Abstract
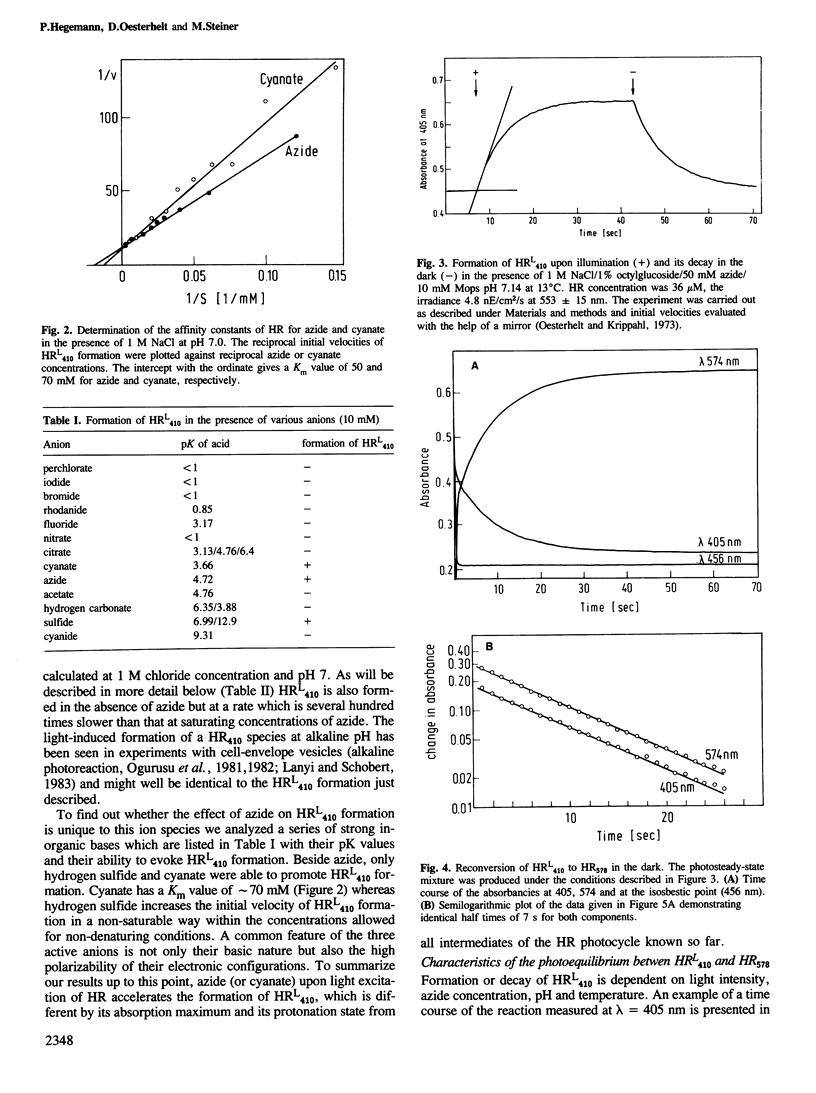
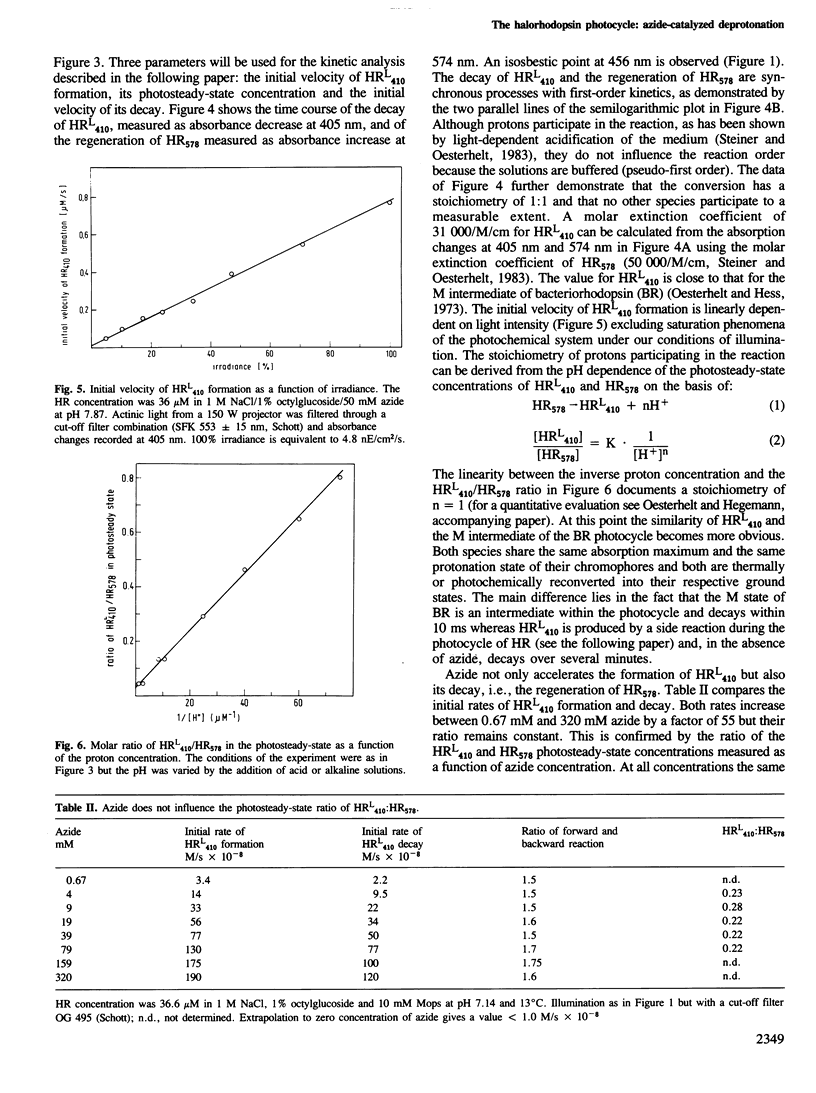
Halorhodopsin, the light-driven chloride pump of halobacteria, undergoes a photochemical cycle in the 10 ms range. Two intermediates, HR640 and HR520, accumulate in the photosteady state after short times (within 100 ms) of illumination. Upon prolonged illumination a third species, HRL410 accumulates, which is formed from HR520/HR640 by deprotonation of the chromophore in a side reaction of the photocycle. In the dark, HRL410 requires several minutes to reconvert thermally to HR578. Thus, molecules in the HRL410 state must be inactive pumps since their maximal turnover number could only be a few per hour. Inorganic bases, such as azide, catalyze the deprotonation of HR520/HR640 as well as the reprotonation of HRL410. Both reactions are accelerated several hundred times by azide but the photosteady-state concentration of HRL410 remains unchanged.
Keywords: halorhodopsin, light-driven chloride pump, azide catalysis, regulation
Full text
PDF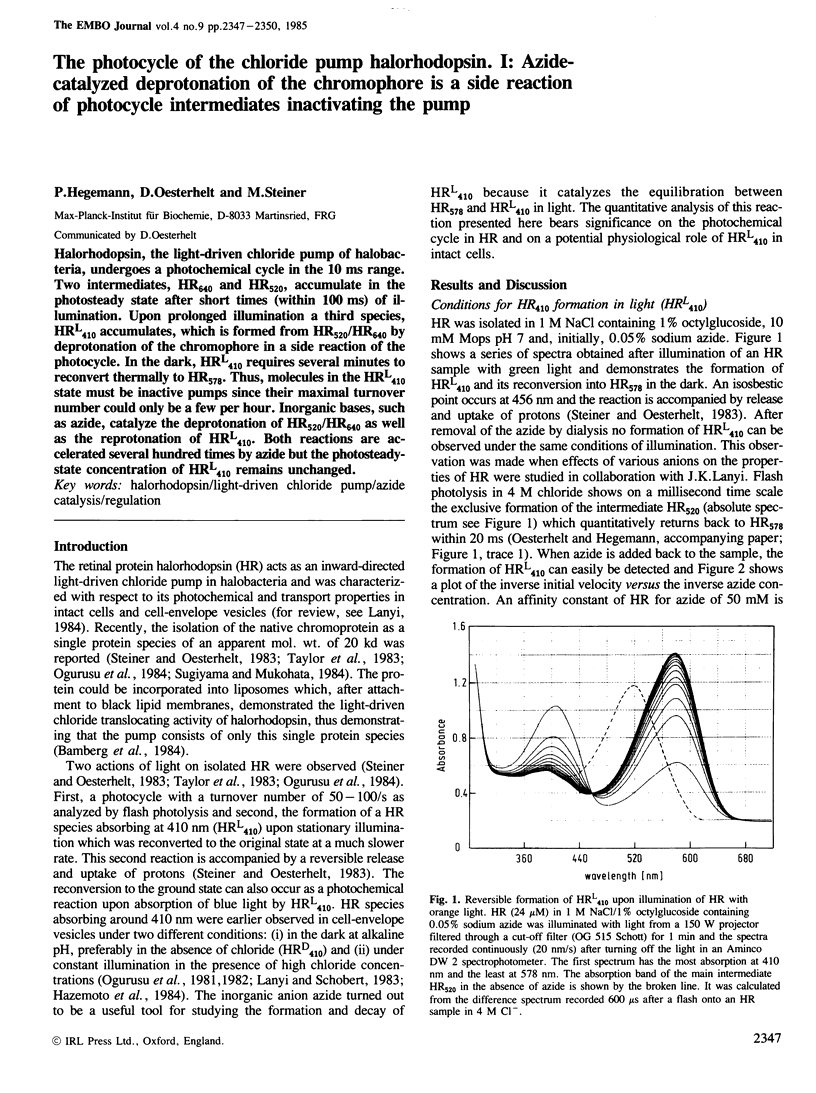

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hazemoto N., Kamo N., Kobatake Y. Suggestion of existence of two forms of halorhodospin in alkaline solution. Biochem Biophys Res Commun. 1984 Jan 30;118(2):502–507. doi: 10.1016/0006-291x(84)91331-7. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Hegemann P., Tittor J. The photocycle of the chloride pump halorhodopsin. II: Quantum yields and a kinetic model. EMBO J. 1985 Sep;4(9):2351–2356. doi: 10.1002/j.1460-2075.1985.tb03938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesterhelt D., Hess B. Reversible photolysis of the purple complex in the purple membrane of Halobacterium halobium. Eur J Biochem. 1973 Aug 17;37(2):316–326. doi: 10.1111/j.1432-1033.1973.tb02990.x. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Krippahl G. Light inhibition of respiration in Halobacterium halobium. FEBS Lett. 1973 Oct 1;36(1):72–76. doi: 10.1016/0014-5793(73)80339-4. [DOI] [PubMed] [Google Scholar]
- Ogurusu T., Maeda A., Sasaki N., Yoshizawa T. Light-induced reaction of halorhodopsin prepared under low salt conditions. J Biochem. 1981 Nov;90(5):1267–1273. doi: 10.1093/oxfordjournals.jbchem.a133591. [DOI] [PubMed] [Google Scholar]
- Ogurusu T., Maeda A., Yoshizawa T. Absorption spectral properties of purified halorhodopsin. J Biochem. 1984 Apr;95(4):1073–1082. doi: 10.1093/oxfordjournals.jbchem.a134695. [DOI] [PubMed] [Google Scholar]
- Steiner M., Oesterhelt D. Isolation and properties of the native chromoprotein halorhodopsin. EMBO J. 1983;2(8):1379–1385. doi: 10.1002/j.1460-2075.1983.tb01595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama Y., Mukohata Y. Isolation and characterization of halorhodopsin from Halobacterium halobium. J Biochem. 1984 Aug;96(2):413–420. doi: 10.1093/oxfordjournals.jbchem.a134852. [DOI] [PubMed] [Google Scholar]
- Taylor M. E., Bogomolni R. A., Weber H. J. Purification of photochemically active halorhodopsin. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6172–6176. doi: 10.1073/pnas.80.20.6172. [DOI] [PMC free article] [PubMed] [Google Scholar]


