Abstract
Previous studies have shown that activation of A1 adenosine receptors results in renal vasoconstriction at submicromolar concentrations of N6-cyclohexyladenosine (CHA) followed by relative vasodilation at higher concentrations. The present data confirm these findings and demonstrate that Na loading enhances the vasoconstrictor effects of CHA in the isolated rat kidney perfused at constant flow. Furthermore, adenosine receptor antagonism with both theophylline and the A1-selective antagonist, xanthine amine congener (8-{4-[(2-aminoethyl)-aminocarbonylmethyloxy]phenyl}-1,3-dipropylxanthine), produced a rightward and apparently parallel shift in the dose response to CHA. Determination of the inhibitory constants for both antagonists revealed that xanthine amine congenar was three orders of magnitude more potent than theophylline in antagonizing CHA-induced renal vasoconstriction. Other investigators have hypothesized that angiotensin II mediates adenosine-induced renal vasoconstriction. However, we have been able to show that A1 receptor activation can result in renal vasoconstriction in the isolated perfused rat kidney devoid of renin substrate. Moreover, a competitive inhibitor of angiotensin II (saralasin) failed to attenuate the hemodynamic effects of CHA at doses that completely blocked the effects of angiotensin II itself. Taken together, these data are consistent with the hypothesis that A1 receptor activation in the kidney leads to vasoconstriction, a response that is enhanced by Na loading, and that A1 adenosine receptors and angiotensin II receptors are separate and distinct biocheical entities. Independent activation of either receptor leads to renal vasoconstriction, which can be prevented by its respective antagonist.
That adenosine can elicit renal vasoconstriction in vivo has been known since 1929 (Drury and Szent-Gyorgyi). Thurau (1964) and subsequently several others (Osswald, 1975, 1984; Osswald et al., 1975, 1980; Spielman and Osswald, 1979; Spielman and Thompson, 1982; Arend et al., 1984, 1985; Hall et al., 1985; Sinclair et al., 1985; Hall and Granger 1986) have hypothesized that adenosine-induced renal vasoconstriction is mediated by intrarenally generated angiotensin II. Consistently, Na loading and Na deprivation suppress and potentiate, respectively, both the activity of the renin-angiotensin system (Tobian, 1960) and adenosine-induced renal vasoconstriction in vivo (Thurau, 1964; Osswald, 1975, 1984; Osswald et al., 1975, 1980; Spielman and Osswald, 1979; Spielman et al., 1980; Arend et al., 1984, 1985). Moreover, exogenous angiotensin II restores adenosine-induced vasoconstriction in Na-loaded rats (Osswald et al., 1975), and high concentrations of either a competitive angiotensin receptor antagonist (Spielman and Osswald, 1979) or a converting enzyme inhibitor (Hall et al., 1985) attenuates adenosine-induced renal vasoconstriction in vivo.
Murray and Churchill (1984, 1985) have shown that adenosine-induced renal vasoconstriction is mediated by the A1 subclass of adenosine receptors. Their studies were performed using the nonrecirculating isolated perfused rat kidney preparation, thus avoiding any accumulation of endogenously released adenosine, and using adenosine analogs that are adenosine receptor agonists but are substrates for neither cellular uptake nor metabolism. CHA, selective at low concentrations for the A1 subclass of adenosine receptors, produced a concentration-dependent vasoconstriction. The present studies were designed to examine the effects of chronic Na loading, adenosine receptor antagonism and angiotensin II receptor antagonism on CHA-induced renal vasoconstriction in the nonrecirculating isolated perfused rat kidney.
Methods
Adult male Sprague-Dawley rats (250–300 g) were used for these experiments. They were housed in a constant temperature room with a light-dark cycle (L:D, 12:12) and cared for in accordance with the principles of the Guide for the Care and Use of Laboratory Animals (Department of Health, Education and Welfare No. 86-23). All animals had free access to Purina Rat Chow. Na-loaded rats were given 0.9% NaCl for a minimum of 3 weeks before the experiments; all other rats were given tap water.
The kidney was isolated according to methods described by Nishiitsutsuji-Uwo et al. (1967). Rats were anesthetized with Na pentobarbital (50 mg/kg i.p.) and given heparin (24 mg/kg) via a femoral vein. Subcostal midline and bilateral incisions were made to expose the gut, allowing isolation and catheterization of the right ureter with polyethylene tubing. After isolating the superior mesenteric and renal arteries, a glass cannula was inserted into the superior mesenteric artery, the perfusion pump was started and the cannula was advanced into the right renal artery and tied into place. The kidney was removed, cleaned of perirenal tissue and placed in a constant temperature perfusion chamber.
The perfusion method used in these studies has been described in detail previously (Murray and Churchill, 1984). Briefly, kidneys were perfused in a single-pass, nonrecirculating system using a Krebs-Henseleit buffer containing 3.5 g/100 ml of Ficoll 70 (Sigma Chemical Co., St. Louis, MO) and 1.0 g/100 ml of bovine serum albumin (fraction V; Miles Laboratories, Inc., Kankakee, IL). The composition of the buffer in millimoles per liter was: NaCI, 120, NaHCO3, 25; KCI, 2.8; CaCl2, 2.5; KH2PO4, 1.2 and MgSO4, 0.84. In addition, the perfusate contained (in miliigrams/100 ml): dextrose, 180; β-alanine, 89; creatinine, 50 and urea, 36. Before use, the perfusate was filtered through a 0.45-μ membrane filter (Millipore Filter Corp., Bedford, MA). The perfusate was oxygenated using a C-DAK 135 SCE hollow fiber dialyzer (Cordis Dow, Miami, FL); medium was passed through the blood compartment of the dialyzer while 95% 02–5% CO2 was passed through the dialysate compartment. Perfusate flow was controlled by a Harvard peristaltic pump (model 1203; Harvard Apparatus Co., Inc., Natick, MA). Before reaching the kidney, the medium was filtered through an 8.0-μ inline membrane filter. Kidneys were perfused at 37°C and pH 7.4.
Perfusion pressure was monitored using a pressure transducer (P231D; Gould Statham, Oxnard, CA) connected to a pressure monitor (SP 1405; Gould Statham) and a strip chart recorder (1701 BM; Hewlett-Packard Co., Palo Alto, CA). An inline flow probe connected to a blood flowmeter (SP2202; Gould Statham) was used to monitor the perfusate flow.
In all protocols, the kidney was perfused at a constant pressure of 100 mm Hg for the first 45 min; the flow rate required to generate 100 mm Hg was then maintained constant, allowing the pressure to vary for the remainder of the experiment. Kidneys were rejected if the perfusion pressure dropped by 15 mm Hg during the initial 15-min period of perfusion at constant flow.
Protocols
1) CHA dose-response curves were generated in kidneys from control and Na-loaded rats. CHA was infused into the perfusion line for 5 min such that the final concentration was 10−9 M; then vehicle without CHA was infused, and 5 min later, CHA was infused to produce a concentration 10 times higher. This was repeated until a maximum final concentration of 10−5 M CHA was achieved. 2) Antagonism of CHA-induced vasoconstriction by theophylline and XAC (8-{4-[(2-aminoethyl)aminocarbonylmethyloxy]phenyl}-1,3-dipropylxanthine) [Jacobson et al., 1985) was Studied in kidneys from control rats. CHA was infused at 0.01 and 0.03 µM in the absence of theophylline, then at 0.02 and 0.06 µM in the presence of 50 µM theophylline. Similar experiments were performed in which other kidneys were perfused in the absence and presence of 0.02 µM XAC. 3) Angiotensin II-induced vasoconstriction and saralasin antagonism were studied in kidneys from control rats. Angiotensin was infused before, during and after infusing saralasin at the concentration of angiotensin; each of these infusion periods was of 5-min duration, and each of the periods was separated by a 5-min period during which only vehicle was infused. 4) Saralasin antagonism of CHA-induced vasoconstriction was studied in kidneys from control rats. CHA was infused at 0.01 and 0.03 µM in the absence of saralasin, then at 0.02 and 0.06 µM in the presence of 10 pM saralasin.
Drug infusions were made through a needle inserted directly into the perfusion line; the rate of infusion of drugs into the perfusion line was controlled by the same pump that controlled the rate of perfusion, such that a constant concentration of drug was achieved at any flow rate. Stock solutions of CHA (Calbiochem-Behring Corp., La Jolla, CA), theophylline (Sigma), XAC, angiotensin II (Sigma) and saralasin (P-113; Peninsula Laboratories, Inc., San Carlos, CA) were all prepared in 3.8% ethanol. Dilutions were prepared such that the final concentration of ethanol infused into the perfusion line was constant at 0.08% in all experiments, including the vehicle control.
Calculations
Drug-induced changes in renal perfusion pressure were calculated as the difference between the average pressure during the final 3 min of drug infusion and the average of the pressures before and after the drug infusion period. Results are expressed as mean ± S.E.M. Analysis of Variance and Scheffe contrasts were used to assess the statistical significance of differences (Wallenstein et al., 1980). The four-point assay test was used to calculate inhibitory constants of antagonists (Staff of the Dept. of Pharmacology, Univ. of Edinburgh, 1970).
Results
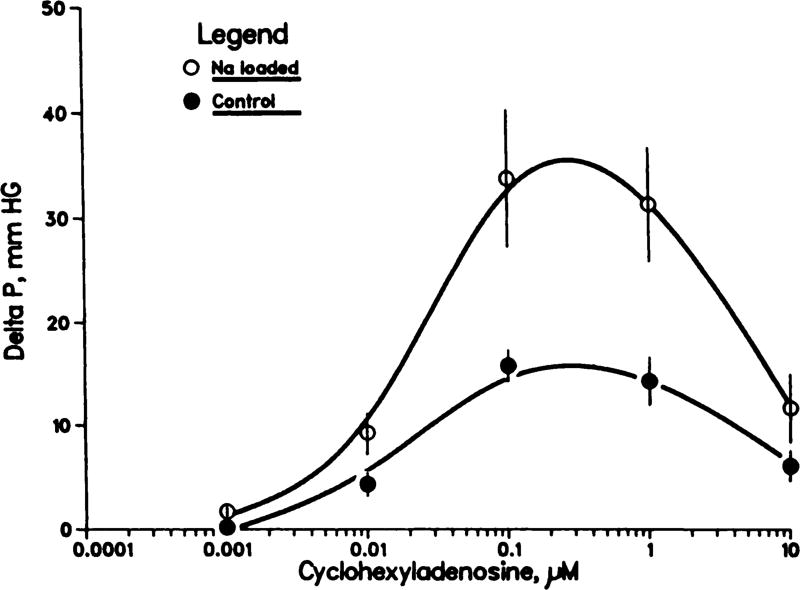
As can be seen in figure 1, the response of control kidneys to CHA was biphasic, with concentration-dependent vasoconstriction over the range 0.001 to 0.1 µM, followed at higher concentrations with a relative vasodilation (reduction in the extent of vasoconstriction). The vasoconstrictive responses were similar, but potentiated, in kidneys from Na-loaded rats; the CHA-induced increases in pressure were significantly greater at both 0.1 and 1.0 µM (P < .05 maximum).
Fig. 1.
Effects of CHA on perfusion pressure of isolated kidneys from rats on a control diet (n = 10) and from chronically Na-loaded rats (n = 11). Mean ± S.E.M.; perfusion pressure increased more in Na loaded than in control kidneys in response to 0.001, 0.1 and 1.0 µM CHA (P < .05, maximum).
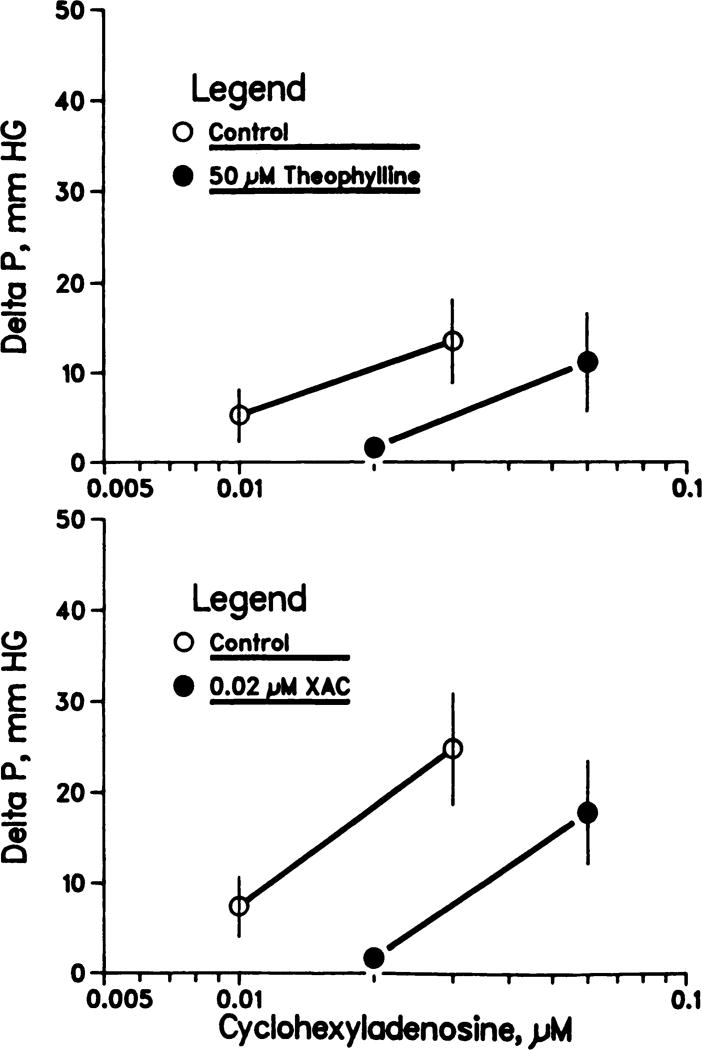
The results of the adenosine receptor antagonist studies are shown in figure 2. Theophylline (50 µM) and XAC (0.02 µM) produced similar, apparently parallel, displacements to the right in the CHA dose-response (vasoconstriction) curves. The calculated inhibitory constants, Ki, for theophylline and XAC were 2.6 × 10−5 and 1 × 10−8 M, respectively; thus, XAC is approximately three orders of magnitude more potent than theophylline in antagonizing CHA-induced renal vasoconstriction.
Fig. 2.
Theophylline (upper panel) and XAC (lower panel) antagonism of CHA-induced increases in perfusion pressure in isolated rat kidneys. Mean ± S.E.M.; n = 8 (upper panel) and n = 9 (lower panel).
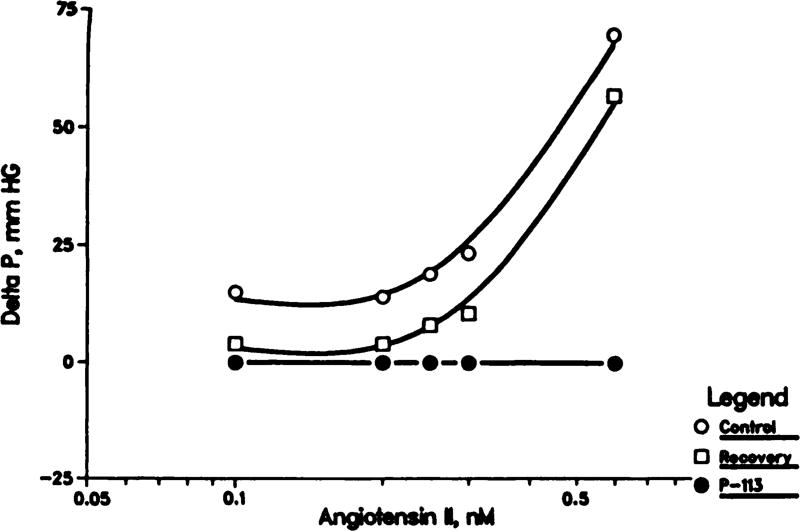
The results in figure 3 show that angiotensin II produces a concentration-dependent vasoconstriction (increase in pressure at constant flow). At each concentration of angiotensin, saralasin at that concentration completely abolished the effect (e.g., 10, 20, 25, 30 and 60 pM saralasin blocked the effects of 0.1, 0.2, 0.25, 0.30 and 0.60 nM angiotensin II, respectively).
Fig. 3.
Saralasin (P-113) antagonism of angiotensin II-induced increases in perfusion pressure in isolated rat kidneys. Angiotensin was given before (control), during and after (recovery) inclusion of saralasin in the perfusate at the concentration of anglotensin.
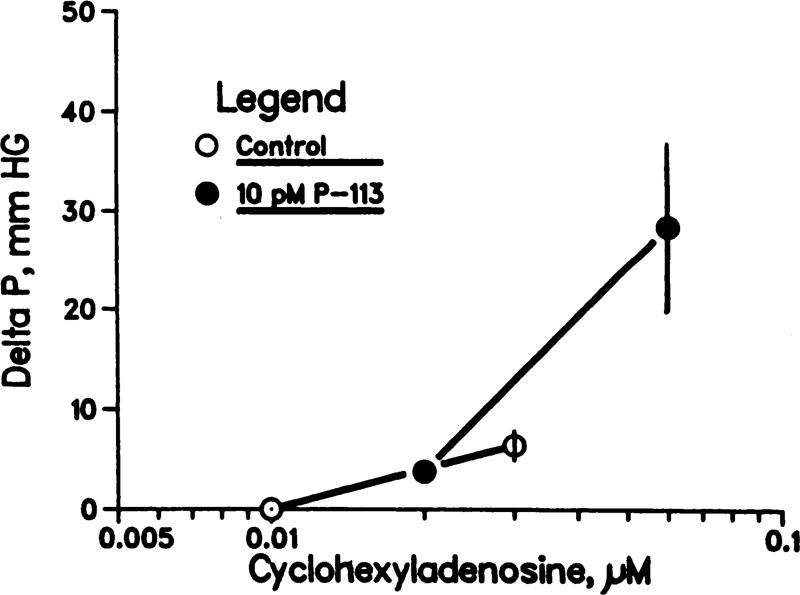
Comparison of the results in figures 1 and 3 suggests that angiotensin II is much more potent than CHA in producing renal vasoconstriction; the response to 0.1 nM angiotensin is as large as that produced by 0.1 µM CHA. This observation, taken together with the observation that 10 pM saralasin abolishes the effect of 0.1 nM angiotensin, suggests that if CHA-induced vasoconstriction is mediated by angiotensin, then 10 pM saralasin should abolish the response to CHA. To the contrary, the results in figure 4 demonstrate that 10 pM saralasin failed even to attenuate CHA-induced vasoconstriction.
Fig. 4.
Saralasin (P-113) does not antagonize CHA-induced increases in perfusion pressure in isolated rat kidneys. Mean ± S.E.M.; n = 5.
Discussion
Murray and Churchill (1984, 1985) have reported the renovascular effects of adenosine and several analogs in the nonrecirculating isolated perfused rat kidney preparation. Both the order of potency of the agonists and the concentration ranges over which the agonists acted were consistent with the hypothesis that constriction and dilation are mediated by A1 and A2 subclasses of adenosine receptor, respectively. Because low concentrations of CHA selectively activate A1 receptors (Daly, 1982), the present observations are consistent: concentration-dependent vasoconstriction at submicromolar CHA concentrations is followed by relative vasodilation at higher concentrations. In the previous studies (Murray and Churchill, 1985), perfusion pressure actually fell below base line in response to even higher CHA concentrations.
It is well known that dietary Na intake has a profound effect on vascular smooth muscle contractility, both in vivo and in vitro. Increases in Na intake enhance the renal vascular responses to norepinephrine and angiotensin II in vivo (Oliver and Cannon, 1978). Similar responses have been reported with respect to the isolated rat kidney, perfused at a constant flow rate; that is, the increases in perfusion pressure in response to norepinephrine, vasopressin and angiotensin II (Berecek et al., 1980, 1982) are greater in kidneys from chronically Na-loaded rats than in kidneys from controls. The present studies show, for the first time in any vascular bed, that CHA-induced vasoconstriction is enhanced by previous Na loading. The mechanisms responsible for this enhanced vascular smooth muscle contractility in Na-loaded animals remain to be elucidated fully.
Adenosine per se has been used in many previous kidney studies, by several different investigators (Drury and Szent-Gyorgyi, 1929 Ono et al., 1966; Osswald, 1975, 1984; Osswald et al., 1975, 1978a,b; Spielman et al., 1980 Haas and Osswald, 1981; Premen et al., 1985; Hall et al., 1985; Hall and Granger, 1986). However, Daly (1982) and others (Clanachan and Muller, 1980; Buckle and Spence, 1982) have recommended strongly that adenosine analogs should be used to elucidate the cellular effects of adenosine receptor activation. Extracellular adenosine can be metabolized (Holland et al., 1985) as well as transported into cells (Plagemann and Wohlhueter, 1982). Intracellular adenosine is a substrate for many enzymes (Holland et al., 1985) and an agonist at the internal P-site or receptor (Wolff et al., 1978; Londos et al., 1980). In contrast, many adenosine analogs act as A1 and/or A2 receptor agonists, but they are not metabolized and they are not taken up by cells; therefore, they are not agonists at the P-site (Daly, 1982). Moreover, inasmuch as some analogs are selective for one or the other of the A1 or A2 subclasses of receptors, the order of potency of analogs can be used to characterize the receptor type. For these reasons, CHA-induced renal vasoconstriction can be taken as evidence that adenosine-induced vasoconstriction is mediated by occupation of plasma membrane adenosine receptors of the A1 subclass.
The antagonistic effects of theophylline and XAC that we found provide even further evidence for this assertion. Both substances antagonize plasma membrane adenosine receptors in other tissues (Ukena et al., 1986), but neither has been shown to affect the internal P-site (Daly, 1983). Whereas theophylline is a relatively weak and nonselective antagonist of both A1 and A2 receptors (Fredholm and Persson, 1982), XAC has been shown to bind to the central A1 receptors with 41-fold greater selectivity and 12,000-fold greater potency than theophylline (Jacobson et al., 1985, 1986). The present results are consistent with these findings. In fact, the Ki for XAC inhibition of CHA-induced renal vasoconstriction corresponds closely to that for XAC binding to rat brain membranes (Jacobson et al., 1986).
It should be stressed that although theophylline can produce cellular effects via phosphodiesterase inhibition, millimolar concentrations are required (Fredholm et al., 1978). Micromolar concentrations antagonize adenosine receptors (Ukena et al., 1986). The concentration of theophylline used in the experiments shown in figure 2 is an order of magnitude less than the IC50 for theophylline inhibition of renal phosphodiesterase activity (Fredholm et al., 1978). Collectively, then, our results provide strong evidence that adenosine-induced renal vasoconstriction is mediated by activation of A1 receptors.
Our previous (Murray and Churchill, 1984, 1985; Churchill and Churchill, 1985) and present results provide several arguments against the hypothesis that angiotensin mediates the adenosine-induced renal vasoconstriction. First, in both the previous (Murray and Churchill, 1985) and present studies, CHA elicited vasoconstriction in isolated kidneys perfused with a nonrecirculating medium devoid of renin substrate. Second, adenosine actually inhibits renin secretion (Tagawa and Vander, 1970 Osswald et al., 1978a) and we have shown that this inhibitory effect is mediated by A1 adenosine receptors (Churchill and Churchill, 1985; Murray and Churchill, 1985). Thus, A1-mediated inhibition of renin secretion and A1-mediated vasoconstriction are simultaneous events. It is difficult to understand how a decrease in renin secretion, even if substrate were present, could account for an angiotensin-induced increase in contractility. Third, Na loading, which is known to suppress both renal tissue renin content and the secretion of renin, actually enhanced CHA-induced vasoconstriction. Finally, both CHA and angiotensin elicited vasoconstriction, and at equipotent concentrations, saralasin completely blocked angiotensin’s effect but failed even to attenuate (and, if anything, enhanced) CHA’s effect.
It is unclear why increased dietary Na potentiates CHA-induced renal vasoconstriction in isolated perfused rat kidneys but blocks adenosine-induced renal vasoconstriction in vivo. A reasonable explanation might be that the renal adenosine receptors in Na-loaded animals are already fully occupied by endogenously released adenosine, because Na loading is known to increase renal adenosine production and release (Haas and Osswald, 1981). In any case, the observation that Na loading attenuates both the activity of the renin angiotensin system and adenosine-induced renal vasoconstriction in vivo cannot be taken as evidence of a causal relationship; aortic clamping is one of the most potent stimulators of the renin angiotensin system, yet this maneuver, like Na loading, blocks adenosine-induced renal vasoconstriction in vivo (Haas and Osswald, 1981). Moreover, the renal hemodynamic effects of 2-chloroadenosine have been shown to be completely independent of tissue renin concentration in the two-kidney, one-clip Goldblatt rat (Churchill et al., 1984).
In summary, activation of A1 adenosine receptors by submicromolar concentrations of CHA resulted in renal vasoconstriction and Na loading enhanced this response. This hemodynamic effect was inhibited by the adenosine receptor antagonists, theophylline and XAC; XAC was three orders of magnitude more potent than theophylline. Despite complete blockade of angiotensin II-induced renal vasoconstriction, saralasin failed to attenuate the renal vascular response to CHA. These data support the concept that A1 adenosine receptors and angiotensin II receptors are separate and distinct biochemical entities, and that independent activation of either receptor leads to renal vasoconstriction.
Acknowledgments
This work was supported by the Hutzel Hospital Research Fund and by the National Institutes of Health (HL 24880).
ABBREVIATIONS
- CHA
N6-cyclohexyIadenosine
- XAC
xanthine amine congenar
References
- Arend LJ, Haramati A, Thompson CJ, Spielman WS. Adenosine-induced decrease in renin release: Dissociation from hemodynamic effects. Am. J. Physiol. 1984;247:F447–F452. doi: 10.1152/ajprenal.1984.247.3.F447. [DOI] [PubMed] [Google Scholar]
- Arend LJ, Thompson CI, Spielman WS. Dipyridamole decreases glomerular filtration in the sodium-depleted dog. Evidence for mediation by intrarenal adenosine. Circ. Res. 1985;56:242–251. doi: 10.1161/01.res.56.2.242. [DOI] [PubMed] [Google Scholar]
- Berecek KH, Murray RD, Gross F. Significance of sympathetic innervation and central adrenergic structures on renal responsiveness in DOCA-treated rats. Circ. Res. 1980;47:675–683. doi: 10.1161/01.res.47.5.675. [DOI] [PubMed] [Google Scholar]
- Berecek KH, Murray RD, Gross F, Brody J. Vasopressin and vascular reactivity in the development of DOCA hypertension in rats with hereditary diabetes insipidus. Hypertension. 1982;4:3–12. doi: 10.1161/01.hyp.4.1.3. [DOI] [PubMed] [Google Scholar]
- Buckle PJ, Spence I. The actions of adenosine and some analogues on evoked and potassium stimulated release at skeletal and autonomic neuromuscular junctions. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1982;319:130–135. doi: 10.1007/BF00503925. [DOI] [PubMed] [Google Scholar]
- Churchill PC, Bidani AK, Churchill MC, Prada J. Renal effects of 2-chloroadenosine in the two-kidney Goldblatt rat. J. Pharmacol. Exp. Ther. 1984;230:302–306. [PubMed] [Google Scholar]
- Churchill PC, Churchill MC. A1 and A2 adenosine receptor activation inhibits and stimulates renin secretion of rat renal cortical slices. J. Pharmacol. Exp. Ther. 1985;232:589–594. [PubMed] [Google Scholar]
- Clanachan AS, Muller MJ. Effect of adenosine uptake inhibition on the nature and potency of theophylline as a presynaptic adenosine receptor antagonist. Can. J. Physiol. Pharmacol. 1980;58:805–809. [Google Scholar]
- Daly JW. Adenosine receptors: Targets for future drugs. J. Med. Chem. 1982;25:197–207. doi: 10.1021/jm00345a001. [DOI] [PubMed] [Google Scholar]
- Daly JW. Adenosine receptors: Characterization with radioactive ligands. In: Daly JW, Kuroda Y, Phillis JW, Shimizu H, Ui M, editors. Physiology and Pharmacology of adenosine derivatives. Raven Press; New York: 1983. pp. 59–69. [Google Scholar]
- Drury AN, Szent-Gyorgyi A. The physiological activity of adenine compounds with special reference to their action upon the mammalian heart. J. Physiol (Lond.) 1929;68:213–237. doi: 10.1113/jphysiol.1929.sp002608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredholm BB, Hedqvist P, Vernet L. Effect of theophylline and other drugs on rabbit renal cyclic nucleotide phosphodiesterase, 5′-nucleotidase and adenosine deaminase. Biochem. Pharmacol. 1978;27:2845–2850. doi: 10.1016/0006-2952(78)90199-5. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Persson CG. Xanthine derivatives as adenosine receptor antagonists. Eur. J. Pharmacol. 1982;81:673–677. doi: 10.1016/0014-2999(82)90359-4. [DOI] [PubMed] [Google Scholar]
- Haas JA, Osswald H. Adenosine-induced fall in glomerular capillary pressure. Effect of ureteral obstruction and aortic constriction in the Munich-Wistar rat kidney. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1981;317:86–89. doi: 10.1007/BF00506263. [DOI] [PubMed] [Google Scholar]
- Hall JE, Granger JP. Renal hemodynamics and arterial pressure during chronic intrarenal adenosine infusion in conscious dogs. Am. J. Physiol. 1986;250:F32–F39. doi: 10.1152/ajprenal.1986.250.1.F32. [DOI] [PubMed] [Google Scholar]
- Hall JE, Granger JP, Hester RL. Interactions between adenosine and angiotensin II in controlling glomerular filtration. Am. J. Physiol. 1985;248:F340–F346. doi: 10.1152/ajprenal.1985.248.3.F340. [DOI] [PubMed] [Google Scholar]
- Holland MJC, Murphy E, Kelleher JK. Adenosine metabolism in human skin fibroblasts. Am. J. Physiol. 1985;248:C21–C26. doi: 10.1152/ajpcell.1985.248.1.C21. [DOI] [PubMed] [Google Scholar]
- Jacobson KA, Kirk KL, Padgett W, Daly JW. Functionalized congeners of 1,3-dialkylxanthines: Preparation of analogues with high affinity for adenosine receptors. J. Med. Chem. 1985;28:1334–1340. doi: 10.1021/jm00147a038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson KA, Ukena D, Kirk K, Daly JW. [3H]Xanthine amine congener of 1,3-dipropyl-8-phenylxanthine: An antagonist radioligand for adenosine receptors. Proc. Natl. Acad. Sci. U.S.A. 1986;83:4089–4093. doi: 10.1073/pnas.83.11.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Londos C, Cooper DMF, Wolff J. Subclasses of external adenosine receptors. Proc. Natl. Acad. Sci. U.S.A. 1980;77:2551–2554. doi: 10.1073/pnas.77.5.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray RD, Churchill PC. The effects of adenosine receptor agonists in the isolated, perfused rat kidney. Am. J. Physiol. 1984;247:H343–H348. doi: 10.1152/ajpheart.1984.247.3.H343. [DOI] [PubMed] [Google Scholar]
- Murray RD, Churchill PC. Concentration dependency of the renal vascular and renin secretory responses to adenosine receptor agonists. J. Pharmacol. Exp. Ther. 1985;232:189–193. [PubMed] [Google Scholar]
- Nishiitsutsuji-Uwo JM, Ross BD, Krebs HA. Metabolic activities of the Isolated perfused rat kidney. Biochem. J. 1967;103:852–862. doi: 10.1042/bj1030852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver JA, Cannon PJ. The effect of altered sodium balance upon renal vascular reactivity to angiotensin II and norepinephrine in the dog. J. Clin. Invest. 1978;61:610–623. doi: 10.1172/JCI108972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono H, Inagaki K, Hashimoto K. A pharmacologic approach to the nature of the autoregulation of the renal blood flow. Jpn. J. Pharmacol. 1966;16:625–634. doi: 10.2170/jjphysiol.16.625. [DOI] [PubMed] [Google Scholar]
- Osswald H. Renal effects of adenosine and their inhibition by theophylline in dogs. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1975;288:79–86. doi: 10.1007/BF00501815. [DOI] [PubMed] [Google Scholar]
- Osswald H. The role of adenosine in the regulation of glomerular filtration rate and renin secretion. Trends Pharmacol. Sci. 1984;5:94–97. [Google Scholar]
- Osswald H, Nabakowski G, Hermes H. Adenosine as a possible mediator of metabolic control of glomerular filtration rate. Int. J. Biochem. 1980;12:263–267. doi: 10.1016/0020-711x(80)90082-8. [DOI] [PubMed] [Google Scholar]
- Osswald H, Schmitz H-J, Heidenreich O. Adenosine response of the rat kidney after saline loading, sodium restriction and hemorrhagia. Pflügers Arch. 1975;357:323–333. doi: 10.1007/BF00585986. [DOI] [PubMed] [Google Scholar]
- Osswald H, Schmitz H-J, Kemper R. Renal action of adenosine: Effect on renin secretion in the rat. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1978a;303:95–99. doi: 10.1007/BF00496190. [DOI] [PubMed] [Google Scholar]
- Osswald H, Spielman WS, Knox FG. Mechanism of adenosine-mediated decreases in glomerular filtration rate in dogs. Circ. Res. 1978b;43:465–469. doi: 10.1161/01.res.43.3.465. [DOI] [PubMed] [Google Scholar]
- Plagemann PGW, Wohlhueter RM. Nucleoside transport in mammalian cells and interaction with intracellular metabolism. In: Berne RM, Rall TW, Rubio R, editors. Regulatory Function of Adenosine. Martinus Nijhoff; Boston: 1982. pp. 179–201. [Google Scholar]
- Premen AJ, Hall JE, Mizelle HL, Cornell JE. Maintenance of renal autoregulation during infusion of aminophylline or adenosine. Am. J. Physiol. 1985;248:F366–F373. doi: 10.1152/ajprenal.1985.248.3.F366. [DOI] [PubMed] [Google Scholar]
- Sinclair RJ, Randall JR, Wise GE, Jones CE. Response of isolated renal artery rings to adenosine and inosine. Drug Dev. Res. 1985;6:391–396. [Google Scholar]
- Spielman WS, Britton S, Fiksen-Olsen MJ. Effect of adenosine on the distribution of renal blood flow in dogs. Circ. Res. 1980;46:449–456. doi: 10.1161/01.res.46.3.449. [DOI] [PubMed] [Google Scholar]
- Spielman WS, Osswald H. Blockade of postocclusive renal vasoconstriction by an angiotensin II antagonist: Evidence for an angiotensin-adenosine interaction. Am. J. Physiol. 1979;237:F463–F467. doi: 10.1152/ajprenal.1979.237.6.F463. [DOI] [PubMed] [Google Scholar]
- Spielman WS, Thompson CI. A proposed role for adenosine in the regulation of renal hemodynamics and renin release. Am. J. Physiol. 1982;242:F423–F435. doi: 10.1152/ajprenal.1982.242.5.F423. [DOI] [PubMed] [Google Scholar]
- Staff of the Department of Pharmacology of the University of Edinburgh. Pharmacological Experiments on Isolated Preparations. Livingstone, London: 1970. p. 26. [Google Scholar]
- Tagawa H, Vander AJ. Effects of adenosine compounds on renal function and renin secretion in dogs. Circ. Res. 1970;26:327–338. doi: 10.1161/01.res.26.3.327. [DOI] [PubMed] [Google Scholar]
- Thurau K. Renal hemodynamics. Am. J. Med. 1964;36:698–719. doi: 10.1016/0002-9343(64)90181-0. [DOI] [PubMed] [Google Scholar]
- Toblan L. Interrelationship of electrolytes, juxtaglomerular cells and hypertension. Physiol. Rev. 1960;40:280–312. doi: 10.1152/physrev.1960.40.2.280. [DOI] [PubMed] [Google Scholar]
- Ukena D, Daly JW, Kirk KL, Jacobson KA. Functionalized congeners of 1,3-dipropyl-8-phenylxanthine: Potent antagonists for adenosine receptors that modulate membrane adenylate cyclase in phenochromocytoma cells, platelets and fat cells. Life Sci. 1986;38:797–807. doi: 10.1016/0024-3205(86)90596-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallenstein S, Zucker CL, Fleiss JL. Some Statistical methods useful in Circulation Research. Circ. Res. 1980;47:1–9. doi: 10.1161/01.res.47.1.1. [DOI] [PubMed] [Google Scholar]
- Wolff J, Londos C, Cook GH. Adenosine interactions with thyroid adenylate cyclase. Arch. Biochem. Biophys. 1978;191:161–168. doi: 10.1016/0003-9861(78)90078-4. [DOI] [PubMed] [Google Scholar]