted in eventual contact between the foot and knee. Thus, it is possible that the true dorsiflexion ROM is greater than reported in this study due to anatomical hindrance. Despite this potential systematic limitation, the torque cutoffs used in the current and previous work allow us to be confident in the accuracy of the dorsiflexion stiffness calculations, given that the threshold used included a sufficient portion of the linear region for robust bilinear fit analysis (Fryhofer et al., 2016).
In conclusion, this study begins to define the role of aging in Achilles tendon biomechanics and ankle function in order to better understand the heightened injury risk in an increasingly active middle aged population. Specifically, we utilized a highly controlled animal model to investigate how Achilles tendon mechanical, structural, and functional properties vary from young to old age through a battery of in vivo and ex vivo assays. Key deficits in tendon material properties with increasing age were identified, which may help explain the increased rupture incidence of the middle aged population, especially when considered in tandem with the functional impairments detected in this study. Macroscale differences in tendon organization and composition were not observed, suggesting that other factors are primarily responsible for age-related deterioration of tendon mechanical strength. Additionally, our results provide a foundation for use of this animal model in future studies on the potential age-specific response of the Achilles tendon-muscle unit to loading or injury.
Acknowledgments
The authors thank R Leiphart, C Hillin, C Riggin, and G Fryhofer for their contributions and the NIH/NIAMS (R01AR064216S2, P30AR050950) and NSF GRFP for funding support.
Grant Support: This study was funded by NIH/NIAMS (R01AR064216S2, P30AR050950) and the NSF GRFP.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Study approved by: University of Pennsylvania IACUC.
Author Contributions: All authors were fully involved in the study and preparation of the manuscript. The manuscript has been read and approved by all of the authors.
Conflicts of Interest
The authors have no conflicts of interest to report.
References
- Cury DP, Dias FJ, Miglino MA, Watanabe IS. Structural and Ultrastructural Characteristics of Bone-Tendon Junction of the Calcaneal Tendon of Adult and Elderly Wistar Rats. PLoS One. 2016;11:e0153568. doi: 10.1371/journal.pone.0153568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danos N, Holt NC, Sawicki GS, Azizi E. Modeling age-related changes in muscle-tendon dynamics during cyclical contractions in the rat gastrocnemius. J Appl Physiol (1985) 2016 doi: 10.1152/japplphysiol.00396.2016. jap 00396 0 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devita P, Fellin RE, Seay JF, Ip E, Stavro N, Messier SP. The Relationships between Age and Running Biomechanics. Med Sci Sports Exerc. 2016;48:98–106. doi: 10.1249/MSS.0000000000000744. [DOI] [PubMed] [Google Scholar]
- Doherty TJ. Invited review: Aging and sarcopenia. J Appl Physiol (1985) 2003;95:1717–1727. doi: 10.1152/japplphysiol.00347.2003. [DOI] [PubMed] [Google Scholar]
- Dudhia J, Scott CM, Draper ER, Heinegard D, Pitsillides AA, Smith RK. Aging enhances a mechanically-induced reduction in tendon strength by an active process involving matrix metalloproteinase activity. Aging Cell. 2007;6:547–556. doi: 10.1111/j.1474-9726.2007.00307.x. [DOI] [PubMed] [Google Scholar]
- Franz JR. The Age-Associated Reduction in Propulsive Power Generation in Walking. Exerc Sport Sci Rev. 2016;44:129–136. doi: 10.1249/JES.0000000000000086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman BR, Gordon JA, Bhatt PB, Pardes AM, Thomas SJ, Sarver JJ, Riggin CN, Tucker JJ, Williams AW, Zanes RC, Hast MW, Farber DC, Silbernagel KG, Soslowsky LJ. Nonsurgical treatment and early return to activity leads to improved Achilles tendon fatigue mechanics and functional outcomes during early healing in an animal model. J Orthop Res. 2016 doi: 10.1002/jor.23253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryhofer GW, Freedman BR, Hillin CD, Salka NS, Pardes AM, Weiss SN, Farber DC, Soslowsky LJ. Post-Injury Biomechanics of Achilles Tendon Vary By Sex and Hormone Status. J Appl Physiol (1985) 2016 doi: 10.1152/japplphysiol.00620.2016. jap 00620 0 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttunen TT, Kannus P, Rolf C, Fellander-Tsai L, Mattila VM. Acute achilles tendon ruptures: incidence of injury and surgery in Sweden between 2001 and 2012. Am J Sports Med. 2014;42:2419–2423. doi: 10.1177/0363546514540599. [DOI] [PubMed] [Google Scholar]
- Joseph MF, Lillie KR, Bergeron DJ, Cota KC, Yoon JS, Kraemer WJ, Denegar CR. Achilles tendon biomechanics in response to acute intense exercise. J Strength Cond Res. 2014;28:1181–1186. doi: 10.1519/JSC.0000000000000361. [DOI] [PubMed] [Google Scholar]
- Karamanidis K, Arampatzis A. Mechanical and morphological properties of human quadriceps femoris and triceps surae muscle-tendon unit in relation to aging and running. J Biomech. 2006;39:406–417. doi: 10.1016/j.jbiomech.2004.12.017. [DOI] [PubMed] [Google Scholar]
- Ko SU, Stenholm S, Metter EJ, Ferrucci L. Age-associated gait patterns and the role of lower extremity strength — results from the Baltimore Longitudinal Study of Aging. Arch Gerontol Geriatr. 2012;55:474–479. doi: 10.1016/j.archger.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostrominova TY, Brooks SV. Age-related changes in structure and extracellular matrix protein expression levels in rat tendons. Age (Dordr) 2013;35:2203–2214. doi: 10.1007/s11357-013-9514-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo K, Morimoto M, Komuro T, Tsunoda N, Kanehisa H, Fukunaga T. Age-related differences in the properties of the plantar flexor muscles and tendons. Med Sci Sports Exerc. 2007;39:541–547. doi: 10.1249/01.mss.0000247006.24965.74. [DOI] [PubMed] [Google Scholar]
- Langberg H, Olesen J, Skovgaard D, Kjaer M. Age related blood flow around the Achilles tendon during exercise in humans. Eur J Appl Physiol. 2001;84:246–248. doi: 10.1007/s004210170013. [DOI] [PubMed] [Google Scholar]
- Lantto I, Heikkinen J, Flinkkila T, Ohtonen P, Leppilahti J. Epidemiology of Achilles tendon ruptures: increasing incidence over a 33-year period. Scand. J Med Sci Sports. 2015;25:e133–138. doi: 10.1111/sms.12253. [DOI] [PubMed] [Google Scholar]
- Larsson L, Grimby G, Karlsson J. Muscle strength and speed of movement in relation to age and muscle morphology. J Appl Physiol Respir Environ Exerc Physiol. 1979;46:451–456. doi: 10.1152/jappl.1979.46.3.451. [DOI] [PubMed] [Google Scholar]
- Lenskjold A, Kongsgaard M, Larsen JO, Nielsen RH, Kovanen V, Aagaard P, Kjaer M, Magnusson SP. The influence of physical activity during youth on structural and functional properties of the Achilles tendon. Scand J Med Sci Sports. 2015;25:25–31. doi: 10.1111/sms.12143. [DOI] [PubMed] [Google Scholar]
- Leppilahti J, Puranen J, Orava S. Incidence of Achilles tendon rupture. Acta Orthop Scand. 1996;67:277–279. doi: 10.3109/17453679608994688. [DOI] [PubMed] [Google Scholar]
- Maffulli N, Ewen SW, Waterston SW, Reaper J, Barrass V. Tenocytes from ruptured and tendinopathic achilles tendons produce greater quantities of type III collagen than tenocytes from normal achilles tendons. An in vitro model of human tendon healing. Am J Sports Med. 2000;28:499–505. doi: 10.1177/03635465000280040901. [DOI] [PubMed] [Google Scholar]
- Mannava S, Plate JF, Whitlock PW, Callahan MF, Seyler TM, Koman LA, Smith TL, Tuohy CJ. Evaluation of in vivo rotator cuff muscle function after acute and chronic detachment of the supraspinatus tendon: an experimental study in an animal model. J Bone Joint Surg Am. 2011;93:1702–1711. doi: 10.2106/JBJS.J.00184. [DOI] [PubMed] [Google Scholar]
- Menz HB. Biomechanics of the Ageing Foot and Ankle: A Mini-Review. Gerontology. 2015;61:381–388. doi: 10.1159/000368357. [DOI] [PubMed] [Google Scholar]
- Menz HB, Morris ME, Lord SR. Foot and ankle characteristics associated with impaired balance and functional ability in older people. J Gerontol A Biol Sci Med Sci. 2005;60:1546–1552. doi: 10.1093/gerona/60.12.1546. [DOI] [PubMed] [Google Scholar]
- Mian OS, Thom JM, Ardigo LP, Minetti AE, Narici MV. Gastrocnemius muscle-tendon behaviour during walking in young and older adults. Acta Physiol (Oxf) 2007;189:57–65. doi: 10.1111/j.1748-1716.2006.01634.x. [DOI] [PubMed] [Google Scholar]
- Mian OS, Thom JM, Ardigo LP, Narici MV, Minetti AE. Metabolic cost, mechanical work, and efficiency during walking in young and older men. Acta Physiol (Oxf) 2006;186:127–139. doi: 10.1111/j.1748-1716.2006.01522.x. [DOI] [PubMed] [Google Scholar]
- Ng N, Soderman K, Norberg M, Ohman A. Increasing physical activity, but persisting social gaps among middle-aged people: trends in Northern Sweden from 1990 to 2007. Glob Health Action. 2011;4:6347. doi: 10.3402/gha.v4i0.6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onambele GL, Narici MV, Maganaris CN. Calf muscle-tendon properties and postural balance in old age. J Appl Physiol (1985) 2006;100:2048–2056. doi: 10.1152/japplphysiol.01442.2005. [DOI] [PubMed] [Google Scholar]
- Pardes AM, Freedman BR, Fryhofer GW, Salka NS, Bhatt PR, Soslowsky LJ. Males have Inferior Achilles Tendon Material Properties Compared to Females in a Rodent Model. Ann Biomed Eng. 2016 doi: 10.1007/s10439-016-1635-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peffers MJ, Fang Y, Cheung K, Wei TK, Clegg PD, Birch HL. Transcriptome analysis of ageing in uninjured human Achilles tendon. Arthritis Res Ther. 2015;17:33. doi: 10.1186/s13075-015-0544-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peffers MJ, Thorpe CT, Collins JA, Eong R, Wei TK, Screen HR, Clegg PD. Proteomic analysis reveals age-related changes in tendon matrix composition, with age- and injury-specific matrix fragmentation. J Biol Chem. 2014;289:25867–25878. doi: 10.1074/jbc.M114.566554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plate JF, Wiggins WF, Haubruck P, Scott AT, Smith TL, Saul KR, Mannava S. Normal aging alters in vivo passive biomechanical response of the rat gastrocnemius-Achilles muscle-tendon unit. J Biomech. 2013;46:450–455. doi: 10.1016/j.jbiomech.2012.11.007. [DOI] [PubMed] [Google Scholar]
- Quinn R. Comparing rat’s to human’s age: how old is my rat in people years? Nutrition. 2005;21:775–777. doi: 10.1016/j.nut.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Riggin CN, Sarver JJ, Freedman BR, Thomas SJ, Soslowsky LJ. Analysis of collagen organization in mouse achilles tendon using high-frequency ultrasound imaging. J Biomech Eng. 2014;136:021029. doi: 10.1115/1.4026285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney SI, Baskin R, Torino DJ, Vafa RP, Khandekar PS, Kuntz AF, Soslowsky LJ. Ibuprofen differentially affects supraspinatus muscle and tendon adaptations to exercise in a rat model. Am J Sports Med. 2016;44:2237–2245. doi: 10.1177/0363546516646377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz A, Silder A, Heiderscheit B, Mahoney J, Thelen DG. Differences in lower-extremity muscular activation during walking between healthy older and young adults. J Electromyogr Kinesiol. 2009;19:1085–1091. doi: 10.1016/j.jelekin.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slane LC, DeWall R, Martin J, Lee K, Thelen DG. Middle-aged adults exhibit altered spatial variations in Achilles tendon wave speed. Physiol Meas. 2015;36:1485–1496. doi: 10.1088/0967-3334/36/7/1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slane LC, Martin J, DeWall R, Thelen D, Lee K. Quantitative ultrasound mapping of regional variations in shear wave speeds of the aging Achilles tendon. Eur Radiol. 2016 doi: 10.1007/s00330-016-4409-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slane LC, Thelen DG. Achilles tendon displacement patterns during passive stretch and eccentric loading are altered in middle-aged adults. Med Eng Phys. 2015;37:712–716. doi: 10.1016/j.medengphy.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LR, Barton ER. SMASH — semi-automatic muscle analysis using segmentation of histology: a MATLAB application. Skelet Muscle. 2014;4:21. doi: 10.1186/2044-5040-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RK, Birch HL, Goodman S, Heinegard D, Goodship AE. The influence of ageing and exercise on tendon growth and degeneration—hypotheses for the initiation and prevention of strain-induced tendinopathies. Comp Biochem Physiol A Mol Integr Physiol. 2002;133:1039–1050. doi: 10.1016/s1095-6433(02)00148-4. [DOI] [PubMed] [Google Scholar]
- Spink MJ, Fotoohabadi MR, Wee E, Hill KD, Lord SR, Menz HB. Foot and ankle strength, range of motion, posture, and deformity are associated with balance and functional ability in older adults. Arch Phys Med Rehabil. 2011;92:68–75. doi: 10.1016/j.apmr.2010.09.024. [DOI] [PubMed] [Google Scholar]
- Stenroth L, Peltonen J, Cronin NJ, Sipila S, Finni T. Age-related differences in Achilles tendon properties and triceps surae muscle architecture in vivo. J Appl Physiol (1985) 2012;113:1537–1544. doi: 10.1152/japplphysiol.00782.2012. [DOI] [PubMed] [Google Scholar]
- Suchak AA, Bostick G, Reid D, Blitz S, Jomha N. The incidence of Achilles tendon ruptures in Edmonton, Canada. Foot Ankle Int. 2005;26:932–936. doi: 10.1177/107110070502601106. [DOI] [PubMed] [Google Scholar]
- Suydam SM, Buchanan TS, Manal K, Silbernagel KG. Compensatory muscle activation caused by tendon lengthening post-Achilles tendon rupture. Knee Surg Sports Traumatol Arthrosc. 2015;23:868–874. doi: 10.1007/s00167-013-2512-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson RB, Heinemeier KM, Couppe C, Kjaer M, Magnusson SP. The effect of aging and exercise on the tendon. J Appl Physiol (1985) 2016 doi: 10.1152/japplphysiol.00328.2016. jap 00328 0 2016. [DOI] [PubMed] [Google Scholar]
- Thornton GM, Lemmex DB, Ono Y, Beach CJ, Reno CR, Hart DA, Lo IK. Aging affects mechanical properties and lubricin/PRG4 gene expression in normal ligaments. J Biomech. 2015;48:3306–3311. doi: 10.1016/j.jbiomech.2015.06.005. [DOI] [PubMed] [Google Scholar]
- Thorpe CT, Peffers MJ, Simpson D, Halliwell E, Screen HR, Clegg PD. Anatomical heterogeneity of tendon: Fascicular and interfascicular tendon compartments have distinct proteomic composition. Sci Rep. 2016;6:20455. doi: 10.1038/srep20455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood LK, Arruda EM, Brooks SV. Regional stiffening with aging in tibialis anterior tendons of mice occurs independent of changes in collagen fibril morphology. J Appl Physiol (1985) 2011;111:999–1006. doi: 10.1152/japplphysiol.00460.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu TY, Pang JH, Wu KP, Chen MJ, Chen CH, Tsai WC. Aging is associated with increased activities of matrix metalloproteinase-2 and -9 in tenocytes. BMC Musculoskelet Disord. 2013;14:2. doi: 10.1186/1471-2474-14-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaseck LW, Miller RA, Brooks SV. Rapamycin Attenuates Age-associated Changes in Tibialis Anterior Tendon Viscoelastic Properties. J Gerontol A Biol Sci Med Sci. 2016;71:858–865. doi: 10.1093/gerona/glv307. [DOI] [PMC free article] [PubMed] [Google Scholar]


