Abstract
Sudden unexpected death in epilepsy (SUDEP) is a devastating epilepsy complication, and no effective preventive strategies are currently available for this fatal disorder. Clinical and animal studies of SUDEP demonstrate that seizure-induced respiratory arrest (S-IRA) is the primary event leading to death after generalized seizures in many cases. Enhancing brain levels of serotonin reduces S-IRA in animal models relevant to SUDEP, including the DBA/1 mouse. Given that serotonin in the brain plays an important role in modulating respiration and arousal, these findings suggest that deficits in respiration and/or arousal may contribute to S-IRA. It is well known that norepinephrine is an important neurotransmitter that modulates respiration and arousal in the brain as well. Therefore, we hypothesized that enhancing noradrenergic neurotransmission suppresses S-IRA. To test this hypothesis, we examined the effect of atomoxetine, a norepinephrine reuptake inhibitor (NRI), on S-IRA evoked by either acoustic stimulation or pentylenetetrazole in DBA/1 mice. We report the original observation that atomoxetine specifically suppresses S-IRA without altering the susceptibility to seizures evoked by acoustic stimulation, and atomoxetine also reduces S-IRA evoked by pentylenetetrazole in DBA/1 mice. Our data suggest that the noradrenergic signaling is importantly involved in S-IRA, and that atomoxetine, a medication widely used to treat attention deficit hyperactivity disorder (ADHD), is potentially useful to prevent SUDEP.
Keywords: SUDEP, NRI, noradrenergic neurotransmission, pentylenetetrazole, therapeutics
1. Introduction
Sudden unexpected death in epilepsy (SUDEP) is a major burden on public health compared with other common neurological disorders, as it is the main contributor to premature deaths in the population with epilepsy [1-3]. Studies in animal models and SUDEP patients indicate that seizure-induced respiratory arrest (S-IRA) is the primary instigator that leads to death in many cases [4-7]. Thus, investigating the mechanisms and treatment of S-IRA will help develop preventive strategies against SUDEP. Enhancing the levels of brain serotonin, a monoamine that is involved in respiration and arousal [8, 9], by several selective serotonin reuptake inhibitors (SSRIs) [6, 10-12] and 5-hydroxytryptophan [7] reduces S-IRA in animal models relevant to SUDEP, including the DBA/1 mouse. These findings suggest that deficits in respiration and/or arousal may contribute to S-IRA. Norepinephrine, another monoamine, is also importantly involved in modulating respiration and arousal [13, 14]. Thus, we hypothesized that enhancing noradrenergic neurotransmission is effective in preventing S-IRA. In the current study, we tested this hypothesis by examining the effect of atomoxetine, a norepinephrine reuptake inhibitor (NRI) that enhances the levels of norepinephrine in the synaptic cleft, on the incidence of S-IRA in DBA/1 mice.
We tested the effect of atomoxetine on S-IRA in DBA/1 mice evoked by either acoustic stimulation or pentylenetetrazole (PTZ), a chemoconvulsant widely used to model human generalized tonic-clonic seizures [15]. Our data showed that S-IRA was suppressed by atomoxetine in DBA/1 mice, independent of seizure induction methods. These observations suggest that the noradrenergic neurotransmission is importantly involved in the pathogenesis of S-IRA, and that atomoxetine, a drug commonly used to treat attention deficit hyperactivity disorder (ADHD), may have significant translational potential to prevent SUDEP in at-risk patients.
2. Material and methods
2.1. Animals
All experimental procedures were approved by the Institutional Animal Care and Use Committee of Massachusetts General Hospital. DBA/1 mice were housed and bred in the Massachusetts General Hospital Center for Comparative Medicine animal facility and provided with rodent food and water ad libitum. Some DBA/1 mice were “primed” starting from postnatal day 26-28 by daily subjecting to acoustic stimulation for 3-4 days to establish consistent susceptibility to S-IRA [7]. Primed DBA/1 mice at approximately 1-2 months of age were used in the seizure model induced by acoustic stimulation, and nonprimed DBA/1 at approximately 2 months of age were used in the seizure model evoked by PTZ.
2.2. S-IRA evoked by different seizure models (acoustic stimulation vs. PTZ)
Generalized seizures and S-IRA were evoked either by acoustic stimulation or by intraperitoneal (IP) administration of PTZ in DBA/1 mice, as previously described [7]. Briefly, in acoustic stimulation model, each DBA/1 mouse was placed in a cylindrical plexiglass chamber in a sound-isolated room, and audiogenic seizures were evoked using an electric bell (96 dB SPL, UC4-150, Zhejiang People's Electronics, China). The acoustic stimulus was given for a maximum duration of 60 s or until the mouse exhibited tonic seizures and S-IRA in most cases. Mice with S-IRA were resuscitated using a rodent respirator (Harvard Apparatus 680, Holliston, MA). S-IRA was also induced in all nonprimed DBA/1 mice by IP administration of a single dose of PTZ (Cat # P6500; Sigma-Aldrich, St. Louis, MO) at 75 mg/kg.
2.3. The effect of atomoxetine on S-IRA
A vehicle control group and treatment groups with different dosages of atomoxetine were included in testing of S-IRA in acoustic stimulation model and PTZ model, respectively. S-IRA was confirmed 24 hr before atomoxetine or vehicle administration in the acoustic stimulation model. Atomoxetine (1-50 mg/kg for acoustic stimulation model or 5-15 mg/kg for PTZ model) or vehicle was administered IP in DBA/1 mice 2 hr prior to acoustic stimulation or IP injection of PTZ. The occurrence of S-IRA was videotaped for offline analysis. Atomoxetine (Cat # Y0001586; Sigma-Aldrich) was dissolved in saline for IP administration.
2.4. Statistical analysis
The incidence of S-IRA was compared among drug and control groups using Wilcoxon Signed Rank test, as these data are nonparametric [7, 16]. The data on the duration of tonic seizures are parametric, which were reported as mean ± SD. One-way ANOVA and post-hoc Tukey's test were used to compare the duration of tonic seizures among drug and control groups. Statistical significance was inferred if p < 0.05.
3. Results
3.1. The effect of atomoxetine on S-IRA evoked by acoustic stimulation
Audiogenic seizures in DBA/1 mice are characterized by wild running episodes and generalized tonic-clonic seizures [2]. The incidence of S-IRA was significantly lower in the groups treated with the NRI atomoxetine at 10 mg/kg (54.5%, n = 11; p < 0.05) and 15 mg/kg (10%, n = 10; p < 0.01) as compared with vehicle control group (100%, n = 7) in primed DBA/1 mice. S-IRA was suppressed by atomoxetine at 20 mg/kg in all 7 mice tested, which was significantly different from vehicle control group (p < 0.01). As atomoxetine dosages were further increased, its effect on S-IRA became less effective, although a significantly lower incidence of S-IRA was still observed at 40 mg/kg atomoxetine (44.4%, n = 9; p < 0.05) as compared with vehicle control group. Atomoxetine at lower dosages (1 and 5 mg/kg) and at the highest dosage (50 mg/kg) tested did not significantly suppress S-IRA in these mice (Fig 1).
Figure 1. Atomoxetine reduces S-IRA evoked by acoustic stimulation in DBA/1 mice.
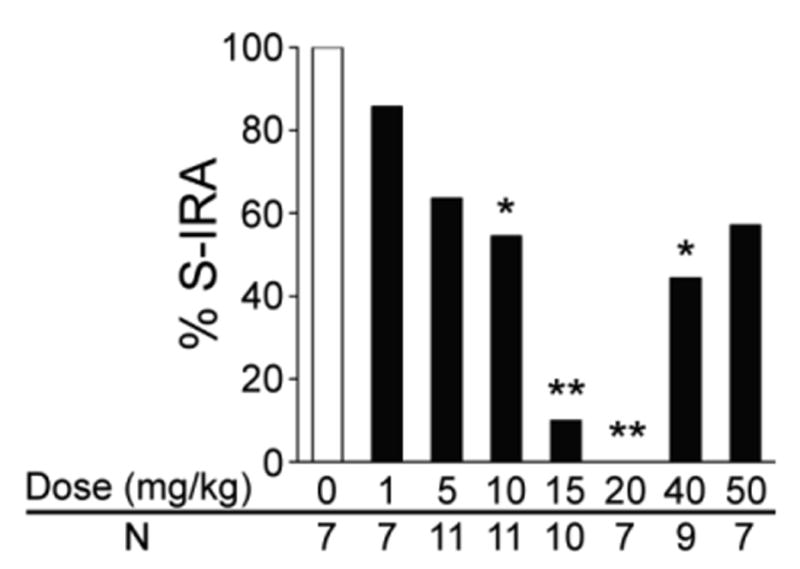
Compared with that of the control group treated with vehicle (saline), the incidence of S-IRA evoked by acoustic stimulation was significantly lower in groups treated with atomoxetine at 10-40 mg/kg in primed DBA/1 mice. The incidence of S-IRA in groups treated with lower dosages of atomoxetine (1 and 5 mg/kg) and the highest dosage tested (50 mg/kg) was not significantly different from that in vehicle control group. Atomoxetine was administered IP 2 hr prior to induction of audiogenic seizures.
* p < 0.05; ** p < 0.01: Significantly different from the vehicle control group.
In the effective dosage range (10-40 mg/kg), atomoxetine specifically suppressed S-IRA without interrupting any seizure behaviors in most of the mice that did not exhibit S-IRA. Although atomoxetine at 15 mg/kg and 40 mg/kg blocked generalized tonic-clonic seizures and tonic seizures in one DBA/1 mouse, respectively, these two mice still exhibited wild running and/or clonic audiogenic seizures. As S-IRA always follows tonic seizures in DBA/1 mice, we also compared the duration of tonic seizures in the absence and presence of atomoxetine. The duration of tonic seizures at 10 mg/kg (12.2 +1.3 sec), 15 mg/kg (10.9 ±4.0 sec), 20 mg/kg (12.7 ± 1.5 sec) or 40 mg/kg (10.8 ± 4.2 sec) atomoxetine was not significantly different from that in vehicle control group (11.7 + 1.1 sec) (Fig 2). Administration of atomoxetine did not result in any obvious behavioral changes in the absence of seizures in DBA/1 mice.
Figure 2. The effect of atomoxetine on the duration of tonic audiogenic seizures in DBA/1 mice.
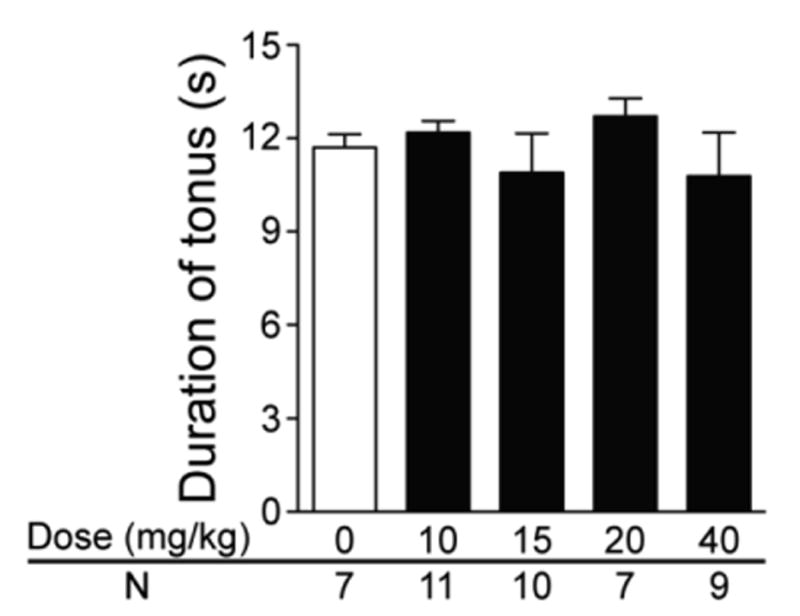
Compared with that of the vehicle control group, the duration of tonic audiogenic seizures was not significantly altered by atomoxetine at 10-40 mg/kg in primed DBA/1 mice, although S-IRA was suppressed by atomoxetine in these animals. Atomoxetine was administered IP 2 hr prior to induction of audiogenic seizures.
3.2. The effect of atomoxetine on S-IRA evoked by PTZ
To exclude the possibility that atomoxetine action on S-IRA is dependent on seizure models, we also tested the effect of atomoxetine on S-IRA evoked by PTZ in nonprimed DBA/1 mice, never exposed to acoustic stimulation. As compared with the incidence of S-IRA evoked by PTZ in vehicle control group (100%, n = 6), the incidence of S-IRA was significantly lower in the groups treated with atomoxetine at 10 mg/kg (28.6%, n = 7; p < 0.05) and 15 mg/kg (28.6%, n = 7; p < 0.05). Atomoxetine at 5 mg/kg did not significantly suppress S-IRA in these mice (Fig 3).
Figure 3. Atomoxetine suppresses S-IRA evoked by PTZ in DBA/1 mice.
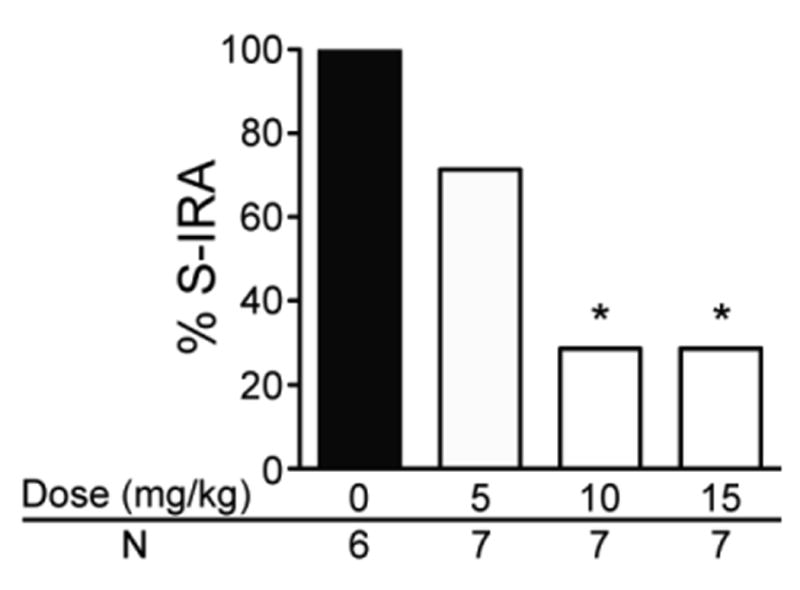
The incidence of S-IRA evoked by PTZ was significantly lower in groups treated with atomoxetine at 10 mg/kg and 15 mg/kg as compared with that of the control group treated with vehicle in nonprimed DBA/1 mice. Atomoxetine was administered IP 2 hr prior to injection of PTZ (75 mg/kg).
* p < 0.05: Significantly different from the vehicle control group.
4. Discussion
In the current study, we demonstrate that atomoxetine, an NRI, suppresses S-IRA in DBA/1 mice, regardless of seizure induction methods. The suppressant effect of atomoxetine on S-IRA evoked by acoustic stimulation is dose-dependent. The maximal reduction in the incidence of S-IRA was observed at 20 mg/kg atomoxetine. Further increase in the dosages diminished the suppressant effect of atomoxetine, probably due to activation of presynaptic adrenergic a2 autoreceptors that inhibit norepinephrine release [14]. Although atomoxetine was reported to produce protective effect against seizures at higher dosages in animal models [17], and atomoxetine at relatively lower dosages is associated with reduced seizure risk in patients [18], we observed that atomoxetine at 10-40 mg/kg reduced S-IRA without interfering with audiogenic seizure behaviors in most of animals tested. The duration of tonic phase of audiogenic seizures in atomoxetine treatment group was not significantly different from that in control group, indicating that atomoxetine specifically blocks S-IRA without affecting the severity of audiogenic seizures. It is not known why generalized tonic and/or clonic seizures were suppressed by atomoxetine in two DBA/1 mice.
Foregoing studies implicate serotonergic signaling in the pathogenesis of S-IRA [19]. For example, several SSRIs specifically suppress S-IRA without altering seizure susceptibility in animal models relevant to SUDEP [10-12, 16, 20]. Our recent study shows that 5-hydroxytryptophan, a chemical precursor for serotonin synthesis, selectively reduces S-IRA without interfering with audiogenic seizure susceptibility in DBA/1 mice [7, 21]. However, the contribution of the other neurotransmission systems to S-IRA is unknown. Our current study, for the first time, demonstrated that atomoxetine, an NRI, specifically suppresses S-IRA without affecting audiogenic seizure behaviors. Given that systemic administration of atomoxetine enhances norepinephrine levels throughout the brain [22], our finding suggests that the noradrenergic neurotransmission plays an important role in the pathophysiology of S-IRA. It should be noted that, although atomoxetine is highly selective to norepinephrine transporters, it also binds to dopamine and serotonin transporters with low affinity [23, 24]. However, microdialysis studies show that atomoxetine does not elevate serotonin levels in the brain [23, 25], and atomoxetine does not enhance dopamine availability in dopamine-rich regions such as the nucleus accumbens and striatum, although it increases that in the prefrontal cortex [24]. Future studies are required to examine if the limited enhancement of dopamine in the prefrontal cortex contributes to S-IRA.
The mechanisms underlying atomoxetine suppression of S-IRA are unknown. Like serotonergic neurotransmission, noradrenergic neurotransmission in the brain modulates respiration [13] and arousal [14]. Thus, stimulation of medullary noradrenergic neurons alters arousal state and respiratory frequency [26]. It is possible that atomoxetine suppresses S-IRA in DBA/1 mice by enhancing respiration and/or arousal. Further studies are needed to explore these possibilities. Interestingly, noradrenergic neurotransmission interacts with serotonergic neurotransmission in certain brain areas [27, 28]. For example, stimulation of the 5-HT3 receptor, a serotonin receptor that is involved in S-IRA in DBA/1 mice [29], elevates norepinephrine level in the locus coeruleus [14]. Better understanding the interaction of noradrenergic and serotonergic neurotransmission in S-IRA will shed significant light on the mechanisms of SUDEP.
Atomoxetine is a medication widely used to treat ADHD around the world [30]. Our study suggests that NRIs such as atomoxetine merit further translational investigation as potential medications to prevent SUDEP. The DBA/1 mouse has been demonstrated to be an animal model relevant to SUDEP, as the pathophysiology and putative therapeutics in this model are generalizable to other animal models and humans [5, 6, 31]. However, like other animal models of provoked seizures, no consistent spontaneous seizures are reported in DBA/1 mice, which always occur in epileptic patients. It is important to confirm our results in other animal models with spontaneous seizures and in clinical trials prior to use in patients.
Co-morbidity of ADHD and epilepsy is not uncommon in pediatric patients [17]. It is not known if ADHD is associated with SUDEP or not. However, the effectiveness of atomoxetine in controlling both ADHD and S-IRA suggests that ADHD and SUDEP may share similar pathophysiology to some extent. Elucidating the pathophysiological mechanisms of atomoxetine in suppressing S-IRA may help to better understand the mechanism and treatment of ADHD.
Taken together, our data demonstrate that enhancing noradrenergic neurotransmission by the NRI atomoxetine suppresses S-IRA evoked by different models of seizures in DBA/1 mice. This finding suggests that the noradrenergic neurotransmission is importantly involved in S-IRA, and that atomoxetine, as well as other NRIs, can be potentially used to prevent SUDEP in patents with epilepsy.
Highlights.
Atomoxetine reduces S-IRA independent of seizure models
Noradrenergic neurotransmission is importantly involved in S-IRA
NRIs have translational potential to prevent SUDEP
Acknowledgments
The authors thank Dr. Srinivasan Tupal (in Dr. Carl Faingold's lab) for statistical help. This work was supported by R03NS078591, R21NS101311, CURE (Citizens United for Research in Epilepsy) foundation and Massachusetts General Hospital Department of Anesthesia, Critical Care and Pain Medicine. Haiting Zhao is a recipient of fellowship from China Scholarship Council (CSC 201506370075).
Abbreviations
- ADHD
Attention deficit hyperactivity disorder
- IP
Intraperitoneal(ly)
- PTZ
Pentylenetetrazol(e)
- S-IRA
Seizure-induced respiratory arrest
- NRI
Norepinephrine reuptake inhibitor
- SSRI
Selective serotonin reuptake inhibitor
- SUDEP
Sudden unexpected death in epilepsy
Footnotes
Conflicts of interest: None of the authors has any conflict of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Thurman DJ, Hesdorffer DC, French JA. Sudden unexpected death in epilepsy: assessing the public health burden. Epilepsia. 2014;55:1479–85. doi: 10.1111/epi.12666. [DOI] [PubMed] [Google Scholar]
- 2.Feng HJ, Faingold CL. Abnormalities of serotonergic neurotransmission in animal models of SUDEP. Epilepsy Behav. 2015 Aug 10; doi: 10.1016/j.yebeh.2015.06.008. pii: S1525-5050(15)00339-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rugg-Gunn F, Duncan J, Hjalgrim H, Seyal M, Bateman L. From unwitnessed fatality to witnessed rescue: Nonpharmacologic interventions in sudden unexpected death in epilepsy. Epilepsia. 2016;57(Suppl 1):26–34. doi: 10.1111/epi.13231. [DOI] [PubMed] [Google Scholar]
- 4.Faingold CL, Randall M, Tupal S. DBA/1 mice exhibit chronic susceptibility to audiogenic seizures followed by sudden death associated with respiratory arrest. Epilepsy Behav. 2010;17:436–40. doi: 10.1016/j.yebeh.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 5.Ryvlin P, Nashef L, Lhatoo SD, Bateman LM, Bird J, Bleasel A, Boon P, Crespel A, Dworetzky BA, Hogenhaven H, Lerche H, Maillard L, Malter MP, Marchal C, Murthy JM, Nitsche M, Pataraia E, Rabben T, Rheims S, Sadzot B, Schulze-Bonhage A, Seyal M, So EL, Spitz M, Szucs A, Tan M, Tao JX, Tomson T. Incidence and mechanisms of cardiorespiratory arrests in epilepsy monitoring units (MORTEMUS): a retrospective study. Lancet Neurol. 2013;12:966–77. doi: 10.1016/S1474-4422(13)70214-X. [DOI] [PubMed] [Google Scholar]
- 6.Buchanan GF, Murray NM, Hajek MA, Richerson GB. Serotonin neurones have anti-convulsant effects and reduce seizure-induced mortality. J Physiol. 2014;592:4395–410. doi: 10.1113/jphysiol.2014.277574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang H, Zhao H, Yang X, Xue Q, Cotten JF, Feng HJ. 5-Hydroxytryptophan, a precursor for serotonin synthesis, reduces seizure-induced respiratory arrest. Epilepsia. 2016;57:1228–35. doi: 10.1111/epi.13430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hodges MR, Richerson GB. Contributions of 5-HT neurons to respiratory control: neuromodulatory and trophic effects. Respir Physiol Neurobiol. 2008;164:222–32. doi: 10.1016/j.resp.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Richerson GB, Buchanan GF. The serotonin axis: Shared mechanisms in seizures, depression, and SUDEP. Epilepsia. 2011;52(Suppl 1):28–38. doi: 10.1111/j.1528-1167.2010.02908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tupal S, Faingold CL. Evidence supporting a role of serotonin in modulation of sudden death induced by seizures in DBA/2 mice. Epilepsia. 2006;47:21–6. doi: 10.1111/j.1528-1167.2006.00365.x. [DOI] [PubMed] [Google Scholar]
- 11.Faingold CL, Tupal S, Randall M. Prevention of seizure-induced sudden death in a chronic SUDEP model by semichronic administration of a selective serotonin reuptake inhibitor. Epilepsy Behav. 2011;22:186–90. doi: 10.1016/j.yebeh.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 12.Zeng C, Long X, Cotten JF, Forman SA, Solt K, Faingold CL, Feng HJ. Fluoxetine prevents respiratory arrest without enhancing ventilation in DBA/1 mice. Epilepsy Behav. 2015;45:1–7. doi: 10.1016/j.yebeh.2015.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doi A, Ramirez JM. Neuromodulation and the orchestration of the respiratory rhythm. Respir Physiol Neurobiol. 2008;164:96–104. doi: 10.1016/j.resp.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berridge CW, Schmeichel BE, Espana RA. Noradrenergic modulation of wakefulness/arousal. Sleep Med Rev. 2012;16:187–97. doi: 10.1016/j.smrv.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.White HS. Clinical significance of animal seizure models and mechanism of action studies of potential antiepileptic drugs. Epilepsia. 1997;38(Suppl 1):S9–17. doi: 10.1111/j.1528-1157.1997.tb04523.x. [DOI] [PubMed] [Google Scholar]
- 16.Faingold CL, Randall M. Effects of age, sex, and sertraline administration on seizure-induced respiratory arrest in the DBA/1 mouse model of sudden unexpected death in epilepsy (SUDEP) Epilepsy Behav. 2013;28:78–82. doi: 10.1016/j.yebeh.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 17.Torres AR, Whitney J, Gonzalez-Heydrich J. Attention-deficit/hyperactivity disorder in pediatric patients with epilepsy: review of pharmacological treatment. Epilepsy Behav. 2008;12:217–33. doi: 10.1016/j.yebeh.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 18.McAfee AT, Landon J, Jones M, Bangs ME, Acharya N, Hornbuckle K, Wong J. A cohort study of the risk of seizures in a pediatric population treated with atomoxetine or stimulant medications. Pharmacoepidemiol Drug Saf. 2013;22:386–93. doi: 10.1002/pds.3390. [DOI] [PubMed] [Google Scholar]
- 19.Richerson GB, Boison D, Faingold CL, Ryvlin P. From unwitnessed fatality to witnessed rescue: Pharmacologic intervention in sudden unexpected death in epilepsy. Epilepsia. 2016;57(Suppl 1):35–45. doi: 10.1111/epi.13236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Faingold CL, Kommajosyula SP, Long X, Plath K, Randall M. Serotonin and sudden death: Differential effects of serotonergic drugs on seizure-induced respiratory arrest in DBA/1 mice. Epilepsy Behav. 2014;37:198–203. doi: 10.1016/j.yebeh.2014.06.028. [DOI] [PubMed] [Google Scholar]
- 21.Buchanan GF, Richerson GB. Epilepsy: A dietary supplement for SUDEP prevention? Nat Rev Neurol. 2016;12:495–6. doi: 10.1038/nrneurol.2016.114. [DOI] [PubMed] [Google Scholar]
- 22.Swanson CJ, Perry KW, Koch-Krueger S, Katner J, Svensson KA, Bymaster FP. Effect of the attention deficit/hyperactivity disorder drug atomoxetine on extracellular concentrations of norepinephrine and dopamine in several brain regions of the rat. Neuropharmacology. 2006;50:755–60. doi: 10.1016/j.neuropharm.2005.11.022. [DOI] [PubMed] [Google Scholar]
- 23.Bymaster FP, Katner JS, Nelson DL, Hemrick-Luecke SK, Threlkeld PG, Heiligenstein JH, Morin SM, Gehlert DR, Perry KW. Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: a potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology. 2002;27:699–711. doi: 10.1016/S0893-133X(02)00346-9. [DOI] [PubMed] [Google Scholar]
- 24.Heal DJ, Cheetham SC, Smith SL. The neuropharmacology of ADHD drugs in vivo: insights on efficacy and safety. Neuropharmacology. 2009;57:608–18. doi: 10.1016/j.neuropharm.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 25.Koda K, Ago Y, Cong Y, Kita Y, Takuma K, Matsuda T. Effects of acute and chronic administration of atomoxetine and methylphenidate on extracellular levels of noradrenaline, dopamine and serotonin in the prefrontal cortex and striatum of mice. J Neurochem. 2010;114:259–70. doi: 10.1111/j.1471-4159.2010.06750.x. [DOI] [PubMed] [Google Scholar]
- 26.Burke PG, Abbott SB, Coates MB, Viar KE, Stornetta RL, Guyenet PG. Optogenetic stimulation of adrenergic C1 neurons causes sleep state-dependent cardiorespiratory stimulation and arousal with sighs in rats. Am J Respir Crit Care Med. 2014;190:1301–10. doi: 10.1164/rccm.201407-1262OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Massari VJ, Tizabi Y, Jacobowitz DM. Potential noradrenergic regulation of serotonergic neurons in the median raphe nucleus. Exp Brain Res. 1979;34:177–82. doi: 10.1007/BF00238350. [DOI] [PubMed] [Google Scholar]
- 28.Maeda T, Kojima Y, Arai R, Fujimiya M, Kimura H, Kitahama K, Geffard M. Monoaminergic interaction in the central nervous system: a morphological analysis in the locus coeruleus of the rat. Comp Biochem Physiol C. 1991;98:193–202. doi: 10.1016/0742-8413(91)90195-y. [DOI] [PubMed] [Google Scholar]
- 29.Faingold CL, Randall M, Zeng C, Peng S, Long X, Feng HJ. Serotonergic agents act on 5-HT3 receptors in the brain to block seizure-induced respiratory arrest in the DBA/1 mouse model of SUDEP. Epilepsy Behav. 2016;64:166–170. doi: 10.1016/j.yebeh.2016.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martinez-Raga J, Knecht C, Szerman N, Martinez MI. Risk of serious cardiovascular problems with medications for attention-deficit hyperactivity disorder. CNS Drugs. 2013;27:15–30. doi: 10.1007/s40263-012-0019-9. [DOI] [PubMed] [Google Scholar]
- 31.Bateman LM, Li CS, Lin TC, Seyal M. Serotonin reuptake inhibitors are associated with reduced severity of ictal hypoxemia in medically refractory partial epilepsy. Epilepsia. 2010;51:2211–4. doi: 10.1111/j.1528-1167.2010.02594.x. [DOI] [PubMed] [Google Scholar]


