Abstract
Liraglutide, a Glucagon-Like Peptide 1 (GLP-1) receptor agonist, is used as a treatment for Type 2 Diabetes Mellitus and obesity because it improves glycemia and decreases food intake. Here, we tested whether chronic activation of the GLP-1 receptor system with liraglutide would induce decreases in intake accompanied by changes in proportional food or macronutrient intake similar to those seen following RYGB in rats when a variety of palatable food options are available. A “cafeteria diet” was used that included: laboratory rodent chow, refried beans (low-fat/low-sugar), low-fat yogurt (low-fat/high-sugar), peanut butter (high-fat/low-sugar) and sugar-fat whip (high-fat/high-sugar). Liraglutide (1 mg/kg daily, sc, n=6) induced significant reductions in body weight and total caloric intake compared to saline–injected control rats (n=6). Although access to a cafeteria diet induced increases in caloric intake in both groups relative to chow alone, liraglutide still effectively decreased intake compared with saline-injected rats suggesting that chronic GLP-1 activation competes with the energy density and palatability of available food options in modulating ingestive behavior. Even with the substantial effects on overall intake, liraglutide did not change food choice or relative macronutrient intake when compared to pre-treatment baseline. When drug treatment was discontinued, the liraglutide group increased caloric intake and rapidly gained body weight to match that of the saline group. These results demonstrate that, while liraglutide effectively decreases caloric intake and body weight in rats, it does not cause adjustments in relative macronutrient consumption. Our data also show that drug-induced decreases in intake and body weight are not maintained following termination of treatment.
Keywords: GLP-1, Taste Preference, Food Choice, Weight Loss, Roux-en Y Gastric Bypass
1. Introduction
Obesity is a prominent health concern worldwide which increases the risk of Type 2 diabetes mellitus (T2DM), cardiovascular disease, and some cancers, among other health problems, and increases mortality while decreasing overall quality of life [see 1]. One treatment strategy drawing significant research attention is the activation of the Glucagon-Like Peptide-1 (GLP-1) receptor system. GLP-1 is an incretin hormone released by enteroendocrine cells that inhibits gastric acid secretion [2,3], enhances glucose-mediated insulin release from the pancreas [4], and increases satiation, all of which help to decrease food intake and promote normal glycemia [5–7]. GLP-1 is also expressed in taste buds [8] and in distinct clusters of neurons in the central nervous system, namely the nucleus of the solitary tract [9,10], and the olfactory bulb [11]. Interventions that prolong the positive effects of GLP-1 receptor activation have been adopted as treatment options because, when released endogenously into the periphery, the hormone itself only has a half-life of about 2 min due to protease degradation [see reviews 5,12,13].
Liraglutide (Saxenda), a synthetic GLP-1 receptor agonist used as a T2DM treatment, helps regulate blood glucose by activating GLP-1 receptors, which, in turn, improves glycemic control through its incretin effects [see reviews 7,13,14]. Due to liraglutide’s affinity for albumin, which protects it from rapid enzymatic degradation, the biological half-life of the drug is extended to about 13 hours [13,14]. Because of its effectiveness at promoting weight loss [e.g. 13,15,16], liraglutide has recently been approved in the United States as a bariatric intervention [16,17]
In the rodent model, liraglutide has also been shown to be effective at promoting weight loss and decreasing food intake [e.g. 18–21]. Some studies have even shown that liraglutide can affect food selection; when treated with the drug and given food options, rats will decrease their intake of foods high in sugar and/or fat relative to laboratory chow [20,21]. Similar effects have been reported following peripheral administration of exenatide, another GLP-1R agonist, which appears to have selective effects on “sweet” taste given that its administration in rats maintained on an obesogenic diet causes decreases in preference for sucrose solutions in long-term two-bottle intake tests (vs. water) but has no effect on preferences observed to compounds representing other basic taste qualities [22]. Here we took advantage of a more complex cafeteria diet paradigm in which four food items that varied in their fat and sugar content were offered to rats, in addition to laboratory chow, to investigate whether rats would systematically and progressively modify their relative macronutrient consumption when treated with liraglutide at a dose that is known to cause significant reductions in total caloric intake. This same cafeteria diet paradigm was recently used to demonstrate that Roux-en-Y gastric bypass (RYGB) not only decreases the number of calories consumed, but also causes progressive increases in the relative proportion of calories taken from complex carbohydrates, but not sugar, and progressive decreases in the relative proportion of calories taken from fat [23].
In humans, RYGB is one of the most perennially effective interventions for the treatment of obesity and its comorbidities [24]. The surgery causes weight loss of approximately 25% and significantly reduces caloric intake [e.g. 25,26]. This exceptional weight loss is accompanied by decreases in overall caloric intake and robust increases in postprandial levels of some endogenous gut hormones including GLP-1 and Peptide Tyrosine Tyrosine (PYY), among others [e.g. 25,27,28]. Increases in these gut peptides are thought to be at the root of the effectiveness of RYGB in the treatment of T2DM via their influence on insulin release [29,30]. There is also a prevailing view that after RYGB, some patients experience changes in food preference, choosing to eat fruits and vegetables compared with more calorically dense foods [25,31]. Yet, RYGB-induced changes in relative macronutrient intake reported in the literature are generally small or transient [31] and the majority of these food intake data are from dietary recall or self-report paradigms which are vulnerable to inaccuracies [see 32–34].
In rodents, food intake and choice can be directly, objectively, and quantitatively assessed and the outcomes cannot be influenced by dietary counseling. Therefore, in the study reported here, we sought to identify whether liraglutide-induced decreases in intake would be accompanied by modulations in relative macronutrient intake when calorically dense and highly palatable food options were available, as is seen after RYGB in a rat model. We also sought to determine whether some degree of drug-induced suppression of total caloric intake and/or potential changes in food selection would be maintained following the termination of treatment.
2. Materials and Method
2.1 Subjects
For these experiments, 12 (n=6/group) male Sprague Dawley adult rats were used over 6 experimental phases. At the beginning of the experiment, animals weighed between 360–397g. Animals were housed individually in cages with chipped wood bedding and a stainless steel Rattle Round (Otto Environmental) toy. In the presence of cafeteria diet, no extra enrichment was offered (i.e. no Rattle Rounds in the cage); however, in phases of the experiment when the animals only had access to chow, the Rattle Rounds were present in the cage. Removing the Rattle Round toys prevented the animals from placing the toy in the jars of food and thus limited spillage and allowed for more accurate intake recordings. Animals were housed in a vivarium on an automated 12:12 light:dark cycle that was controlled for temperature and humidity. Throughout each phase of the study, the rats had 24-h access to standard lab chow (Purina 5001), presented in suspended stainless steel hoppers, and deionized (DI) water. All procedures done in this experiment were approved by the Animal Care and Use Committee at Florida State University.
2.2 Diet
In addition to chow, rats were given selective access to a “cafeteria diet”. The diet options chosen represented one of four basic macronutrient categories: Low-Fat/Low-Sugar (Old EL Paso® Traditional Refried Beans), Low-Fat/High-Sugar (Yoplait® Low-Fat Vanilla Yogurt), High-Fat/Low-Sugar (JIF® Creamy Peanut butter), or High-Fat/High-Sugar (Sugar-Fat Whip [SFW]; prepared in our laboratory, see Table 1 for recipe). The macronutrient and caloric composition of these foods are presented in Table 1. For all phases of this project involving the cafeteria diet, the food options were presented to the animals in pre-weighed 4-oz glass jars. The jars were tethered to a cage corner via stainless steel wire. At the same time each day, 24-h intake was recorded and food jars were replaced with new jars, filled with the respective fresh food, and jar positions were rotated counterclockwise to a new cage corner. We also recorded overnight intake of chow and water and recorded daily body weight.
Table 1.
The comprehensive breakdown of the food items in the cafeteria diet by macronutrient composition. This diet was presented to both groups before, during, and after drug exposure.
| Diet | Caloric Density1 | Fat (g/kg; %kcal) | Carbohydrate2 (g/kg; %kcal) | Sugar (g/kg; %kcal) | Protein (g/kg; %kcal) | Fiber (g/kg) |
|---|---|---|---|---|---|---|
| Chow | 3.36 | −13.5 | −58.0 | −6.2 | −28.0 | 5.1 |
| Refried Beans (Low Sugar/Low Fat) | 0.92 | 20.8; 20.4 | 133.2; 57.9 | 0; 0 | 49.9; 21.7 | 5 |
| Vanilla Yogurt (High Sugar/Low Fat) | 0.93 | 6.7; 6.4 | 186.4; 80.2 | 133.2; 57.3 | 31.1; 13.4 | 0 |
| Peanut Butter (Low Sugar/High Fat) | 6.40 | 500.4; 70.6 | 250.3; 15.7 | 93.8; 5.9 | 218.9; 13.7 | 6.3 |
| Sugar - Fat Whip3 (High Sugar/High Fat) | 5.80 | 369.2; 57.3 | 423.4; 29.2 | 399.2; 27.5 | 195.8; 13.5 | 0 |
Caloric Density calculated using an approximate 9 kcal/g for fat, 4 kcal/g for carbohydrate and protein, and 0 kcal/g of fiber.
Carbohydrate measure includes sugar.
Sugar Fat Whip was made by combining 208g each of corn oil (Winn Dixie) and vegetable shortening (Winn Dixie), 300g whey protein (Jarrows Formulas), and 480g of powdered sugar (Winn Dixie).
2.3 Drug Acclimation
The animals were acclimated to a 1.0 mg/kg dose (s.c.) of liraglutide (Novo Nordisk; Saxenda; diluted in 0.9% sterile saline) by starting at 0.1 mg/kg and incrementing the daily dose (always in 1.0 ml/kg BW) by 0.1 mg/kg over 13 days. This was done to adapt the animal to higher doses, minimizing potential adverse reactions, and eventually reach an effective circulating level of the drug that would be maintained throughout much of the test period after a single injection. Control animals were injected with 0.9% saline at a volume (1.0 ml/kg BW) based on the dosing regimen for liraglutide. Following the dose of 0.2 mg/kg liraglutide, there was an excessive decrease in body weight, fluid intake, and food intake, and a criterion was implemented in which the liraglutide group had to consume an average of at least 50% of their pre-drug baseline chow intake before being moved to the next dose in the schedule. This criterion was met for all increases in drug dose except one day in which the animals received a premature increase in dose (from 0.4 to 0.5 mg/kg) due to a statistical calculation error, but this appeared to have little consequence. The target dose of 1.0 mg/kg was chosen because of the documented effectiveness of liraglutide, at this and lower doses (0.225–0.5mg/kg) to decrease total caloric intake in rats [18,35,36] as well as an attempt to maintain a prolonged and effective level of serum liraglutide after only a single daily injection [37].
2.4 Procedure
After arrival, the animals were given 10 days of acclimation to the vivarium and daily handling before being introduced to the foods. Then, along with their chow, rats were presented with 24-h access for 4 days each of beans, then yogurt, then peanut butter, and finally SFW for a total of 16 consecutive days. Immediately following the introduction of the individual foods, all rats received access to all cafeteria foods plus chow for 8 consecutive days (Pre-Drug Acclimation phase; Days 1–8).
After food acclimation, the animals had 18 consecutive days with 24-h access to only chow and water (Chow-Only Break phase; Days 9–26). This break was included to emulate the 18-day surgery/recovery period that rats received between pre-surgical and postsurgical testing in our prior RYGB study [23].
Following this Chow-Only Break, the rats were assigned to either the liraglutide or control group in a counterbalanced fashion based on average body weight, average total caloric intake, average proportional intake of each food in the cafeteria diet, and average proportional intake of each macronutrient (including sugar) from the final 2 days of each respective baseline period (baselines described below). They were then started on the drug acclimation schedule, described above, which lasted for 13 days, during which animals only had access to chow and water (Drug Acclimation phase; Days 27–39).
After the liraglutide group had reached the 1.0 mg/kg dose, both groups continued receiving once daily injections of either 1.0 mg/kg liraglutide (1.0 ml/kg) or 1.0 ml/kg saline and were given access to the cafeteria diet as described above for 12 days (Cafeteria Diet Drug phase; Days 40–51). The groups then received chow only while still getting once-daily injections for an additional 10 days (Chow-Only Drug phase; Days 52–61) after which the daily injections ceased and the rats were given 24-h access to the cafeteria diet for the final 22 days of the experiment (Cafeteria Diet No Drug phase; Days 62–83).
2.5 Data Analysis
The total caloric intake and the body weight of the final 2 days of the 18-day Chow-Only Break were averaged for each animal and used as the baseline measures for the Drug Acclimation and Chow-Only Drug phases during treatment. For the remaining phases in which animals had access to the cafeteria diet, with or without liraglutide, baseline was taken as the average total caloric intake and the average proportion caloric intake from each food type and macronutrient (and sugar) from the final 2 days of the 8-day Pre-Drug Acclimation phase. On the final 2 days of each respective pre-drug baseline period, there were no statistically significant group differences in: intake of any of the cafeteria food options, proportional intake of any of the macronutrients measured, or body weight (all ps>0.3).
For all intake measures, overnight intake was taken from the difference in offset weight from onset weight in grams. Intake was then broken down into macronutrient components of each food item. Caloric density was found using the grams of each macronutrient on the nutritional information labels associated with each food and an approximate 9 kcal/g of fat, 4 kcal/g of carbohydrate (including sugar) and protein, 0 kcal/g of fiber (Table 1). Calories consumed were calculated by multiplying the grams consumed for each food by its caloric density. Proportional caloric intake for each food was found by dividing caloric intake of that food each day by the total calories consumed from all foods. Proportional caloric intake of each macronutrient was found by summing the products of the daily proportional caloric intake for each food item times the proportion of calories of a given macronutrient in that food (see Table 1).
Statistical analysis included mixed repeated-measures group x day 2-way ANOVAs of body weight, total caloric intake, proportional intake of each food and macronutrient measured (including sugar), for each experimental phase. We also performed mixed repeated-measures group x day 2-way ANOVAs of the difference in proportional intake of each food and macronutrient (and sugar) from baseline over a specific treatment phase in the experiment. In some cases, two-tailed t-tests were used to compare measures from the first day of one phase with a) the final day of that same phase b) the final day of the previous phase, and c) the pre-drug baseline. When appropriate, Bonferroni corrections were made as indicated.
3. Results
3.1 Chow intake decreased during liraglutide Drug Acclimation phase
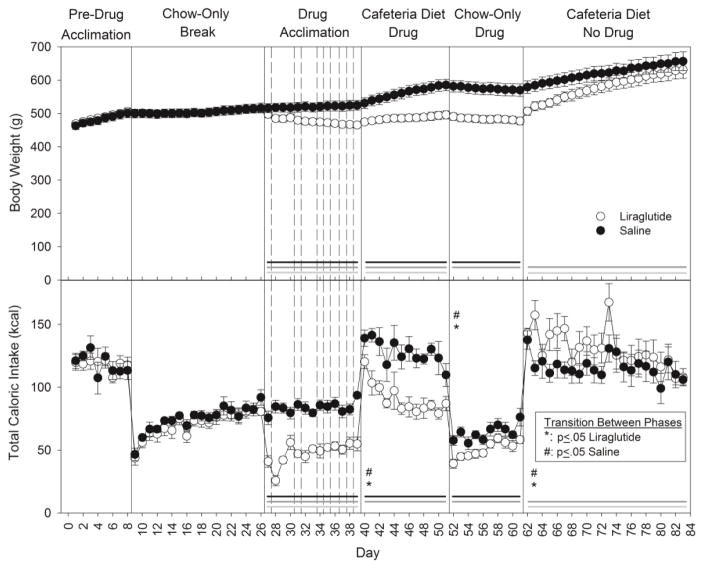
Liraglutide drug acclimation began following the 18-day Chow-Only Break after the pre-drug exposure to the cafeteria diet. Animals were initially administered the 0.1 mg/kg dose and then the dose was systematically increased to 1.0 mg/kg over the next 13 days during which time the rats had 24-h access to only chow and water. Over this time period, the total caloric intake from chow was significantly lower (F(1,10)=89.84, p<0.001) in the liraglutide group compared to the control group and there was also a significant effect of day (F(12,120) = 5.26, p<0.001) and day x group interaction (F(12,120)=4.10, p<0.001; Figure 1).
Figure 1.
Mean ± SE body weight (top) and total caloric intake (bottom) for the duration of the experiment. Shaded line denotes statistical difference (black: group effect; dark grey: day effect; light grey: day x group interaction; p≤ 0.05). Vertical solid lines separate experimental phases. Vertical dashed lines represent dose increments in the Drug Acclimation phase. For total caloric intake, differences between the days at a phase transition within each group are indicated (Liraglutide [*] or Saline [#]; p≤ 0.05). Additional corresponding statistics can be found in Tables 3 and 5. N=6/group.
3.2 The presence of palatable and calorically dense diet options caused increases in caloric intake even in liraglutide-treated rats
On the first day of the reintroduction of the cafeteria diet (Day 40), both the drug-treated and control rats increased their total caloric intake relative to the last day of drug acclimation (Day 39) when they had only chow available (saline vs. liraglutide: F(1,10)=25.60, p<0.001); day: F(1,10)=260.46, p<0.001); interaction (F(1,10)=8.42, p<0.016; Figure 1). Bonferroni-corrected t-tests confirmed that both groups increased their total caloric intake from Day 39 to Day 40 (both ps<0.001). In fact, on the first day of the Cafeteria Diet Drug phase, the total caloric intake of the animals in the liraglutide group returned to pre-drug baseline (t(5)=0.9, p>0.41) while the saline group consumed significantly more (Saline Baseline vs. Day 40: t(5)=4.98, Bonferroni-corrected p<0.04; Figure 1).
Over the duration of Cafeteria Diet Drug phase (Days 40–51); the liraglutide group did consume significantly less total calories compared to control animals (Table 3; Figure 1). Within each group, when the total caloric intake on the first (Day 40) and last (Day 51) day of this phase were compared, an overall decrease was detected in the liraglutide group (t(5)=6.06, Bonferroni-corrected p<0.02); however, the decrease in the saline group did not survive Bonferroni correction (t(5)=4.02, p=0.01, Bonferroni-corrected p>0.10). Even with the decrease across days, the total caloric intake on the last day of this phase (Day 51) remained significantly greater than that seen on the last day of the Drug Acclimation phase (Day 39), when rats were being presented with only chow, for both groups (group: F(1,10)=26.55, p<0.001; day: F(1,10)=22.45, p<0.001; day x group: F(1,10)=2.43, p>0.15; Figure 1).
Table 3.
Two-way ANOVA statistics for the total caloric intake and the proportional caloric intake (kcal) from each food item in the cafeteria diet during the Cafeteria Diet Drug phase. Bolded values indicate statistical significance (p≤0.05). See corresponding data in Figures 1 and 3.
| Total kcal | Bean | Yogurt | Peanut Butter | SFW | Chow | |
|---|---|---|---|---|---|---|
| By Group | F(1,10)=20.76, p<0.001 | F(1,10)=1.47, p>0.25 | F(1,10)=0.88, p>0.37 | F(1,10)=1.04, p>0.33 | F(1,10)=0.90, p>0.37 | F(1,10)=3.65, p>0.09 |
| By Day | F(11,110)=6.45, p<0.001 | F(11,110)=6.65, p<0.001 | F(11,110)=4.03, p<0.001 | F(11,110)=1.34, p>0.22 | F(11,110)=2.06, p<0.03 | F(11,110)=1.47, p>0.15 |
| Day x Group | F(11,110)=1.24, p>0.27 | F(11,110)=1.29, p>0.24 | F(11,110)=1.32, p>0.23 | F(11,110)=0.54, p>0.87 | F(11,110)=1.42, p>0.17 | F(11,110)=0.89, p>0.56 |
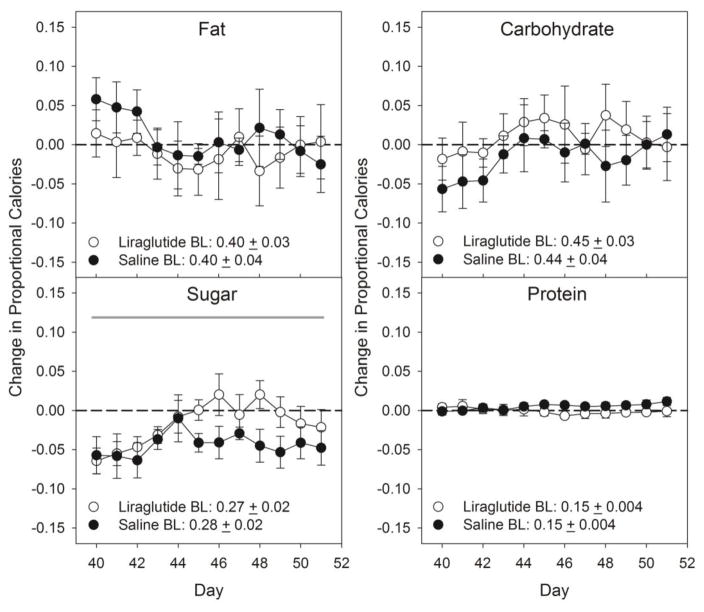
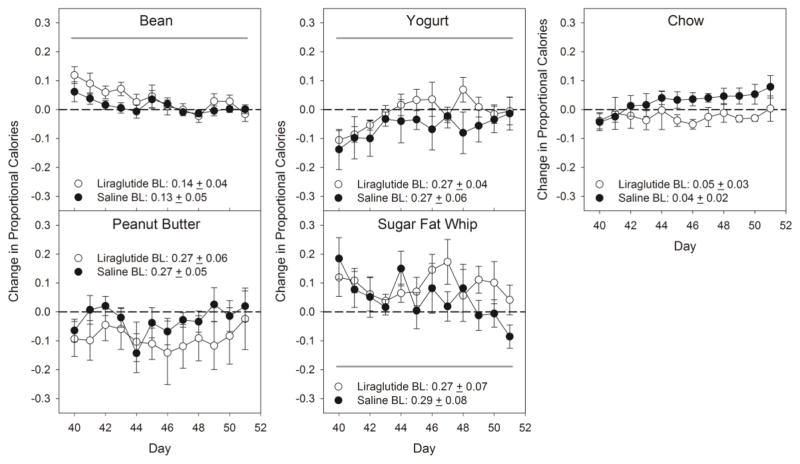
There were no differences between the groups and no group x day interactions when the change in the proportion of total caloric intake relative to pre-drug baseline was examined for each food or each macronutrient category and sugar (Table 2, 3; Figure 2, 3). There were some significant effects across days detected in this measure for refried beans, yogurt, SFW, and sugar, but, as can be seen in Figures 2 and 3, these were relatively minor and/or nonsystematic (Tables 2 & 3), and notably, affected both groups. Thus, the changes seen in total caloric intake as a function of drug treatment were not accompanied by adjustments in the relative proportion of calories taken from the various foods and macronutrients when the cafeteria diet was available.
Table 2.
Two-way ANOVA statistics for the proportional caloric intake (kcal) from each macronutrient measured (including sugar) in the cafeteria diet during the Cafeteria Diet Drug phase. Bolded values indicate statistical significance (p≤0.05). See corresponding data in Figure 2.
| Fat | Carbohydrate | Sugar | Protein | |
|---|---|---|---|---|
| By Group | F(1,10)=0.28 p>0.61 |
F(1,10)=0.61, p>0.46 | F(1,10)=1.79, p>0.21 | F(1,10) =1.75, p>0.22 |
| By Day | F(11,110) =1.37, p>0.19 | F(11,110) =1.58, p>0.12 | F(11,110) =4.15, p<0.001 | F(11,110) =0.31, p>0.98 |
| Day x Group | F(11,110) =0.63, p>0.80 | F(11,110) =0.59, p>0.83 | F(11,110)=1.77, p>0.07 | F(11,110) =1.48, p>0.15 |
Figure 2.
Mean ± SE change in proportional caloric intake from individual macronutrients and sugar in the cafeteria diet foods during the Cafeteria Diet Drug phase. Shaded line denotes statistical difference (black: group effect; dark grey: day effect; light grey: day x group interaction; p≤ 0.05). Additional corresponding statistics can be found in Table 2. N=6/group.
Figure 3.
Mean ± SE change in proportional calories from the cafeteria foods during the Cafeteria Diet Drug phase. Shaded line denotes statistical difference (black: group effect; dark grey: day effect; light grey: day x group interaction; p≤ 0.05). Additional corresponding statistics can be found in Table 3. N=6/group.
When the cafeteria diet was once again removed and only chow was available, both groups dropped their caloric intake on the first day (Day 52) of chow-only exposure relative to the last day of the previous phase (Day 51) when the cafeteria diet was available. A two-way mixed repeated-measures ANOVA of total caloric intake indicated a significant effect of group (saline vs. liraglutide: F(1,10)=9.89, p<0.01) and day (Day 51 vs. Day 52: F(1,10)=150.36, p<0.001) with no interaction (F(1,10)=0.28, p>0.61). Additionally, the caloric intake seen on Day 52 was also significantly less relative to the caloric intake on the last day (Day 39) of the Drug Acclimation phase for both groups (liraglutide: t(5)=4.72, Bonferroni-corrected p<0.04, saline: t(5)=10.44, Bonferroni-corrected p<0.001). By the final day of the Chow-Only Drug phase (Day 61), animals in the saline group were consuming similar total calories relative to the chow-only pre-drug baseline (t(5)=1.27, p>0.26) while the liraglutide-treated animals were consuming significantly less (t(5)=6.17, Bonferroni-corrected p<0.016). Nonetheless, when analyzing all ten days of the Chow-Only Drug phase, the liraglutide group ate significantly less chow than the control group (F(1,10)=9.81, p<0.01), and these initial drops in intake were followed by a progressive increase across the 10 days (Days 52–61: F(9,90)=13.16, p<0.001) with no group x day interaction (F(9,90)=1.39, p>0.20; Figure 1).
3.3 Liraglutide-induced differences in total caloric intake of the cafeteria diet disappeared soon after termination of treatment
In this final phase, Cafeteria Diet No Drug, access to the cafeteria diet (Days 62–83) was returned to the rats and liraglutide and saline treatments were discontinued. A two-way repeated-measures mixed ANOVA revealed that both groups consumed significantly more on the first day of this phase (Day 62) than on the final day of the Chow-Only Drug phase (Day 61) (group: F(1,10)=0.90, p>0.37, day: F(1,10)=527.04, p<0.001, day x group: F(1,10)=13.57, p<0.004; Figure 1). This was confirmed with paired sampled t-tests (liraglutide: t(5)=22.00, Bonferroni-corrected p<0.001; saline: t(5)=12.11, Bonferroni-corrected p<0.001). Moreover, on the first day of this phase, both the liraglutide and saline groups increased their caloric intake above pre-drug baseline, but the comparison in the former group failed to survive Bonferroni correction (Baseline vs. Day 62 liraglutide group: t(5)=3.46, Bonferroni- corrected p>0.14, uncorrected p=0.018; Baseline vs. Day 62 saline group: t(5)=6.23, Bonferroni- corrected p<0.016).
After the initial overshoots in caloric intake, both groups eventually decreased overall caloric intake across days with a significant day x group interaction (Table 5; Figure 1). By the final day of this phase (Day 83) animals from both the saline- and liraglutide-injected group were consuming a similar amount of calories as they had been during pre-drug baseline (liraglutide: t(5)=1.24, p>0.27; saline: t(5)=4.04, Bonferroni-corrected p>0.08).
Table 5.
Two-way ANOVA statistics showing the total caloric intake and the proportional caloric intake (kcal) from each food item in the cafeteria diet during the Cafeteria Diet No Drug phase. Bolded values indicate statistical significance (p≤0.05). See corresponding data in Figures 1 and 5.
| Total kcal | Bean | Yogurt | Peanut Butter | SFW | Chow | |
|---|---|---|---|---|---|---|
| By Group | F(1,10)= 1.90, p>0.20 | F(1,10)= 0.008, p>0.93 | F(1,10)= 2.55, p>0.14 | F(1,10)= 0.04, p>0.84 | F(1,10)= 0.44, p>0.52 | F(1,10)= 12.31, p<0.006 |
| By Day | F(21,210)= 4.98 p<0.001 |
F(21,210)= 5.17, p<0.001 | F(21,210)= 3.04, p<0.001 | F(21,210)= 0.83, p>0.68 | F(21,210)= 1.84, p<0.02 | F(21,210)= 2.02, p<0.007 |
| Day By Group | F(21,210)= 2.01, p< 0.007 | F(21,210)= 1.02, p>0.45 | F(21,210)= 1.10, p>0.35 | F(21,210)= 1.01, p>0.45 | F(21,210)= 1.14, p>0.31 | F(21,210)= 1.40, p>0.12 |
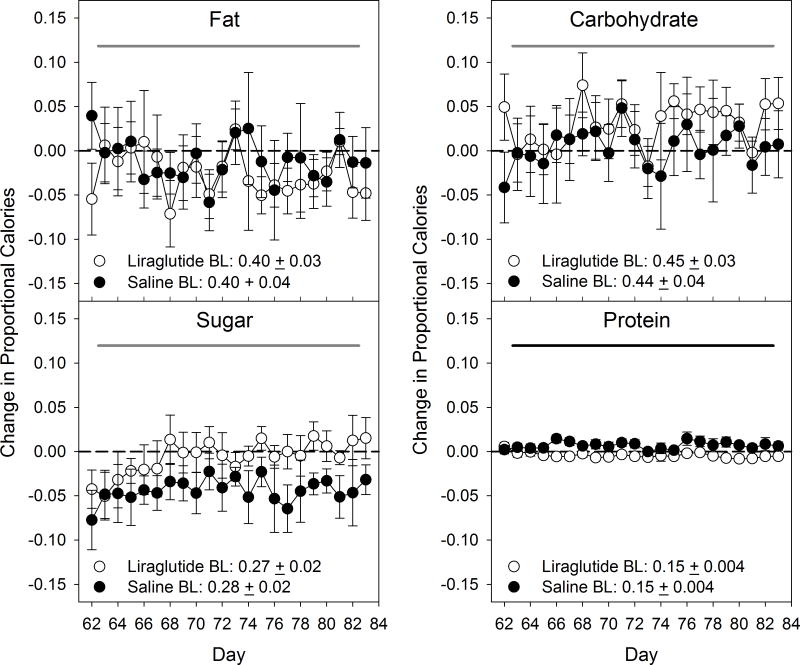
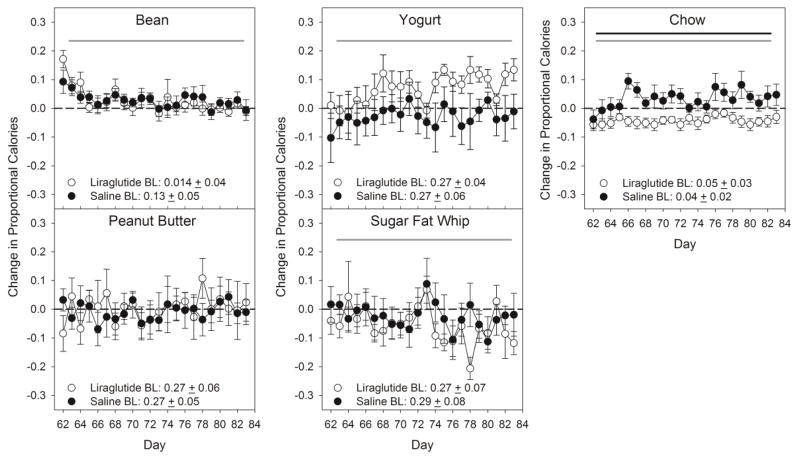
A two-way mixed repeated-measures ANOVA indicated that there was a group difference in the change in proportional calories consumed from chow and from protein relative to the pre-drug baseline, with the animals from the liraglutide group consuming less of each than did the saline group (Tables 4 & 5; Figures 4 & 5), but these differences were relatively small. There were no main effects of group or group x day interactions for proportion of calories taken from any of the other food items, fat, carbohydrate, or sugar relative to pre-drug baseline (Tables 4 & 5; Figures 4 & 5). Across the 22 days there were also significant differences in the change relative to pre-drug baseline of proportional caloric intake of refried beans, yogurt, SFW, chow, fat, carbohydrate, and sugar (Tables 4 & 5; Figures 4 & 5).
Table 4.
Two-way ANOVA statistics showing the proportional caloric intake (kcal) from each macronutrient (including sugar) in the cafeteria diet during the Cafeteria Diet No Drug phase. Bolded values indicate statistical significance (p≤0.05). See corresponding data in Figure 4.
| Fat | Carbohydrate | Sugar | Protein | |
|---|---|---|---|---|
| By Group | F(1,10)=0.14, p>0.72 | F(1,10)=0.48, p>0.50 | F(1,10)=2.39, p>0.15 | F(1,10)=9.58, p<0.01 |
| By Day | F(21,210)=1.64, p<0.04 | F(21,210)=1.72, p<0.03 | F(21,210)=2.05, p< 0.006 | F(21,210)=1.25, p>0.21 |
| Day x Group | F(21,210) =1.03, p>0.43 | F(21,210)=0.95, p>0.53 | F(21,210)=0.63, p>0.90 | F(21,210)=1.57, p>0.06 |
Figure 4.
Mean ± SE change in proportional caloric intake from individual macronutrients and sugar in the cafeteria diet foods during the Cafeteria Diet No Drug phase. Shaded line denotes statistical difference (black: group effect; dark grey: day effect; light grey: day x group interaction; p≤ 0.05). Additional corresponding statistics can be found in Table 4. N=6/group.
Figure 5.
Mean ± SE change in proportional calories from the cafeteria foods during the Cafeteria Diet No Drug phase. Shaded line denotes statistical difference (black: group effect; dark grey: day effect; light grey: day x group interaction; p≤ 0.05). Additional corresponding statistics can be found in Table 5. N=6/group.
3.4 Changes in total caloric intake were accompanied by more gradual changes in body weight
After only the first day (Day 27) of liraglutide treatment, the body weight of the experimental animals decreased from pre-treatment baseline (t(5)=9.57, Bonferroni corrected p<0.001). Over the duration of the Drug Acclimation Phase (Days 27–39), liraglutide-treated rats had lower body weight than saline-treated animals (F(1,10)=7.94, p<0.018). The initial decreases in body weight in the liraglutide group continued across days (F(12,120)=7.68, p<0.001), as supported by a significant day by group interaction (F(12,120)=22.79, p<0.001; Figure 1).
Once the desired dose of liraglutide was reached, 1.0 mg/kg, animals were re-exposed to the cafeteria diet. Although both groups gained some weight relative to the previous night, the liraglutide-treated animals still weighed significantly less than the saline group, and, as body weight of the saline animals progressively increased across days, liraglutide-treated animals maintained a lower body weight creating the significant interaction (group: F(1,10)=17.51, p<0.002, day: F(11,110)=50.46, p<0.001, group x day: F(11,110)=12.60, p<0.001; Figure 1). By the final day of the Cafeteria Diet Drug phase (Day 51), the saline animals weighed significantly more (t(5)=12.85, Bonferroni corrected p<0.001) than pre-drug baseline while the liraglutide animals maintained their baseline body weight (p>0.50).
Following 12 days of access to the cafeteria diet, the animals were given access to chow-only diet and kept on either saline or drug (Chow-Only Drug phase). The weight difference between the groups was maintained with the liraglutide-treated animals weighing significantly less than controls (group: F(1,10)=22.48, p<0.001, day: F(9, 90)=13.40, p<0.001, day x group F (9,90)=0.65, p=0.75; Figure 1).
After only one night of having access to the cafeteria diet without drug treatment (Day 62), the body weight of the animals which had been receiving liraglutide increased from the previous day (Day 61) (liraglutide: t(5)=20.86, Bonferroni corrected p<0.001; Figure 1). This body weight increase continued and by the final day (Day 83) animals in both groups weighed significantly more than they had on the first day (Day 62) of this phase (liraglutide: t(5)=8.59, Bonferroni corrected p<0.001, saline: t(5)=6.08, Bonferroni corrected p<0.016; Figure 1). Across the 22 days, the liraglutide-treated animals closed the gap in body weight between the groups (F(1,10)=2.59, p>0.14) as all animals continued to progressively increase in body weight (F(21,210)=97.94, p<0.001) with a group x day interaction (F(21,210)=5.41, p<0.001). By the final day of this phase (Day 83), animals from both treatment groups weighed significantly more than their pre-drug baseline body weight (liraglutide: t(5)=6.78, Bonferroni corrected p<0.008; saline: t(5)=8.03, Bonferroni corrected p<0.001; Figure 1) and there was no longer a statistical difference in body weight between the control and liraglutide groups (t(10)=0.74, p>0.48).
4. Discussion
Activation of the GLP-1 receptor system via chronic administration of 1.0 mg/kg liraglutide induced significant reductions in overall caloric intake and in body weight. However, this treatment failed to induce significantly meaningful modulations in proportional caloric intake from any specific food or macronutrient measured in these experiments.
During the Drug Acclimation phase, the effects of liraglutide treatment on weight loss and intake were immediate. Over the 13-day drug acclimation period, the liraglutide group lost an average of 48g (9%) of their pre-treatment body weight. Once the 1.0 mg/kg dose was reached and the animals were switched to the cafeteria diet, the body weight of liraglutide-treated animals ceased to drop, but the difference between the two groups continued. However, when the chow-only condition was reintroduced after the cafeteria diet during drug treatment, both the control and liraglutide groups had a parallel decrease in their body weight that was likely caused by the drop in total caloric intake seen after removal of the palatable and energy dense diet options. Thus, liraglutide proved to be an effective treatment in inducing and maintaining lower body weight relative to controls across dietary conditions.
When given access to the choices in the cafeteria diet, after having only chow during the Drug Acclimation phase, both the control and liraglutide groups significantly increased their total caloric intake on the first day. This increase was followed by a decline in caloric consumption by both groups across the 12 days of the Cafeteria Diet Drug phase; nevertheless, by the end of this phase, overall intake in both groups was still greater than what was seen on the last day of Drug Acclimation phase when only chow was available. Throughout this phase the difference in total calories consumed between the two groups was maintained. Thus, although the liraglutide group reliably consumed fewer calories than the saline-treated rats, the effectiveness of liraglutide to lower caloric intake appeared to compete with the energy density and/or palatability of the diet options available.
Despite the significant group difference in total calories consumed, we found no drug-related change in relative intake of any specific food or macronutrient. This suggests that chronic activation of the GLP-1 receptor system, with liraglutide, did not affect the relative preference of the diet options available. These results differ from the well documented changes in intake following rodent RYGB [38–44], even in a similar cafeteria diet paradigm [23], and are dissimilar from other liraglutide studies. Recently, liraglutide treatment in rodents has been shown to induce changes in food choice, increasing relative intake of chow compared with candy options [21] or a sugar- and fat-rich palatable gubra diet [20]. In both of those studies, though, rats had been maintained on these diets for many weeks before drug treatment started. In our study, the rats only had limited access to the cafeteria diet before drug treatment. It is also possible that the complexity and number of diet options available in addition to chow was a critical factor in our experiment. These are not mutually exclusive possibilities and the basis for the difference in the outcomes of the former studies and ours remains to be identified. Nevertheless, it is clear that within the context of the food options offered and with a dose of liraglutide that had significant attenuating effects on total caloric intake and body weight, relative food and macronutrient selection were largely unaffected.
In addition to these reported changes in food intake and preference, and the increases in serum GLP-1 levels [see reviews 25,27,28], there are a variety of other hormonal, anatomical, and physiological consequences of RYGB. Moreover, the pattern of release of GLP-1 following RYGB is fundamentally different from a single injection of a 1.0 mg/kg dose of liraglutide [14]. Our data suggest that the complexity of the RYGB model cannot be explained through chronic manipulation of a single peptide system and that other surgical consequences must be complicit in driving the changes in relative macronutrient intake. Nevertheless, activation of the GLP-1 system potently affected caloric intake and body weight and should not be disregarded as a potential bariatric intervention.
The behavior following termination of the injections is somewhat reminiscent of what has been seen in human patients upon discontinuation of treatment [16]. The lower body weight that was maintained relative to controls in the liraglutide-injected rats began to disappear within days once drug treatment ceased and the rats had access to palatable and calorically dense food options. Moreover, following discontinuation of treatment, animals from the liraglutide group consumed proportionally fewer calories from chow relative to pre-drug baseline, whereas the saline group consumed similar proportional calories from all of the other diet options as they were during pre-drug baseline. The overshoots in intake from the animals which had been receiving liraglutide were driven primarily from the 4 additional diet options (Table 5; Figure 1 & 5). Even with this group difference in chow intake, the formerly liraglutide-treated animals still did not differ in proportional intake of fat, carbohydrate, or sugar relative to their pre-drug baseline compared to the saline-treated group.
5. Conclusion
Collectively, the profile of results presented here suggests that chronic stimulation of the GLP-1 system does not induce any meaningful changes in relative macronutrient intake or food preference, at least with respect to the specific parameters of the cafeteria diet paradigm used here, in contrast to what is observed in a rat model of RYGB [e.g. 23,38–44]. In the context of obesity treatment, our results propose that liraglutide would be most effective when used with diet modification that lowers the caloric density of food options and leads to an overall reduction in daily caloric intake, but importantly when both the drug and diet are stopped weight gain appears inevitable.
Highlights.
Liraglutide effectively maintained decreased weight across diet conditions.
Liraglutide competes with palatability/energy density of diet to influence intake.
Decreased intake was not accompanied by changes in relative macronutrient intake.
Terminating treatment erased differences in body weight and intake between groups.
Acknowledgments
Portions of this work were presented at the Annual Meeting of the Society for the Study of Ingestive Behavior in Porto, Portugal in July 2016. Also, portions of this manuscript appear in a Thesis authored by Kellie Hyde in partial fulfillment of a Master of Science degree in Psychobiology at the Florida State University.
Funding
This work was supported in part by a grant from the National Institute on Deafness and Other Communication Disorders (NIH R21-DC012751) to ACS.
Footnotes
Disclosures
ClR received honoraria from NovoNordisk, Johnson&Johnson and Herbalife for serving on specialist advisory boards and as speaker during industry sponsored symposia, but no company had any role in this study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cefalu WT, Bray GA, Home PD, Garvey WT, Klein S, Pi-Sunyer FX, Hu FB, Raz I, Van Gaal L, Wolfe BM, Ryan DH. Advances in the science, treatment, and prevention of the disease of obesity: reflections from a diabetes care editors’ expert forum. Diabetes Care. 2015;38:1567–1582. doi: 10.2337/dc15-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wettergren A, Wøjdemann M, Meisner S, Stadil F, Holst JJ. The inhibitory effect of glucagon-like peptide-1 (GLP-1) 7–36 amide on gastric acid secretion in humans depends on an intact vagal innervation. Gut. 1997;40:597–601. doi: 10.1136/gut.40.5.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schjoldager BT, Mortensen PE, Christiansen J, Orskov C. GLP-1 (glucagon-like peptide 1) and truncated GLP-1, fragments of human proglucagon, inhibit gastric acid secretion in humans. Dig Dis Sci. 1989;34:703–708. doi: 10.1007/BF01540341. [DOI] [PubMed] [Google Scholar]
- 4.Komatsu R, Matsuyama T, Namba M, Watanabe N, Itoh H, Kono N, Tarui S. Glucagonostatic and insulinotropic action of glucagonlike peptide I-(7–36)-amide. Diabetes. 1989;38:902–905. doi: 10.2337/diab.38.7.902. [DOI] [PubMed] [Google Scholar]
- 5.Hayes MR, De Jonghe BC, Kanoski SE. Role of the glucagon-like-peptide-1 receptor in the control of energy balance. Physiol Behav. 2010;100:503–510. doi: 10.1016/j.physbeh.2010.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flint A, Raben A, Astrup A, Holst JJ. Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. J Clin Invest. 1998;101:515–520. doi: 10.1172/JCI990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lovshin JA, Drucker DJ. Incretin-based therapies for type 2 diabetes mellitus. Nat Rev Endocrinol. 2009;5:262–269. doi: 10.1038/nrendo.2009.48. [DOI] [PubMed] [Google Scholar]
- 8.Feng XH, Liu XM, Zhou LH, Wang J, Liu GD. Expression of glucagon-like peptide-1 in the taste buds of rat circumvallate papillae. Acta Histochem. 2008;110:151–154. doi: 10.1016/j.acthis.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 9.Larsen PJ, Tang-Christensen M, Holst JJ, Ørskov C. Distribution of glucagon-like peptide-1 and other preproglucagon-derived peptides in the rat hypothalamus and brainstem. Neuroscience. 1997;77:257–270. doi: 10.1016/s0306-4522(96)00434-4. [DOI] [PubMed] [Google Scholar]
- 10.Jin SL, Han VK, Simmons JG, Towle AC, Lauder JM, Lund PK. Distribution of glucagonlike peptide I (GLP-I), glucagon, and glicentin in the rat brain: an immunocytochemical study. J Comp Neurol. 1988;271:519–532. doi: 10.1002/cne.902710405. [DOI] [PubMed] [Google Scholar]
- 11.Merchenthaler I, Lane M, Shughrue P. Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. J Comp Neurol. 1999;403:261–280. doi: 10.1002/(sici)1096-9861(19990111)403:2<261::aid-cne8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 12.Williams DL. Neural integration of satiation and food reward: role of GLP-1 and orexin pathways. Physiol Behav. 2014;136:194–199. doi: 10.1016/j.physbeh.2014.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drucker DJ, Dritselis A, Kirkpatrick P. Liraglutide. Nat Rev Drug Discov. 2010;9:267–268. doi: 10.1038/nrd3148. [DOI] [PubMed] [Google Scholar]
- 14.Nuffer WA, Trujillo JM. Liraglutide: a new option for the treatment of obesity. Pharmacother. 2015;35:926–934. doi: 10.1002/phar.1639. [DOI] [PubMed] [Google Scholar]
- 15.Astrup A, Carraro R, Finer N, Harper A, Kunesova M, Lean MEJ, Niskanen L, Rasmussen MF, Rissanen A, Rössner S, Savolainen MJ, Van Gaal L. Safety, tolerability and sustained weight loss over 2 years with the once-daily human GLP-1 analog, liraglutide. Int J Obes. 2012;36:843–854. doi: 10.1038/ijo.2011.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pi-Sunyer X, Astrup A, Fujioka K, Greenway F, Halpern A, Krempf M, Lau DC, le Roux CW, Violante Ortiz R, Jensen CB, Wilding JP. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015;373:11–22. doi: 10.1056/NEJMoa1411892. [DOI] [PubMed] [Google Scholar]
- 17.Food and Drug Administration. FDA approves weight-management drug Saxenda. FDA News Release. 2014 https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm427913.htm.
- 18.Hayes MR, Kanoski SE, Alhadeff AL, Grill HJ. Comparative effects of the long-acting GLP-1 receptor ligands, liraglutide and exendin-4, on food intake and body weight suppression in rats. Obes. 2011;19:1342–1349. doi: 10.1038/oby.2011.50. [DOI] [PubMed] [Google Scholar]
- 19.Kanoski SE, Fortin SM, Arnold M, Grill HJ, Hayes MR. Peripheral and central GLP-1 receptor populations mediate the anorectic effects of peripherally administered GLP-1 receptor agonists, liraglutide and exendin-4. Endocrinology. 2011;152:3103–3112. doi: 10.1210/en.2011-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hansen G, Jelsing J, Vrang N. Effects of liraglutide and sibutramine on food intake, palatability, body weight and glucose tolerance in the gubra DIO-rats. Acta Pharmacol Sin. 2012;33:194–200. doi: 10.1038/aps.2011.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raun K, von Voss P, Gotfredsen CF, Golozoubova V, Rolin B, Knudsen LB. Liraglutide, a long-acting glucagon-like peptide-1 analog, reduces body weight and food intake in obese candy-fed rats, whereas a dipeptidyl peptidase-IV inhibitor, vildagliptin, does not. Diabetes. 2007;56:8–15. doi: 10.2337/db06-0565. [DOI] [PubMed] [Google Scholar]
- 22.Zhang X, Wang Y, Long Y, Wang L, Li Y, Gao F, Tian H. Alteration of sweet taste in high-fat diet induced obese rats after 4 weeks treatment with exenatide. Peptides. 2013;47:115–123. doi: 10.1016/j.peptides.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 23.Mathes CM, Letourneau C, Blonde GD, le Roux CW, Spector AC. Roux-en-Y gastric bypass in rats progressively decreases the proportion of fat calories selected from a palatable cafeteria diet. Am J Physiol - Regul Integr Comp Physiol. 2016;310:952–959. doi: 10.1152/ajpregu.00444.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sjöström L. Surgical intervention as a strategy for treatment of obesity. Endocrine. 2000;13:213–230. doi: 10.1385/ENDO:13:2:213. [DOI] [PubMed] [Google Scholar]
- 25.Lutz TA, Bueter M. The physiology underlying Roux-en-Y gastric bypass: a status report. Am J Physiol Regul Integr Comp Physiol. 2014;307:1275–1291. doi: 10.1152/ajpregu.00185.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sjöström L, Lindroos AK, Peltonen M, Torgerson J, Bouchard C, Carlsson B, Dahlgren S, Larsson B, Narbro K, Sjöström CD, Sullivan M, Wedel H. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2009;351:2683–2693. doi: 10.1056/NEJMoa035622. [DOI] [PubMed] [Google Scholar]
- 27.Pournaras DJ, le Roux CW. Obesity, gut hormones, and bariatric surgery. World J Surg. 2009;33:1983–1988. doi: 10.1007/s00268-009-0080-9. [DOI] [PubMed] [Google Scholar]
- 28.Pournaras DJ, Osborne A, Hawkins SC, Mahon D, Ghatei MA, Bloom SR, Welbourn R, le Roux CW. The gut hormone response following roux-en-y gastric bypass: cross-sectional and prospective study. Obes Surg. 2010;20:56–60. doi: 10.1007/s11695-009-9989-1. [DOI] [PubMed] [Google Scholar]
- 29.Madsbad S, Dirksen C, Holst JJ. Mechanisms of changes in glucose metabolism and bodyweight after bariatric surgery. Lancet Diabetes Endocrinol. 2014;2:152–164. doi: 10.1016/S2213-8587(13)70218-3. [DOI] [PubMed] [Google Scholar]
- 30.Jørgensen NB, Dirksen C, Bojsen-Møller KN, Jacobsen SH, Worm D, Hansen DL, Kristiansen VB, Naver L, Madsbad S, Holst JJ. Exaggerated glucagon-like peptide 1 response is important for improved β-cell function and glucose tolerance after Roux-en-Y gastric bypass in patients with type 2 diabetes. Diabetes. 2013;62:3044–3052. doi: 10.2337/db13-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mathes CM, Spector AC. Food selection and taste changes in humans after Roux-en-Y gastric bypass surgery: a direct-measures approach. Physiol Behav. 2012;107:476–483. doi: 10.1016/j.physbeh.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 32.Hill RJ, Davies PS. The validity of self-reported energy intake as determined using the doubly labelled water technique. Br J Nutr. 2001;85:415–430. doi: 10.1079/bjn2000281. [DOI] [PubMed] [Google Scholar]
- 33.Schoeller D. Limitations in the assessment of dietary energy intake by self-report. Metab. 1995;44:18–22. doi: 10.1016/0026-0495(95)90204-x. [DOI] [PubMed] [Google Scholar]
- 34.Livingstone MB. Assessment of food intakes: are we measuring what people eat? Br J Biomed Sci. 1995;52:58–67. [PubMed] [Google Scholar]
- 35.Vrang N, Jelsing J, Simonsen L, Jensen AE, Thorup I, Søeborg H, Knudsen LB. The effects of 13 wk of liraglutide treatment on endocrine and exocrine pancreas in male and female ZDF rats: a quantitative and qualitative analysis revealing no evidence of drug-induced pancreatitis. Am J Physiol Endocrinol Metab. 2012;303:253–264. doi: 10.1152/ajpendo.00182.2012. [DOI] [PubMed] [Google Scholar]
- 36.Hansen HH, Fabricius K, Barkholt P, Mikkelsen JD, Jelsing J, Pyke C, Knudsen LB, Vrang N. Characterization of liraglutide, a glucagon-like peptide-1 (GLP-1) receptor agonist, in rat partial and full nigral 6-hydroxydopamine lesion models of Parkinson’s disease. Brain Res. 2016;1646:354–365. doi: 10.1016/j.brainres.2016.05.038. [DOI] [PubMed] [Google Scholar]
- 37.Knudsen LB, Nielsen PF, Huusfeldt PO, Johansen NL, Madsen K, Pedersen FZ, Thøgersen H, Wilken M, Agersø H. Potent derivatives of glucagon-like peptide-1 with pharmacokinetic properties suitable for once daily administration. J Med Chem. 2000;43:1664–1669. doi: 10.1021/jm9909645. [DOI] [PubMed] [Google Scholar]
- 38.Bueter M, Miras AD, Chichger H, Fenske W, Ghatei MA, Bloom SR, Unwin RJ, Lutz TA, Spector AC, Le Roux CW. Alterations of sucrose preference after Roux-en-Y gastric bypass. Physiol Behav. 2011;104:709–721. doi: 10.1016/j.physbeh.2011.07.025. [DOI] [PubMed] [Google Scholar]
- 39.Hajnal A, Kovacs P, Ahmed T, Meirelles K, Lynch CJ, Cooney RN. Gastric bypass surgery alters behavioral and neural taste functions for sweet taste in obese rats. Am J Physiol Gastrointest Liver Physiol. 2010;299:967–979. doi: 10.1152/ajpgi.00070.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zheng H, Shin AC, Lenard NR, Townsend RL, Patterson LM, Sigalet DL, Berthoud HR. Meal patterns, satiety, and food choice in a rat model of Roux-en-Y gastric bypass. Am J Physiol Regul Integr Comp Physiol. 2009;297:1273–1282. doi: 10.1152/ajpregu.00343.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seyfried F, Miras AD, Bueter M, Prechtl CG, Spector AC, le Roux CW. Effects of preoperative exposure to a high-fat versus a low-fat diet on ingestive behavior after gastric bypass surgery in rats. Surg Endosc. 2013;27:4192–4201. doi: 10.1007/s00464-013-3020-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shin AC, Zheng H, Pistell PJ, Berthoud HR. Roux-en-Y gastric bypass surgery changes food reward in rats. Int J Obes (Lond) 2011;35:642–651. doi: 10.1038/ijo.2010.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saeidi N, Nestoridi E, Kucharczyk J, Uygun MK, Yarmush ML, Stylopoulos N. Sleeve gastrectomy and Roux-en-Y gastric bypass exhibit differential effects on food preferences, nutrient absorption and energy expenditure in obese rats. Int J Obes (Lond) 2012;36:1396–1402. doi: 10.1038/ijo.2012.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mathes CM, Bohnenkamp RA, Blonde GD, Letourneau C, Corteville C, Bueter M, Lutz TA, le Roux CW, Spector AC. Gastric bypass in rats does not decrease appetitive behavior towards sweet or fatty fluids despite blunting preferential intake of sugar and fat. Physiol Behav. 2015;142:179–188. doi: 10.1016/j.physbeh.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]