Abstract
Although commonly interpreted as a marker of episodic memory during neuropsychological exams, relatively little is known regarding the neurobehavior of “total learning” immediate recall scores. Medial temporal lobes are clearly associated with delayed recall performances, yet immediate recall may necessitate networks beyond traditional episodic memory. We aimed to operationalize cognitive and neuroanatomic correlates of total immediate recall in several aging syndromes. Demographically-matched neurologically normal adults (n=91), individuals with Alzheimer’s disease (n=566), logopenic variant primary progressive aphasia (PPA) (n=34), behavioral variant frontotemporal dementia (n=97), semantic variant PPA (n=71), or nonfluent/agrammatic variant PPA (n=39) completed a neurocognitive battery, including the CVLT-Short Form trials 1–4 Total Immediate Recall; a majority subset also completed a brain MRI. Regressions covaried for age and sex, and MMSE in cognitive and total intracranial volume in neuroanatomic models. Neurologically normal adults demonstrated a heterogeneous pattern of cognitive associations with total immediate recall (executive, speed, delayed recall), such that no singular cognitive or neuroanatomic correlate uniquely predicted performance. Within the clinical cohorts, there were syndrome-specific cognitive and neural associations with total immediate recall; e.g., semantic processing was the strongest cognitive correlate in svPPA (partial r=0.41), while frontal volumes was the only meaningful neural correlate in bvFTD (partial r=0.20). Medial temporal lobes were not independently associated with total immediate recall in any group (ps > 0.05). Multiple neurobehavioral systems are associated with “total learning” immediate recall scores that importantly differ across distinct clinical syndromes. Conventional memory networks may not be sufficient or even importantly contribute to total immediate recall in many syndromes. Interpreting learning scores as equivalent to episodic memory may be erroneous.
Keywords: Alzheimer disease, Primary progressive aphasia, Frontotemporal lobar degeneration, Immediate memory, Executive functions, Neuropsychology
1. Introduction
Converging neurobiological and behavioral data support the notion of multiple, dissociable memory systems that are broadly divided into encoding, storage, and retrieval stages (Perani et al., 1993; Shallice et al., 1994; Squire, 2004). Parcellation of the individual components of memory processing has deepened our understanding of the neural and cognitive systems supporting mnemonic abilities and the mechanisms by which these may become disrupted and/or enhanced (e.g., Delis, 1991; Kramer et al., 2005; Weintraub et al., 2004). Indeed, a substantial body of literature focused on delayed recall processes (i.e., storage and retrieval) consistently supports the critical role of medial temporal and frontal lobe networks that, when affected, demonstrate predictable patterns of memory impairment across distinct clinical syndromes (Delis, 1991; Wheeler et al., 1997; Zola-Morgan et al., 1986). However, delineation of “encoding” processes has received relatively less attention and its neural and cognitive underpinnings are subsequently not as well understood (Friedman and Johnson, 2000).
Total immediate recall during learning trials is the most commonly used measure of “encoding” and is frequently interpreted as an overall marker of clinical episodic memory abilities, comparable to delayed recall scores (e.g., Albert et al., 2001). These total learning scores are among the most psychometrically reliable metrics in memory paradigms (Benedict et al., 1998; Lacritz et al., 2001; Woods et al., 2006), and are therefore particularly well positioned for application in memory research and clinical assessment of patients longitudinally. Yet, clinical lesion studies have long demonstrated a double-dissociation between immediate and delayed recall such that some densely amnestic patients are still able to perform within normative limits on immediate recall tasks, and there are cases of patients with intact long term memory yet impaired immediate recall (Shallice and Warrington, 1970; Vallar, 1990). More recent work additionally supports preservation of immediate recall despite damage to the medial temporal lobes suggesting (at least partial) independence from traditional medial and diencephalic memory structures (Squire, 2004). Though many functional imaging studies have focused analyses on increased hippocampal formation activation (e.g.,(Szaflarski et al., 2004), there is clear appreciation of the role of the prefrontal cortex, particularly the left inferior gyrus (Habib et al., 2003), and potentially even more broadly distributed networks (e.g., parietal-temporal, cerebellum)(Sperling, 2007; Woodruff-Pak et al., 2001) during learning paradigms. Yet, clinical neuropsychologists continue to commonly interpret total learning scores as memory reflecting medial temporal lobe functioning. Taken in the context of the clinical lesion and functional imaging works, total immediate recall may draw upon substantially disparate cognitive and neural systems than delayed recall, raising the question if immediate recall can then be accurately interpreted as “memory,” or if this may be a misnomer. A better understanding of which cognitive and neural factors are associated with immediate recall total learning scores with will both enhance our understanding of memory processing as well as our ability to more accurately interpret the neurobehavioral systems affected in clinical syndromes with immediate recall impairment.
Drawing on theory-based framework of information processing posited by Baddeley and Hitch, we hypothesized multiple cognitive networks may importantly contribute to successful total immediate recall performances (Baddeley, 2003, 2001; Baddeley and Hitch, 1974). Initially, incoming information may be held in a brief echoic store (acoustic store) requiring attentional processes wherein the trace is actively rehearsed (phonological loop) concurrently drawing upon basic phonological processes (e.g., fluency). Additionally, strength of existing semantic knowledge facilitates contextual integration during initial processing. For example, when linguistic processing is disrupted in children with language disorders (e.g., reduced vocabularies) or experimental speech sound manipulation, immediate verbal recall capacities are significantly reduced (Gathercole et al., 1999; Page and Norris, 2003). Not surprisingly, integrity of language-based neural systems, including the left inferior frontal gyrus and inferior parietal lobule, have also been linked to successful immediate echoic recall (Gathercole et al., 1999; Papagno and Vallar, 1992; Thorn and Gathercole, 1999). Following phonological processing, incoming information may be simultaneously organized and manipulated by central executive cognitive processes in an interactive manner with previously learned information in order to be stored for long-term use (Baddeley, 2003). Consistent with this theory, seminal experimental work demonstrates the beneficial effects of depth of information processing via organization during learning (e.g., chunking), supporting the role of cognitive control during immediate recall trials (Mandler and Parker, 1976; Hayes et al., 2007). Relatedly, both functional neuroimaging and clinical lesion studies support involvement of the dorsolateral prefrontal cortices as a major contributing system during the transfer of episodic information into long-term memory (Alexander, 2003; Fletcher et al., 1998; Tulving et al., 1994). Lastly, greater length of time between item presentations (inter-interval presentation) and reduced rate of covert rehearsal during initial learning negatively impacts subsequent immediate recall, suggesting there is also an important speeded cognitive component during verbal encoding (Baddeley, 1986; Cowan, 1992; Cowan et al., 1992). In a complementary framework, Squire and colleagues (2004) additionally suggested that immediate memory processing may in fact be modal-specific, occurring within the neural system(s) where the long-term store will eventually be processed, in conjunction with medial temporal systems. This latter theory provides further support of the need for potentially whole-brain cognitive networks during initial information processing, depending on the type of information to be learned. Together, these models highlight the multifaceted neurobehavioral systems, beyond traditional information storage and medial temporal networks, that may importantly impact total learning scores.
Given its relative complexity, total immediate recall may become disrupted following changes at any one point of the multiple ability areas involved. Understanding how “learning” can manifest in the context of distinct neurological etiologies will aid in disentangling the unique cognitive and neural substrates that differentially contribute to total immediate recall. Therefore, we aimed to characterize the correlates of total immediate recall across demographically-matched cohorts of neurologically normal older adults and several clinical neurodegenerative syndromes – Alzheimer’s disease, logopenic variant primary progressive aphasia (lvPPA), behavioral variant frontotemporal dementia (bvFTD), semantic variant primary progressive aphasia (svPPA), and nonfluent/agrammatic variant primary progressive aphasia (nfvPPA). While traditional memory and medial temporal systems are primarily affected in Alzheimer’s disease (Rabinovici et al., 2007b), individuals with bvFTD demonstrate particular vulnerability of the frontal and executive networks with relative sparing of long-term memory stores (Bott et al., 2014). On the other hand, distinct language-based networks are disrupted in each primary progressive aphasia syndrome. Individuals with lvPPA exhibit fluent but empty speech with impaired echoic recall and poor word retrieval associated with left posterior temporal and inferior parietal atrophy, svPPA is characterized by fluent speech but prominent semantic (i.e., word meaning) loss and anterior temporal lobe atrophy, while individuals with nfvPPA demonstrate apraxic, effortful, agrammatic speech and impaired complex syntactic understanding with left inferior frontal and insular atrophy (Gorno-Tempini et al., 2011, 2004).
Our primary study aim is to operationalize the neurocognitive processes associated with total immediate recall during a list learning paradigm, as illustrated by a schematic model in Fig. 1. Additionally, given that a subset of study participants completed structural neuroimaging, we secondarily aimed to explore potential neuroanatomic correlates (volumetric regions of interest) of total immediate recall performances within each of the study cohorts. We hypothesized that each of the multifaceted cognitive domains examined and a wide network of brain regions involving frontotemporal (given their involvement in delayed recall) but also parietal systems (e.g., Jonides et al., 1998) would contribute to total immediate recall among neurologically normal older adults. On the other hand, given their clinical presentation of rapid forgetting and severe hippocampal dysfunction, we hypothesized immediate recall would also draw upon these traditional memory systems in individuals with AD. Among individuals with bvFTD, we anticipated executive control and frontal neural systems to be the primary point of disruption during immediate recall with relatively less episodic memory store involvement. Lastly, while we hypothesized overlap in the contribution of language and left temporal networks in all of the aphasia groups, we anticipated slightly distinct patterns. Individuals with lvPPA have disrupted phonological processing and echoic store so we hypothesized primary contributions from echoic recall; conversely, among svPPA individuals, we hypothesized poor semantic knowledge (i.e., limited contextual knowledge) to primarily be associated with immediate recall during learning trials. In nfvPPA, we hypothesized reduced fluency and verbal speed to demonstrate the largest correlation with total immediate recall performance. Identification of the neurobehavioral processes associated with repetitive trial learning may reveal networks beyond traditional memory systems that are important to consider when interpreting total learning scores, and ultimately enhance our understanding of initial information processing pathways.
Fig. 1.
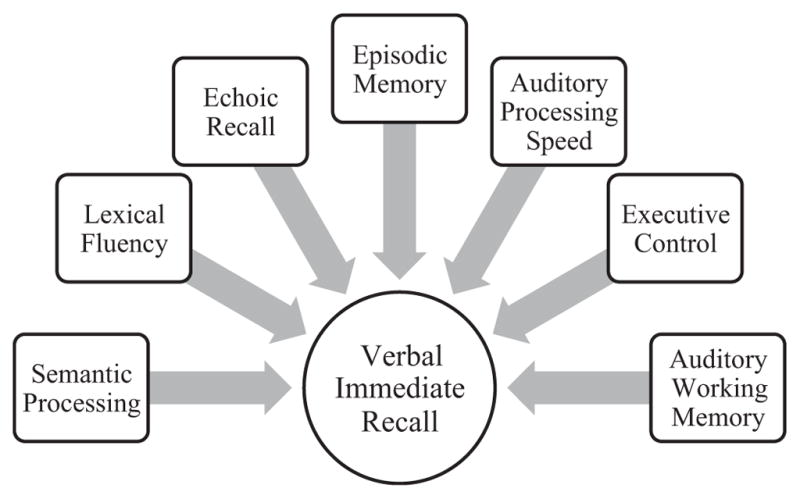
Schematic diagram illustrating the hypothesized contributing cognitive correlates of total immediate recall during learning trials on a verbal list learning paradigm.
2. Materials and methods
2.1. Participants
We drew the first study visit at which the California Verbal Learning Test, short form (CVLT-SF) was administered from the University of California, San Francisco Memory and Aging Center database across diagnostic groups. Participant inclusion criteria included Mini Mental Status Examination ≥18 (MMSE)(Folstein et al., 1975) and English as a primary language. Diagnoses were determined based on comprehensive neurobehavioral evaluations that included a neurological history and exam, caregiver/informant interview, and neuropsychological and language assessment (see Kramer et al., 2003 for protocol details). AD and bvFTD participants met “probable” criteria according to established clinical research guidelines (McKhann et al., 2011; Rascovsky et al., 2011). PPA participants were assessed by the UCSF MAC language team and also classified according to consensus research criteria (Gorno-Tempini et al., 2011). A cohort of normal aging adults were also included as a comparison group. Participants in the control comparison group were community-dwelling older adults who evidenced normal neurological and neuropsychological examinations per research consensus (McKhann et al., 2011) and did not have major memory concerns or a diagnosed memory condition. Exclusionary criteria included a history of another neurological condition (or any neurological condition for normal adults) or medical condition affecting the central nervous system, a metabolic disorder or major organ dysfunction, alcohol abuse or dependence within 5 years, head trauma with loss of consciousness > 30 min, or deteriorating cardiovascular disease. Participants were matched on age, education, and sex across diagnostic groups. Our final sample included 91 neurologically normal older adults, and 566 CE, 34 lvPPA, 97 bvFTD, 71 svPPA, and 39 nfvPPA individuals. This project was conducted in accordance to the Helsinki Declaration; written informed consent was obtained from all participants via a protocol that covered all testing sites approved by the institutional review board at University of California, San Francisco.
2.2. Neurocognitive assessment
2.2.1. Total immediate verbal recall
All participants completed the California Verbal Learning Test, short form (CVLT-SF) to determine total immediate verbal recall performances. On the CVLT-SF, participants were read a list of 9 words (one word/second) and asked to recall the words in any order; the same list of words was repeated across four trials. Each learning trial was presented immediately following participant response. Total immediate recall was calculated by summing the the total number of words recalled across the four trials (range 0–36; Delis et al., 2000).
2.2.2. Other cognitive measures
Participants also completed a brief neuropsychological battery assessing domains hypothesized to be important for total verbal immediate recall, including semantic processing, echoic recall, auditory working memory, lexical fluency, executive control, verbal processing speed, and episodic memory (visual delayed recall); this brief standardized battery has been previously described and validated to be neuroantomically sensitive to age-related neurodegeneration (Kramer et al., 2003; Possin et al., 2011).
In brief, semantic processing included confrontation naming (15-item short form of the Boston Naming Test, BNT), receptive vocabulary (16-item modified version of the Peabody Picture Vocabulary Test, PPVT-R), and semantic fluency (animals generated in 60-seconds). Sample-based z-scores for the BNT, PPVT-R, and animal fluency were calculated and averaged to create a semantic processing composite score. Echoic recall was determined via digit span forward length, while auditory working memory was assessed via digit span backward length. Lexical fluency was determined by the number of D-words generated in 60-seconds. Executive control was measured utilizing a modified version of the Trail Making Test which required participants to serially alternate between numbers and days of the week (total time to complete) and a Stroop interference task (correct items/60”). Sample-based z-scores were created for each of these measures and average together to create an executive control composite. Verbal processing speed performance was determined using the color naming condition of the Stroop (correct items/60”).
To assess delayed recall independent from the CVLT-SF, participants were asked to draw the modified Benson figure from memory after a 10-min delay (scored on accuracy, range=0–17 points; Possin et al., 2011). We specifically selected a visual task to measure delayed recall for conceptual purposes. Our overarching theoretical goal was to determine the extent to which verbal immediate recall represents episodic memory. Given that three of our clinical groups of interest had aphasic syndromes, we selected a visual task to measure “true” delayed recall abilities versus the confounding impact of language abilities. Although ours is a verbal immediate recall task, these list learning scores are commonly utilized to represent memory abilities even in aphasia syndromes or syndromes with language processing difficulties (e.g., semantic processing deficits in Alzheimer’s disease). In these cases, poor performances on a verbal delayed recall may in fact be related to poor speech output or lack of semantic contextual knowledge (e.g., essentially learning nonsense words) versus forgetting. These language-related problems are circumvented by using a visual delayed recall task.
2.3. Neuroimaging data
A subset of participants (n = 259) also received a 1.5-T Magnetom Vision or 3-T Magnetom Vision TIM Trio system (Siemens, Iselin, NJ) magnetic resonance imaging (MRI) scan within 90 days of their CVLT-SF admininstration. T1 weighted whole-brain images were acquired via volumetric magnetization prepared rapid gradient-echo MRI (MPRAGE, TR/TE/TI = 2300/2.98/900 ms) with a 15-degree flip angle, coronal orientation perpendicular to the double spin-echo sequence, 1.0 × 1.0 mm in-plan resolution and 1.5 mm slab thickness. 1 × 1 × 1 mm voxel size; FOV = 256 × 240 mm and 160 slices, TR = 2300 ms, TE = 3 ms, FA = 9°. Participants who completed MRI were slightly younger (M=66.8 vs 64.8 years, p < 0.01), and demonstrated mild but significantly higher total MMSE (M = 24.5 vs. 25.6, p < 0.01) and immediate recall performances (M=18.9 vs. 20.7 words, p < 0.01) compared to those who did not complete neuroimaging; however, those who completed neuroimaging did not differ on sex or educational levels compared to those who did not (ps > 0.05). Given that they were younger and demonstrated mildly better cognitive performances, the MRI analyses may represent the neuroanatomic correlates of immediate recall among mildly higher functioning individuals.
2.4. Freesurfer analyses
T1 MPRAGE structural images were analyzed using Freesurfer version 5.1, which is freely available online (http://surfer.nmr.mgh.harvard.edu/). In brief, Freesurfer averages multiple volumetric T1-weighted images correcting for motion, removes non-brain tissue via a hybrid watershed/surface deformation procedure (Segonne et al., 2004), and applies intensity normalization (Sled et al., 1998). Images were then transformed using automated Talairach and segmented into cortical and subcortical white matter and deep gray matter volumetric structures (e.g., hippocampus). Total intracranial volume was calculated via atlas normalization procedures (Buckner et al., 2004). Once the cortical models were completed, the surfacing algorithm corrected for topological defects and completed surface inflation, registration to a spherical atlas, parcellation of cerebral cortex into regions of interest based on gyral and sulcal structure. Surface-based data were created utilizing both intensity and continuity information from the entire three-dimensional MR volume. Images were individually quality checked for accuracy of segmentation, and manual edits were made to correct for geometric inaccuracies in white matter and pial surfaces, as needed.
A priori regions of interest included cortical areas hypothesized to be important to repeated verbal immediate recall, including the frontal cortex (sum of bilateral rostral and caudal middle frontal, superior frontal gyri, pars opercularis, pars orbitalis, pars triangularis, frontal poles, and lateral and medial orbitofrontal cortex, and rostral and caudal anterior cingulate cortex), left medial temporal lobe (sum of left entorhinal, parahippocampal, and hippocampal cortex), lateral left temporal cortex (sum of left inferior, superior, and middle temporal gyri and left temporal pole), and parietal cortex (sum of bilateral inferior and superior parietal cortex).
2.5. Statistical analyses
We examined syndrome-specific demographic and immediate recall differences via analyses of variance (ANOVA), followed up by Tukey’s HSD pairwise analyses. Next, we examined overall (i.e., collapsed across groups) and within-group correlates of total immediate recall performances via linear regression modeling with partial correlation effect sizes reported adjusting for age, sex, and MMSE for cognitive and age, sex, and total intracranial volumes for volumetric analyses. In the cognitive analyses, we elected to control for age and sex due to the extant literature delineating the effects of these demographics (e.g., Heaton et al., 2004); education was not controlled for given the lack of significant assocatiation between education and immediate recall across these groups (ps > 0.05) and because MMSE was instead selected as a covariate. Though all eligible participants demonstrated MMSE≥18 (inclusion criteria), we additionally covaried for total MMSE in order to adjust for effects of syndrome severity and highlight the signal of the relationships between each of the cognitive domains and immediate recall. Simiarly, age, sex, and total intracranial volume were covaried in volumetric analyses to adjust for nonspecific factors that are associated with brain volumes. Of note, we opted to emphasize effect sizes instead of significance in these initial analyses to examine the relative patterns of associations and to reduce issues of multiple comparison (type I error) particularly given the relatively smaller sample sizes within syndromes. However, the relationship between traditional memory systems (i.e., delayed recall and medial temporal lobe) and immediate recall were of particular interest; therefore, we conducted within-samples effect size boostrapping (100 samples) to determing if the magnitude of the relationship between the tradition memory system correlates differed from the other correlates examined. The subsequent multivariable models (described below) provide statistical significance parameters for all relavant immediate recall correlates.
We then conducted simultaneous multivariable linear regression models to determine the strongest, independent factors associated with total immediate recall. To increase parsimony, we only included those cognitive and anatomic correlates that demonstrated meaningful univariable effect sizes with total immediate recall according to Cohen’s (1992) criteria (i.e., partial r’s≥0.20). These regression analyses additionally covaried for age, sex, and MMSE for cognitive models, and age, sex, and total intracranial volumes for volumetric models.
3. Results
Table 1 presents demographic and MMSE performances by diagnostic group. The diagnostic groups were statistically comparable across age, education, and sex. However, MMSE scores differed such that neurologically normal adults performed better than all clinical groups (ps < 0.001), while the the AD and lvPPA cohorts performed the poorest but were comparable to one another (p = 0.85). MMSE performances across individuals with bvFTD, svPPA and nfvPPA did not differ (ps > 0.62).
Table 1.
Clinico-demographic characteristics across demographically-matched diagnostic groups.
| Neurologically normal older adults (n=91) (a) | Alzheimer’s disease (n=566) (b) | Logopenic variant PPA (n=34) (c) | Behavioral variant FTD (n=97) (d) | Semantic variant PPA (n=71) (e) | Nonfluent variant PPA (n=39) (f) | p-value | |
|---|---|---|---|---|---|---|---|
| Neuroimaging within 90 days | n=56 | n=86 | n=20 | n=36 | n=36 | n=25 | |
| Age, y | 66.0 (8.4) | 66.6 (8.5) | 64.5 (7.7) | 65.2 (6.7) | 64.7 (7.4) | 68.1 (6.3) | 0.13 |
| Education, y | 16.7 (2.6) | 15.9 (2.8) | 16.5 (2.8) | 16.1 (2.5) | 16.3 (2.7) | 16.0 (2.4) | 0.12 |
| Sex (%M, n) | 44.0% (40) | 45.1% (255) | 57.9% (18) | 57.3% (55) | 54.9% (39) | 46.2% (18) | 0.19 |
| Total MMSE | 29.2 (1.0) | 23.8 (3.2) | 23.2 (3.3) | 25.5 (3.0) | 25.5 (3.0) | 26.4 (2.0) | < 0.001 a > b–f; d–f > b,c |
Note. PPA = primary progressive aphasia; FTD = frontotemporal dementia; MMSE = Mini-Mental Status Exam.
3.1. Total immediate verbal recall group differences
The demographically-matched cohorts significantly differed in their CVLT total immediate recall performances after controlling for MMSE (F(5, 896) = 109.2, p < 0.001; see Fig. 2). Neurologically normal older adults recalled the most words across the four learning trials (M = 28.4 words, SD = 4.0) compared to any clinical group (ps < 0.001), and fell within expectations when compared to normative standards (Delis et al., 2000). Adjusting for MMSE, participants with AD (M = 18.1 words, SD = 5.3), bvFTD (M=20.4 words, SD=6.7) and nfvPPA (M = 21.7 words, SD=6.1) demonstrated comparable total immediate recall performances (ps > 0.05), which were better than individuals with svPPA (M = 17.4 words, SD = 6.5) (ps < 0.004). Individuals with lvPPA (M = 15.9 words, SD = 7.2) and svPPA did not significantly differ in their CVLT total immediate recall performances (p = 0.99).
Fig. 2.
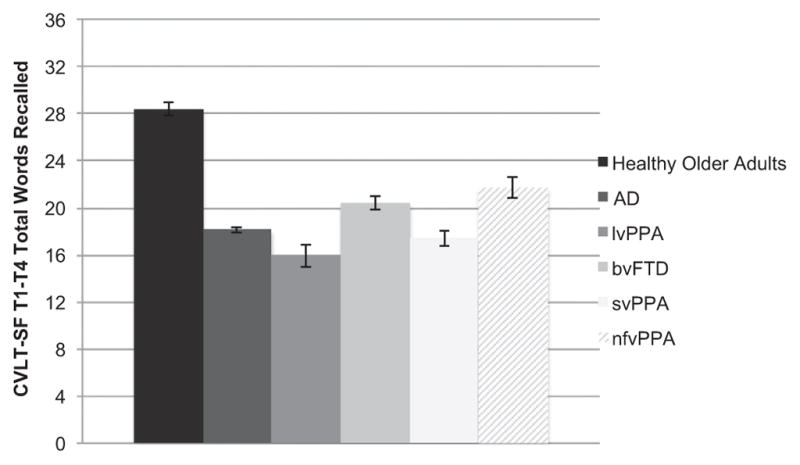
Total repeated immediate recall performance across demographically-matched diagnostic groups. Note. CVLT-SF T1–T4 = California Verbal Learning Test-short form Trials 1–4; AD = Alzheimer’s disease; lvPPA = logopenic variant primary progressive aphasia; bvFTD = behavioral variant frontotemporal dementia; svPPA = semantic variant primary progressive aphasia; nfvPPA = nonfluent variant primary progressive aphasia.
Notably, the median intrusion error rate in the whole sample was 0 (IQR = 0, 2), and the median intrusions within each clinical syndrome was also 0 (normal IQR=0, 1, range=0–7; bvFTD IQR = 0, 1, range = 0–8; lvPPA IQR = 0, 1, range = 0–4; nfvPPA IQR = 0, 1, range = 0–6), with the exception of AD (median = 1, IQR = 0, 2, range = 0–10) and svPPA (median = 1, IQR = 0, 3, range = 0–10).
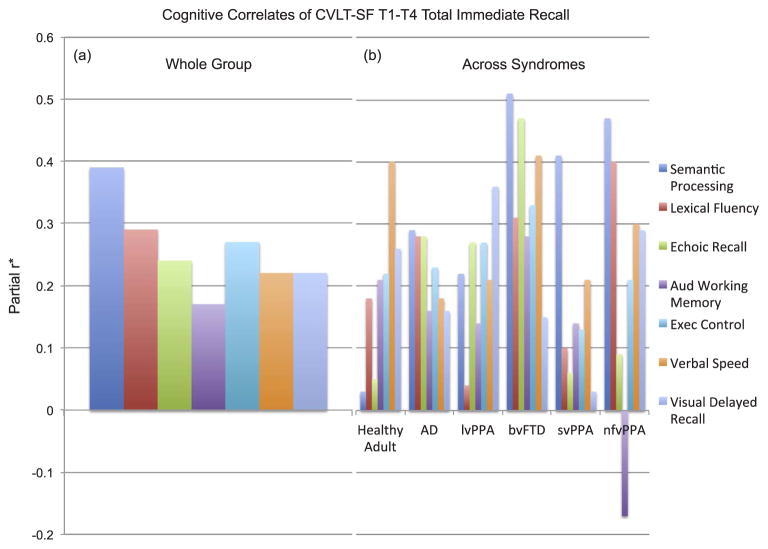
3.2. Cognitive correlates of total immediate verbal recall
Adjusting for age, sex, and MMSE and collapsed across all syndromes, almost all of the cognitive ability areas examined (with the exception of working memory) were meaningfully associated (partial r ≥ 0.20) with CVLT total immediate recall scores. Notably, Benson figure recall (visual delayed recall) was among the weakest correlate of CVLT immediate recall; statistically, the executive control composite demonstrated a significantly larger association with CVLT immediate recall than the relationship between CVLT immediate recall and Benson figure recall (difference 0.09, 95%CI, 0.02, 0.17).
Within the neurologically normal adults, the verbal processing speed composite demonstrated the strongest association with CVLT total immediate recall (partial r = 0.40), with auditory working memory and executive control composites, as well as Benson figure recall showing small-to-medium effects (partial r’s = 0.21 to 0.26; Fig. 3). Interestingly, Benson figure recall demonstrated significantly larger associations with CVLT immediate recall than Digits forward (echoic recall) (difference 0.32, 95%CI 0.05, 0.58) and the semantic processing composite (difference 0.30, 95%CI 0.06, 0.59), but demonstrate statistically the same contribution to CVLT immediate recall as all other cognitive correlates (ps > 0.05). Participants with AD showed a different pattern of effect sizes such that Benson figure recall, verbal processing speed composite, and Digits backward (auditory working memory) (partial r’s = 0.16 to 0.18) were among the weakest associations while the semantic processing composite, letter fluency, Digits forward, and the executive control composite demonstrated larger contributions (partial r’s = 0.28 to 0.29). The correlation between Benson figure recall and CVLT immediate recall was not statistically different from the other cognitive correlates of CVLT immediate recall in AD (ps > 0.05). Among bvFTD individuals, all domains examined with the exception of Benson figure recall (partial r = 0.15) demonstrated medium effect sizes with CVLT total immediate recall performances. The relationship between CVLT immediate recall and Benson figure recall was in fact significantly smaller than the relationship between CVLT immediate recall and Digits forward (difference 0.47, 95%CI −0.76, −0.19), letter fluency (difference 0.23, 95%CI 0.50, 0.04), and animal fluency (difference 0.36, 95%CI 0.58, 0.14).
Fig. 3.
Distinct cognitive abilities are associated with CVLT-SF T1-T4 Total Immediate Recall performances both within (a) and across (b) diagnostic groups. Note. (a) The cognitive correlates of immediate recall are highly multidimensional demonstrating relatively largest associations with language processing and among the smallest correlations with visual delayed recall abilities (Benson Figure). (b) Importantly, these cognitive correlates are syndrome-specific; for example, in AD, a highly distributed pattern of cognitive correlates is evidenced, whereas in svPPA, semantic processing is the primary cognitive correlate of immediate recall performances. *Partial r adjusted for age, sex, MMSE. CVLT-SF T1-T4 = California Verbal Learning Test-short form Trials 1–4; AD = Alzheimer’s disease; lvPPA = logopenic variant primary progressive aphasia; bvFTD = behavioral variant frontotemporal dementia; svPPA = semantic variant primary progressive aphasia; nfvPPA = nonfluent variant primary progressive aphasia.
Individuals with PPA syndromes demonstrated similarities in their patterns, such that the semantic processing composite was among the strongest association with CVLT immediate verbal recall, yet there were also clear differences. For instance, in svPPA individuals, the semantic processing composite was by far the single most strongly associated cognitive correlate of CVLT total immediate recall (partial r = 0.41), while Benson figure recall was among the weakest correlate of CVLT immediate recall in svPPA. The relationship between Benson figure recall and CVLT immediate recall was significantly smaller than the relationship between CVLT immediate recall and the executive control (difference 0.27, 95%CI 0.49, 0.01) and semantic processing (difference = 0.46, 95%CI 0.73, 0.21) composites. On the other hand, Digits forward was relatively more strongly associated with CVLT total immediate recall in the lvPPA cohort than svPPA or nfvPPA (partial r = 0.27 vs. 0.06 vs. 0.09, respectively), whereas letter fluency (partial r = 0.40 vs. 0.04 vs. 0.10) and the verbal processing speed composite (partial r = 0.30 vs. 0.21 vs. 0.21) were relatively more strongly associated with CVLT total immediate recall in nfvPPA compared to the other aphasia groups. Interestingly, the size of the association between Benson figure recall and CVLT immediate recall did not statistically differ from any of the other cognitive correlates of CVLT immediate recall within the lvPPA or nfvPPA groups (ps > 0.05).
Multivariable linear regression models were subsequently developed in which cognitive correlates that demonstrated meaningful associations (partial r’s ≥ 0.20) with CVLT total immediate recall were simultaneously entered covarying for age, sex, and MMSE. Among neurologically normal older adults, while the overall model was significant, none of the individual cognitive correlates uniquely predicted CVLT total immediate recall (see Table 2). Individuals with AD demonstrated independent, significant associations between the semantic processing composite and Digits forward with CVLT total immediate recall, whereas only Benson figure recall was associated with CVLT total immediate recall for lvPPA individuals. In bvFTD, the semantic processing composite and Digits forward emerged as unique cognitive predictors of CVLT total immediate recall. Only the semantic processing composite independently predicted CVLT total immediate recall performances among participants with svPPA. While the model examining cognitive predictors in nfvPPA was significant (p = 0.048), none of the individual cognitive parameters reached significance.
Table 2.
Final multivariable regression models evaluating the unique cognitive correlates of total immediate recall during learning trials within diagnostic groups (covarying for age, sex, and Mini Mental Status Exam).
| Adj. R2 | F | Total df | VIF | Std. β | Partial r | p-value | |
|---|---|---|---|---|---|---|---|
| Neurologically Normal Older Adults | |||||||
| Model | 0.36 | 3.2 | 28 | 0.02 | |||
| Aud Working Memory | 1.9 | 0.18 | 0.09 | 0.40 | |||
| Executive Control | 2.6 | 0.19 | 0.07 | 0.43 | |||
| Verbal Processing Speed | 2.5 | 0.09 | 0.10 | 0.71 | |||
| Visual Delayed Recall | 1.8 | 0.41 | 0.24 | 0.055 | |||
| Alzheimer’s Disease | |||||||
| Model | 0.30 | 15.8 | 240 | < 0.001 | |||
| Semantic Processing | 1.3 | 0.21 | 0.20 | < 0.001 | |||
| Lexical Fluency | 1.3 | 0.0.09 | 0.13 | 0.14 | |||
| Echoic Recall | 1.2 | 0.20 | 0.20 | < 0.001 | |||
| Executive Control | 1.3 | 0.12 | 0.12 | 0.051 | |||
| Logopenic variant PPA | |||||||
| Model | 0.46 | 3.3 | 21 | 0.03 | |||
| Semantic Processing | 2.9 | 0.31 | 0.29 | 0.28 | |||
| Echoic Recall | 2.6 | 0.01 | 0.01 | 0.97 | |||
| Executive Control | 1.8 | 0.18 | 0.19 | 0.40 | |||
| Processing Speed | 1.6 | 0.15 | 0.15 | 0.47 | |||
| Visual Delayed Recall | 1.3 | 0.43 | 0.47 | 0.03 | |||
| Behavioral variant Frontotemporal Dementia | |||||||
| Model | 0.72 | 9.96 | 32 | < 0.001 | |||
| Semantic Processing | 1.5 | 0.35 | 0.49 | 0.008 | |||
| Lexical Fluency | 2.3 | 0.02 | 0.16 | 0.84 | |||
| Echoic Recall | 2.4 | 0.39 | 0.40 | 0.02 | |||
| Aud Working Memory | 2.1 | 0.18 | 0.10 | 0.20 | |||
| Executive Control | 1.9 | 0.14 | 0.23 | 0.28 | |||
| Verbal Processing Speed | 3.2 | −0.01 | −0.18 | 0.99 | |||
| Semantic variant PPA | |||||||
| Model | 0.39 | 6.5 | 42 | < 0.001 | |||
| Semantic Processing | 1.9 | 0.60 | 0.38 | < 0.001 | |||
| Verbal Processing Speed | 1.7 | 0.01 | 0.02 | 0.93 | |||
| Nonfluent variant PPA | |||||||
| Model | 0.26 | 2.4 | 32 | 0.048 | |||
| Semantic Processing | 4.1 | 0.51 | 0.23 | 0.11 | |||
| Lexical Fluency | 3.5 | 0.11 | 0.15 | 0.69 | |||
| Executive Control | 3.7 | −0.11 | −0.04 | 0.70 | |||
| Verbal Processing Speed | 4.5 | −0.05 | −0.03 | 0.87 | |||
| Visual Delayed Recall | 1.6 | 0.15 | 0.23 | 0.45 | |||
Note. PPA = primary progressive aphasia; Aud = auditory.
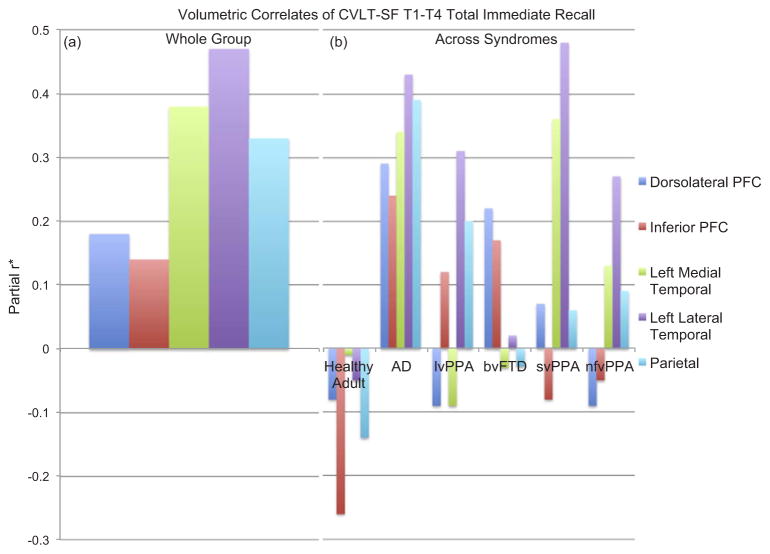
3.3. Neuroanatomic correlates of total immediate verbal recall
Adjusting for age, sex, and total intracranial volumes and collapsed across all study groups, temporal and parietal volumes were most strongly associated with immediate recall scores. Notably, the lateral temporal lobe demonstrated the strongest association with immediate recall performances comparable with medial temporal and parietal correlates (ps > 0.05), and these associations were larger than the relationships between immediate recall and prefrontal volumes (DLPFC vs. MTL difference 0.20, 95%CI 0.07, 0.32; IFG vs. MTL difference 0.22, 95%CI 0.10, 0.36). However, this pattern was highly dependent on the study group examined. In neurologically normal adults, none of the individual neuroanatomic regions demonstrated meaningful relationships with repeated immediate recall (partial r’s < 0.20). In contrast, in individuals with AD, all neuroanatomic volumetric ROIs examined demonstrated at least small to moderate relationships with immediate recall scores (partial r’s ≥ 0.21) (Fig. 4). Notably, larger left lateral temporal and parietal volumes were most strongly associated with total immediate recall performances in individuals with AD, while medial temporal volumes was among the weakest asscoiation, though this did not statistically differ from the other correlates (differences range 0.005–0.20, ps > 0.05). In bvFTD, only frontal volumes demonstrated a meaningful, albeit small, associations with total immediate recall (partial rs = 0.17 and 0.22); though the association between medial temporal lobe volumes and immediate recall was descriptively smaller than all other anatomic correlates, this did not reach statistical differnence (difference range 0.02–0.17, ps > 0.05). Among individuals with PPA syndromes, larger left lateral temporal volumes demonstrated consistent, medium-sized associations with better total immediate verbal recall (partial r’s ≥ 0.27). However, among lvPPA individuals, larger parietal cortical volumes also demonstrated a small but meaningful, positive relationship with total immediate recall (partial r = 0.20), which was not the case for either svPPA (partial r = 0.08) or nfvPPA (partial r=0.05) individuals. Interestingly, among the PPA syndromes, the magnitude of the relationship between the medial temporal lobe volume and immediate recall did not statistically differ from the other anatomic correlates (lvPPA difference range 0.02–0.22; svPPA difference range 0.03–0.22; nfvPPA difference range 0.01–0.14, ps > 0.05).
Fig. 4.
Distinct neuroanatomic correlates are associated with CVLT-SF T1-T4 Total Immediate Recall Performances both within (a) and across (b) diagnostic groups. Note. (a) The neural correlates of immediate recall are also multifaceted, though demonstrating the largest associations with temporal-parietal regions. (b) However, these neuroanatomic patterns vary highly depending on the clinical syndrome examined; for example, in AD, a largely dispersed pattern of anatomic regions is evidenced, whereas in bvFTD, only frontal regions are associated with immediate recall performances. *Partial r adjusted for age, sex, and total intracranial volumes; CVLT-SF T1-T4 = California Verbal Learning Test – short form Trials 1–4; AD = Alzheimer’s disease; lvPPA = logopenic variant primary progressive aphasia; bvFTD = behavioral variant frontotemporal dementia; svPPA = semantic variant primary progressive aphasia; nfvPPA = nonfluent variant primary progressive aphasia.
Parallel multivariable linear regression models examining the constellation of volumetric ROIs associated with total immediate recall (≥0.20) covarying for age, sex, and total intracranial volume were then conducted within each diagnostic group. Given that none of the indivdual neural regions examined were meaningfully associated with total immediate recall in neurologically normal adults, we did not develop a model in this cohort. In AD, although all cortical regions were entered into the model, none of the individual regions reached significance, with only left lateral temporal volumes approaching significance in predicting total immediate recall (p = 0.07; see Table 3). The neuroanatomic regression models in lvPPA and svPPA did not reach significance (ps > 0.07). Individuals with bvFTD and nvPPA only demonstrated meaningful relationships with one neuroantomic region each so multivariable models were not conducted in these groups.
Table 3.
Final multivariable regression models evaluating the unique neuroanatomic correlates of total immediate recall during learning trials within AD and lvPPA individuals (covarying for age, sex, and total intracranial volume).
| Adj. R2 | F | Total df | Std. β | Partial r | p-value | |
|---|---|---|---|---|---|---|
| Alzheimer’s Disease | ||||||
| Model | 0.20 | 4.0 | 85 | < 0.001 | ||
| Frontal | −0.17 | −0.11 | 0.34 | |||
| Left Medial Temporal | 0.16 | 0.13 | 0.25 | |||
| Left Temporal | 0.40 | 0.21 | 0.07 | |||
| Parietal | 0.16 | 0.12 | 0.31 | |||
| Logopenic variant PPA | ||||||
| Model | 0.14 | 1.6 | 19 | 0.22 | ||
| Left Temporal | 0.26 | 0.25 | 0.45 | |||
| Parietal | 0.02 | 0.02 | 0.94 | |||
| Semantic variant PPA | ||||||
| Model | 0.16 | 2.2 | 35 | 0.07 | ||
| Left Medial Temporal | 0.15 | 0.13 | 0.48 | |||
| Left Temporal | 0.48 | 0.36 | 0.05 | |||
Note. PPA = primary progressive aphasia.
4. Discussion
The neurobehavioral substrates associated with immediate recall performance across learning trials reflected a multifaceted set of neurocognitive domains that were then highly specific to the clinical syndrome examined. Even in cases in which the demographically-matched cohorts demonstrated comparable objective performances (e.g., bvFTD and nfvPPA), the pattern of cognitive and neuroanatomic associations varied widely. Of interest in the broader context of memory research, delayed recall only emerged as a unique significant predictor in one group (i.e., lvPPA), and the medial temporal lobes, a region with well-established ties to the storage and retrieval of novel information (Squire, 2004), did not independently correlate with total immediate recall performances in any of our study groups. In fact, within each of the individual syndromes, delayed recall and medial temporal lobe volumes demonstrated either statistically comparable or smaller associations with immediate recall compared to the other cognitive and anatomic correlates. These data suggest that immediate recall “learning” scores may not in fact be a direct marker of our conventional understanding of episodic memory, per se, but instead reflect a more nuanced and fluid construct of the multiple pathways by which initial information processing can occur.
As a normative comparison, neurologically normal aging adults demonstrated a heterogeneous constellation of cognitive correlates of total immediate recall, evidencing the largest effects with verbal processing speed but also associations with executive function processes (executive control and working memory) and delayed recall. The large correlation between speed and immediate recall is commensurate with the cognitive aging literature, which suggests that while other ability areas change, cognitive speed may be particularly specific to aging effects and impact other cognitive ability areas (Salthouse, 2017). Notably, while the omnibus multivariable model was significant, none of the individual cognitive parameters emerged as independent predictors of total immediate recall among the neurologically normal adults; this may suggest that the combination of these cognitive abilities, including but not limited to information storage and retrieval, is important for immediate recall trial performances in neurologically normal adults. Interestingly in this group, none of the regional brain volumes examined were meaningfully related to total immediate recall. Again, these null neural correlates may reflect a lack of specificity of one singular brain region that importantly correlates with total immediate recall versus a more distrbuted network or, likely, multiple contributing networks (Sperling, 2007). Notably, the lack of association between brain volume and cognitive functions in otherwise healthy adults, particularly with regard to limbic regions, is documented in prior work and may be related to the highly variable nature of brain structure among normal aging adults (Raz et al., 1998). These data suggest multiple points of information processing may contribute to normal adult total learning scores, particularly highlighing the roles of efficiency (i.e., speed) and central executive organizational abilities in addition to information storage capacity (Friedman and Johnson, 2000).
On the other hand, although all diagnosed with a progressive aphasia syndrome and demonstrating prominent associations with left lateral temporal lobe volumes (Gorno-Tempini et al., 2011, 2004), individuals with lvPPA, svPPA, or nfvPPA each revealed highly distinct cognitive correlates of total immediate verbal recall. Among those with svPPA, better semantic processing was the strongest correlate of total immediate recall with episodic memory contributing little-to-none. This pattern is consistent with the clinical features of svPPA which is characterized by fluent speech but with prominently impaired semantic memory. Therefore, individuals with svPPA may have a more circumscribed difficulty integrating novel verbal information to be learned with pre-existing verbal knowledge. In other words, incoming verbal material may lack contextual meaning (semantics) among svPPA individuals making it difficult to process and encode for later recall, suggesting that poor verbal episodic memory performance in svPPA may reflect, at least in part, a semantic impairment rather than an episodic memory deficit per se. Interestingly, while left lateral temporal volumes were the strongest anatomic correlate, left medial temporal volumes were also meaningfully associated with immediate recall. Widespread temporal lobe changes can be observed in svPPA, including hippocampal atrophy, suggesting that more global temporal networks may impact immediate recall in this group. Though notably, when modeled together, lateral temporal volumes approached significance while medial temporal volumes were no longer meaningfully associated with immediate recall highlighting the relative strength of lateral over medial temporal lobe networks.
However, in lvPPA and nfvPPA, while semantic processing emerged a common correlate, several other cognitive abilities were additionally meaningfully associated with total immediate recall. For instance, as differentials, echoic recall was more strongly associated with total immediate recall among individuals with lvPPA, while fluency and speeded cognitive processes emerged as relatively stronger predictors among nfvPPA individuals. These dissociations are again consistent with the distinctive clinical features of lvPPA (i.e., limited phonological processing storage) versus nfvPPA (i.e., apraxic, effortful, slowed speech output). Interestingly, in both groups, executive control and episodic memory were also associated with immediate recall, which is consistent with the pattern observed among neurologically normal adults. However, the relatively major contributions from semantic and verbal processing among the aphasia groups meaningfully contrast them from the neurologically normal older adults in which these language-based factors demonstrated little-to-no impact. Among indivivduals with aphasia, verbal “learning” scores may therefore be an equal or potentially better indicator of initial phonological processing involving left lateral temporal neural networks rather than traditional episodic memory systems.
Relatedly, and commensurate with the strong link to AD pathology (Mesulam et al., 2008; Rabinovici et al., 2007a, b), individuals with lvPPA and AD demonstrated comparable objective performances and similarities in some of the cognitive and neuroanatomic correlates of total immediate recall. In both lvPPA and AD, semantic processing, immediate echoic recall and executive control, as well as greater left lateral temporal and parietal lobe volumes were among the strongest associations with total immediate recall. These relationships are consistent with the pattern of dorsal cortical disruption commonly observed in AD pathology (Scahill et al., 2002). However, among individuals with typical amnestic AD presentations, frontal and medial temporal volumes were each meaningfully associated with total immediate recall, which were relatively weaker correlates among lvPPA individuals. Therefore, individuals with typical AD demonstrated a highly distributed pattern of cognitive factors associated with total immediate recall including language-based processing (semantic processing and fluency) and attentional and executive control with meaningful associations with all brain regions examined. Notably, despite vulnerability of the memory systems and in contrast with our hypotheses and functional neuroimaging data (e.g., (Sperling, 2007), delayed recall and medial temporal volumes were among the least strongly associated factors with total immediate recall in AD. Instead, disturbance in initial phonological processing and word retrieval difficulties commonly observed in AD (anomia associated with temporal-parietal dysfunction; Taler and Phillips, 2008) and poor organization of information (e.g., via the central executive) may more accurately account for total immediate recall performances among individuals with amnestic AD. Interestingly, on the other hand, while it was not a major predictor among individuals with AD, visual episodic memory emerged as a unique cognitive correlate of total immediate recall among individuals with lvPPA. Although basic visuospatial processing is commonly spared in lvPPA (Magnin et al., 2013), there are verbally-mediated aspects to visual delayed recall abilities (Paivio, 1986). Given the severe rapid phonological forgetting in lvPPA, it is possible that disruption in these language-based processes impact visual memory tasks as well. Therefore, the association between visual delayed recall and list learning immediate recall may represent parallel verbally-mediated encoding processes in lvPPA. Nonetheless, this finding was not hypothesized and given our small lvPPA cohort (n=34), it is also possible that this is simply a spurious result that would need to be replicated and explored in a larger sample.
Lastly, individuals with bvFTD demonstrated among the most heterogeneous and robust associations (partial r’s≥0.28) between total immediate recall and all cognitive domains examined, with the exception of episodic memory. Despite the breadth of cognitive correlates, neuroanatomically, only greater frontal cortical volumes were meaningfully associated (partial r=0.21) with list learning immediate recall performances. This pattern of associations is consistent with the primary frontal pattern of neural atrophy in bvFTD, compared to the other neurodegenerative conditions (Glosser et al., 2002), and highlights the wide-reaching network effects of frontal lobe dysfunction to a multitude of cogntive processes. During immediate recall, disruption particularly in medial fronto-insular circuits impacting motivation, inhibition, and sustained cognitive attentional control are commonly observed in bvFTD and may represent interruption in the central executive component of information processing (Bott et al., 2014). On the other hand, it is also possible that frontal lobe atrophy and immediate recall scores are simply both markers of overall disease severity in bvFTD and therefore correlate together. Interestingly, language-based cognitive abilities, including semantic processing, was also associated with total immediate recall in this group. Although there is relative sparing of language networks in bvFTD, even early in the disease process, performances on confrontational naming and fluency differ from those of healthy controls (Ranasinghe et al., 2016; Seeley et al., 2008). These differences may represent true changes in semantic processing areas (e.g., anterior temporal lobe pathology; Rabinovici and Miller, 2010) that disrupt contextual integration of novel verbal information during immediate verbal recall. In contrast, given their relative strengths in episodic memory (Bott et al., 2014; Ranasinghe et al., 2016) and supporting our hypotheses, the lack of association between delayed recall and total immediate recall highlights the dissociation between initial learning scores versus information storage/retrieval among individuals with bvFTD. In sum, list learning immediate recall scores may more strongly represent verbally-mediated executive control and semantic processes rather than episodic memory storage in bvFTD.
Our findings are not without limitations. These data are cross-sectional and associative, therefore determining the mechanistic nature (e.g., directionality) of how these cognitive and neuroanatomic processes interact with total immediate recall is not possible. That is, particularly within the clinical cohorts, the sequence of and threshold at which the component processes of immediate recall may disrupt overall performance is not clear. Additionally, while our neuroanatomic correlates reflect cortical atrophy patterns, they do not directly measure concurrent neural “activation” or network recruitment during immediate recall performances. Therefore, we are limited in extrapolating how these neural sytems are directly used during recall on list learning tasks; however, the size of the relationships between anatomic volumes and immediate recall, especially relative to our neurologically normal cohort, suggests comparative patterns of systems that may play a role in the observed behavioral outcomes. Additionally, our sample sizes, especially within the neuroimaging subset were modest. Future replication of these findings is needed to help support their validity. Relatedly, only a subset of our samples completed neuroimaging and those individuals were younger and demonstrated mildly better cognition, limiting the generalizability of our neuroanatomic analyses to older and more impaired individuals. However, we would expect these relationships to become more prominent with increasing disease severity; therefore, our presented analyses represent a conservative estimate of these relationships. Lastly, within the clinical syndromes, while diagnoses were determined by experts (i.e., language/speech pathology team, neurologists, neuropsychologists) using evidence-based consensus criteria, the underlying pathology within each of the clinical groups may be varied and ultimately reveal disparate neural mechanisms. However, given the similar behavioral presentation of each syndrome, each diagnosis likely reflects similar current underlying cognitive and neural disruption at least at the time of their total immediate recall performance measurement.
Taken together, we demonstrated: 1) multiple distinct cognitive and neural systems extending far beyond conventional memory networks were important for immediate recall during learning trials, and 2) the neurobehavior related to immediate recall performance differs and depends on the brain organization of the presenting individual. These neurobehavioral components likely represent the separable stages of how humans process and encode novel information into long-term memory as posited by cognitive psychology models (e.g., Baddeley, 2003). While involvement of traditional memory systems is clearly necessary for later recall of information (Squire et al., 2004), their involvement may not be sufficient, or even significantly contribute to, immediate recall performances during “learning” itself. These patterns underscore the highly integrated nature of cognitive and brain networks and their relatively plastic contribution to behavioral outcomes following injury or disease. Therefore, use of total “learning” scores as an indicator of episodic memory, or even “encoding”, may in fact be quite misleading and at times, inaccurate. Instead, interpretation of this multidimensional construct appears to be best aided by examination of the cognitive strengths and weaknesses of the individual, beyond information storage capacity, that may contribute to performance manifestation. These data also have important implications for how to best integrate repeated learning scores into diagnostic conceptualizations. Namely, if a clinician is aiming to capture traditional episodic memory (e.g., suspected Alzheimer’s disease), greater weight and focused interpretation of the delayed and not the immediate recall component may be most appropriate. On the other hand, if bvFTD is on the differential for example, clinicians might consider the pattern of total learning scores alongside other attentional and executive measures as an indicator of frontally-mediated dysfunction. Ultimately, accurate interpretation of the processes that neuropsychological scores are reflecting (particularly when using multifaceted measures such as repeated immediate recall) is critical to avoid misdiagnosis and optimize recommendations and treatment approaches.
Acknowledgments
This study was supported by National Institute on Aging (NIA 1R01AG032289 PI: Kramer, R01AG048234 PI: Kramer, UCSF ADRC P50 AG023501, and UCSF PPG P01 AG019724). Our work was also supported by Larry L. Hillblom Network Grant for the Prevention of Age-Associated Cognitive Decline (2014-A-004-NET PI: Kramer).
Footnotes
Disclosures
J. H. Kramer receives royalties from Psychological Assessment Resources, Inc. for the California Verbal Learning Test.
References
- Albert MS, Moss MB, Tanzi R, Jones K. Preclinical prediction of AD using neuropsychological tests. J Int Neuropsychol Soc. 2001;7(5):631–639. doi: 10.1017/s1355617701755105. [DOI] [PubMed] [Google Scholar]
- Alexander A. CFA of the test of memory and learning in a TBI sample. Arch Clin Neuropsychol. 2003;18(7):739–739. [Google Scholar]
- Baddeley A. Working memory: looking back and looking forward. Nat Rev Neurosci. 2003;4(10):829–839. doi: 10.1038/nrn1201. http://dx.doi.org/10.1038/nrn1201. [DOI] [PubMed] [Google Scholar]
- Baddeley AD. Is working memory still working? Am Psychol. 2001;56(11):851–864. doi: 10.1037/0003-066x.56.11.851. [DOI] [PubMed] [Google Scholar]
- Baddeley AD. Working Memory. Clarendon Press; Oxford: 1986. [Google Scholar]
- Baddeley AD, Hitch GJ. Recent Advances in learning and Motivation. Academic; New York: 1974. [Google Scholar]
- Benedict RHB, Schretlen D, Groninger L, Brandt J. Hopkins Verbal Learning Test Revised: normative data and analysis of inter-form and test-retest reliability. Clin Neuropsychol. 1998;12(1):43–55. http://dx.doi.org/10.1076/Clin.12.1.43.1726. [Google Scholar]
- Bott NT, Radke A, Stephens ML, Kramer JH. Frontotemporal dementia: diagnosis, deficits and management. Neurodegener Dis Manag. 2014;4(6):439–454. doi: 10.2217/nmt.14.34. http://dx.doi.org/10.2217/nmt.14.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Head D, Parker J, Fotenos AF, Marcus D, Morris JC, Snyder AZ. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. NeuroImage. 2004;23(2):724–738. doi: 10.1016/j.neuroimage.2004.06.018. http://dx.doi.org/10.1016/j.neuroimage.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Cohen J. A power primer. Psychol Bull. 1992;112(1):155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- Cowan N. Verbal memory span and the timing of spoken recall. J Mem Lang. 1992;31(5):668–684. http://dx.doi.org/10.1016/0749-596x(92)90034-U. [Google Scholar]
- Cowan N, Day L, Saults JS, Keller TA, Johnson T, Flores L. The role of verbal output time in the effects of word-length on immediate memory. J Mem Lang. 1992;31(1):1–17. http://dx.doi.org/10.1016/0749-596x(92)90002-F. [Google Scholar]
- Delis DC, Massman PJ, Butters N, Salmon DP, Cermak LS, Kramer JH. Profiles of demented and amnesic patients on the California Verbal Learning Test: implications for the assessment of memory disorders. Psychol Assess. 1991;3(1):19–26. [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. CVLT-II: California verbal learning test: adult version. Psychological Corporation; 2000. [Google Scholar]
- Fletcher PC, Shallice T, Dolan RJ. The functional roles of prefrontal cortex in episodic memory - I. Encoding. Brain. 1998;121:1239–1248. doi: 10.1093/brain/121.7.1239. http://dx.doi.org/10.1093/Brain/121.7.1239. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, Mchugh PR. Mini-mental state - practical method for grading cognitive state of patients for clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. http://dx.doi.org/10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Friedman D, Johnson R., Jr Event-related potential (ERP) studies of memory encoding and retrieval: a selective review. Microsc Res Tech. 2000;51(1):6–28. doi: 10.1002/1097-0029(20001001)51:1<6::AID-JEMT2>3.0.CO;2-R. http://dx.doi.org/10.1002/1097-0029(20001001)51:1<6::AID-JEMT2>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Gathercole SE, Service E, Hitch GJ, Adams AM, Martin AJ. Phonological short-term memory and vocabulary development: Further evidence on the nature of the relationship. Appl Cogn Psychol. 1999;13(1):65–77. http://dx.doi.org/10.1002/(Sici)1099-0720(199902)13:1<65::Aid-Acp548>3.0.Co;2-O. [Google Scholar]
- Glosser G, Gallo JL, Clark CM, Grossman M. Memory encoding and retrieval in frontotemporal dementia and Alzheimer’s disease. Neuropsychology. 2002;16(2):190–196. [PubMed] [Google Scholar]
- Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, Grossman M. Classification of primary progressive aphasia and its variants. Neurology. 2011;76(11):1006–1014. doi: 10.1212/WNL.0b013e31821103e6. http://dx.doi.org/10.1212/Wnl.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Murray RC, Rankin KP, Weiner MW, Miller BL. Clinical, cognitive and anatomical evolution from nonfluent progressive aphasia to corticobasal syndrome: a case report. Neurocase. 2004;10(6):426–436. doi: 10.1080/13554790490894011. http://dx.doi.org/10.1080/13554790490894011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habib R, Nyberg L, Tulving E. Hemispheric asymmetries of memory: the HERA model revisited. Trends Cogn Sci. 2003;7(6):241–245. doi: 10.1016/s1364-6613(03)00110-4. [DOI] [PubMed] [Google Scholar]
- Hayes SM, Nadel L, Ryan L. The effect of scene context on episodic object recognition: parahippocampal cortex mediates memory encoding and retrieval success. Hippocampus. 2007;17(9):873–889. doi: 10.1002/hipo.20319. http://dx.doi.org/10.1002/hipo.20319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Miller SW, Taylor MJ, Grant I. Revised comprehensive norms for an expanded Halstead-Reitan Battery: Demographically adjusted neuropsychological norms for African American and Caucasian adults. Lutz, FL: Psychological Assessment Resources; 2004. [Google Scholar]
- Jonides J, Schumacher EH, Smith EE, Koeppe RA, Awh E, Reuter-Lorenz PA, Willis CR. The role of parietal cortex in verbal working memory. J Neurosci. 1998;18(13):5026–5034. doi: 10.1523/JNEUROSCI.18-13-05026.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer JH, Jurik J, Sha SJ, Rankin KP, Rosen HJ, Johnson JK, Miller BL. Distinctive neuropsychological patterns in frontotemporal dementia, semantic dementia, and Alzheimer disease. Cogn Behav Neurol. 2003;16(4):211–218. doi: 10.1097/00146965-200312000-00002. [DOI] [PubMed] [Google Scholar]
- Kramer JH, Rosen HJ, Du AT, Schuff N, Hollnagel C, Weiner MW, Delis DC. Dissociations in hippocampal and frontal contributions to episodic memory performance. Neuropsychology. 2005;19(6):799–805. doi: 10.1037/0894-4105.19.6.799. http://dx.doi.org/10.1037/0894-4105.19.6.7999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacritz LH, Cullum CM, Weiner MF, Rosenberg RN. Comparison of the hopkins verbal learning test-revised to the California verbal learning test in Alzheimer’s disease. Appl Neuropsychol. 2001;8(3):180–184. doi: 10.1207/S15324826AN0803_8. http://dx.doi.org/10.1207/S15324826AN0803_8. [DOI] [PubMed] [Google Scholar]
- Magnin E, Chopard G, Ferreira S, Sylvestre G, Dariel E, Ryff I, Rumbach L. Initial neuropsychological profile of a series of 20 patients with logopenic variant of primary progressive aphasia. J Alzheimers Dis. 2013;36(4):799–808. doi: 10.3233/JAD-122335. http://dx.doi.org/10.3233/JAD-122335. [DOI] [PubMed] [Google Scholar]
- Mandler JM, Parker RE. Memory for descriptive and spatial information in complex pictures. J Exp Psychol-Human Learn Mem. 1976;2(1):38–48. http://dx.doi.org/10.1037//0278-7393.2.1.38. [PubMed] [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, Phelps CH. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association work-groups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263–269. doi: 10.1016/j.jalz.2011.03.005. http://dx.doi.org/10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam M, Wicklund A, Johnson N, Rogalski E, Leger GC, Rademaker A, Bigio EH. Alzheimer and frontotemporal pathology in subsets of primary progressive aphasia. Ann Neurol. 2008;63(6):709–719. doi: 10.1002/ana.21388. http://dx.doi.org/10.1002/ana.21388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page MPA, Norris DG. The irrelevant sound effect: what needs modelling, and a tentative model. Q J Exp Psychol Sect a-Human Exp Psychol. 2003;56(8):1289–1300. doi: 10.1080/02724980343000233. http://dx.doi.org/10.1080/02724980343000233. [DOI] [PubMed] [Google Scholar]
- Paivio A. Dual coding and episodic memory: Subjective and objective sources of memory trace components. Human Memory and Cognitive Capabilities: Mechanisms and performances. 1986:225–236. [Google Scholar]
- Papagno C, Vallar G. Phonological Short-Term-Memory and the Learning of Novel Words - the Effect of Phonological Similarity and Item Length. Q J Exp Psychol Sect a-Human Exp Psychol. 1992;44(1):47–67. [Google Scholar]
- Perani D, Bressi S, Cappa SF, Vallar G, Alberoni M, Grassi F, et al. Evidence of multiple memory systems in the human brain. A [18F]FDG PET metabolic study. Brain. 1993;116(Pt 4):903–919. doi: 10.1093/brain/116.4.903. [DOI] [PubMed] [Google Scholar]
- Possin KL, Laluz VR, Alcantar OZ, Miller BL, Kramer JH. Distinct neuroanatomical substrates and cognitive mechanisms of figure copy performance in Alzheimer’s disease and behavioral variant frontotemporal dementia. Neuropsychologia. 2011;49(1):43–48. doi: 10.1016/j.neuropsychologia.2010.10.026. http://dx.doi.org/10.1016/j.neuropsychologia.2010.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovici GD, Furst AJ, O’Neil JP, Racine CA, Mormino EC, Baker SL, Jagust WJ. C-11-PIB PET imaging in Alzheimer disease and frontotemporal lobar degeneration. Neurology. 2007a;68(15):1205–1212. doi: 10.1212/01.wnl.0000259035.98480.ed. http://dx.doi.org/10.1212/01.Wnl.0000259035.98480.Ed. [DOI] [PubMed] [Google Scholar]
- Rabinovici GD, Seeley WW, Kim EJ, Gorno-Tempini ML, Rascovsky K, Pagliaro TA, Rosen HJ. Distinct MRI atrophy patterns in autopsy-proven Alzheimer’s disease and frontotemporal lobar degeneration. Am J Alzheimers Dis Other Dement. 2007b;22(6):474–488. doi: 10.1177/1533317507308779. http://dx.doi.org/10.1177/1533317507308779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovici GD, Miller BL. Frontotemporal lobar degeneration epidemiology, pathophysiology, diagnosis and management. CNS Drugs. 2010;24(5):375–398. doi: 10.2165/11533100-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranasinghe KG, Rankin KP, Lobach IV, Kramer JH, Sturm VE, Bettcher BM, Miller BL. Cognition and neuropsychiatry in behavioral variant frontotemporal dementia by disease stage. Neurology. 2016;86(7):600–610. doi: 10.1212/WNL.0000000000002373. http://dx.doi.org/10.1212/WNL.0000000000002373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, Miller BL. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134:2456–2477. doi: 10.1093/brain/awr179. http://dx.doi.org/10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Gunning-Dixon FM, Head D, Dupuis JH, Acker JD. Neuroanatomical correlates of cognitive aging: evidence from structural magnetic resonance imaging. Neuropsychology. 1998;12(1):95–114. doi: 10.1037//0894-4105.12.1.95. http://dx.doi.org/10.1037/0894-4105.12.1.95. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. Contributions of the Individual Differences Approach to Cognitive Aging. J Gerontol B Psychol Sci Soc Sci. 2017;72(1):7–15. doi: 10.1093/geronb/gbw069. http://dx.doi.org/10.1093/geronb/gbw069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scahill RI, Schott JM, Stevens JM, Rossor MN, Fox NC. Mapping the evolution of regional atrophy in Alzheimer’s disease: unbiased analysis of fluid-registered serial MRI. Proc Natl Acad Sci USA. 2002;99(7):4703–4707. doi: 10.1073/pnas.052587399. http://dx.doi.org/10.1073/pnas.052587399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Crawford R, Rascovsky K, Kramer JH, Weiner M, Miller BL, Gorno-Tempini ML. Frontal paralimbic network atrophy in very mild behavioral variant frontotemporal dementia. Archoves Neurol. 2008;65(2):249–255. doi: 10.1001/archneurol.2007.38. http://dx.doi.org/10.1001/archneurol.2007.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segonne F, Dale AM, Busa E, Glessner M, Salat D, Hahn HK, Fischl B. A hybrid approach to the skull stripping problem in MRI. Neuroimage. 2004;22(3):1060–1075. doi: 10.1016/j.neuroimage.2004.03.032. http://dx.doi.org/10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- Shallice T, Fletcher P, Frith CD, Grasby P, Frackowiak RS, Dolan RJ. Brain regions associated with acquisition and retrieval of verbal episodic memory. Nature. 1994;368(6472):633–635. doi: 10.1038/368633a0. http://dx.doi.org/10.1038/368633a0. [DOI] [PubMed] [Google Scholar]
- Shallice T, Warrington EK. Independent functioning of verbal memory stores: a neuropsychological study. Q J Exp Psychol. 1970;22(2):261–273. doi: 10.1080/00335557043000203. http://dx.doi.org/10.1080/00335557043000203. [DOI] [PubMed] [Google Scholar]
- Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17(1):87–97. doi: 10.1109/42.668698. http://dx.doi.org/10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- Sperling R. Functional MRI studies of associative encoding in normal aging, mild cognitive impairment, and Alzheimer’s disease. Ann N Y Acad Sci. 2007;1097:146–155. doi: 10.1196/annals.1379.009. http://dx.doi.org/10.1196/annals.1379.009. [DOI] [PubMed] [Google Scholar]
- Squire LR. Memory systems of the brain: a brief history and current perspective. Neurobiol Learn Mem. 2004;82(3):171–177. doi: 10.1016/j.nlm.2004.06.005. http://dx.doi.org/10.1016/j.nlm.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Squire LR, Stark CE, Clark RE. The medial temporal lobe. Annu Rev Neurosci. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. http://dx.doi.org/10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- Szaflarski JP, Holland SK, Schmithorst VJ, Dunn RS, Privitera MD. High-resolution functional MRI at 3T in healthy and epilepsy subjects: hippocampal activation with picture encoding task. Epilepsy Behav. 2004;5(2):244–252. doi: 10.1016/j.yebeh.2004.01.002. http://dx.doi.org/10.1016/j.yebeh.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Taler V, Phillips NA. Language performance in Alzheimer’s disease and mild cognitive impairment: a comparative review. J Clin Exp Neuropsychol. 2008;30(5):501–556. doi: 10.1080/13803390701550128. http://dx.doi.org/10.1080/13803390701550128. [DOI] [PubMed] [Google Scholar]
- Thorn ASC, Gathercole SE. Language-specific knowledge and short-term memory in bilingual and non-bilingual children. Q J Exp Psychol Sect a-Human Exp Psychol. 1999;52(2):303–324. doi: 10.1080/713755823. http://dx.doi.org/10.1080/027249899391089. [DOI] [PubMed] [Google Scholar]
- Tulving E, Kapur S, Markowitsch HJ, Craik FIM, Habib R, Houle S. Neuroanatomical correlates of retrieval in episodic memory - auditory sentence recognition. Proc Natl Acad Sci USA. 1994;91(6):2012–2015. doi: 10.1073/pnas.91.6.2012. http://dx.doi.org/10.1073/Pnas.91.6.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallar G, Shallice T. Neuropsychological Impairments of Short-term Memory. Cambridge University Press; Cambridge, UK: 1990. [Google Scholar]
- Weintraub D, Moberg PJ, Culbertson WC, Duda JE, Stern MB. Evidence for impaired encoding and retrieval memory profiles in Parkinson disease. Cogn Behav Neurol. 2004;17(4):195–200. [PubMed] [Google Scholar]
- Wheeler MA, Stuss DT, Tulving E. Toward a theory of episodic memory: the frontal lobes and autonoetic consciousness. Psychol Bull. 1997;121(3):331–354. doi: 10.1037/0033-2909.121.3.331. [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Vogel RW, 3rd, Ewers M, Coffey J, Boyko OB, Lemieux SK. MRI-assessed volume of cerebellum correlates with associative learning. Neurobiol Learn Mem. 2001;76(3):342–357. doi: 10.1006/nlme.2001.4026. http://dx.doi.org/10.1006/nlme.2001.4026. [DOI] [PubMed] [Google Scholar]
- Woods SP, Delis DC, Scott JC, Kramer JH, Holdnack JA. The California Verbal Learning Test–second edition: test-retest reliability, practice effects, and reliable change indices for the standard and alternate forms. Arch Clin Neuropsychol. 2006;21(5):413–420. doi: 10.1016/j.acn.2006.06.002. http://dx.doi.org/10.1016/j.acn.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Zola-Morgan S, Squire LR, Amaral DG. Human amnesia and the medial temporal region: enduring memory impairment following a bilateral lesion limited to field CA1 of the hippocampus. J Neurosci. 1986;6(10):2950–2967. doi: 10.1523/JNEUROSCI.06-10-02950.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]