Abstract
Iron is an essential element required for many processes within the cell. Dysregulation in iron homeostasis due to iron overload is detrimental. This nutrient is postulated to contribute to the initiation of cancer; however, the mechanisms by which this occurs remain unclear. Defining how iron promotes the development of ovarian cancers from precursor lesions is essential for developing novel therapeutic strategies. In this review, we discuss (1) how iron overload conditions may initiate ovarian cancer development, (2) dysregulated iron metabolism in cancers, (3) the interplay between bacteria, iron, and cancer, and (4) chemotherapeutic strategies targeting iron metabolism in cancer patients.
Keywords: Endometriosis, fallopian tube fimbrial epithelium, microbiome, ovarian surface epithelium, therapy
1. Introduction
Iron is an essential trace element that is required for maintenance of healthy mammalian cells. There exists between 3–5 grams of iron in the average adult with the large majority being associated with hemoglobin and the remainder stored within ferritin (the iron storage protein complex) in hepatocytes and macrophages (Andrews, 1999). When iron is needed, it can be released via ferritinophagy which is an autophagic process promoting iron release from ferritin (Mancias et al., 2014). Iron is needed to support important cellular processes including (1) oxygen transport via hemoglobin, (2) metabolic reactions, and (3) synthesis of DNA (Wang and Pantopoulos, 2011). Seminal discoveries identifying transferrin receptor 1 (CD71), iron regulation via iron-regulatory binding protein (IRP) binding to iron responsive elements (IRE) in specific mRNA transcripts, as well as the hepcidin (HAMP)/ferroportin (FPN1) axis are significant to our current understanding of iron regulation. Nonetheless, we have yet to fully understand iron metabolism thoroughly, particularly with respect to the biology of cancers.
Due to the role of iron in redox reactions and the absence of mechanisms to eliminate excess iron, iron homeostasis needs to be maintained carefully by a variety of molecules involved in transportation, storage, and degradation which are discussed herein. If iron homeostasis is disrupted, iron engages in Fenton reactions (ferrous (Fe2+) iron reduces hydrogen peroxide (H2O2) to generate hydroxyl radicals (OH•)) (Winterbourn, 1995; Wang and Pantopoulos, 2011); this event leads to the formation of reactive oxygen species (ROS) which may promote ferroptosis, a morphologically distinct form of non-apoptotic cell death (Dixon et al., 2012). Overexpression of nuclear co-activator 4 (NCOA4), a recently identified receptor involved in iron release from ferritin (Mancias et al., 2014), contributes to the ferroptotic response (Hou et al., 2016). Thus, modulation of the ferroptotic and ferritinophagic processes may be potential targets for development of novel therapeutic treatment strategies for cancer in addition to iron chelators which deplete intracellular iron essential for cancer cell survival (Lui et al., 2015).
In this review, we discuss how ovarian cancer may be initiated in response to iron overload conditions and how cancer cells may become dependent on altered iron metabolism. We present data from the Cancer Genome Atlas (TCGA) illustrating dysregulated expression of iron molecules in ovarian cancer. Finally, we discuss potential treatment strategies involving targeting of these pathways.
2. Initiation of Ovarian Cancer by Persistent Oxidative Stress Mediated by Iron Overload
Chronic iron overload can lead to a number of diseases including (1) cancer (Lagergren et al., 2016), (2) retinal degeneration (He et al., 2007), (3) neurodegenerative diseases (Berg and Youdim, 2006), (4) hyperferritinemia (Stein et al., 2010), (5) hereditary hemochromatosis (Liu et al., 2016; Powell et al., 2016), and (6) β-thalassemia (Liu et al., 2016). Ovarian cancers are characterized by elevated genomic mutations which is proposed to result from oxidative damage (i.e., DNA adducts, lipid peroxidation, and 8-OHdG) (Yamada et al., 2011). Menstrual effluent (via retrograde menstruation) is suggested to contribute to endometriosis and development of rarer types (though often more chemoresistant in relation to the more common serous ovarian carcinoma (Itamochi et al., 2008)) of ovarian cancers such as endometrioid and clear cell ovarian cancers (Defrere et al., 2006). It is indicated that the pelvic region has an elevated iron level due to retrograde menstruation which may thus promote endometrial survival and implantation at ectopic sites (Van Langendonckt et al., 2002). Iron deposits (i.e., hemosiderin) have not only been identified in endometriotic lesions but also within the fallopian tube of patients diagnosed with serous epithelial ovarian cancers (Yamaguchi et al., 2008; Seidman, 2013). In addition to ovulation, another potential contributor to ovarian cancer pathogenesis is follicular fluid (Emori and Drapkin, 2014). In particular, follicular fluid contains hormones such as estradiol (Emori and Drapkin, 2014), reactive oxygen species (ROS), and transferrin (Shigeta et al., 2016). Additionally, iron and DNA adducts are increased in follicular fluid of endometriosis patients compared to infertile controls (Singh et al., 2013; Da Broi et al., 2016). Iron and transcript levels of ferritin and transferrin receptor were increased in follicles close to an endometrioma (characterized by elevated iron levels (Yamaguchi et al., 2008)) affecting oocyte retrieval (Sanchez et al., 2014) although another study did not identify any differences in iron content or ferritin levels (Benaglia et al., 2015). Exposure of fallopian epithelium to follicular fluid leads to a small increase in cell proliferation along with IL-8 cytokine levels (Bahar-Shany et al., 2014). High ROS containing follicular fluid was capable of inducing early onset B-cell lymphoma in mammary fat pads in mice lacking p53 (Huang et al., 2015).
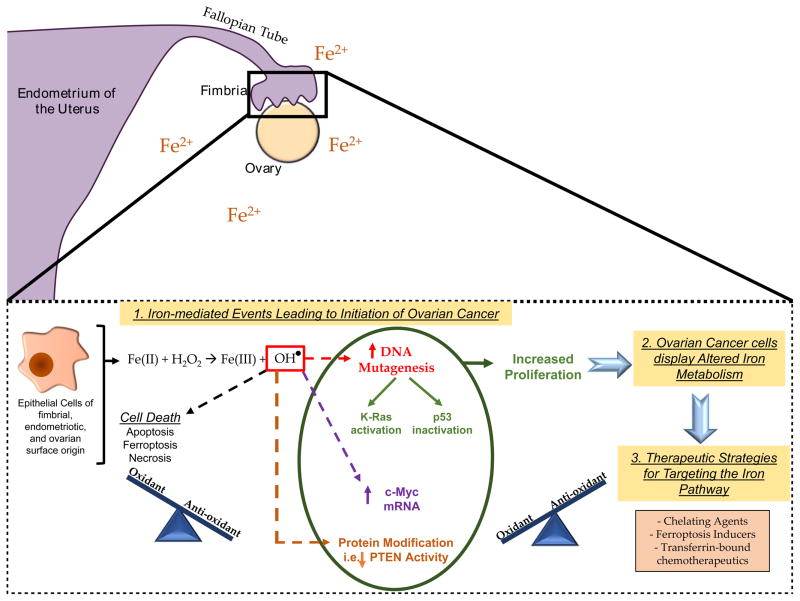
The detailed molecular mechanism underlying initiation of ovarian cancers remains unclear (Figure 1). Since iron induces ROS via its participation in Fenton reactions (Winterbourn, 1995; Yamada et al., 2011), iron overload may initiate carcinogenesis by increasing oxidative stress levels and thus mutagenesis (Vercellini et al., 2011). Precursor cells postulated to contribute to specific subtypes of ovarian cancers (i.e., endometriotic cells, fimbrial secretory epithelial cells, and ovarian epithelial cells) may be exposed to a sublethal dose of oxidative stress and thus may have the potential to undergo tumorigenesis due to persistent anti-oxidant defenses (Kobayashi, 2016). Ferritin, nuclear factor (erythroid-derived 2)-like 2 (NRF2), as well as heme oxygenase 1 (HO-1) amongst others (i.e., NAD(P)H:Quinone Oxidoreductase 1 (NQO1), glutathione S-Transferase Pi 1 (GSTP1), and glutathione-dependent peroxidases (GPX)) contribute to such anti-oxidant defenses and may therefore participate in the tumorigenesis process along with persistent DNA damaging events (Iwabuchi et al., 2015; Kobayashi, 2016). This would enable a proliferative environment for survival advantage (Iwabuchi et al., 2015). Indeed, for example, the incidence of hepatocellular carcinoma (HCC) is elevated 200-fold in patients with hereditary hemochromatosis; the resulting increase in ROS coinciding with unbalanced anti-oxidant defenses is suggested to promote tumorigenesis (Marrogi et al., 2001). In endometriotic cysts, iron was measured to be ~100mM and was associated with lipid peroxide, 8-OHdG positivity, and evidence of iron deposits (via Prussian blue staining) (Yamaguchi et al., 2008). Iron deposits have also been identified in the fallopian tube (Seidman, 2013). Fimbrial secretory epithelial cells treated with increasing doses of iron elicited increased cellular proliferation along with changes in p53, MAPK, AKT, and c-Myc proteins, as well as increased ROS species (0.05–100mM) (Lattuada et al., 2015). Furthermore, vitamin D3 could oppose the oxidative stress-induced events mediated by iron in these fimbrial cells (Uberti et al., 2016). In support of the transition from precursor cells to cancer, it is notable that a clear cell ovarian cancer gene signature was induced upon iron treatment in immortalized ovarian surface epithelial cells; this was regulated partially by DNA methylation (Yamaguchi et al., 2010).
Figure 1. Model of the Contribution of Iron to Ovarian Cancer Development.
Iron is elevated in the peritoneal cavity of women with endometriosis, in endometriotic cysts, and in fallopian tube fimbriae. Iron engages in Fenton reactions resulting in ROS such as hydroxyl radicals. An excess of ROS can promote cell death such as ferroptosis. However, sublethal levels of ROS may lead to increased proliferative capacity (if balanced with an increase in anti-oxidant response) thereby promoting tumorigenesis. ROS can lead to increased c-Myc transcripts, directly reduce PTEN activity, and cause DNA mutations leading to inactivation of p53 and activation of K-Ras. Tumor cells are characterized by increased iron dependence. Therefore, therapeutic strategies targeting components of the iron pathway may prove useful in treating ovarian cancer patients.
ROS damages not only DNA but also proteins and lipids; products of lipid peroxidation can themselves damage DNA (Luczaj and Skrzydlewska, 2003). DNA damaging events could lead to (1) its repair unless the damage is extensive leading to cell death or (2) survival of mutant cells if other changes are present leading to an accumulation of mutations (Tak et al., 2000). ROS cause numerous types of DNA damage such as strand breakages, formation of apurinic/apyrimidinic sites, base modifications (8-OHdG), thymidine glycols, and ring-opened based products (Toyokuni, 1996). There are likely “specific” sites that are susceptible to such DNA damage (Tanaka et al., 1999). These can be mutagenic resulting in Ras activation and p53 inactivation (Du et al., 1994). Indeed, K-Ras and the MAPK signaling cascade have been shown to be hyperactivated in response to oxidative stress (Yamada et al., 2011). Furthermore, PTEN can be inactivated directly by hydrogen peroxide via oxidation which was accompanied by elevated PIP3 levels and AKT activation (Leslie et al., 2003). Mutations in K-Ras (a codon 12 G to T transversion) can be induced by nickel (similar chemical and physical properties to iron (Valko et al., 2006)) in renal sarcomas and mutations in p53 (G to T transversion) can be induced by iron in renal cancers (Toyokuni, 1996). Further, K-Ras was identified to be mutated (GGT to GCT mutation at codon 12) at 8 weeks in one rat out of 26 exposed to cigarette smoke for a 1 hour time twice a day (8, 12, or 20 weeks) (Maehira et al., 1999). Oxidative stress can also lead to marked increases in c-Myc (a known mediator of cell proliferation) transcripts (Toyokuni, 1996; Li and Spector, 1997; Elouil et al., 2005). Altogether, these events may contribute to iron-induced persistent oxidative stress events, thereby leading to precursor lesions transitioning to various subtypes of ovarian cancer (Yamada et al., 2011).
It is well-established that estrogen is associated with an increased risk of tumorigenesis in ovarian cancers (Liehr and Jones, 2001; Mungenast and Thalhammer, 2014; Jeon et al., 2016; Kyriakidis and Papaioannidou, 2016) as well as endometrial and breast cancers (Brown and Hankinson, 2015). Strikingly, there are links between estrogen and iron which stems from a hamster kidney tumor model in which the animals were implanted with estrogen and provided either normal chow or a diet with elevated iron levels which doubled tumor incidence (Wyllie and Liehr, 1998). There are also notable changes in intracellular levels of iron upon estrogen treatment in breast cancer cells (Liehr and Jones, 2001). Specifically, metabolic estrogen components generates ROS which promotes iron release from ferritin in the Fe+2 form (Liehr and Jones, 2001) and furthermore, there is evidence that estrogen metabolites can themselves form DNA adducts (Cavalieri and Rogan, 2016). Since c-Myc can control gene expression of molecules involved in iron metabolism (i.e., ferritin heavy chain (FTH1) and IRP-2), it is interesting that estrogen can modulate expression of c-Myc (Liehr and Jones, 2001). Balance of intracellular iron is tightly controlled by the HAMP/FPN1 axis (Hou et al., 2012) and both mediators are regulated via estrogen response elements (ERE) within their promoter regions (Hou et al., 2012; Qian et al., 2015). Estrogen also can increase transferrin mRNA by binding to specific elements within the promoter region of transferrin via an atypical (non-consensus) ERE sequence (Vyhlidal et al., 2002).
3. Dysregulation of Iron Metabolism in Cancer
Iron mediates its effects via complex mechanisms of regulation including transcriptional and post-transcriptional means (i.e., via IRP) (Liu et al., 2005) and changes in iron metabolism are a property of tumors (Miller et al., 2011). The pathways of iron metabolism that are altered in cancer include (1) iron uptake/export processes, (2) storage, and (3) regulation (Zhang and Zhang, 2015). Indeed, iron is critical for a number of cellular processes including heme biosynthesis and the production of iron-sulphur clusters, which regulate a large array of enzymatic activities required for sustaining increased cellular proliferation and metabolic functions (Rouault, 2015). The number of molecules identified to be involved in iron metabolism has increased exponentially in the last decade (Hower et al., 2009). Indeed, a network of pathways integrating these molecules has been developed for 107 reactions/transport processes (using CellDesigner version 3.5.2) for various cell types including intestine, liver (important in uptake and storage), as well as reticulocytes and macrophages (utilization and recycling) (Hower et al., 2009). To comprehend the complexity of iron regulation, global gene profiling approaches such as Affymetrix oligonucleotide arrays have been used to define patterns of genes that are altered (Liu et al., 2005). For example, an Iron Regulatory Gene Signature (IRGS) has been utilized to identify high risk cancer patients (Miller et al., 2011). This signature is comprised of 16 iron regulatory genes including CYBRD1 (cytochrome b reductase 1), STEAP1 (six transmembrane epithelial antigen of the prostate 1), STEAP2 (six transmembrane epithelial antigen of the prostate 2), HFE (hemochromatosis), SCARA5 (scavenger receptor class A, member 5 (putative)), LTF (lactotransferrin), TFRC (transferrin receptor 1 (CD71)), SLC40A1 (solute carrier family 40 (iron-regulated transporter), member 1), ISCU (iron-sulfur cluster scaffold homolog), SFXN1 (sideroflexin 1), EPAS1 (endothelial PAS domain protein 1), SLC25A37 (solute carrier family 25, member 37), ABCG2 (ATP-binding cassette, subfamily G (WHITE), member 2), SFXN5 (sideroflexin 5), HIF1AN (hypoxia inducible factor 1, alpha subunit inhibitor), and ALAD (aminolevulinate dehydrogenase) (Miller et al., 2011). This signature was demonstrated to predict breast cancer recurrence as well as subclassify molecular subtypes of breast cancer, which may aid in determination of patient outcomes (Miller et al., 2011).
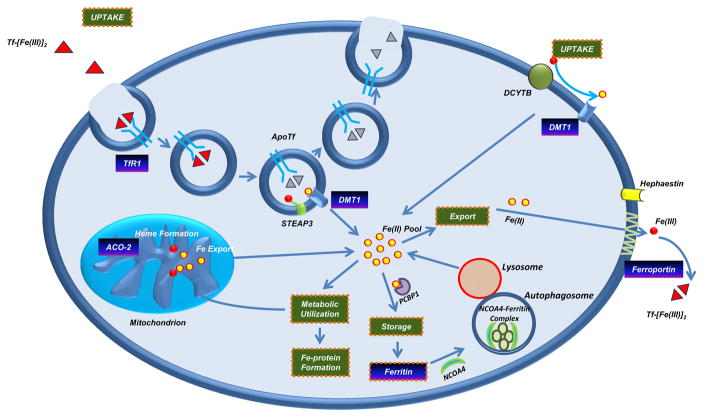
Figure 2 summarizes the major mediators in iron metabolism. Briefly, in enterocytes, duedonal cytochrome b (DCYTB) reduces Fe+3 to Fe+2, which can then be imported into the cell by the divalent metal transporter 1 (DMT1) (Canonne-Hergaux et al., 1999; McKie et al., 2001). The ferric Fe+3 can also bind to transferrin (holo-transferrin); the iron-bound transferrin then binds to CD71 on the plasma membrane and enters cells via endocytosis (Byrne et al., 2010; Wang and Pantopoulos, 2011). Iron is then released from transferrin within the endosomal compartment and reduced to Fe+2 by six-transmembrane epithelial antigen of prostate 3 (STEAP3) (Zhang et al., 2012), allowing its transport to the cytosol via DMT1 (Anderson et al., 2013). Fe+2 becomes part of the labile iron pool (LIP) which may then be used for generating iron-dependent protein complexes, iron-sulfur clusters, heme, or used in metabolic pathways as an enzymatic cofactor (Wang and Pantopoulos, 2011). If in excess, iron may then be stored within the ferritin complex or exported by FPN1 (Donovan et al., 2000; Shi et al., 2008). Elevated serum iron levels promote production of the 84 amino acid protein hormone HAMP in the liver which initiates endocytosis of FPN1 on the surface of hepatocytes, macrophages, enterocytes, and placental cells (Pigeon et al., 2001; Anderson et al., 2002). FPN1 is the only known exporter channel for intracellular iron in the Fe+2 form (Ward and Kaplan, 2012). Ferroportin disease is characterized by mutations in FPN1 leading to hyperferritinemia and macrophage iron loading (Mayr et al., 2010) which may be due to altered trafficking of FPN1 to the cell surface (rate of synthesis, rate of internalization, and/or rate of degradation) (Ward and Kaplan, 2012). FPN1 levels can also be modulated via mechanisms distinct from HAMP such as post-transcriptionally by the IRP/IRE system (Ross et al., 2012; Miseta et al., 2015) as well as transcriptionally by (1) hypoxia, (2) transition metals and heme, as well as (3) inflammation (Ward and Kaplan, 2012). The presence of HAMP reduces circulating iron levels leading to intracellular sequestration of iron (Miseta et al., 2015). HAMP/FPN1 axis is critical to body iron homeostasis; HAMP is upregulated by a variety of mechanisms involving transferrin receptor-2, hemochromatosis (HFE), bone morphogenic protein 6 (BMP6), and hemojuvelin (iron sensing extracellular system) (Miseta et al., 2015). Erythropoietin negatively influences HAMP gene expression (Miseta et al., 2015). Furthermore, IL-6 (which is upregulated in cancers and promotes cellular proliferation in human breast carcinoma (Sansone et al., 2007)) signals through the JAK/STAT signaling cascade and upregulates HAMP expression (Miseta et al., 2015). As indicated earlier, estrogen, vitamin D, and other hormones also influence HAMP expression (Miseta et al., 2015). Finally, iron regulatory proteins (IRPs) regulate translation of iron transport proteins (such as FTH1 and CD71), through their interactions with 3′-UTR or 5′-UTR iron responsive elements (Weiss et al., 1997).
Figure 2. The Iron Regulatory Pathway.
Iron bound transferrin is endocytosed into the cell via CD71. Iron is then reduced by STEAP3 from the Fe+3 to Fe+2 state, which then is shuttled out of the endosome via DMT1. Holo-transferrin and CD71 are recycled back to the cell surface. Non-transferrin bound iron may also be reduced to Fe+2 by DCYTB and transported into the cell by DMT1. Intracellular redox active iron may then be used for metabolic processes, formation of iron-sulphur clusters, or shuttled into the mitochondria for heme synthesis. If intracellular iron is in excess, it can be stored (via the chaperone protein PCBP1) in the ferritin storage complex. When needed, iron can then be released from the ferritin complex through the process of ferritinophagy, mediated by NCOA4. Iron may also be exported from the cell by FPN1, following oxidation to Fe+3 by HEPH.
A new mediator in iron metabolism, namely NCOA4 (previously known as androgen-associated protein 70 (ARA70) (Ligr et al., 2010)), was identified via a quantitative proteomics approach and demonstrated to be involved in the process of ferritinophagy (ferritin breakdown in lysosomes) (Dowdle et al., 2014; Mancias et al., 2014; Mancias et al., 2015). NCOA4 interacts with both the heavy and light chain of ferritin (Dowdle et al., 2014). Reduction of NCOA4 protein hindered co-localization of FTH1 with LC3B/LAMP1; furthermore, NCOA4 reduces IRP-2 and CD71 protein (Mancias et al., 2014). Functions independent of ferritinophagy include NCOA4’s ability to regulate DNA replication via binding to MCM7; further, deficiency of NCOA4 activates DNA replication origin activation via CMG helicase regulation (Bellelli et al., 2014). Additionally, NCOA4 mediates DNA damage/replication stress events (i.e., fork stalling, inhibition of fork speed, and premature senescence) (Bellelli et al., 2014). Using a NCOA4 knockout mouse model, the ferritinophagy receptor was demonstrated to be involved in promoting iron balance (Bellelli et al., 2016). In addition to interacting with the androgen receptor, NCOA4 can bind to the estrogen receptor (ERα) (Lanzino et al., 2005). While these studies refer to the full-length form (NCOA4α/ARA70α), an alternative splice variant (NCOA4β/ARA70β) was identified lacking an internal sequence of 985bp (Alen et al., 1999). The two NCOA4 isoforms elicit distinct roles in breast and prostate cancer cells; in particular, NCOA4α elicits tumor suppressive activity whereas NCOA4β elicits oncogenic activity (Peng et al., 2008; Ligr et al., 2010; Wu et al., 2011). It is presently unclear how these two isoforms mediate distinct functional activities; however, based on functional domains that have so far been identified, the AhR and FTH binding domains are lacking in the shorter variant (Kollara and Brown, 2006; Mancias et al., 2015). Aside from one report demonstrating that NCOA4/ARA70 mRNA expression was increased in human ovarian cancers compared to normal ovarian surface epithelium via in situ hybridization, further investigations are needed to fully comprehend the role of NCOA4 (as a ferrinophagic mediator and a nuclear co-activator) in the biology of ovarian cancers (Shaw et al., 2001).
SNPs in the germline for cellular transport genes was found to be associated with epithelial ovarian cancer risk (Chornokur et al., 2015). Specifically, one gene that was associated with serous epithelial ovarian cancers was the Hephaestin (HEPH) gene which is involved in catalyzing redox-active iron to its non-redox active form for transport from the enterocytes to the blood (Chornokur et al., 2015). Furthermore, we analyzed The Cancer Genome Atlas (TCGA) via cBIOPORTAL (Cerami et al., 2012; Gao et al., 2013) for alterations in iron pathway-mediating genes in ovarian cancers and this is presented in Table 1. Several studies have presented evidence implicating transferrin in ovarian cancer biology. For example, OVCAR-3 responded to transferrin with increased proliferation (Martinez et al., 2000). Transferrin was found to antagonize the cell death response induced by FTH1 and c-Myc in the N.1 human ovarian carcinoma cell line (Fassl et al., 2003). Treatment of fallopian epithelial cells with transferrin increased phosphorylation of H2AX in a CD71 and ROS-dependent manner (Shigeta et al., 2016). DNA double strand breaks were also observed in response to transferrin treatment in murine fallopian tubes (ex vivo) (Shigeta et al., 2016). Finally, there exists clinical data implicating transferrin as a biomarker to classify ovarian cancers (Ahmed et al., 2005; Kozak et al., 2005; Hogdall et al., 2011; Macuks et al., 2012; Moore et al., 2012). In addition, the transferrin receptor 2 appears to play a role in mediating iron homeostasis; it is highly expressed in ovarian cancer cell lines and inversely associated with CD71 expression (Calzolari et al., 2007). Elevated redox active iron, elevated CD71, and lowered FPN1 levels were present in ectopic endometrial stromal cells resulting in increased intracellular labile iron pool compared to normal eutopic endometria (Mori et al., 2015). The human hemochromatosis protein has two prevalent mutations at H63D and C282Y that are associated with iron overload diseases such as type I hemochromatosis (Gannon et al., 2011). Interestingly, the C282Y mutation was more frequent in patients with epithelial ovarian cancer and associated with a poor patient outcome (Gannon et al., 2011). Contradictory findings are reported for the iron storage protein, ferritin. Reduced FTH1 mRNA was identified in ovarian cancer specimens and associated with a poor patient outcome (Lobello et al., 2016) whereas its expression was elevated in metastatic ovarian cancer specimens relative to primary tumors (Tripathi and Chatterjee, 1996). However, upon FTH1 knockdown, SKOV3 ovarian cancer cells were more proliferative and migratory (Lobello et al., 2016) and furthermore, FTH1 was induced by c-Myc expression leading to increased cell survival (Fassl et al., 2003).
Table 1.
Summary of alterations in the iron metabolic pathway in ovarian cancers obtained from the TCGA via cBIOPORTAL. Information on the chromosomal location of the genes listed was obtained from Genecard (http://www.genecards.org/).
| Gene | Symbol | GCID | Chromosome Location | Genomic Location (base pairs) | Mutations | Deletions | Amplifications |
|---|---|---|---|---|---|---|---|
| Aconitase 1; Iron-Responsive Element Binding Protein 1 | ACO1; IRP1 | GC09P032374 | 9p21.1 | Start: 32,384,603 bp | 2/311 (0.6%) | 1/311 (0.3%) | 5/311 (1.6%) |
| End: 32,454,769 bp | |||||||
| Ferritin Heavy Chain | FTH1 | GC11M061959 | 11q12.3 | Start: 61,959,718 bp | 1/311 (0.3%) | 0/311 (0%) | 6/311 (1.9%) |
| End: 61,967,660 bp | |||||||
| Ferritin Light Chain | FTL | GC19P048965 | 19q13.33 | Start: 48,965,301 bp | 0/311 (0%) | 3/311 (1%) | 2/311 (0.6%) |
| End: 48,966,879 bp | |||||||
| Hepcidin Antimicrobial Peptide | HAMP | GC19P035281 | 19q13.12 | Start: 35,280,716 bp | 0/311 (0%) | 0/311 (0%) | 30/311 (9.6%) |
| End: 35,285,143 bp | |||||||
| Iron-Responsive Element Binding Protein 2 | IREB2; IRP2 | GC15P078437 | 15q25.1 | Start: 78,437,431 bp | 0/311 (0%) | 1/311 (0.3%) | 5/311 (1.6%) |
| End: 78,501,456 bp | |||||||
| Nuclear Receptor Coactivator4 | NCOA4 | GC10M046005 | 10q11.22 | Start: 46,005,088 bp | 0/311 (0%) | 0/311 (0%) | 3/311 (1%) |
| End: 46,030,714 bp | |||||||
| Transferrin | TF | GC03P133663 | 3q22.1 | Start: 133,661,998 bp | 1/311 (0.3%) | 0/311 (0%) | 23/311 (7.4%) |
| End: 133,779,006 bp | |||||||
| Transferrin Receptor | TFRC; CD71 | GC03M196027 | 3q29 | Start: 196,027,183 bp | 1/311 (0.3%) | 1/311 (0.3%) | 76/311 (24.4%) |
| End: 196,082,189 bp | |||||||
| Solute Carrier Family 11 (Proton-Coupled Divalent Metal Ion Transporter), Member 2 | SLC11A2; DMT1 | GC12M050979 | 12q13.12 | Start: 50,979,401 bp | 0/311 (0%) | 1/311 (0.3%) | 2/311 (0.6%) |
| End: 51,028,566 bp | |||||||
| Solute Carrier Family 40 (Iron-Regulated Transporter), Member 1 | SLC40A1; FPN1 | GC02M189560 | 2q32.2 | Start: 189,560,579 bp | 1/311 (0.3%) | 0/311 (0%) | 25/311 (8%) |
| End: 189,583,758 bp |
4. Bacteria, Iron, and Cancer
It is hypothesized that changes in the microbiome of the pelvic region may be associated with the development of gynecological cancers including ovarian cancers (Chase et al., 2015). It is thus interesting that the microbiome of the gut is altered with increased dietary iron (Ng, 2016). This metal is critical for promoting pathogenic bacteria virulence, growth, and colonization (Ng, 2016). Evidence suggests that the protective microorganisms (Bifidobacterium longum and Lactabacillus acidophilus) which reduce oxidative stress in the gut become overwhelmed by pathogenic bacteria (Ng, 2016). Furthermore, utilization of antibiotics or breeding microorganism-free mice are associated with reduced colon cancer (Ng, 2016). Mutations in genes involved in iron metabolism (i.e., IRP-2) promotes a protective microbiome (Ng, 2016). In addition, heme may damage the colonic surface epithelium leading to crypt cell hyperproliferation which fills-in the denuded area; heme mediates these effects by altering the levels of Bacteroidetes and Firmicutes in the colonic environment (N et al., 2012). Lipid peroxidation mediated by heme was reduced in rats treated with antibiotics, which was accompanied by decreased crypt cell proliferation (Martin et al., 2015). Siderophores enable bacteria to solubilize iron from their environment to promote their cellular proliferation (Ellermann and Arthur, 2016). For example, uropathogenic E. coli (UPEC) bacteria depend on iron for growth and the ferritinophagy pathway was recently demonstrated to contribute to its increased survival (Bauckman and Mysorekar, 2016). It is suggested that epigenetic changes (i.e., methylation of CDKN2A) in the host uroepithelial cells due to UPEC infection may lead to bladder cancer (Tolg et al., 2011).
5. Targeting the Iron Pathway in Cancer and Future Perspectives
Since there is no mechanism to eliminate excess iron from the body, iron levels need to be tightly controlled; if dysregulated, disease conditions develop (Blanchette et al., 2016). Thus, modulating any of the processes involved in iron metabolism could be feasible targeting strategies for cancer treatment (Zhang and Zhang, 2015). There is evidence that phlebotomy may have potential as a cancer treatment strategy (Zacharski et al., 2008; Assi and Baz, 2014; Chifman et al., 2014; Nirei et al., 2015; Distante et al., 2016; Inati et al., 2017). Since HAMP levels are critical in regulating body iron levels, therapeutic strategies such as antagonists (via altering function (binding properties to FPN1) or modulation of its levels (via regulatory mechanisms)) to target this molecule would be worthwhile (Blanchette et al., 2016). Curcumin, a primary component of turmeric associated with anti-inflammatory properties (Funk et al., 2006; Jiao et al., 2009), is an iron-chelating agent that can also inhibit HAMP production thereby altering body iron levels (Jiao et al., 2009). Additionally, a variety of iron chelating agents exists, some of which have been approved for clinical use to treat iron overload disorders though not yet in use for treatment of cancer (Lui et al., 2015). For example, deferrioxamine (DFO) is the accepted chelator for use in treatment of β-thalassemia (Lui et al., 2015). However, it has a short half-life in plasma and thus requires long period of infusion (8–12 hours) resulting in poor patient compliance (Lui et al., 2015). Therefore, oral chelators would be superior in this regard; indeed, deferasirox (EXJADE) has not only improved tolerance for such iron overload disorders but also elicits higher anti-proliferative activity (Lui et al., 2015). Thiosemicarbazone chelators (3-AP, PIH analogs, DpT analogs) not only target iron metabolism but also innumerable signaling pathways; therefore, they appear to have more potency and thus may be beneficial in their use as a cancer treatment strategy although not yet in use for this purpose (Lui et al., 2015) (Heath et al., 2013). Curcumin can also modulate IRP activity leading to changes in FTH1 and CD71 expression (Jiao et al., 2006; Hatcher et al., 2008). Since CD71 is markedly elevated in cancers (Daniels et al., 2006), it can be targeted with monoclonal antibodies which diminish cellular proliferation leading to an increased cell death response in vitro (Daniels et al., 2006). Currently used as efficient targeting agents, there is evidence of the therapeutic benefit of transferrin-conjugates with therapeutic compounds such as doxorubicin, cisplatin, and tirapazamine thus far in mouse xenograft models although these have been unsuccessful in clinical trials (Tortorella and Karagiannis, 2014).
We previously reported that iron treatment in a series of gynecological cell lines resulted in cell death initiated by the MAPK pathway (and dependent on Ras mutational status) leading to increased autophagosome and lysosomes (Bauckman et al., 2013); these changes were accompanied by increased ROS and mitochondrial damage (Bauckman et al., 2015). The cell death induced by FAC appeared to be distinct from the recently described ferroptosis; this is a cell death pathway distinct from apoptosis, necrosis, and autophagy though with features similar to glutamate-induced death (Dixon et al., 2012). Cells that are rapidly dividing are seemingly addicted to higher levels of iron and thus may be more responsive to changes in ROS levels resulting in cell death such as ferroptosis (Tarangelo and Dixon, 2016). Interestingly, C′ (Cornell) dots (poly(ethylene glycol)-coated silica nanoparticles) can eliminate cancer cells in a growth restricted medium similar to the environmental conditions found in vivo for a tumor (Tarangelo and Dixon, 2016). These dots bind to extracellular iron and following their entry into cells, they increase intracellular redox active iron and ROS leading to a ferroptotic mechanism of cell death (Tarangelo and Dixon, 2016). The use of C′ dots in in vivo mouse xenograft models led to regression of tumors (Tarangelo and Dixon, 2016). Via a screening approach, Ferrostatin-1 was identified to be a specific inhibitor for ferroptosis which involves inhibition of cysteine uptake via the cysteine/glutamate antiporter system (xc-) which decreases the anti-oxidant response (Dixon et al., 2012; Skouta et al., 2014). Further, Sorafenib induces cell death by chelation of intracellular iron, targeting of the xc- system, and increasing ROS; these events could be blocked with ferrostatin-1 (Louandre et al., 2013; Dixon et al., 2014). Strengthening of the ferroptotic response was promoted by Rb deficiency and p53 which transcriptionally upregulates SLC7A11 (a cysteine/glutamate antiporter component) to reduce cysteine uptake (Jiang et al., 2015; Louandre et al., 2015). Pancreatic ductal adenocarcinomas (PDAC), which are generally insensitive to apoptosis, were sensitized to Artesunate (ART) via induction of ferroptosis, specifically in cells with constitutive K-Ras activity (Eling et al., 2015). In this regard, it is suggested that profiling of patient tissues for ferroptotic genes may identify responsiveness to ferroptosis-inducing agents (Eling et al., 2015). ART also hindered growth of ovarian cancer cell lines and tumors in a mouse xenograft model via induction of ROS leading to DNA damage and a ferroptotic response (Greenshields et al., 2016). The ferroptotic response is associated with a reduction in glutathione (GSH) levels via GPX enzymes (Yang et al., 2014). Negative regulators of the ferroptotic response include (1) HSPB1 (Heat Shock Protein Family B Member 1) in an HSF-dependent manner (Sun et al., 2015) (2) CISD1 (CDGSH iron sulfur domain 1) which is an iron-containing outer mitochondrial membrane protein implicating mitochondrial lipid damage (via peroxidation) (Yuan et al., 2016), (3) NRF2 via activation of the p62-Keap1 signaling pathway (key regulators of the antioxidant response) (Sun et al., 2016) in which its activation leads to increased HO-1, FTH1, as well as NQO1 transcripts (Sun et al., 2016), (4) reduction of autophagy markers (ATG5 or ATG7) thereby decreasing autophagic flux turnover (Hou et al., 2016), and (5) NCOA4 depletion (Hou et al., 2016).
Ovarian cancer remains one of the deadliest gynecological cancers in women in the United States. Regrettably, the disease is typically only identified once the cancer has already progressed to advanced stages. Although considerable knowledge has thus far been obtained regarding iron metabolism, further work is direly needed to clarify the mechanisms how this essential nutrient leads to ovarian cancers from precursor lesions. This will be essential for developing improved therapeutic strategies for these ovarian cancer patients.
Acknowledgments
This work was supported by funding from the National Cancer Institute to MN (R21 CA178468-01). We apologize to those in this area of research whose work we have not cited.
References
- Ahmed N, Oliva KT, Barker G, Hoffmann P, Reeve S, Smith IA, Quinn MA, Rice GE. Proteomic tracking of serum protein isoforms as screening biomarkers of ovarian cancer. Proteomics. 2005;5:4625–4636. doi: 10.1002/pmic.200401321. [DOI] [PubMed] [Google Scholar]
- Alen P, Claessens F, Schoenmakers E, Swinnen JV, Verhoeven G, Rombauts W, Peeters B. Interaction of the putative androgen receptor-specific coactivator ARA70/ELE1alpha with multiple steroid receptors and identification of an internally deleted ELE1beta isoform. Mol Endocrinol. 1999;13:117–128. doi: 10.1210/mend.13.1.0214. [DOI] [PubMed] [Google Scholar]
- Anderson ER, Taylor M, Xue X, Ramakrishnan SK, Martin A, Xie L, Bredell BX, Gardenghi S, Rivella S, Shah YM. Intestinal HIF2alpha promotes tissue-iron accumulation in disorders of iron overload with anemia. Proc Natl Acad Sci U S A. 2013;110:E4922–4930. doi: 10.1073/pnas.1314197110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson GJ, Frazer DM, Wilkins SJ, Becker EM, Millard KN, Murphy TL, McKie AT, Vulpe CD. Relationship between intestinal iron-transporter expression, hepatic hepcidin levels and the control of iron absorption. Biochem Soc Trans. 2002;30:724–726. doi: 10.1042/bst0300724. [DOI] [PubMed] [Google Scholar]
- Andrews NC. Disorders of iron metabolism. N Engl J Med. 1999;341:1986–1995. doi: 10.1056/NEJM199912233412607. [DOI] [PubMed] [Google Scholar]
- Assi TB, Baz E. Current applications of therapeutic phlebotomy. Blood Transfus. 2014;12(Suppl 1):s75–83. doi: 10.2450/2013.0299-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahar-Shany K, Brand H, Sapoznik S, Jacob-Hirsch J, Yung Y, Korach J, Perri T, Cohen Y, Hourvitz A, Levanon K. Exposure of fallopian tube epithelium to follicular fluid mimics carcinogenic changes in precursor lesions of serous papillary carcinoma. Gynecol Oncol. 2014;132:322–327. doi: 10.1016/j.ygyno.2013.12.015. [DOI] [PubMed] [Google Scholar]
- Bauckman K, Haller E, Taran N, Rockfield S, Ruiz-Rivera A, Nanjundan M. Iron alters cell survival in a mitochondria-dependent pathway in ovarian cancer cells. Biochem J. 2015;466:401–413. doi: 10.1042/BJ20140878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauckman KA, Haller E, Flores I, Nanjundan M. Iron modulates cell survival in a Ras- and MAPK-dependent manner in ovarian cells. Cell Death Dis. 2013;4:e592. doi: 10.1038/cddis.2013.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauckman KA, Mysorekar IU. Ferritinophagy drives uropathogenic Escherichia coli persistence in bladder epithelial cells. Autophagy. 2016;12:850–863. doi: 10.1080/15548627.2016.1160176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellelli R, Castellone MD, Guida T, Limongello R, Dathan NA, Merolla F, Cirafici AM, Affuso A, Masai H, Costanzo V, et al. NCOA4 transcriptional coactivator inhibits activation of DNA replication origins. Mol Cell. 2014;55:123–137. doi: 10.1016/j.molcel.2014.04.031. [DOI] [PubMed] [Google Scholar]
- Bellelli R, Federico G, Matte A, Colecchia D, Iolascon A, Chiariello M, Santoro M, De Franceschi L, Carlomagno F. NCOA4 Deficiency Impairs Systemic Iron Homeostasis. Cell Rep. 2016;14:411–421. doi: 10.1016/j.celrep.2015.12.065. [DOI] [PubMed] [Google Scholar]
- Benaglia L, Paffoni A, Mangiarini A, Restelli L, Bettinardi N, Somigliana E, Vercellini P, Fedele L. Intrafollicular iron and ferritin in women with ovarian endometriomas. Acta Obstet Gynecol Scand. 2015;94:646–653. doi: 10.1111/aogs.12647. [DOI] [PubMed] [Google Scholar]
- Berg D, Youdim MB. Role of iron in neurodegenerative disorders. Top Magn Reson Imaging. 2006;17:5–17. doi: 10.1097/01.rmr.0000245461.90406.ad. [DOI] [PubMed] [Google Scholar]
- Blanchette NL, Manz DH, Torti FM, Torti SV. Modulation of hepcidin to treat iron deregulation: potential clinical applications. Expert Rev Hematol. 2016;9:169–186. doi: 10.1586/17474086.2016.1124757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SB, Hankinson SE. Endogenous estrogens and the risk of breast, endometrial, and ovarian cancers. Steroids. 2015;99:8–10. doi: 10.1016/j.steroids.2014.12.013. [DOI] [PubMed] [Google Scholar]
- Byrne SL, Chasteen ND, Steere AN, Mason AB. The unique kinetics of iron release from transferrin: the role of receptor, lobe-lobe interactions, and salt at endosomal pH. J Mol Biol. 2010;396:130–140. doi: 10.1016/j.jmb.2009.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calzolari A, Oliviero I, Deaglio S, Mariani G, Biffoni M, Sposi NM, Malavasi F, Peschle C, Testa U. Transferrin receptor 2 is frequently expressed in human cancer cell lines. Blood Cells Mol Dis. 2007;39:82–91. doi: 10.1016/j.bcmd.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Canonne-Hergaux F, Gruenheid S, Ponka P, Gros P. Cellular and subcellular localization of the Nramp2 iron transporter in the intestinal brush border and regulation by dietary iron. Blood. 1999;93:4406–4417. [PubMed] [Google Scholar]
- Cavalieri EL, Rogan EG. Depurinating estrogen-DNA adducts, generators of cancer initiation: their minimization leads to cancer prevention. Clin Transl Med. 2016;5:12. doi: 10.1186/s40169-016-0088-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase D, Goulder A, Zenhausern F, Monk B, Herbst-Kralovetz M. The vaginal and gastrointestinal microbiomes in gynecologic cancers: a review of applications in etiology, symptoms and treatment. Gynecol Oncol. 2015;138:190–200. doi: 10.1016/j.ygyno.2015.04.036. [DOI] [PubMed] [Google Scholar]
- Chifman J, Laubenbacher R, Torti SV. A systems biology approach to iron metabolism. Adv Exp Med Biol. 2014;844:201–225. doi: 10.1007/978-1-4939-2095-2_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chornokur G, Lin HY, Tyrer JP, Lawrenson K, Dennis J, Amankwah EK, Qu X, Tsai YY, Jim HS, Chen Z, et al. Common Genetic Variation In Cellular Transport Genes and Epithelial Ovarian Cancer (EOC) Risk. PLoS One. 2015;10:e0128106. doi: 10.1371/journal.pone.0128106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Broi MG, de Albuquerque FO, de Andrade AZ, Cardoso RL, Jordao Junior AA, Navarro PA. Increased concentration of 8-hydroxy-2′-deoxyguanosine in follicular fluid of infertile women with endometriosis. Cell Tissue Res. 2016;366:231–242. doi: 10.1007/s00441-016-2428-4. [DOI] [PubMed] [Google Scholar]
- Daniels TR, Delgado T, Rodriguez JA, Helguera G, Penichet ML. The transferrin receptor part I: Biology and targeting with cytotoxic antibodies for the treatment of cancer. Clin Immunol. 2006;121:144–158. doi: 10.1016/j.clim.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Defrere S, Van Langendonckt A, Vaesen S, Jouret M, Gonzalez Ramos R, Gonzalez D, Donnez J. Iron overload enhances epithelial cell proliferation in endometriotic lesions induced in a murine model. Hum Reprod. 2006;21:2810–2816. doi: 10.1093/humrep/del261. [DOI] [PubMed] [Google Scholar]
- Distante S, Eikeland J, Pawar T, Skinnes R, Hoie K, You P, Morkrid L, Eide L. Blood removal therapy in hereditary hemochromatosis induces a stress response resulting in improved genome integrity. Transfusion. 2016;56:1435–1441. doi: 10.1111/trf.13588. [DOI] [PubMed] [Google Scholar]
- Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon SJ, Patel DN, Welsch M, Skouta R, Lee ED, Hayano M, Thomas AG, Gleason CE, Tatonetti NP, Slusher BS, et al. Pharmacological inhibition of cystine-glutamate exchange induces endoplasmic reticulum stress and ferroptosis. Elife. 2014;3:e02523. doi: 10.7554/eLife.02523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan A, Brownlie A, Zhou Y, Shepard J, Pratt SJ, Moynihan J, Paw BH, Drejer A, Barut B, Zapata A, et al. Positional cloning of zebrafish ferroportin1 identifies a conserved vertebrate iron exporter. Nature. 2000;403:776–781. doi: 10.1038/35001596. [DOI] [PubMed] [Google Scholar]
- Dowdle WE, Nyfeler B, Nagel J, Elling RA, Liu S, Triantafellow E, Menon S, Wang Z, Honda A, Pardee G, et al. Selective VPS34 inhibitor blocks autophagy and uncovers a role for NCOA4 in ferritin degradation and iron homeostasis in vivo. Nat Cell Biol. 2014;16:1069–1079. doi: 10.1038/ncb3053. [DOI] [PubMed] [Google Scholar]
- Du MQ, Carmichael PL, Phillips DH. Induction of activating mutations in the human c-Ha-ras-1 proto-oncogene by oxygen free radicals. Mol Carcinog. 1994;11:170–175. doi: 10.1002/mc.2940110308. [DOI] [PubMed] [Google Scholar]
- Eling N, Reuter L, Hazin J, Hamacher-Brady A, Brady NR. Identification of artesunate as a specific activator of ferroptosis in pancreatic cancer cells. Oncoscience. 2015;2:517–532. doi: 10.18632/oncoscience.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellermann M, Arthur JC. Siderophore-mediated iron acquisition and modulation of host-bacterial interactions. Free Radic Biol Med. 2016 doi: 10.1016/j.freeradbiomed.2016.10.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elouil H, Cardozo AK, Eizirik DL, Henquin JC, Jonas JC. High glucose and hydrogen peroxide increase c-Myc and haeme-oxygenase 1 mRNA levels in rat pancreatic islets without activating NFkappaB. Diabetologia. 2005;48:496–505. doi: 10.1007/s00125-004-1664-4. [DOI] [PubMed] [Google Scholar]
- Emori MM, Drapkin R. The hormonal composition of follicular fluid and its implications for ovarian cancer pathogenesis. Reprod Biol Endocrinol. 2014;12:60. doi: 10.1186/1477-7827-12-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassl S, Leisser C, Huettenbrenner S, Maier S, Rosenberger G, Strasser S, Grusch M, Fuhrmann G, Leuhuber K, Polgar D, et al. Transferrin ensures survival of ovarian carcinoma cells when apoptosis is induced by TNFalpha, FasL, TRAIL, or Myc. Oncogene. 2003;22:8343–8355. doi: 10.1038/sj.onc.1207047. [DOI] [PubMed] [Google Scholar]
- Funk JL, Oyarzo JN, Frye JB, Chen G, Lantz RC, Jolad SD, Solyom AM, Timmermann BN. Turmeric extracts containing curcuminoids prevent experimental rheumatoid arthritis. J Nat Prod. 2006;69:351–355. doi: 10.1021/np050327j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon PO, Medelci S, Le Page C, Beaulieu M, Provencher DM, Mes-Masson AM, Santos MM. Impact of hemochromatosis gene (HFE) mutations on epithelial ovarian cancer risk and prognosis. Int J Cancer. 2011;128:2326–2334. doi: 10.1002/ijc.25577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6:pl1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenshields AL, Shepherd TG, Hoskin DW. Contribution of reactive oxygen species to ovarian cancer cell growth arrest and killing by the anti-malarial drug artesunate. Mol Carcinog. 2016 doi: 10.1002/mc.22474. [DOI] [PubMed] [Google Scholar]
- Hatcher H, Planalp R, Cho J, Torti FM, Torti SV. Curcumin: from ancient medicine to current clinical trials. Cell Mol Life Sci. 2008;65:1631–1652. doi: 10.1007/s00018-008-7452-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Hahn P, Iacovelli J, Wong R, King C, Bhisitkul R, Massaro-Giordano M, Dunaief JL. Iron homeostasis and toxicity in retinal degeneration. Prog Retin Eye Res. 2007;26:649–673. doi: 10.1016/j.preteyeres.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath JL, Weiss JM, Lavau CP, Wechsler DS. Iron deprivation in cancer--potential therapeutic implications. Nutrients. 2013;5:2836–2859. doi: 10.3390/nu5082836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogdall C, Fung ET, Christensen IJ, Nedergaard L, Engelholm SA, Petri AL, Risum S, Lundvall L, Yip C, Pedersen AT, et al. A novel proteomic biomarker panel as a diagnostic tool for patients with ovarian cancer. Gynecol Oncol. 2011;123:308–313. doi: 10.1016/j.ygyno.2011.07.018. [DOI] [PubMed] [Google Scholar]
- Hou W, Xie Y, Song X, Sun X, Lotze MT, Zeh HJ, 3rd, Kang R, Tang D. Autophagy promotes ferroptosis by degradation of ferritin. Autophagy. 2016;12:1425–1428. doi: 10.1080/15548627.2016.1187366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Y, Zhang S, Wang L, Li J, Qu G, He J, Rong H, Ji H, Liu S. Estrogen regulates iron homeostasis through governing hepatic hepcidin expression via an estrogen response element. Gene. 2012;511:398–403. doi: 10.1016/j.gene.2012.09.060. [DOI] [PubMed] [Google Scholar]
- Hower V, Mendes P, Torti FM, Laubenbacher R, Akman S, Shulaev V, Torti SV. A general map of iron metabolism and tissue-specific subnetworks. Mol Biosyst. 2009;5:422–443. doi: 10.1039/b816714c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang HS, Chu SC, Hsu CF, Chen PC, Ding DC, Chang MY, Chu TY. Mutagenic, surviving and tumorigenic effects of follicular fluid in the context of p53 loss: initiation of fimbria carcinogenesis. Carcinogenesis. 2015;36:1419–1428. doi: 10.1093/carcin/bgv132. [DOI] [PubMed] [Google Scholar]
- Inati A, Kahale M, Sbeiti N, Cappellini MD, Taher AT, Koussa S, Nasr TA, Musallam KM, Abbas HA, Porter JB. One-year results from a prospective randomized trial comparing phlebotomy with deferasirox for the treatment of iron overload in pediatric patients with thalassemia major following curative stem cell transplantation. Pediatr Blood Cancer. 2017;64:188–196. doi: 10.1002/pbc.26213. [DOI] [PubMed] [Google Scholar]
- Itamochi H, Kigawa J, Terakawa N. Mechanisms of chemoresistance and poor prognosis in ovarian clear cell carcinoma. Cancer Sci. 2008;99:653–658. doi: 10.1111/j.1349-7006.2008.00747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwabuchi T, Yoshimoto C, Shigetomi H, Kobayashi H. Oxidative Stress and Antioxidant Defense in Endometriosis and Its Malignant Transformation. Oxid Med Cell Longev. 2015;2015:848595. doi: 10.1155/2015/848595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon SY, Hwang KA, Choi KC. Effect of steroid hormones, estrogen and progesterone, on epithelial mesenchymal transition in ovarian cancer development. J Steroid Biochem Mol Biol. 2016;158:1–8. doi: 10.1016/j.jsbmb.2016.02.005. [DOI] [PubMed] [Google Scholar]
- Jiang L, Kon N, Li T, Wang SJ, Su T, Hibshoosh H, Baer R, Gu W. Ferroptosis as a p53-mediated activity during tumour suppression. Nature. 2015;520:57–62. doi: 10.1038/nature14344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao Y, Wilkinson Jt, Christine Pietsch E, Buss JL, Wang W, Planalp R, Torti FM, Torti SV. Iron chelation in the biological activity of curcumin. Free Radic Biol Med. 2006;40:1152–1160. doi: 10.1016/j.freeradbiomed.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Jiao Y, Wilkinson Jt, Di X, Wang W, Hatcher H, Kock ND, D’Agostino R, Jr, Knovich MA, Torti FM, Torti SV. Curcumin, a cancer chemopreventive and chemotherapeutic agent, is a biologically active iron chelator. Blood. 2009;113:462–469. doi: 10.1182/blood-2008-05-155952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H. Potential scenarios leading to ovarian cancer arising from endometriosis. Redox Rep. 2016;21:119–126. doi: 10.1179/1351000215Y.0000000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollara A, Brown TJ. Functional interaction of nuclear receptor coactivator 4 with aryl hydrocarbon receptor. Biochem Biophys Res Commun. 2006;346:526–534. doi: 10.1016/j.bbrc.2006.05.148. [DOI] [PubMed] [Google Scholar]
- Kozak KR, Su F, Whitelegge JP, Faull K, Reddy S, Farias-Eisner R. Characterization of serum biomarkers for detection of early stage ovarian cancer. Proteomics. 2005;5:4589–4596. doi: 10.1002/pmic.200500093. [DOI] [PubMed] [Google Scholar]
- Kyriakidis I, Papaioannidou P. Estrogen receptor beta and ovarian cancer: a key to pathogenesis and response to therapy. Arch Gynecol Obstet. 2016;293:1161–1168. doi: 10.1007/s00404-016-4027-8. [DOI] [PubMed] [Google Scholar]
- Lagergren K, Wahlin K, Mattsson F, Alderson D, Lagergren J. Haemochromatosis and gastrointestinal cancer. Int J Cancer. 2016;139:1740–1743. doi: 10.1002/ijc.30229. [DOI] [PubMed] [Google Scholar]
- Lanzino M, De Amicis F, McPhaul MJ, Marsico S, Panno ML, Ando S. Endogenous coactivator ARA70 interacts with estrogen receptor alpha (ERalpha) and modulates the functional ERalpha/androgen receptor interplay in MCF-7 cells. J Biol Chem. 2005;280:20421–20430. doi: 10.1074/jbc.M413576200. [DOI] [PubMed] [Google Scholar]
- Lattuada D, Uberti F, Colciaghi B, Morsanuto V, Maldi E, Squarzanti DF, Molinari C, Boldorini R, Bulfoni A, Colombo P, et al. Fimbrial cells exposure to catalytic iron mimics carcinogenic changes. Int J Gynecol Cancer. 2015;25:389–398. doi: 10.1097/IGC.0000000000000379. [DOI] [PubMed] [Google Scholar]
- Leslie NR, Bennett D, Lindsay YE, Stewart H, Gray A, Downes CP. Redox regulation of PI 3-kinase signalling via inactivation of PTEN. EMBO J. 2003;22:5501–5510. doi: 10.1093/emboj/cdg513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DW, Spector A. Hydrogen peroxide-induced expression of the proto-oncogenes, c-jun, c-fos and c-myc in rabbit lens epithelial cells. Mol Cell Biochem. 1997;173:59–69. doi: 10.1023/a:1006828402225. [DOI] [PubMed] [Google Scholar]
- Liehr JG, Jones JS. Role of iron in estrogen-induced cancer. Curr Med Chem. 2001;8:839–849. doi: 10.2174/0929867013372931. [DOI] [PubMed] [Google Scholar]
- Ligr M, Li Y, Zou X, Daniels G, Melamed J, Peng Y, Wang W, Wang J, Ostrer H, Pagano M, et al. Tumor suppressor function of androgen receptor coactivator ARA70alpha in prostate cancer. Am J Pathol. 2010;176:1891–1900. doi: 10.2353/ajpath.2010.090293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Sun B, Yin H, Liu S. Hepcidin: A Promising Therapeutic Target for Iron Disorders: A Systematic Review. Medicine (Baltimore) 2016;95:e3150. doi: 10.1097/MD.0000000000003150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Popovich Z, Templeton DM. Global genomic approaches to the iron-regulated proteome. Ann Clin Lab Sci. 2005;35:230–239. [PubMed] [Google Scholar]
- Lobello N, Biamonte F, Pisanu ME, Faniello MC, Jakopin Z, Chiarella E, Giovannone ED, Mancini R, Ciliberto G, Cuda G, et al. Ferritin heavy chain is a negative regulator of ovarian cancer stem cell expansion and epithelial to mesenchymal transition. Oncotarget. 2016 doi: 10.18632/oncotarget.11495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louandre C, Ezzoukhry Z, Godin C, Barbare JC, Maziere JC, Chauffert B, Galmiche A. Iron-dependent cell death of hepatocellular carcinoma cells exposed to sorafenib. Int J Cancer. 2013;133:1732–1742. doi: 10.1002/ijc.28159. [DOI] [PubMed] [Google Scholar]
- Louandre C, Marcq I, Bouhlal H, Lachaier E, Godin C, Saidak Z, Francois C, Chatelain D, Debuysscher V, Barbare JC, et al. The retinoblastoma (Rb) protein regulates ferroptosis induced by sorafenib in human hepatocellular carcinoma cells. Cancer Lett. 2015;356:971–977. doi: 10.1016/j.canlet.2014.11.014. [DOI] [PubMed] [Google Scholar]
- Luczaj W, Skrzydlewska E. DNA damage caused by lipid peroxidation products. Cell Mol Biol Lett. 2003;8:391–413. [PubMed] [Google Scholar]
- Lui GY, Kovacevic Z, Richardson V, Merlot AM, Kalinowski DS, Richardson DR. Targeting cancer by binding iron: Dissecting cellular signaling pathways. Oncotarget. 2015;6:18748–18779. doi: 10.18632/oncotarget.4349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macuks R, Baidekalna I, Donina S. An ovarian cancer malignancy risk index composed of HE4, CA125, ultrasonographic score, and menopausal status: use in differentiation of ovarian cancers and benign lesions. Tumour Biol. 2012;33:1811–1817. doi: 10.1007/s13277-012-0440-1. [DOI] [PubMed] [Google Scholar]
- Maehira F, Miyagi I, Asato T, Eguchi Y, Takei H, Nakatsuki K, Fukuoka M, Zaha F. Alterations of protein kinase C, 8-hydroxydeoxyguanosine, and K-ras oncogene in rat lungs exposed to passive smoking. Clin Chim Acta. 1999;289:133–144. doi: 10.1016/s0009-8981(99)00163-1. [DOI] [PubMed] [Google Scholar]
- Mancias JD, Wang X, Gygi SP, Harper JW, Kimmelman AC. Quantitative proteomics identifies NCOA4 as the cargo receptor mediating ferritinophagy. Nature. 2014;509:105–109. doi: 10.1038/nature13148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancias JD, Pontano Vaites L, Nissim S, Biancur DE, Kim AJ, Wang X, Liu Y, Goessling W, Kimmelman AC, Harper JW. Ferritinophagy via NCOA4 is required for erythropoiesis and is regulated by iron dependent HERC2-mediated proteolysis. Elife. 2015;4 doi: 10.7554/eLife.10308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrogi AJ, Khan MA, van Gijssel HE, Welsh JA, Rahim H, Demetris AJ, Kowdley KV, Hussain SP, Nair J, Bartsch H, et al. Oxidative stress and p53 mutations in the carcinogenesis of iron overload-associated hepatocellular carcinoma. J Natl Cancer Inst. 2001;93:1652–1655. doi: 10.1093/jnci/93.21.1652. [DOI] [PubMed] [Google Scholar]
- Martin OC, Lin C, Naud N, Tache S, Raymond-Letron I, Corpet DE, Pierre FH. Antibiotic suppression of intestinal microbiota reduces heme-induced lipoperoxidation associated with colon carcinogenesis in rats. Nutr Cancer. 2015;67:119–125. doi: 10.1080/01635581.2015.976317. [DOI] [PubMed] [Google Scholar]
- Martinez MB, Ruan M, Fitzpatrick LA. Altered response to thyroid hormones by breast and ovarian cancer cells. Anticancer Res. 2000;20:4141–4146. [PubMed] [Google Scholar]
- Mayr R, Janecke AR, Schranz M, Griffiths WJ, Vogel W, Pietrangelo A, Zoller H. Ferroportin disease: a systematic meta-analysis of clinical and molecular findings. J Hepatol. 2010;53:941–949. doi: 10.1016/j.jhep.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKie AT, Barrow D, Latunde-Dada GO, Rolfs A, Sager G, Mudaly E, Mudaly M, Richardson C, Barlow D, Bomford A, et al. An iron-regulated ferric reductase associated with the absorption of dietary iron. Science. 2001;291:1755–1759. doi: 10.1126/science.1057206. [DOI] [PubMed] [Google Scholar]
- Miller LD, Coffman LG, Chou JW, Black MA, Bergh J, D’Agostino R, Jr, Torti SV, Torti FM. An iron regulatory gene signature predicts outcome in breast cancer. Cancer Res. 2011;71:6728–6737. doi: 10.1158/0008-5472.CAN-11-1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miseta A, Nagy J, Nagy T, Poor VS, Fekete Z, Sipos K. Hepcidin and its potential clinical utility. Cell Biol Int. 2015;39:1191–1202. doi: 10.1002/cbin.10505. [DOI] [PubMed] [Google Scholar]
- Moore LE, Pfeiffer RM, Zhang Z, Lu KH, Fung ET, Bast RC., Jr Proteomic biomarkers in combination with CA 125 for detection of epithelial ovarian cancer using prediagnostic serum samples from the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial. Cancer. 2012;118:91–100. doi: 10.1002/cncr.26241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori M, Ito F, Shi L, Wang Y, Ishida C, Hattori Y, Niwa M, Hirayama T, Nagasawa H, Iwase A, et al. Ovarian endometriosis-associated stromal cells reveal persistently high affinity for iron. Redox Biol. 2015;6:578–586. doi: 10.1016/j.redox.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mungenast F, Thalhammer T. Estrogen biosynthesis and action in ovarian cancer. Front Endocrinol (Lausanne) 2014;5:192. doi: 10.3389/fendo.2014.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIJ, Derrien M, van Doorn GM, Rijnierse A, van den Bogert B, Muller M, Dekker J, Kleerebezem M, van der Meer R. Dietary heme alters microbiota and mucosa of mouse colon without functional changes in host-microbe cross-talk. PLoS One. 2012;7:e49868. doi: 10.1371/journal.pone.0049868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng O. Iron, microbiota and colorectal cancer. Wien Med Wochenschr. 2016;166:431–436. doi: 10.1007/s10354-016-0508-4. [DOI] [PubMed] [Google Scholar]
- Nirei K, Matsuoka S, Nakamura H, Matsumura H, Moriyama M. Incidence of hepatocellular carcinoma reduced by phlebotomy treatment in patients with chronic hepatitis C. Intern Med. 2015;54:107–117. doi: 10.2169/internalmedicine.54.2715. [DOI] [PubMed] [Google Scholar]
- Peng Y, Li CX, Chen F, Wang Z, Ligr M, Melamed J, Wei J, Gerald W, Pagano M, Garabedian MJ, et al. Stimulation of prostate cancer cellular proliferation and invasion by the androgen receptor co-activator ARA70. Am J Pathol. 2008;172:225–235. doi: 10.2353/ajpath.2008.070065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pigeon C, Ilyin G, Courselaud B, Leroyer P, Turlin B, Brissot P, Loreal O. A new mouse liver-specific gene, encoding a protein homologous to human antimicrobial peptide hepcidin, is overexpressed during iron overload. J Biol Chem. 2001;276:7811–7819. doi: 10.1074/jbc.M008923200. [DOI] [PubMed] [Google Scholar]
- Powell LW, Seckington RC, Deugnier Y. Haemochromatosis. Lancet. 2016;388:706–716. doi: 10.1016/S0140-6736(15)01315-X. [DOI] [PubMed] [Google Scholar]
- Qian Y, Yin C, Chen Y, Zhang S, Jiang L, Wang F, Zhao M, Liu S. Estrogen contributes to regulating iron metabolism through governing ferroportin signaling via an estrogen response element. Cell Signal. 2015;27:934–942. doi: 10.1016/j.cellsig.2015.01.017. [DOI] [PubMed] [Google Scholar]
- Ross SL, Tran L, Winters A, Lee KJ, Plewa C, Foltz I, King C, Miranda LP, Allen J, Beckman H, et al. Molecular mechanism of hepcidin-mediated ferroportin internalization requires ferroportin lysines, not tyrosines or JAK-STAT. Cell Metab. 2012;15:905–917. doi: 10.1016/j.cmet.2012.03.017. [DOI] [PubMed] [Google Scholar]
- Rouault TA. Mammalian iron-sulphur proteins: novel insights into biogenesis and function. Nat Rev Mol Cell Biol. 2015;16:45–55. doi: 10.1038/nrm3909. [DOI] [PubMed] [Google Scholar]
- Sanchez AM, Papaleo E, Corti L, Santambrogio P, Levi S, Vigano P, Candiani M, Panina-Bordignon P. Iron availability is increased in individual human ovarian follicles in close proximity to an endometrioma compared with distal ones. Hum Reprod. 2014;29:577–583. doi: 10.1093/humrep/det466. [DOI] [PubMed] [Google Scholar]
- Sansone P, Storci G, Tavolari S, Guarnieri T, Giovannini C, Taffurelli M, Ceccarelli C, Santini D, Paterini P, Marcu KB, et al. IL-6 triggers malignant features in mammospheres from human ductal breast carcinoma and normal mammary gland. J Clin Invest. 2007;117:3988–4002. doi: 10.1172/JCI32533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman JD. The presence of mucosal iron in the fallopian tube supports the “incessant menstruation hypothesis” for ovarian carcinoma. Int J Gynecol Pathol. 2013;32:454–458. doi: 10.1097/PGP.0b013e31826f5ce2. [DOI] [PubMed] [Google Scholar]
- Shaw PA, Rittenberg PV, Brown TJ. Activation of androgen receptor-associated protein 70 (ARA70) mRNA expression in ovarian cancer. Gynecol Oncol. 2001;80:132–138. doi: 10.1006/gyno.2000.6068. [DOI] [PubMed] [Google Scholar]
- Shi H, Bencze KZ, Stemmler TL, Philpott CC. A cytosolic iron chaperone that delivers iron to ferritin. Science. 2008;320:1207–1210. doi: 10.1126/science.1157643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigeta S, Toyoshima M, Kitatani K, Ishibashi M, Usui T, Yaegashi N. Transferrin facilitates the formation of DNA double-strand breaks via transferrin receptor 1: the possible involvement of transferrin in carcinogenesis of high-grade serous ovarian cancer. Oncogene. 2016;35:3577–3586. doi: 10.1038/onc.2015.425. [DOI] [PubMed] [Google Scholar]
- Singh AK, Chattopadhyay R, Chakravarty B, Chaudhury K. Markers of oxidative stress in follicular fluid of women with endometriosis and tubal infertility undergoing IVF. Reprod Toxicol. 2013;42:116–124. doi: 10.1016/j.reprotox.2013.08.005. [DOI] [PubMed] [Google Scholar]
- Skouta R, Dixon SJ, Wang J, Dunn DE, Orman M, Shimada K, Rosenberg PA, Lo DC, Weinberg JM, Linkermann A, et al. Ferrostatins inhibit oxidative lipid damage and cell death in diverse disease models. J Am Chem Soc. 2014;136:4551–4556. doi: 10.1021/ja411006a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein P, Yu H, Jain D, Mistry PK. Hyperferritinemia and iron overload in type 1 Gaucher disease. Am J Hematol. 2010;85:472–476. doi: 10.1002/ajh.21721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Ou Z, Xie M, Kang R, Fan Y, Niu X, Wang H, Cao L, Tang D. HSPB1 as a novel regulator of ferroptotic cancer cell death. Oncogene. 2015;34:5617–5625. doi: 10.1038/onc.2015.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Ou Z, Chen R, Niu X, Chen D, Kang R, Tang D. Activation of the p62-Keap1-NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells. Hepatology. 2016;63:173–184. doi: 10.1002/hep.28251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tak PP, Zvaifler NJ, Green DR, Firestein GS. Rheumatoid arthritis and p53: how oxidative stress might alter the course of inflammatory diseases. Immunol Today. 2000;21:78–82. doi: 10.1016/s0167-5699(99)01552-2. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Iwasa Y, Kondo S, Hiai H, Toyokuni S. High incidence of allelic loss on chromosome 5 and inactivation of p15INK4B and p16INK4A tumor suppressor genes in oxystress-induced renal cell carcinoma of rats. Oncogene. 1999;18:3793–3797. doi: 10.1038/sj.onc.1202707. [DOI] [PubMed] [Google Scholar]
- Tarangelo A, Dixon SJ. Nanomedicine: An iron age for cancer therapy. Nat Nanotechnol. 2016;11:921–922. doi: 10.1038/nnano.2016.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolg C, Sabha N, Cortese R, Panchal T, Ahsan A, Soliman A, Aitken KJ, Petronis A, Bagli DJ. Uropathogenic E. coli infection provokes epigenetic downregulation of CDKN2A (p16INK4A) in uroepithelial cells. Lab Invest. 2011;91:825–836. doi: 10.1038/labinvest.2010.197. [DOI] [PubMed] [Google Scholar]
- Tortorella S, Karagiannis TC. Transferrin receptor-mediated endocytosis: a useful target for cancer therapy. J Membr Biol. 2014;247:291–307. doi: 10.1007/s00232-014-9637-0. [DOI] [PubMed] [Google Scholar]
- Toyokuni S. Iron-induced carcinogenesis: the role of redox regulation. Free Radic Biol Med. 1996;20:553–566. doi: 10.1016/0891-5849(95)02111-6. [DOI] [PubMed] [Google Scholar]
- Tripathi PK, Chatterjee SK. Elevated expression of ferritin H-chain mRNA in metastatic ovarian tumor. Cancer Invest. 1996;14:518–526. doi: 10.3109/07357909609076897. [DOI] [PubMed] [Google Scholar]
- Uberti F, Morsanuto V, Lattuada D, Colciaghi B, Cochis A, Bulfoni A, Colombo P, Bolis G, Molinari C. Protective effects of vitamin D3 on fimbrial cells exposed to catalytic iron damage. J Ovarian Res. 2016;9:34. doi: 10.1186/s13048-016-0243-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact. 2006;160:1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Van Langendonckt A, Casanas-Roux F, Donnez J. Iron overload in the peritoneal cavity of women with pelvic endometriosis. Fertil Steril. 2002;78:712–718. doi: 10.1016/s0015-0282(02)03346-0. [DOI] [PubMed] [Google Scholar]
- Vercellini P, Crosignani P, Somigliana E, Vigano P, Buggio L, Bolis G, Fedele L. The ‘incessant menstruation’ hypothesis: a mechanistic ovarian cancer model with implications for prevention. Hum Reprod. 2011;26:2262–2273. doi: 10.1093/humrep/der211. [DOI] [PubMed] [Google Scholar]
- Vyhlidal C, Li X, Safe S. Estrogen regulation of transferrin gene expression in MCF-7 human breast cancer cells. J Mol Endocrinol. 2002;29:305–317. doi: 10.1677/jme.0.0290305. [DOI] [PubMed] [Google Scholar]
- Wang J, Pantopoulos K. Regulation of cellular iron metabolism. Biochem J. 2011;434:365–381. doi: 10.1042/BJ20101825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward DM, Kaplan J. Ferroportin-mediated iron transport: expression and regulation. Biochim Biophys Acta. 2012;1823:1426–1433. doi: 10.1016/j.bbamcr.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss G, Houston T, Kastner S, Johrer K, Grunewald K, Brock JH. Regulation of cellular iron metabolism by erythropoietin: activation of iron-regulatory protein and upregulation of transferrin receptor expression in erythroid cells. Blood. 1997;89:680–687. [PubMed] [Google Scholar]
- Winterbourn CC. Toxicity of iron and hydrogen peroxide: the Fenton reaction. Toxicol Lett. 1995;82–83:969–974. doi: 10.1016/0378-4274(95)03532-x. [DOI] [PubMed] [Google Scholar]
- Wu X, Chen F, Sahin A, Albarracin C, Pei Z, Zou X, Singh B, Xu R, Daniels G, Li Y, et al. Distinct function of androgen receptor coactivator ARA70alpha and ARA70beta in mammary gland development, and in breast cancer. Breast Cancer Res Treat. 2011;128:391–400. doi: 10.1007/s10549-010-1131-5. [DOI] [PubMed] [Google Scholar]
- Wyllie S, Liehr JG. Enhancement of estrogen-induced renal tumorigenesis in hamsters by dietary iron. Carcinogenesis. 1998;19:1285–1290. doi: 10.1093/carcin/19.7.1285. [DOI] [PubMed] [Google Scholar]
- Yamada Y, Shigetomi H, Onogi A, Haruta S, Kawaguchi R, Yoshida S, Furukawa N, Nagai A, Tanase Y, Tsunemi T, et al. Redox-active iron-induced oxidative stress in the pathogenesis of clear cell carcinoma of the ovary. Int J Gynecol Cancer. 2011;21:1200–1207. doi: 10.1097/IGC.0b013e318222cfdd. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K, Mandai M, Toyokuni S, Hamanishi J, Higuchi T, Takakura K, Fujii S. Contents of endometriotic cysts, especially the high concentration of free iron, are a possible cause of carcinogenesis in the cysts through the iron-induced persistent oxidative stress. Clin Cancer Res. 2008;14:32–40. doi: 10.1158/1078-0432.CCR-07-1614. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K, Mandai M, Oura T, Matsumura N, Hamanishi J, Baba T, Matsui S, Murphy SK, Konishi I. Identification of an ovarian clear cell carcinoma gene signature that reflects inherent disease biology and the carcinogenic processes. Oncogene. 2010;29:1741–1752. doi: 10.1038/onc.2009.470. [DOI] [PubMed] [Google Scholar]
- Yang WS, SriRamaratnam R, Welsch ME, Shimada K, Skouta R, Viswanathan VS, Cheah JH, Clemons PA, Shamji AF, Clish CB, et al. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156:317–331. doi: 10.1016/j.cell.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan H, Li X, Zhang X, Kang R, Tang D. CISD1 inhibits ferroptosis by protection against mitochondrial lipid peroxidation. Biochem Biophys Res Commun. 2016;478:838–844. doi: 10.1016/j.bbrc.2016.08.034. [DOI] [PubMed] [Google Scholar]
- Zacharski LR, Chow BK, Howes PS, Shamayeva G, Baron JA, Dalman RL, Malenka DJ, Ozaki CK, Lavori PW. Decreased cancer risk after iron reduction in patients with peripheral arterial disease: results from a randomized trial. J Natl Cancer Inst. 2008;100:996–1002. doi: 10.1093/jnci/djn209. [DOI] [PubMed] [Google Scholar]
- Zhang C, Zhang F. Iron homeostasis and tumorigenesis: molecular mechanisms and therapeutic opportunities. Protein Cell. 2015;6:88–100. doi: 10.1007/s13238-014-0119-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Tao Y, Zhang Z, Guo X, An P, Shen Y, Wu Q, Yu Y, Wang F. Metalloreductase Steap3 coordinates the regulation of iron homeostasis and inflammatory responses. Haematologica. 2012;97:1826–1835. doi: 10.3324/haematol.2012.063974. [DOI] [PMC free article] [PubMed] [Google Scholar]