Abstract
Purpose
To simultaneously measure concentration, T1, and T2 values of metabolites in human brain in a single scan session.
Methods
A new pulse sequence with multiple variable acquisition parameters was proposed to encode metabolite T1 and T2 information into the acquired data. A linear combination fitting algorithm was developed in-house to simultaneously determine metabolite concentrations and relaxation times.
Results
Concentration, T1 and T2 values of N-acetyl-aspartate, total creatine, total choline, and glutamate were reliably measured in the frontal grey matter and white matter regions of nine healthy volunteers at 7 T in less than ten minutes of scan time per voxel. T1 and T2 values of glutamine as well as T1 of glutathione were also measured in the frontal grey matter region with reasonable precision.
Conclusions
The proposed technique allows multiparametric characterization of brain metabolites in a single scan session, making it possible to measure both levels and intracellular microenvironment of brain chemicals in clinical MRS studies.
Keywords: Metabolite, T1, T2, Relaxation, High Field
INTRODUCTION
Many In vivo magnetic resonance spectroscopy (MRS) signals are cell-type specific. For example, N-acetyl-aspartate (NAA) has been considered as a neuronal marker; glutamate (Glu) resides predominantly in glutamatergic neurons whereas glutamine (Gln) in astroglial cells (1). Levels of brain metabolites provide cell-type specific insights into brain function and pathophysiology. In addition, relaxation rates of metabolites contain important information on cellular microenvironment (2,3). Previously, reduced metabolite T2 relaxation times were found in bipolar disorder and schizophrenia patients and were attributed to reduced cell volumes and altered macromolecule structures (4). Although high field (e.g., 7 T) MRS provides increased sensitivity and spectral resolution, the prolonged T1 and shortened T2 relaxation times often make it necessary to characterize metabolite T1 and T2 values in order to obtain accurate quantification of metabolite concentrations and optimize repetition time (TR) and echo time (TE) of a pulse sequence for optimal signal-to-noise per unit time.
Prior MRS studies have measured T1 and T2 relaxation times separately, using inversion-recovery or multi-TR sequences to measure T1 and multi-TE or multi-echo sequences to measure T2 (5-16). In this work, we demonstrate the feasibility of simultaneous determination of brain metabolite levels, T1 and T2 relaxations in one scan session at 7 T. This multiparametric approach may shorten the preparation and total scan time for measuring both T1 and T2, reduce differences in subject and scanner conditions between T1 and T2 measurements, and improve reliability in measuring overlapped metabolite signals. We designed a point resolved spectroscopy (PRESS) sequence with a J-suppression pulse (17) and multiple (e.g., 30) sets of acquisition parameters. The acquisition parameters which include TE values, inversion-recovery time (TI), and the flip angle of the J-suppression pulse were optimized for separating the overlapping metabolite signals and maximizing signal-to-noise ratios (SNRs) of the metabolites of interest. The whole dataset acquired with different acquisition parameters were modeled simultaneously by linear combinations of metabolite basis functions to obtain metabolite concentrations and relaxation times. Each basis function contains the simulated free induction decay (FID) signals of a metabolite for all acquisition parameter sets. The T1 and T2 relaxation effects of all metabolites were incorporated into the fitting program. Our results showed that, in addition to T1 and T2 of NAA, creatine and choline signals, relaxation times of several dilute signals such as Glu, Gln and glutathione (GSH) can also be measured together, making it possible to simultaneously probe many cell-type specific microenvironment within the time constraint of a typical clinical MRS study.
METHODS
Pulse Sequence
As shown in Fig. 1, the main building block of the pulse sequence is a PRESS sequence inserted with a J-suppression pulse between the two 180° refocusing pulses (17). The 90° excitation pulse was an amplitude-modulated pulse with duration = 4.5 ms and full width at half maximum (FWHM) bandwidth = 3.1 kHz. The 180° refocusing pulses were also amplitude-modulated with duration = 8.0 ms and bandwidth = 2.0 kHz. The J-suppression pulse was a frequency selective RF pulse placed at the resonance frequency of the aspartyl CH proton of NAA at 4.38 ppm, thereby altering the J-evolution of the NAA aspartyl CH2 multiplet signals at 2.49 ppm. By optimizing the flip angle of the J-suppression pulse and the TE1 value based on density matrix simulations, the NAA aspartyl multiplet at 2.49 ppm can be suppressed to reduce its interference to the Gln multiplet at 2.45 ppm and the GSH multiplet at 2.54 ppm. Density matrix simulations were also performed to investigate the J-evolution of the Glu and Gln signals as functions of TE values. The following TE values were used to encode T2 decay factors into the data: 60, 70, 80, 90, 100, 110, 120, 130, 170, 220, and 230 ms.
Fig. 1.

Schematics of the pulse sequence with multiple variable acquisition parameters. The second RF pulse is a broadband hyperbolic secant pulse with a variable TI and variable flip angle. TE, TE1, and the flip angle of the J-suppression pulse are also variable.
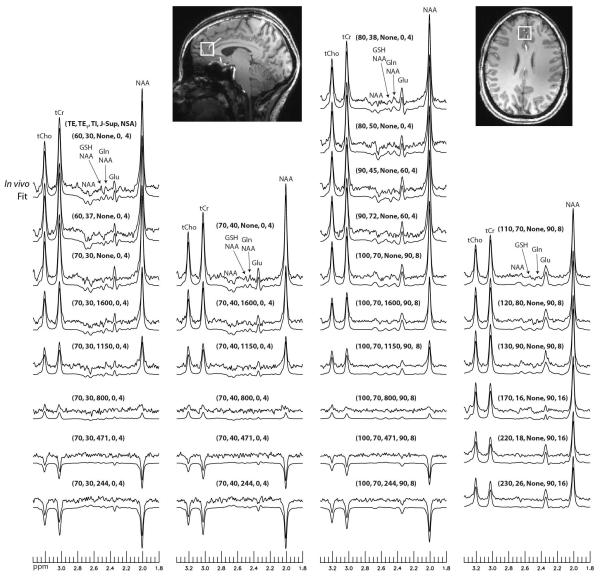
Prior to the PRESS block, eight variable power RF pulses with optimized relaxation delays (VAPOR) were used to suppress water signals (18). Each pulse was a 9 ms sinc-Gauss pulse with a bandwidth of 310 Hz. Before the VAPOR block, two broadband hyperbolic secant pulses were used to null and invert the longitudinal magnetizations of all metabolites. The first 90° hyperbolic secant pulse, which was also the first pulse of the entire pulse sequence, had a nominal flip angle of 90°, duration of 6.8 ms, and a bandwidth of 2.5 kHz. This pulse was used to normalize the longitudinal magnetizations of all metabolites to zero at the beginning of each TR. The 90° excitation pulse of the PRESS block always started 2.5 s after this magnetization nulling pulse. The second hyperbolic secant pulse had the same duration and similar bandwidth as the first pulse. It was used to invert the longitudinal magnetizations of all metabolites for T1 encoding. Six different settings were used for the second hyperbolic secant pulse: (1) no inversion; (2) TI = 1600 ms, nominal flip angle = 160.2°; (3) TI = 1150 ms, nominal flip angle = 160.2°; (4) TI = 800 ms, nominal flip angle = 160.2°; (5) TI = 471 ms, nominal flip angle = 160.2°; and (6) TI = 244 ms, nominal flip angle = 142.6°. For the fifth and sixth settings, the second hyperbolic secant pulse replaced the third and fifth VAPOR pulses, respectively. Because the nominal flip angle of the second hyperbolic secant pulse was the same as the flip angle of the corresponding VAPOR pulse, the effectiveness of VAPOR for water suppression was not affected. The second hyperbolic secant pulse was applied when TE was 70 and 100 ms. The pulse sequence had a total of 30 different sets of acquisition parameters, corresponding to 30 sets of values for TE, TE1, TI, J-suppression flip angle, and number of signal averages (NSA). The parameter values for all 30 sets of acquisition parameters are given in Fig. 4. The total number of metabolite data acquisitions in the pulse sequence was 192. Adding the time for two preparation acquisitions and two acquisitions of unsuppressed water signals, the total scan time for this pulse sequence was 9 min and 48 s when a 3 s TR was used.
Fig. 4.
Stack plots of reconstructed spectra and corresponding fits from the frontal GM voxel of one healthy volunteer. TE (ms), TE1 (ms), TI (ms), J-suppression flip angle (deg), and NSA (number of signal averages) values for the 30 sets of acquisition parameters are listed above the corresponding spectra.
Computation of Basis Functions and Phantom Experiments
Basis functions of NAA acetyl moiety, NAA aspartyl moiety, N-acetylaspartylglutamate (NAAG) acetyl moiety, NAAG aspartyl and glutamate moieties, γ-aminobutyric acid (GABA), Glu, Gln, GSH, aspartate (ASP), total creatine (tCr), total choline (tCho), and myo-inositol (mI) were simulated using GAMMA C++ library (19) for measurement of NAA, tCr, tCho, Glu, Gln, and GSH in the 1.8-3.35 ppm region. Chemical shifts and coupling constants were obtained from the literature (20,21). The simulations used actual excitation and refocusing pulses and three-dimensional spatial localization with 201 × 201 × 201 spatial points (22). Simulated basis function for each metabolite contained free induction decay (FID) signals for all 30 sets of acquisition parameters. Linear phase shift over the 1.8-3.35 ppm region due to the transient Bloch-Siegert effect of the J-suppression pulse was removed from the basis functions using singlets as references.
Phantom experiments were performed using the proposed pulse sequence to calibrate the linear phase shifts of the acquired data. The NAA acetyl and creatine peaks obtained by scanning a phantom were fitted with two Lorentzian shaped peaks to find the linear phase shifts for all 30 sets of acquisition parameters. These linear phase shifts were later used to correct the in vivo data. Unsuppressed water signals were also acquired from the phantom using the proposed pulse sequence, which were later used to perform eddy current correction for the in vivo experiments.
In Vivo Data Acquisition
Nine healthy volunteers (three women and six men; age = 28 ± 8 years) were recruited for measurements of metabolite concentrations and relaxation times in the frontal cortex. Two of the volunteers were scanned twice. No data were excluded and all collected data were used in the analysis. All volunteers gave informed consent in accordance with procedures approved by our local institutional review board. Experiments were performed on a Siemens 7 T scanner equipped with a 32-channel receiver head coil. T1-weighted magnetization prepared rapid gradient echo (MPRAGE) images were acquired with TR = 3 s, TE = 3.9 ms, matrix = 256 × 256 × 256, and resolution = 1 × 1 × 1 mm3 to position the MRS voxel and perform tissue segmentation. For each subject, MRS data were collected from two 2 × 2 × 2 cm3 voxels in the frontal cortex. One voxel was placed in the grey matter (GM) dominant region of prefrontal cortex (PFC) and medial pregenual anterior cingulate cortex (pgACC), both of which have been implicated in several brain disorders (23,24). The other voxel was placed in the white matter (WM) dominant right frontal cortex. B0 field inhomogeneities were minimized by first- and second-order shimming, achieving mean water linewidth of 11.1 Hz and 13.4 Hz for the voxels in the GM and WM regions, respectively. MRS data acquisition for each voxel lasted < 10 min.
Data Analysis
Water suppressed FIDs acquired from 192 acquisitions were averaged over each parameter set to obtain 30 sets of averaged FIDs, each of which had 32 channels. These 32-channel FIDs were combined into single-channel FIDs using a generalized least square method (25), in which coil sensitivities were computed from the unsuppressed water signals acquired in two acquisitions. The previously acquired unsuppressed water signals from the phantom experiments, which contained 30 sets of unsuppressed water signals, were used to correct for the phase errors in the combined single-channel FIDs caused by zero-order eddy currents (26). These 30 sets of FIDs were Fourier transformed into the frequency domain to obtain 30 sets of spectra, each of which corresponds to one set of acquisition parameters (TE, TE1, TI, J-suppression flip angle, NSA). Linear phase corrections were performed on the 30 sets of spectra using linear phase shift values obtained from the phantom experiment. The corrected spectra were subsequently quantified using an in-house developed linear combination fitting program which used a Levenberg-Marquardt least square minimization algorithm. Ideally, the 30 sets of spectra could be fitted at the same time to find all the unknown variables, which include concentration, T1, T2, frequency shift, linewidth, and lineshape for all metabolite peaks, as well as zero-order phases and spline baselines for all 30 sets of spectra. This would generate a very high dimensional parameter space, making it difficult for the nonlinear minimization algorithm to sufficiently sample it. In order to make the fitting process manageable, we split the fitting process into two passages. In the first passage, each of the 30 sets of spectra was fitted individually by linear combinations of the corresponding FIDs of the basis functions to find peak frequency shifts, peak linewidths, the Gaussian-Lorentzian ratio of the Voigt lineshape, the zero-order phase, and a spline baseline. The zero-order phase and spline baseline were then removed from each spectrum. The peak linewidths and Gaussian-Lorentzian ratio were averaged over 30 sets of spectra to give the mean peak linewidths and mean Gaussian-Lorentzian ratio. In the second passage, all 30 sets of corrected spectra were put into one large vector and were fitted together by a linear combination of the basis functions. The peak frequency shifts, mean peak linewidths and mean Gaussian-Lorentzian ratio from the first passage were used as inputs for the second passage fitting. The unknown variables for the second passage fitting were concentration, T1, and T2 values of the metabolites. The T1 scaling factors due to the 90° magnetization nulling pulse and the variable inversion pulse and the T2 scaling factors for different TEs were computed at each step of the iterative fitting process. In the second passage fitting, T2 values were constrained to 60 – 600 ms; the differences between T1 and T2 values were constrained to 0 – 1.7 s. For both passages, only spectral data between 1.8 - 3.35 ppm were fitted because this range contains all major metabolites for our clinical studies. Additionally, the metabolite peaks downfield from 3.8 ppm were partially suppressed by the water suppression pulses that had a bandwidth of ~310 Hz.
Comparison with the Conventional Approach
For comparison purposes, metabolite T1 and T2 values were also computed from the in vivo data using a conventional approach. In our pulse sequence, 15 of the 30 acquisition parameter sets do not have the variable inversion pulse (Fig. 4). Metabolite concentration values were independently determined from each of the 15 acquisition parameter sets and were then fitted by mono-exponential functions to obtain metabolite T2 values. For T1 encoding in our pulse sequence, six different TI settings were used for each of the three (TE, TE1) values, which were (70 ms, 30 ms), (70 ms, 40 ms), and (100 ms, 70 ms). Metabolite concentration values were independently determined for each acquisition parameter set. Six sets of averaged metabolite concentration values corresponding to six different TI settings were computed as the weighted average of metabolite concentrations for the three different (TE, TE1) values, where the inverse of noise SD values were used as the weights. Metabolite T1 values were then determined by fitting the six sets of averaged metabolite concentration values.
The conventional and proposed methods for measuring metabolite T1 and T2 were further compared using Monte Carlo analysis to assess the influence of random noise. For measuring T1 using the conventional method, three inversion recovery scans were simulated, which used TR = 4.944 s, TE = 60 ms, NSA = 24, and TI = none, 500 ms, and 3000 ms, respectively. For measuring T2 using the conventional method, two scans were simulated, which used TR = 2.5 s, NSA = 44, and TE = 80 and 170 ms, respectively. The J-suppression pulses were removed from the proposed method. For both methods, the FIDs were generated by combining basis functions of different metabolites with known concentration, T1, and T2 values, as well as known linewidths and lineshapes. The same amount of noise was added to the FIDs for both methods. The SNR for the NAA singlet peak in a single data acquisition at TE = 60 ms was 44 for both methods, which was similar to the in vivo scans. The total scan time was 576 s for both methods.
Measuring Phantom T1
Metabolite T1 relaxation times of a Braino phantom were measured using the proposed pulse sequence and a conventional inversion recovery PRESS sequence. The scan time for the proposed pulse sequence was cut down to 288 s by reducing the NSA by a factor of 2 compared to the in vivo experiments. The conventional inversion recovery sequence used TR = 8 s, TE = 60 ms, NSA = 6, and TI = none, 3000 ms, 1800 ms, 1100 ms, 471 ms, and 244 ms, which also had a scan time of 288 s. For both sequences, the phantom was measured seven times, with the phantom being taken out and reinserted each time. Because T2 relaxation times are highly dependent on sequence design partly due to incomplete rephrasing caused by spin diffusion (7,15), metabolite T2 values of the phantom were not measured.
RESULTS
Density matrix simulated spectra of Glu and Gln for TE = 30 – 240 ms with a 10 ms increment are displayed in Fig. 2. T2 relaxation was ignored in the simulation. At 7 T, the Glu and Gln peak height and width are mainly functions of total TE, i.e. TE1 + TE2 (21). At low TE values of 30 and 40 ms, the Glu peak at 2.35 ppm and Gln peak at 2.44 ppm have low amplitudes and broad lineshapes, which are difficult to be distinguished from the strong macromolecule baseline signals. This was why shorter TE values were not used in the pulse sequence. The Glu and Gln peaks grow higher and sharper for TE = 60 - 110 ms, keep growing higher but broader when TE = 120 – 130 ms, and become too broad for TE = 140 – 160 ms. After TE of 170 ms, the Glu and Gln peaks grow higher and sharper again. To encode T2 decay factors of metabolites while keeping the Glu and Gln C4 peaks sharp, we used eleven TE values in the pulse sequence, i.e. 60, 70, 80, 90, 100, 110, 120, 130, 170, 220, and 230 ms.
Fig. 2.
Numerically simulated spectra of Glu and Gln for TE = 30 – 240 ms with a 10 ms increment. The concentration ratio was [Glu] : [Gln] = 4 : 1. A 9 Hz line broadening was applied to the spectra. T2 relaxation was ignored here.
Discounting the different TI values used to encode T1 information, the pulse sequence used 15 different sets of TE, TE1, and J-suppression flip angle. Density matrix simulated spectra of NAA at these 15 sets of parameter values are displayed in Fig. 3. T2 relaxation was ignored in the simulation. For TE = 60, 70, and 80 ms, we used two different TE1 values for each TE to alter the NAA aspartyl signals to enhance spectral resolution of the Gln and GSH signals from the overlapping NAA aspartyl signals. When TE was 90 ms and longer, the J-suppression pulse was used to effectively suppress the NAA aspartyl signals at 2.49 ppm by using an optimal set of TE1 and J-suppression flip angle. Fig. 3 shows that the NAA aspartyl signals at 2.49 ppm become very small when TE is 100 – 130 ms and 220 – 230 ms.
Fig. 3.
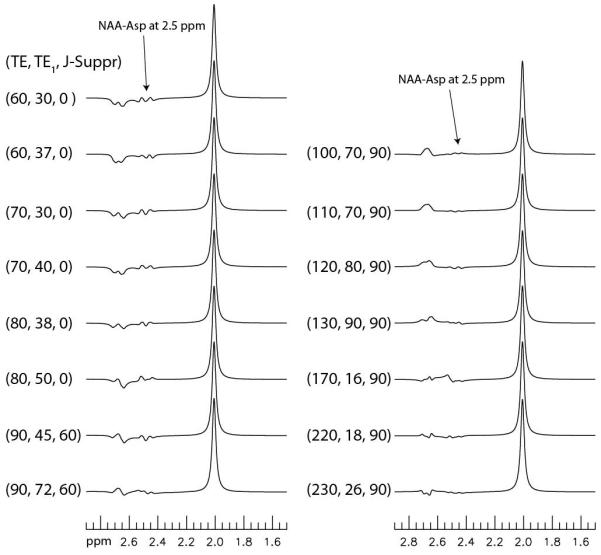
Numerically simulated spectra of NAA at 15 different sets of TE (ms), TE1 (ms), and J-suppression flip angle (deg). A 9 Hz line broadening was applied to the spectra. T2 relaxation was ignored. The NAA aspartyl signals at 2.49 ppm become very small when TE falls into the 100 – 130 ms and 220 – 230 ms ranges.
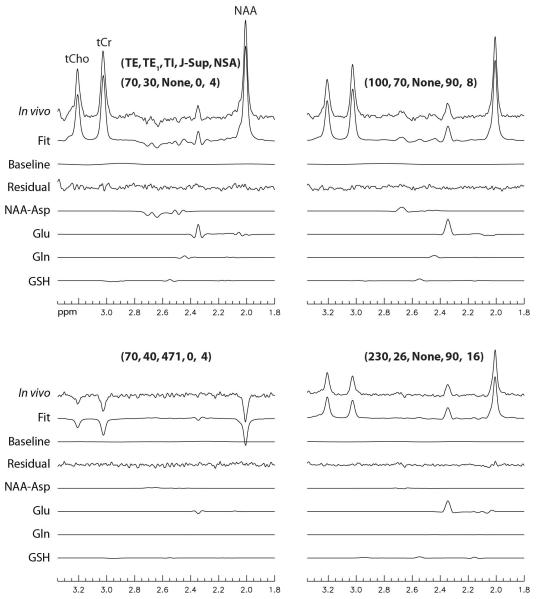
Stack plots of the reconstructed spectra and corresponding fits from the frontal GM voxel of one healthy volunteer are displayed in Fig. 4. Parameter values of TE, TE1, TI, J-suppression flip angle, and NSA for the 30 sets of acquisitions are given above the corresponding spectra. Detailed fitting results for four of the 30 spectra are plotted in Fig. 5. Fig. 4 shows that the signal variations of the in vivo spectra as functions of TE, TE1, TI, and J-suppression flip angle agreed very well with our model. Fig. 5 shows that the fitting residuals were very small.
Fig. 5.
Detailed fitting results for four typical spectra presented in Fig. 4. The parameter values for TE (ms), TE1 (ms), TI (ms), J-suppression flip angle (deg), and NSA are listed above the corresponding spectra.
Using an in-house developed tissue segmentation software, we found that the voxels in the frontal GM region contained (66 ± 8)% GM, (31 ± 8)% WM, and (3 ± 1)% cerebrospinal fluid, whereas voxels in the frontal WM region contained (18 ± 8)% GM, (81 ± 8 )% WM, and (1 ± 1)% cerebrospinal fluid. Metabolite concentration, T1, and T2 values obtained from eight healthy volunteers are given in Table. 1. Very high precision was obtained for NAA, creatine, choline and glutamate. T1 of the NAA singlet in the frontal GM region (1.43 ± 0.02 s) was significantly shorter than in the frontal WM region (1.54 ± 0.05 s). Whereas, T2 of the NAA singlet in the frontal GM region (203 ± 17 ms) was significantly longer than in the frontal WM region (169 ± 16 ms). T1 of the NAA aspartyl multiplets at 2.49 ppm and 2.67 ppm in the frontal GM region (0.94 ± 0.11 s) was similar to that of the frontal WM region (0.99 ± 0.12 s); T2 of the NAA aspartyl multiplets in the frontal GM region (141 ± 17 ms) was significantly longer than in the frontal WM region (122 ± 14 ms). Both T1 and T2 values of the NAA aspartyl multiplets were significantly shorter than those of the NAA singlet in both frontal GM and WM regions. T1 of tCr in the frontal GM region (1.31 ± 0.03 s) was significantly shorter than in the frontal WM region (1.53 ± 0.04 s); T2 of tCr in the frontal GM region (127 ± 6 ms) was significantly longer than in the frontal WM region (109 ± 7 ms). T1 of tCho in the frontal GM region (1.17 ± 0.03 s) was similar to that of the frontal WM region (1.19 ± 0.03 s); T2 of tCho in the frontal GM region (197 ± 11 ms) was significantly longer than in the frontal WM region (130 ± 14 ms). The Glu/tCr ratios in the frontal GM and WM regions were 0.94 ± 0.14 and 0.94 ± 0.11 respectively, which were the same. T1 of Glu in the frontal GM region (1.27 ± 0.12s) was significantly shorter than in the frontal WM region (1.55 ± 0.14 s); T2 of Glu in the frontal GM region (184 ± 18 ms) was significantly longer than in the frontal WM region (139 ± 8 ms). We were also able to measure the concentration, T1, and T2 values of Gln in the frontal GM region. T2 of Gln (84 ± 25 ms) was significantly shorter than that of Glu in the frontal GM region. The T1 and T2 values of Gln in the frontal WM region could not be measured reliably because they reached the lower or upper limits in the fitting process for eight out of the nine samples. Consequently, the Gln concentration in the frontal WM region could not be reliably measured here as it depended on the T1 and T2 relaxation factors. T1 of GSH in the frontal GM region was found to be 0.49 ± 0.11s. T2 of GSH in the frontal GM region could not be measured reliably because two of the nine samples reached the lower or upper limits in the fitting process and the standard deviation was 60% of the mean value. Consequently, the GSH concentration in the frontal GM region could not be reliably measured. In the frontal WM region, neither T1, T2, nor concentration of GSH could be reliably measured due to the large spread of the data values.
Table 1.
Metabolite concentration (/[tCr]), T1, and T2 values in the frontal GM and WM dominant regions of nine healthy volunteers computed using the proposed method.
| Metabolite ratio (/[tCr]) | T1 (s) | T2 (ms) | ||||
|---|---|---|---|---|---|---|
| GM | WM | GM | WM | GM | WM | |
| NAA (CH3) | 1.16 ± 0.10 | 1.32 ± 0.11 | 1.43 ± 0.02 | 1.54 ± 0.05 | 203 ± 17 | 169 ± 16 |
| NAA (CH2) | 1.16 ± 0.10 | 1.32 ± 0.11 | 0.94 ± 0.11 | 0.99 ± 0.12 | 141 ±17 | 122 ± 14 |
| Glu | 0.94 ± 0.14 | 0.94 ± 0.11 | 1.27 ± 0.12 | 1.55 ± 0.14 | 184 ± 18 | 139 ± 8 |
| Gln | 0.40 ± 0.15 | - | 1.04 ± 0.27 | - | 84 ± 25 | - |
| GSH | - | - | 0.49 ± 0.11 | - | - | - |
| tCr | 1 | 1 | 1.31 ± 0.03 | 1.53 ± 0.04 | 127 ± 6 | 109 ± 7 |
| tCho | 0.25 ± 0.04 | 0.30 ± 0.08 | 1.17 ± 0.03 | 1.19 ± 0.03 | 197 ± 11 | 130 ± 14 |
Average coefficient of variation (CV) values for metabolite concentration, T1, and T2 measurements are listed in Table 2, which were computed using the data collected from the two healthy volunteers who had two scans in both the frontal GM and WM regions. The CV values for Glu concentration, T1, and T2 values were found to be 3.5%, 4.2%, and 6.7% in the frontal GM region and 11%, 4.6%, and 5.6% in the frontal WM region, which were fairly low considering the scan time was under ten minutes.
Table 2.
Average inter-scan CV values for metabolite concentration, T1, and T2 values in the frontal GM and WM regions of two healthy volunteers who had two separate scans in each region.
| CV of metabolite ratio (%) |
CV of T1 (%) | CV of T2 (%) | ||||
|---|---|---|---|---|---|---|
| GM | WM | GM | WM | GM | WM | |
| NAA (CH3) | 3.2 | 7.8 | 0.74 | 0.59 | 4.4 | 1.8 |
| NAA (CH2) | 3.2 | 7.8 | 4.5 | 18 | 4.7 | 3.1 |
| Glu | 3.5 | 4.2 | 6.7 | 11 | 4.6 | 5.6 |
| Gln | 5.1 | - | 8.3 | - | 9.7 | - |
| GSH | - | - | 41 | - | - | - |
| tCr | 0 | 0 | 0.91 | 3.4 | 1.7 | 1.9 |
| tCho | 1.7 | 12 | 0.22 | 1.4 | 4.2 | 6.0 |
Metabolite T1 and T2 relaxation times separately computed using conventional methods from data collected from the nine healthy volunteers are given in Table. 3. Relaxation times computed using the proposed method (Table. 1) and conventional methods (Table. 3) agree well for most metabolites. The T1 and T2 values computed using the conventional method have much larger variations because separately fitting T1 and T2 does not take advantage of the additional information from the rest of the data, resulting in less well-determined solutions.
Table 3.
Separately computed metabolite T1 and T2 relaxation times using conventional methods from data collected from the nine healthy volunteers.
| T1 (s) | T2 (ms) | |||
|---|---|---|---|---|
| GM | WM | GM | WM | |
| NAA (CH3) | 1.39 ± 0.08 | 1.56 ± 0.07 | 199 ± 21 | 164 ± 19 |
| NAA (CH2) | 0.86 ± 0.10 | 0.96 ± 0.12 | 226 ± 115 | 140 ± 22 |
| Glu | 1.16 ± 0.11 | 1.40 ± 0.15 | 175 ± 30 | 144 ± 22 |
| Gln | 0.95 ± 0.49 | - | 130 ± 46 | - |
| GSH | 0.32 ± 0.15 | - | - | - |
| tCr | 1.23 ± 0.07 | 1.42 ± 0.10 | 126 ± 12 | 109 ± 10 |
| tCho | 1.10 ± 0.12 | 1.14 ± 0.11 | 179 ± 13 | 135 ± 14 |
Results for the Monte Carlo analysis of the conventional and proposed methods are given in Table 4. The overall performance of the proposed method is significantly better. For Glu, Gln, and GSH, the proposed method dramatically increased the precisions of T1 and T2 measurements compared to the conventional method. For in vivo studies, fitting the entire dataset simultaneously can better suppress errors associated with individual data points, which is important for improving the measurement of weaker signals such as glutamate and glutamine.
Table 4.
Monte Carlo analysis of the conventional and proposed methods with the same total scan time. Metabolite T1 and T2 relaxation times were measured separately in the conventional method and simultaneously in the proposed method.
| Separate | Simultaneous | |||||
|---|---|---|---|---|---|---|
| True value | Mean | CV (%) | Mean | CV (%) | ||
| NAA (CH3) | Concentration | 1.20 | 1.19 | 4.0 | 1.19 | 2.6 |
| T1 (s) | 1.50 | 1.45 | 11 | 1.50 | 1.7 | |
| T2 (ms) | 200 | 199 | 3.8 | 201 | 2.6 | |
| NAA (CH2) | Concentration | 1.20 | 1.18 | 6.2 | 1.19 | 2.6 |
| T1 (s) | 1.00 | 0.97 | 8.6 | 1.00 | 6.2 | |
| T2 (ms) | 135 | 137 | 8.6 | 134 | 4.3 | |
| Glu | Concentration | 0.85 | 0.84 | 6.7 | 0.85 | 4.7 |
| T1 (s) | 1.30 | 1.26 | 11 | 1.29 | 4.2 | |
| T2 (ms) | 180 | 185 | 15 | 181 | 5.2 | |
| Gln | Concentration | 0.35 | 2.9 | 474 | 0.36 | 27 |
| T1 (s) | 1.05 | 1.03 | 26 | 1.03 | 18 | |
| T2 (ms) | 90 | 92 | 72 | 96 | 30 | |
| GSH | Concentration | 0.20 | 0.21 | 16 | 0.20 | 14 |
| T1 (s) | 0.50 | 0.48 | 28 | 0.50 | 11 | |
| T2 (ms) | 200 | 226 | 56 | 215 | 30 | |
| tCr | Concentration | 1.00 | 0.99 | 3.4 | 0.99 | 4.6 |
| T1 (s) | 1.30 | 1.26 | 11 | 1.30 | 2.0 | |
| T2 (ms) | 125 | 125 | 3.8 | 125 | 9.9 | |
| tCho | Concentration | 0.20 | 0.20 | 3.7 | 0.20 | 2.3 |
| T1 (s) | 1.20 | 1.15 | 18 | 1.20 | 1.6 | |
| T2 (ms) | 195 | 199 | 5.7 | 195 | 3.3 | |
Metabolite T1 values of the Braino phantom measured by the conventional inversion recovery and proposed sequences are listed in Table 5. The two methods agree well, which verified that the proposed method can measure T1 reliably even though a relatively short TR of 3 s was used.
Table 5.
Metabolite T1 relaxation times of a Braino phantom measured by a conventional inversion recovery sequence and the proposed pulse sequence.
| Conventional method (s) |
Proposed method (s) |
|
|---|---|---|
| NAA (CH3) | 0.799 ± 0.006 | 0.799 ± 0.005 |
| NAA (CH2) | 0.537 ± 0.015 | 0.556 ± 0.018 |
| Glu | 0.541 ± 0.009 | 0.555 ± 0.009 |
| creatine | 0.420 ± 0.008 | 0.434 ± 0.002 |
| choline | 0.264 ± 0.004 | 0.258 ± 0.001 |
DISCUSSION
We proposed a new pulse sequence with multiple variable acquisition parameters to simultaneously measure metabolite concentration, T1 and T2 values. By varying the acquisition parameters that includes TE, TE1, TI, and J-suppression flip angle, we not only encoded metabolite T1 and T2 information into the acquired data but also generated different metabolite-metabolite and metabolite-baseline interactions. Compared to conventional methods where T1 and T2 are determined separately, the proposed method had smaller variations in computed T1 and T2 values because information from all collected data was utilized in computing both T1 and T2. In this study, six different TI settings were used for TE = 70 and 100 ms to encode T1 information. At these two TE values, both Glu and Gln C4 peaks are relatively high and well defined, so TE values of 70 and 100 ms are good for encoding T1 information of Glu and Gln. If one is only interested in measuring T1 of Glu, it would be better to use multiple TI values for TE = 240 ms because the Glu C4 peak is very high at that TE value and the macromolecule signals are almost zero at such a long TE. Furthermore, we do not have to use all six TI settings at a certain TE. Our linear combination fitting program that simultaneously computes metabolite concentration, T1, and T2 values allows very flexible acquisition schemes. The use of different TI settings can be spread out across different TEs such as using two TI settings for one TE and three TI settings for another TE. The design of the T1 encoding scheme depends on the metabolites of interest and a priori knowledge of metabolite T1, T2, concentration, and J-evolution. Therefore, the T1 and T2 encoding scheme of this pulse sequence can be further optimized for specific metabolite(s) and available scan time for the pulse sequence.
To the best of our knowledge, this is the first time that the metabolite T1 or T2 values are determined simultaneously. Since no previous studies have examined metabolite relaxation in the frontal cortex at 7 T, which is important for psychiatric studies, we have compared our measurements with literature data acquired from other GM and WM regions. The T1 and T2 values of NAA (CH3), tCr, and tCho in the parietal WM region were previously measured at 7 T using separate scan sessions with a total scan time of 22 min (13). Our T1 and T2 values of NAA (CH3), tCr, and tCho in the frontal WM region are within 15% of the corresponding parietal WM values in the previous study. Compared to another 7 T study (14), our T2 values of NAA (CH3), tCr, and tCho in the frontal GM and WM regions agree very well with those in the cerebellum GM and motor cortex WM regions, respectively. The T2 values of Glu in the frontal GM and WM regions in our study are about 30% and 40% longer than the previously reported values in the cerebellum GM and motor cortex WM regions (14), respectively.
In this study, we found that T2 of Gln (84 ± 25 ms) was much shorter than that of Glu (184 ± 18 ms) in the frontal GM region. This explains well why a relatively low Gln/Glu ratio of 0.21 in the frontal GM region was found by our previous study (17), for which a TE of 106 ms was used. In this study, the Gln/Glu ratio in the frontal GM region was found to be 0.42, corresponding to a Gln/Glu ratio of 0.23 without T2 correction at TE = 106 ms. T2 of Gln was previously reported to be significantly shorter than that of Glu at 3 T (27). In this study, T1 of GSH in the frontal GM region was found to be 0.49 ± 0.11 s which was much shorter than the T1 values of other metabolites. This agrees well with a previous study at 3 T (28), in which GSH T1 was found to be 0.40 ± 0.04 s in the fronto-parietal region. The reason for GSH to have very short T1 was thought to be due to the presence of neighboring sulphur in the cysteine moiety of GSH (28).
With the continuous advances in MR technology and especially with the advent of high field techniques, more and more effort has been put to extract metabolite relaxation information in clinical studies. The results presented here demonstrate that it is feasible to simultaneously measure NAA, creatine, choline and glutamate levels and their transverse and longitudinal relaxation times in a single scan session under ten minutes per voxel with high precision. Most previous metabolite relaxation studies have focused on the strong NAA, creatine and choline signals. As glutamate, glutamine, and glutathione (GSH) are increasingly found to play important roles in numerous psychiatric and neurological disorders, it is highly desirable to be able to probe their respective cellular microenvironment within the time constraint of a typical clinical MRS study. Although glutamine T1 and T2 as well as glutathione T1 were measured in GM in this study, their weaker signals in WM have prevented us from measuring them within the ten minutes scan time. It is possible that the information content of our method may be further augmented by significantly increasing the variations of acquisition parameters. It is hoped that the increased information content in the acquired data will translate into more quantifiable clinical MRS parameters in the future.
CONCLUSIONS
A new approach for acquiring and quantifying single voxel MRS data was proposed to allow simultaneous measurement of concentration, T1 and T2 values of metabolites. Instead of only varying TE to measure T2 or varying TI to measure T1, multiple acquisition parameters such as TE, TE1, TI, and J-suppression flip angle were varied during data acquisition to encode both T1 and T2 information while maximizing the reliability of measuring metabolites of interest such as Glu and Gln. The whole dataset that consisted of data acquired with different sets of acquisition parameters were fitted by a single linear combination of basis functions to simultaneously quantify metabolite concentration, T1, and T2 values. In less than ten minutes of scan time per voxel, concentration, T1, and T2 values of NAA, tCr, tCho, and Glu in frontal GM and WM regions were reliably measured. Concentration, T1, and T2 values of Gln, as well as T1 of GSH, were also successfully measured in the frontal GM region. In the frontal GM region, T2 of Gln (84 ± 25 ms) was found to be significantly shorter than that of Glu (184 ± 18 ms). T1 of GSH (0.49 ± 0.11s) was found to be significantly shorter than that of other metabolites.
ACKNOWLEDGEMENTS
We thank Mr. Christopher Johnson and Mrs. Maria Ferraris Araneta for recruiting and caring for the healthy volunteers. This work was supported by the intramural programs of the NIH and NIMH.
REFERENCES
- 1.Erecinska M, Silver IA. Metabolism and Role of Glutamate in Mammalian Brain. Progress in Neurobiology. 1990;35(4):245–296. doi: 10.1016/0301-0082(90)90013-7. [DOI] [PubMed] [Google Scholar]
- 2.Bottomley PA, Hardy CJ, Argersinger RE, Allenmoore G. A Review of H-1 Nuclear-Magnetic-Resonance Relaxation in Pathology - Are T1 and T2 Diagnostic. Medical Physics. 1987;14(1):1–37. doi: 10.1118/1.596111. [DOI] [PubMed] [Google Scholar]
- 3.Frahm J, Bruhn H, Gyngell ML, Merboldt KD, Hanicke W, Sauter R. Localized Proton Nmr-Spectroscopy in Different Regions of the Human-Brain Invivo - Relaxation-Times and Concentrations of Cerebral Metabolites. Magnetic Resonance in Medicine. 1989;11(1):47–63. doi: 10.1002/mrm.1910110105. [DOI] [PubMed] [Google Scholar]
- 4.Ongur D, Prescot AP, Jensen JE, Rouse ED, Cohen BM, Renshaw PF, Olson DP. T(2) Relaxation Time Abnormalities in Bipolar Disorder and Schizophrenia. Magnetic Resonance in Medicine. 2010;63(1):1–8. doi: 10.1002/mrm.22148. [DOI] [PubMed] [Google Scholar]
- 5.Choi CG, Frahm J. Localized proton MRS of the human hippocampus: Metabolite concentrations and relaxation times. Magnetic Resonance in Medicine. 1999;41(1):204–207. doi: 10.1002/(sici)1522-2594(199901)41:1<204::aid-mrm29>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 6.Mlynarik V, Gruber S, Moser E. Proton T-1 and T-2 relaxation times of human brain metabolites at 3 Tesla. Nmr in Biomedicine. 2001;14(5):325–331. doi: 10.1002/nbm.713. [DOI] [PubMed] [Google Scholar]
- 7.Michaeli S, Garwood M, Zhu XH, DelaBarre L, Andersen P, Adriany G, Merkle H, Ugurbil K, Chen W. Proton T-2 relaxation study of water, N-acetylaspartate, and creatine in human brain using Hahn and Carr-Purcell spin echoes at 4T and 7T. Magnetic Resonance in Medicine. 2002;47(4):629–633. doi: 10.1002/mrm.10135. [DOI] [PubMed] [Google Scholar]
- 8.Brief EE, Whittal KP, Li DKB, MacKay A. Proton T-1 relaxation times of cerebral metabolites differ within and between regions of normal human brain. Nmr in Biomedicine. 2003;16(8):503–509. doi: 10.1002/nbm.857. [DOI] [PubMed] [Google Scholar]
- 9.Ethofer T, Mader I, Seeger U, Helms G, Erb M, Grodd W, Ludolph A, Klose U. Comparison of longitudinal metabolite relaxation times in different regions of the human brain at 1.5 and 3 Tesla. Magnetic Resonance in Medicine. 2003;50(6):1296–1301. doi: 10.1002/mrm.10640. [DOI] [PubMed] [Google Scholar]
- 10.Traber F, Block W, Lamerichs R, Gieseke J, Schild HH. H-1 metabolite relaxation times at 3.0 tesla: Measurements of T1 and T2 values in normal brain and determination of regional differences in transverse relaxation. Journal of Magnetic Resonance Imaging. 2004;19(5):537–545. doi: 10.1002/jmri.20053. [DOI] [PubMed] [Google Scholar]
- 11.Kreis R, Slotboom J, Hofmann L, Boesch C. Integrated data acquisition and processing to determine metabolite contents, relaxation times, and macromolecule baseline in single examinations of individual subjects. Magnetic Resonance in Medicine. 2005;54(4):761–768. doi: 10.1002/mrm.20673. [DOI] [PubMed] [Google Scholar]
- 12.Ganji SK, Banerjee A, Patel AM, Zhao YD, Dimitrov IE, Browning JD, Brown ES, Maher EA, Choi CH. T2 measurement of J-coupled metabolites in the human brain at 3T. Nmr in Biomedicine. 2012;25(4):523–529. doi: 10.1002/nbm.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Y, Xu D, Ozturk-Isik E, Lupo J, Chen A, Vigneron D, Nelson S. T1 and T2 Metabolite Relaxation Times in Normal Brain at 3T and 7T. J Mol Imaging Dynam. 2012;S1(002) [Google Scholar]
- 14.Marjanska M, Auerbach EJ, Valabregue R, Van de Moortele PF, Adriany G, Garwood M. Localized H-1 NMR spectroscopy in different regions of human brain in vivo at 7T: T-2 relaxation times and concentrations of cerebral metabolites. Nmr in Biomedicine. 2012;25(2):332–339. doi: 10.1002/nbm.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ronen I, Ercan E, Webb A. Rapid multi-echo measurement of brain metabolite T-2 values at 7 T using a single-shot spectroscopic Carr-Purcell-Meiboom-Gill sequence and prior information. Nmr in Biomedicine. 2013;26(10):1291–1298. doi: 10.1002/nbm.2951. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y, Shen J. Simultaneous quantification of glutamate and glutamine by J-modulated spectroscopy at 3 Tesla. Magn Reson Med. 2016;76(3):725–732. doi: 10.1002/mrm.25922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.An L, Li S, Murdoch JB, Araneta MF, Johnson C, Shen J. Detection of glutamate, glutamine, and glutathione by radiofrequency suppression and echo time optimization at 7 tesla. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2015;73:451–458. doi: 10.1002/mrm.25150. [DOI] [PubMed] [Google Scholar]
- 18.Tkac I, Andersen P, Adriany G, Merkle H, Ugurbil K, Gruetter R. In vivo H-1 NMR spectroscopy of the human brain at 7 T. Magnetic Resonance in Medicine. 2001;46(3):451–456. doi: 10.1002/mrm.1213. [DOI] [PubMed] [Google Scholar]
- 19.Smith SA, Levante TO, Meier BH, Ernst RR. Computer-Simulations in Magnetic-Resonance - an Object-Oriented Programming Approach. Journal of Magnetic Resonance Series A. 1994;106(1):75–105. [Google Scholar]
- 20.Govindaraju V, Young K, Maudsley AA. Proton NMR chemical shifts and coupling constants for brain metabolites. Nmr in Biomedicine. 2000;13(3):129–153. doi: 10.1002/1099-1492(200005)13:3<129::aid-nbm619>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 21.Choi CH, Dimitrov IE, Douglas D, Patel A, Kaiser LG, Amezcua CA, Maher EA. Improvement of resolution for brain coupled metabolites by optimized H-1 MRS at 7 T. Nmr in Biomedicine. 2010;23(9):1044–1052. doi: 10.1002/nbm.1529. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y, An L, Shen J. Fast and Accurate Simulation of Spatially Localized Magnetic Resonance Spectroscopy. EUROMAR; Aarhus, Denmark: 2016. [Google Scholar]
- 23.Devinsky O, Morrell MJ, Vogt BA. Contributions of Anterior Cingulate Cortex to Behaviour. Brain. 1995;118:279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- 24.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 25.An L, van der Veen JW, Li SZ, Thomasson DM, Shen J. Combination of multichannel single-voxel MRS signals using generalized least squares. Journal of Magnetic Resonance Imaging. 2013;37(6):1445–1450. doi: 10.1002/jmri.23941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klose U. Invivo Proton Spectroscopy in Presence of Eddy Currents. Magnetic Resonance in Medicine. 1990;14(1):26–30. doi: 10.1002/mrm.1910140104. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y, Shen J. Simultaneous quantification of glutamate and glutamine by J-modulated spectroscopy at 3 Tesla. Magnetic Resonance in Medicine. 2015 doi: 10.1002/mrm.25922. n/a-n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choi IY, Lee P. Doubly selective multiple quantum chemical shift imaging and T-1 relaxation time measurement of glutathione (GSH) in the human brain in vivo. Nmr in Biomedicine. 2013;26(1):28–34. doi: 10.1002/nbm.2815. [DOI] [PMC free article] [PubMed] [Google Scholar]