Abstract
The family Miridae is one of the most species-rich families of insects. To better understand the diversity and evolution of mirids, we determined the mitogenome of Lygus pratenszs and re-sequenced the mitogenomes of four mirids (i.e., Apolygus lucorum, Adelphocoris suturalis, Ade. fasciaticollis and Ade. lineolatus). We performed a comparative analysis for 15 mitogenomic sequences representing 11 species of five genera within Miridae and evaluated the potential of these mitochondrial genes as molecular markers. Our results showed that the general mitogenomic features (gene content, gene arrangement, base composition and codon usage) were well conserved among these mirids. Four protein-coding genes (PCGs) (cox1, cox3, nad1 and nad3) had no length variability, where nad5 showed the largest size variation; no intraspecific length variation was found in PCGs. Two PCGs (nad4 and nad5) showed relatively high substitution rates at the nucleotide and amino acid levels, where cox1 had the lowest substitution rate. The Ka/Ks values for all PCGs were far lower than 1 (<0.59), but the Ka/Ks values of cox1-barcode sequences were always larger than 1 (1.34 –15.20), indicating that the 658 bp sequences of cox1 may be not the appropriate marker due to positive selection or selection relaxation. Phylogenetic analyses based on two concatenated mitogenomic datasets consistently supported the relationship of Nesidiocoris + (Trigonotylus + (Adelphocoris + (Apolygus + Lygus))), as revealed by nad4, nad5, rrnL and the combined 22 transfer RNA genes (tRNAs), respectively. Taken sequence length, substitution rate and phylogenetic signal together, the individual genes (nad4, nad5 and rrnL) and the combined 22 tRNAs could been used as potential molecular markers for Miridae at various taxonomic levels. Our results suggest that it is essential to evaluate and select suitable markers for different taxa groups when performing phylogenetic, population genetic and species identification studies.
Keywords: Molecular evolution, Phylogeny, Mitochondrial genome, Insect, Heteroptera, Molecular marker, DNA barcoding, Cox1
Introduction
Mirid bugs (Hemiptera: Miridae) are one of the most species-rich families of insects, with approximately 11,000 described species in 1,200 genera (Cassis & Schuh, 2012; Jung & Lee, 2012). Mirid bugs play a key role in natural systems and agroecosystems, with a wide range of food preferences and behaviors (Cassis & Schuh, 2012; Jung & Lee, 2012; Wheeler, 2001). Some mirids are of great economic importance as pests on various cultivated plants (Cassis & Schuh, 2012; Lu et al., 2010), whereas others are beneficial species used as biological control agents (Cassis & Schuh, 2012). In China, several mirids (e.g., Apolygus lucorum, Lygus pratenszs and Adelphocoris lineolatus) are important insect pests on crops, vegetables and forages and recently have extensively increased population density on cotton due to increasing Bt cotton adoption (Lu et al., 2010). However, little is known about interspecific and intraspecific diversity and evolution in these mirids.
Mirids show high morphological diversity and some are difficult to be identified by eye due to small body size, especially closely related species with similar morphological characteristics. The mitochondrial cytochrome c oxidase subunit 1 (cox1) has been widely used as a molecular marker for molecular phylogenetics, population genetics and species identification in animals (Avise, 2009; Hebert, Ratnasingham & Waard, 2003; Jinbo, Kato & Ito, 2011; Simon et al., 2006). The effectiveness of cox1 as a DNA barcoding marker has been widely investigated in many insect groups, such as Lepidoptera (Cameron & Whiting, 2008; DeWaard et al., 2010; Wiemers & Fiedler, 2007); Hemiptera (Abd-Rabou et al., 2012; Foottit et al., 2008; Jung, Duwal & Lee, 2011; Park et al., 2011; Raupach et al., 2014) and Coleoptera (Kubisz et al., 2012; Monaghan et al., 2005; Raupach et al., 2010). These studies showed that cox1 was an effective and suitable DNA barcoding marker for most insect groups, but showed limited ability to identify closely related species for some groups (Chi et al., 2012; Lee et al., 2013; Schmidt & Sperling, 2008). Therefore, it is essential to explore other potential mitochondrial and nuclear markers for these groups, such as nuclear ITS (Park et al., 2010) and other mitochondrial genes (e.g., nad4 and nad5) (Brabec et al., 2015; Levkanicova & Bocak, 2009; Ye et al., 2015; Yu, Kong & Li, 2016).
Insect mitogenome is a circular double-stranded molecule of 15–18 kb in size and usually contains 37 genes and a large non-coding region (known as control region) (Boore, 1999; Cameron, 2014). During the past decades, insect mitogenomes are the most extensively used genetic information for molecular evolutionary, phylogenetic and population genetic studies (Cameron, 2014; Simon et al., 2006). To date, only ten complete or nearly complete mitogenomes have been determined for Miridae. However, the number of sequenced mirid mitogenomes is still very limited compared to the species-richness of Miridae. Therefore, sequencing more mirid mitogenomes is essential for understanding the evolution of Miridae at the genomic level. In particular, all mirid mitogenomes available in GenBank were sequenced for just a single individual per species, which limited our understanding of intraspecific mitogenomic diversity. To date, knowledge about intraspecific evolution of insect mitogenomes is limited, with the notable exception of Drosophila melanogaster(Wolff et al., 2016). Due to the linkage of these mitochondrial genes within the same mtDNA molecule, the same genealogy shared by mitochondrial genes is expected. However, incongruent phylogenetic results were frequently found among different mitochondrial genes (Duchêne et al., 2011; Havird & Santos, 2014; Nadimi, Daubois & Hijri, 2016). In addition, the single or a few concatenated genes could serve as a proxy for the entire mitogenomes (Duchêne et al., 2011; Havird & Santos, 2014; Nadimi, Daubois & Hijri, 2016), which provides a good opportunity to resolve phylogenetic relationships of Miridae that currently lacks sufficient entire mitogenome sequences. However, the performance of the best genes or regions is highly taxa-dependent (Duchêne et al., 2011; Havird & Santos, 2014; Nadimi, Daubois & Hijri, 2016). Therefore, it is needed to evaluate the potential and suitability of single mitochondrial genes as molecular markers within Miridae.
In this study, we sequenced and annotated the mitogenome of L. pratenszs and re-sequenced the mitogenomes of four mirid species (i.e., Apo. lucorum, Ade. suturalis, Ade. fasciaticollis and Ade. lineolatus). These five mirid bugs are important pests on crops, vegetables and forages in China (Lu et al., 2010; Zhang et al., 2016). Combined with ten mirid mitogenomes available from GenBank (Table 1), we provided a comparative mitogenomic analysis at various taxonomic levels. Particularly, we focused on molecular evolution of mitochondrial genes within genera and species. We also evaluated the potential of these mitochondrial genes as molecular markers by genetic distance and phylogenetic analyses. The results will provide useful genetic information for further studies on phylogeny, species identification, phylogeography and population genetics in mirid bugs.
Table 1. List of mirid species analyzed in the study.
| Subfamily | Species | Size (bp) | A+T% | AT-skew | GC-skew | GenBank accession | References |
|---|---|---|---|---|---|---|---|
| Bryocorinae | Nesidiocoris tenuis | 17,544 | 75.0 | 0.10 | −0.11 | NC_022677 | Dai et al. (2012) |
| Mirinae | Adelphocoris fasciaticollis | 15,434 | 77.4 | 0.16 | −0.22 | KJ001714 | Wang et al. (2016a); Wang et al. (2016b) |
| Adelphocoris fasciaticollis_Yuana | 13,587 | 77.0 | 0.17 | −0.21 | KU234536 | This study | |
| Adelphocoris lineolatus | 15,595 | 77.1 | 0.16 | −0.21 | KJ020286 | Wang et al. (2014b) | |
| Adelphocoris lineolatus_Yuan | 15,433 | 76.9 | 0.16 | −0.21 | KU234537 | This study | |
| Adelphocoris nigritylusa | 14,522 | 77.2 | 0.17 | −0.21 | KJ020287 | Wang et al. (2014b) | |
| Adelphocoris suturalisa | 14,327 | 76.8 | 0.17 | −0.20 | KJ020288 | Wang et al. (2014b) | |
| Adelphocoris suturalis_Yuana | 14,106 | 76.8 | 0.17 | −0.21 | KU234538 | This study | |
| Apolygus lucorum | 14,768 | 76.8 | 0.12 | −0.12 | NC_023083 | Wang et al. (2014a) | |
| Apolygus lucorum_Yuan | 15,647 | 76.8 | 0.11 | −0.12 | KU234539 | This study | |
| Lygus hesperus | 17,747 | 75.3 | 0.14 | −0.19 | NC_024641 | Unpublished | |
| Lygus lineolaris | 17,027 | 75.9 | 0.13 | −0.18 | NC_021975 | Roehrdanz et al. (2016) | |
| Lygus pratenszsa | 14,239 | 75.6 | 0.15 | −0.18 | KU234540 | This study | |
| Lygus rugulipennisa | 15,819 | 75.5 | 0.14 | −0.18 | KJ170898 | Wang et al. (2014b) | |
| Trigonotylus caelestialiuma | 15,095 | 74.9 | 0.14 | −0.13 | KJ170899 | Wang et al. (2014b) |
Notes.
Incomplete mitochondrial genomes.
Materials and Methods
Sample and DNA extraction
Adult specimens of five mirid bugs were collected from alfalfa field in Shishe Town, Xifeng District, Qingyang City, Gansu Province, China, in July 2013. Samples and voucher specimens have been deposited in the State Key Laboratory of Grassland Agro-Ecosystems, College of Pastoral Agricultural Science and Technology, Lanzhou University, Lanzhou, China. All specimens were initially preserved in 100% ethanol in the field, and transferred to −20 °C until used for DNA extraction. The total genomic DNA was extracted from thorax muscle of a single specimen using the Omega Insect DNA Kit (Omega Bio-Tek, Norcross, GA, USA) according to the manufacturer’s protocols.
PCR amplification and sequencing
For each mirid species, mitogenome was amplified with 10–13 overlapping fragments by using universal insect mitogenome primers (Simon et al., 2006) and species-specific primers designed from sequenced fragments. All primers used in this study are provided in Table S1 . PCR and sequence reactions were conducted following our previous studies (Yuan et al., 2015a; Yuan et al., 2015b).
Genome annotation and sequence analysis
Sequence files were assembled into contigs with BioEdit 7.0.9.0 (Hall, 1999). Each of the five mirid mitogenomes newly sequenced in the present study was annotated following our previous studies (Yuan et al., 2015a; Yuan et al., 2015b). Nucleotide composition and codon usage were analyzed with MEGA 6.06 (Tamura et al., 2013). For each protein-coding gene (PCG) of all 15 mirid mitogenomes, the number of synonymous substitutions per synonymous site (Ks) and the number of nonsynonymous substitutions per nonsynonymous site (Ka) were calculated with MEGA 6.06 (Tamura et al., 2013). We also calculated the genetic distances for 13 PCGs and two ribosomal RNA genes (rRNAs) with MEGA 6.06 (Tamura et al., 2013) under the Kimura-2-parameter model (K2P). Strand asymmetry was calculated using the formulas: AT-skew = [A−T]/[A+T] and GC-skew = [G−C]/[G+C] (Perna & Kocher, 1995). To determine whether the Ka/Ks values of cox1-barcode sequences were relevant with the scope of sequences used, we downloaded all 7,759 cox1 sequences of Miridae available in GenBank (March 1, 2017). After removing sequences shorter than 658 bp, a total of 2,326 sequences in 144 genera were obtained (Table S7). Except for forty genera with only one sequence, the remaining 104 genera were used to calculate the values of Ka, Ks and Ka/Ks.
Phylogenetic analysis
Complete or nearly complete mitogenome sequences of eleven mirid bugs (15 samples, Table 1) and two outgroups from Pentatomomorpha (Corizus tetraspilus and Eurydema gebleri) (Yuan et al., 2015a; Yuan et al., 2015b) were used to perform phylogenetic analyses. The complete sequences of 13 PCGs (excluding stop codons), two rRNAs and 22 transfer RNA genes (tRNAs) were used for phylogenetic analyses. Each PCG was aligned individually based on codon-based multiple alignments by using the MAFFT algorithm within the TranslatorX (Abascal, Zardoya & Telford, 2010) online platform. Sequences of each rRNA gene were individually aligned using the MAFFT v7.0 online server with G-INS-i strategy (Katoh & Standley, 2013). Alignments of individual genes were then concatenated as a combined matrix with DAMBE 5.3.74 (Xia, 2013). Two datasets were assembled for phylogenetic analyses: (1) nucleotide sequences of 13 PCGs (P123) with 11,133 residues and (2) nucleotide sequences of 13 PCGs, two rRNAs and 22 tRNAs (P123RT) with 14,905 residues. To evaluate phylogenetic potential of single mitochondrial genes, i.e., each of 13 PCGs, rrnL and rrnS, as well as the combined 22 tRNAs, were also used in phylogenetic analyses.
The optimal partitioning schemes and corresponding nucleotide substitution models for each datasets were determined by PartitionFinder v1.1.1 (Lanfear et al., 2012). We created input configuration files that contained pre-defined data blocks by genes and codons, e.g., 39 partitions for P123, 42 partitions for P123RT and 3 partitions for each of 13 PCGs. The “greedy” algorithm with branch lengths estimated as “unlinked” and Bayesian information criterion (BIC) were used to search for the best-fit scheme (Table S2). The best-fit partitioning schemes selected by PartitionFinder were used in all subsequent phylogenetic analyses. We used jModelTest 2.1.7 (Posada, 2008) to determine the best evolutionary model for rrnL, rrnS and the combined 22 tRNAs.
Phylogenetic analyses were conducted with Bayesian inference (BI) and maximum likelihood (ML) methods available on the CIPRES Science Gateway v3.3 (Miller, Pfeiffer & Schwartz, 2010). Bayesian analyses were performed with MrBayes 3.2.3 (Ronquist et al., 2012) on Extreme Science and Engineering Discovery Environment (XSEDE 8.0.24). Two independent runs with four chains (three heated and one cold) each were conducted simultaneously for 1 × 106 generations. Each run was sampled every 100 generations. Stationarity is assumed to be reached when ESS (estimated sample size) value is above 100 and PSRF (potential scale reduction factor) approach 1.0 as suggested in MrBayes 3.2.3 manual (Ronquist et al., 2012). The first 25% samples were discarded as burn-in, and the remaining trees were used to calculate posterior probabilities (PP) in a 50% majority-rule consensus tree. ML analyses were carried out using RAxML-HPC2 (Stamatakis, 2014) on XSEDE 8.0.24 with the GTRGAMMA model, and the node reliability was assessed by 1,000 bootstraps (BS).
Results
General features of mirid mitogenomes
In the present study, we sequenced and annotated the mitogenomes of five mirid bugs: two were completely sequenced, whereas three were nearly complete mitogenomes (lacking sequences of three tRNAs and the putative control region) (Table 1, Table S3). The mitogenome sequences of five mirids have been deposited in GenBank of NCBI underaccession numbers: KU234536 –KU234540. The two completely sequenced mitogenomes contained 37 typical mitochondrial genes (i.e., 13 PCGs, 22 tRNA genes and two rRNAs) and a large non-coding region (putative control region) (Table S3). The order and orientation of the mitochondrial genes were identical to that of the putative ancestral insect mitogenome (Boore, 1999; Cameron, 2014). Gene overlaps and spacers were presented in several conserved positions in the mirid mitogenomes, e.g., trnS2/nad1 (7 bp), trnW/trnC (−8 bp), atp8/atp6 (−7 bp) and nad4/nad4L (−7 bp).
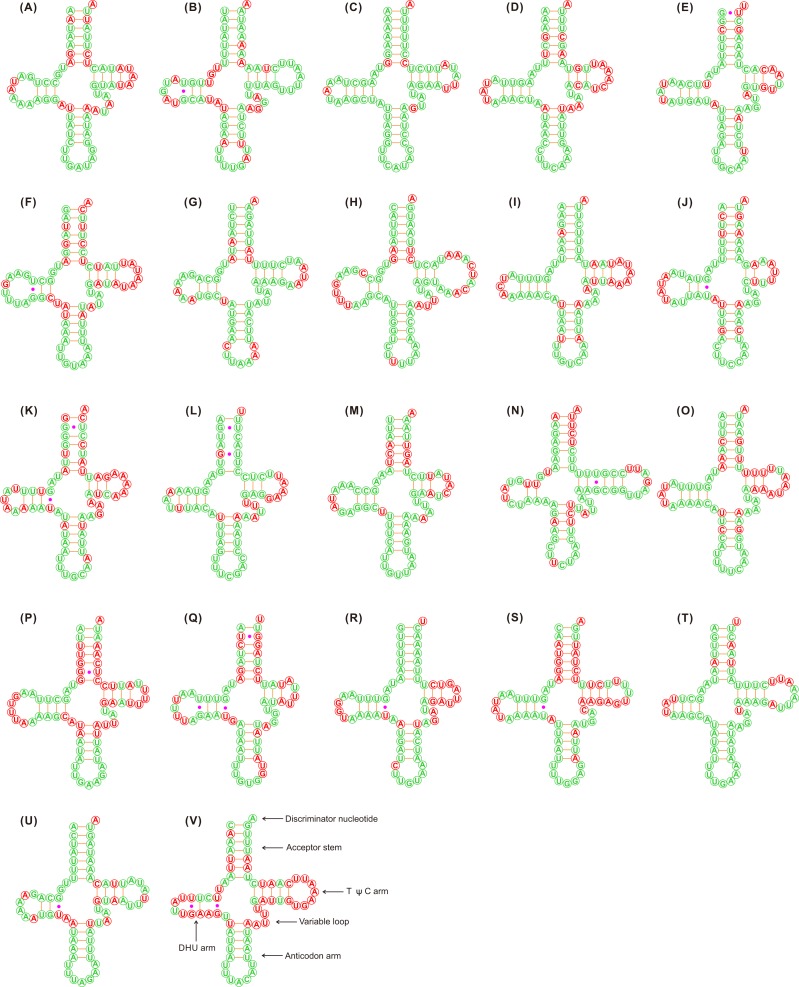
The tRNAs in the three Adelphocoris species could be folded into a classical clover-leaf secondary structure (Fig. 1). However, trnS1 (AGN) in Apo. lucorum and L. pratenszs species lacked the DHU stem-loop structures, as previously observed in many other true bugs (Wang et al., 2014b; Yuan et al., 2015a; Yuan et al., 2015b). All 22 tRNAs in Apolygus and Lygus species used the standard anticodon, whereas two tRNAs in the three Adelphocoris species were exceptions: trnS1 was predicted to have anticodon UCU, whereas trnK had the anticodon UUU (Table S3). The sequences and structures of anticodon arms and aminoacyl acceptor stems were well conserved within Miridae, whereas most of the variations (nucleotide substitutions and indels) were found in the DHU loops, pseudouridine (TψC) arms and variable loops (Fig. 1).
Figure 1. Putative secondary structures of the 22 tRNA genes identified in the mitochondrial genome of Adelphocoris lineolatus.
(A) trnI, (B) trnQ, (C) trnM, (D) trnW, (E) trnC, (F) trnY, (G) trnL2, (H) trnK, (I) trnD, (J) trnG, (K) trnA, (L) trnR, (M) trnN, (N) trnS1, (O) trnE, (P) trnF, (Q) trnH, (R) trnT, (S) trnP, (T) trnS2, (U) trnL1, (V) trnV. All tRNA genes are shown in the order of occurrence in the mitochondrial genome starting from trnI. The nucleotides showing 100% identity in the 15 mirid mitochondrial genomes are marked with green color, and the variable region are marked with red color. Bars indicate Watson-Crick base pairings, and dots between G and U pairs mark canonical base pairings in tRNA.
All of the mirid mitogenomes showed similar nucleotide composition in the J-strand: high A+T content, positive AT- and negative GC-skews (Fig. S1), as is usually observed in insect mitogenomes (Hassanin, Leger & Deutsch, 2005). For each part of mitogenomes, the A+T content, AT- and GC-skews showed low variability among different taxonomic levels (i.e., family, subfamily, genus and species). For AT-skew, a negative value was found in PCGs, rrnL, rrnS and the 2nd codon position of PCGs, whereas the 1st and 3rd codon positions of PCGs had positive AT-skew values. Except for Apo. lucorum, a positive AT-skew value also was found for tRNAs in all mirids. For GC-skew, the 1st codon position of PCGs, rrnL, rrnS and tRNAs were markedly positive, whereas the 2nd and 3rd codon positions of PCGs showed negative values. The pattern of codon usage in all analyzed mirid mitogenomes was consistent with previous findings in insects (e.g., Wang et al., 2015; Yuan et al., 2015a), namely that A+T-rich codons were preferably used (Table S4).
Gene variability in mirid mitogenomes
Among 13 PCGs, four genes (cox1, cox3, nad1 and nad3) had no length variability in the 15 examined mirid mitogenomes (Table S5). Four genes showed size variation only in one species (i.e., atp6, cox2 and nad4 in N. tenuis, cob in L. lineolaris). N. tenuis belonging to Bryocorinae showed the most length differences with species from Mirinae. The length of nad5 was most variable, but conserved within each genus. No length variation was found in the same species, whereas the most variations were found among genera. For the two genera including more than two species, no size variation was present in Adelphocoris, and only cob in L. lineolaris showed size difference with the others in Lygus. For rrnL and rrnS, intra-generic and -specific size differences were slight, whereas large differences were found among genera.
The numbers of variable and informative sites varied among genes and taxa (Table 2). The smallest gene atp8 showed the highest informative sites in Miridae, Mirinae and Mirini, followed by nad2 and nad6, while nad4L in Adelphocoris and nad3 in Lygus. The cox1 gene showed least informative sites in Miridae, Mirinae and Mirini, while nad6 in Adelphocoris and nad4L in Lygus. Generally, rrnL and rrnS had the lower informative sites than most PCGs; rrnL was slightly higher than rrnS, except for Adelphocoris. Compared to the whole cox1, cox1-barcode sequences contained relatively small informative sites in most taxonomic levels (except for Lygus). Except for cox1, other two longest genes (nad4 and nad5) contained moderate informative sites in most taxonomic levels (except for Lygus).
Table 2. Number of variable sites and number of informative sites in Miridae.
| Miridae | Mirinae | Mirini | Adelphocoris | Lygus | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Gene | Number of variable sites (%) | Number of informative sites (%) | Number of variable sites (%) | Number of informative sites (%) | Number of variable sites (%) | Number of informative sites (%) | Number of variable sites (%) | Number of informative sites (%) | Number of variable sites (%) | Number of informative sites (%) |
| atp6 | 319 (47.47) | 212 (31.55) | 260 (38.86) | 203 (30.34) | 227 (33.93) | 199 (29.75) | 39 (5.83) | 23 (3.44) | 50 (7.47) | 9 (1.35) |
| atp8 | 84 (52.83) | 60 (37.74) | 74 (46.54) | 56 (35.22) | 58 (36.48) | 56 (35.22) | 4 (2.56) | 3 (1.92) | 7 (4.40) | 2 (1.26) |
| cob | 412 (36.43) | 273 (24.14) | 350 (30.95) | 263 (23.25) | 296 (26.17) | 254 (22.46) | 51 (4.51) | 39 (3.45) | 117 (10.34) | 19 (1.68) |
| cox1 | 534 (34.83) | 344 (22.44) | 425 (27.72) | 322 (21.00) | 357 (23.29) | 314 (20.48) | 87 (5.68) | 60 (3.91) | 110 (7.18) | 23 (1.50) |
| cox2 | 267 (39.38) | 185 (27.29) | 219 (32.30) | 168 (24.78) | 183 (26.99) | 164 (24.19) | 37 (5.46) | 23 (3.39) | 53 (7.82) | 10 (1.47) |
| cox3 | 311 (39.72) | 204 (26.05) | 252 (32.18) | 191 (24.39) | 212 (27.08) | 189 (24.14) | 36 (4.06) | 25 (3.19) | 62 (7.92) | 11 (1.40) |
| nad1 | 343 (37.12) | 215 (23.27) | 274 (29.65) | 194 (21.00) | 215 (23.27) | 190 (20.56) | 27 (2.92) | 19 (2.06) | 75 (8.12) | 12 (1.30) |
| nad2 | 562 (55.92) | 352 (35.02) | 441 (43.88) | 324 (32.24) | 363 (36.12) | 316 (31.44) | 67 (6.75) | 32 (3.22) | 91 (9.05) | 11 (1.09) |
| nad3 | 168 (47.86) | 103 (29.34) | 132 (37.61) | 96 (27.35) | 109 (31.05) | 94 (26.78) | 14 (3.99) | 10 (2.85) | 32 (9.12) | 7 (1.99) |
| nad4 | 703 (52.96) | 450 (33.86) | 570 (42.99) | 404 (30.47) | 452 (34.09) | 399 (30.09) | 74 (5.58) | 46 (3.47) | 112 (8.45) | 23 (1.73) |
| nad4L | 167 (54.58) | 101 (33.01) | 130 (42.48) | 94 (30.72) | 105 (34.31) | 94 (30.72) | 20 (6.60) | 13 (4.29) | 30 (9.90) | 3 (0.99) |
| nad5 | 828 (48.51) | 541 (31.69) | 678 (39.72) | 506 (29.64) | 550 (32.22) | 496 (29.06) | 69 (4.07) | 56 (3.30) | 120 (7.07) | 19 (1.12) |
| nad6 | 289 (57.68) | 172 (34.33) | 228 (46.63) | 155 (31.70) | 172 (35.17) | 151 (30.88) | 18 (3.68) | 9 (1.84) | 51 (10.49) | 7 (1.44) |
| rrnL | 501 (39.60) | 321 (25.38) | 388 (30.77) | 285 (22.60) | 301 (23.91) | 283 (22.48) | 28 (2.27) | 17 (1.38) | 47 (3.75) | 9 (0.72) |
| rrnS | 494 (55.01) | 211 (23.50) | 205 (23.06) | 198 (22.27) | 205 (23.16) | 193 (21.81) | 36 (4.47) | 12 (1.49) | 34 (3.99) | 4 (0.47) |
| cox1-barcoding sequences | 221 (33.59) | 135 (20.52) | 176 (26.75) | 125 (19.00) | 142 (21.58) | 121 (18.39) | 28 (4.26) | 24 (3.65) | 48 (7.29) | 13 (1.98) |
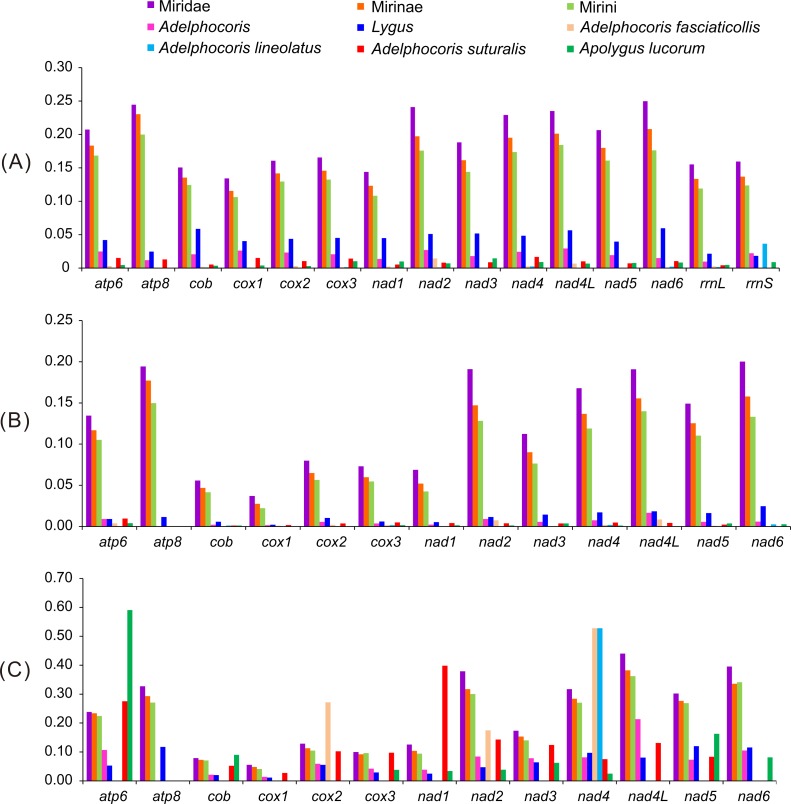
As expected, the largest genetic distances were found among subfamilies, followed by Tribes and genera, whereas the smallest among species (Fig. 2). Some genes (e.g., atp6, cox2, nad1, nad3 and nad6) showed no intraspecific variations at nucleotide and/or amino acid levels, indicating that these genes were highly conserved. The values of K2P and Ka in 13 PCGs of Lygus were larger than that of Adelphocoris, indicating that Lygus had the higher substitution rates. For the K2P distance, nad6 was the highest in Miridae and Lygus, whereas atp8 in Mirinae and Mirini. The K2P distance of nad4L was the highest within Adelphocoris, and relatively high K2P distance was also found in Miridae, Mirinae, Mirini and Lygus. The two rRNAs showed similar K2P distance with those of cox1-3 and cob in Miridae, Mirinae and Mirini, but had the lowest substitution rates in Lygus. For Miridae, Mirinae and Mirini, atp8 showed the highest Ka value, whereas nad4L in Adelphocoris and nad6 in Lygus showed the highest Ka values. Two genes (nad4 and nad5) showed relatively high substitution rates at the nucleotide and amino acid levels. Cox1 showed the lowest substitution rate in Miridae, Mirinae, Mirini, Adelphocoris and Lygus. The Ka/Ks values for all PCGs were far lower than 1 (<0.59) (Fig. 2), suggesting that these genes were evolving under purifying selection. However, we found that the Ka/Ks values for cox1-barcode sequences of the 15 mirids were always larger than 1 (1.34 in Ade. suturalis to 15.20 in Mirini; Table S6). In addition, analyses for cox1-barcode sequences of Miridae from GenBank showed that forty-four genera had no Ka and/or Ks values (Table S7), whereas the values of Ka/Ks for the remaining 61 genera were larger than 1 (1.5–263.0, Table S7), indicating that these sequences may be under positive selection or selection relaxation during the evolutionary process of Miridae. These results suggested that different genes had different substitution rates, whereas the same genes in different taxonomic levels showed large differences.
Figure 2. The K2P genetic distance, Ka and Ka/Ks of 13 protein-coding genes among the 15 mirid mitochondrial genomes.
(A) K2P, the Kimura-2- parameter distance; (B) Ka, the number of nonsynonymous substitutions per nonsynonymous site; (C) Ka/Ks. Ks, the number of synonymous substitutions per synonymous site.
Mirid phylogeny based on combined mitochondrial genes
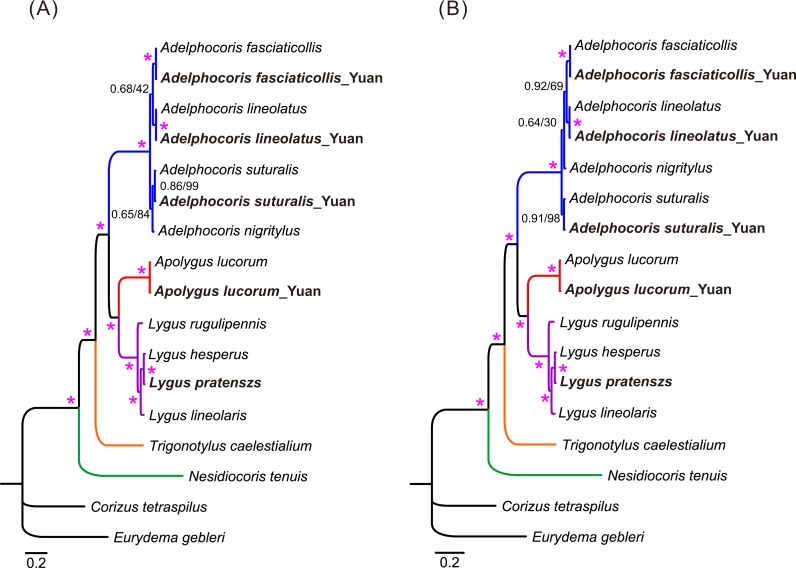
Phylogenetic relationships of 15 mirid mitogenome sequences were inferred using BI and ML methods based on two mitogenomic datasets (P123 and P123RT). The results showed that the two methods with the same dataset resulted in identical tree topology, whereas slight difference was found in the relationships of three species between the two datasets (Fig. 3). For the P123 dataset, Ade. nigritylus was sister to Ade. suturalis (PP = 0.65, BS = 84). For the P123RT dataset, Ade. nigritylus had a closer relationship with Ade. fasciaticolli and Ade. lineolatus (PP = 0.64, BS = 30), which was consistent with previous mirid phylogeny based on mitogenomic data (Wang et al., 2014b). However, low supports were present in both analyses (Fig. 3), indicating that the phylogenetic position of Ade. nigritylus was unstable. All analyses consistently supported the relationship of Nesidiocoris + (Trigonotylus + (Adelphocoris + (Apolygus + Lygus))), as previous mitogenomic analyses (Wang et al., 2014b). For Lygus, the two datasets consistently recovered a phylogeny of (rugulipennis + (lineolaris + (hesperus + pratenszs))). The monophyly of Adelphocoris was strongly supported by all analyses with high supports (PP = 1.0, BS = 100), as was the monophyly of Lygus (PP = 1.0, BS = 100).
Figure 3. The mitochondrial phylogeny of eleven mirid bugs based on the two combined datasets: (A) P123 and (B) P123RT.
Numbers on branches are Bayesian posterior probabilities (PP, before slash) and Bootstrap values (BS, after slash). Asterisk (*) indicates PP = 1.0 and BS = 100. Species sequenced in the present study are bold.
Mirid phylogeny based on single mitochondrial gene
We performed phylogenetic analyses using BI and ML methods with single mitochondrial genes, including each of 13 PCGs, rrnL, rrnS and the combined 22 tRNAs (Figs. S2 and S3). The results showed that the tree topologies were variable among different datasets, indicating incongruent phylogenetic signals among genes, as reported in previous similar studies (Duchêne et al., 2011; Havird & Santos, 2014; Nadimi, Daubois & Hijri, 2016). However, all analyses consistently supported the monophyly of each of Adelphocoris (PP = 0.87–1.0, BS = 82–100) and Lygus (PP = 0.74–1.0, BS = 59–100), and several relationships within each of these two genera were recovered by different individual genes (Table 3, Figs. S2 and S3). Four datasets (nad4, nad5, rrnL and tRNAs) with the two analytical methods supported the phylogeny: Nesidiocoris + (Trigonotylus + (Adelphocoris + (Apolygus + Lygus))), as recovered by the P123 and P123RT datasets. This relationship among the five genera was also supported by BI analyses with two other genes (cox1 and nad2). These four datasets supported the Lygus phylogeny of (rugulipennis + (lineolaris + (hesperus + pratenszs))), as four other genes (cob, cox3, nad1 and nad6). For Adelphocoris, BI and ML analyses of four genes (cox2, cox3, nad5 and rrnL) supported (fasciaticollis + lineolatus) + nigritylus (PP = 0.85–1.0, BS = 34–98), whereas other four genes (atp6, cox1, nad1 and nad4) recovered the sister relationship of nigritylus and suturalis (PP = 0.63–0.9, BS = 53–97). It was notable that the cox1-barcode sequences did not support the sister relationship of Apolygus + Lygus consistently recovered by many independent datasets (Fig. 3 and Figs. S2–S4).
Table 3. The phylogeny for the major clades of Miridae recovered by different mitochondrial datasets and analytical approaches.
| Gene | Adelphocoris | Lygus | Nes+(Tri+ (Ade+(Apo+Lys))) | Af+Al | (Af+Al)+An | An+As | Lr+(Ll+ (Lh+Lp)) |
|---|---|---|---|---|---|---|---|
| atp6 | M/M | M/M | N/N | N/N | N/N | Y/Y | Y/N |
| atp8 | M/M | M/M | N/N | N/N | N/N | N/N | N/N |
| cob | M/M | M/M | N/N | Y/Y | N/N | N/N | Y/Y |
| cox1 | M/M | M/M | Y/N | N/Y | N/N | Y/Y | N/Y |
| cox2 | M/M | M/M | N/N | Y/Y | Y/Y | N/N | Y/N |
| cox3 | M/M | M/M | N/N | Y/Y | Y/Y | N/N | Y/Y |
| nad1 | M/M | M/M | N/N | N/N | N/N | Y/Y | Y/Y |
| nad2 | M/M | M/M | Y/N | N/N | N/N | N/N | N/N |
| nad3 | M/M | M/M | N/N | N/Y | N/N | N/N | N/N |
| nad4 | M/M | M/M | Y/Y | Y/Y | N/N | Y/Y | Y/Y |
| nad4L | M/M | M/P | N/N | N/N | N/N | Y/N | Y/N |
| nad5 | M/M | M/M | Y/Y | Y/Y | Y/Y | N/N | Y/Y |
| nad6 | M/M | M/M | N/N | N/N | N/N | N/N | Y/Y |
| rrnL | M/M | M/M | Y/Y | Y/Y | Y/Y | N/N | Y/Y |
| rrnS | M/M | M/M | N/N | N/N | N/N | N/N | N/N |
| 22 tRNAs | M/M | M/M | Y/Y | Y/Y | N/N | N/Y | Y/Y |
| P123 | M/M | M/M | Y/Y | Y/Y | N/N | Y/Y | Y/Y |
| P123RT | M/M | M/M | Y/Y | Y/Y | Y/Y | N/N | Y/Y |
| cox1-barcoding sequences | M/M | M/M | N/N | Y/Y | N/N | Y/Y | Y/Y |
Notes.
Results from left to right are obtained from Bayesian inference and maximum likelihood, respectively.
- M
- monophyletic
- P
- paraphyletic or polyphyletic
- Y
- yes a phylogeny is supported
- N
- no a phylogeny is not supported.
- Nes
- Nesidiocoris
- Tri
- Trigonotylus
- Ade
- Adelphocoris
- Apo
- Apolygus
- Lys
- Lygus
- Af
- Adelphocoris fasciaticollis
- Al
- Ade. lineolatus
- An
- Ade. nigritylus
- As
- Ade. suturalis
- Lr
- Lygus rugulipennis
- Ll
- L. lineolaris
- Lh
- L. hesperus
- Lp
- L. pratenszs
Discussion
To date, a total of 15 mitogenomes representing 11 species in five genera were sequenced for Miridae. For the seven nearly complete mitogenomes, the undetermined region was the control region characterized by notable base composition bias, high numbers of tandem repeats and stable stem-loop structures. These features may result in disruption of PCR and sequencing reactions, as has been reported in other true bugs (Wang et al., 2014b; Yuan et al., 2015a). The size of completely sequenced mitogenomes greatly varied among genera, ranging from 14,522 bp in Ade. nigritylus and 17,747 bp in L. hesperus, primarily due to the significant size variation of the control region. The complete mitogenome of Apo. lucorum (15,647 bp) re-sequenced in the present study was rather larger than that previously sequenced by Wang et al. (2014a) (14,768 bp), largely due to significant size difference between the two control regions. Sequence alignment of control regions found that Apo. lucorum previously sequenced lacked regions of multiple repeated sequences, implying that the control region may be incomplete. The two completely sequenced mitogenomes of Ade. lineolatus were highly similar in length, with a 162 bp difference. Generally, genome size and total length of spacers and overlaps were more conserved within genus and species than that within subfamily and family, as reported in previous similar studies (Roehrdanz et al., 2016; Wang et al., 2014a; Wang et al., 2014b; Wang et al., 2016a).
The loss of the DHU arm in trnS1 (AGN) has been considered a typical feature of insect mitogenomes (Cameron, 2014). It has been shown that in the nematode Ascaris suum the tRNA genes that lack the DHU arm are functional (Okimoto et al., 1992). We found that trnS1 in Apo. lucorum and L. pratenszs species lacked the DHU arm, but this tRNA in all sequenced mitogenomes of Adelphocoris species had a classical clover-leaf secondary structure, indicating the diversity in the secondary structures of trnS1 within miridae. With the exception of trnS1 and trnK, all the remaining 20 tRNAs used the same anticodon in mirids as in other hemipterans (Wang et al., 2015; Yuan et al., 2015a; Yuan et al., 2015b). In the four Adelphocoris species, trnS1 changed the anticodon GCU with UCU and trnK changed the anticodon CUU with UUU. We re-sequenced the mitogenomes of three Adelphocoris species, confirming that the two anticodons of trnS1 and trnK were genus-specific conserved. Therefore, the variations in structures and anticodons of mirid tRNAs may be genus-specific, and may indicate the high species diversity of Miridae. The mutations in the trnS1 and trnK anticodons were uncommon in hemipteran mitogenomes, which may be correlated with the AGG codon reassignments between serine and lysine (Abascal et al., 2006; Wang et al., 2014b). We also noticed that trnS1 in coleopteran mitogenomes always used the anticodon UCU (Sheffield et al., 2008; Yuan et al., 2016) and trnK used UUU in some beetles (Li et al., 2016; Wang et al., 2016b), suggesting the parallel evolution of AGG codon reassignments and point mutations at the anticodons of trnS1/trnK in insect mitogenomes (Abascal et al., 2006; Wang et al., 2014b). Further study by sequencing more mitogenomes from other genera and species is needed to investigate the evolution of anticodons and structures within Miridae.
Currently, cox1 has been extensively used as DNA barcoding for evaluating and resolving phylogenetic relationships in insects (Hebert, Ratnasingham & Waard, 2003; Jinbo, Kato & Ito, 2011). Although the whole cox1 sequences were evolving under purifying selection, the Ka/Ks values of the cox1-barcode sequences were always larger than 1 at various taxonomic levels within Miridae. Therefore, when we aim to determine the neutral population structure of mirids, the whole cox1 sequences, combined other mitochondrial genes (e.g., nad4 and nad5) as well as other markers (e.g., microsatellites and SNPs), may be preferred. In contrast, the cox1-barcode sequences may have the potential to study the adaptive evolution of mirids in the future. However, further investigations with denser sampling and additional analytical methods for the Ka/Ks ratio (e.g., Bayesian methods) are essential to reveal evolutionary patterns of cox1-barcode sequences within Miridae.
Phylogenetic analyses indicated that individual genes supported different phylogenetic relationships of the 15 mirids despite their linked nature, as reported in previous studies (Duchêne et al., 2011; Havird & Santos, 2014; Nadimi, Daubois & Hijri, 2016; Seixas, Paiva & Russo, 2016). The most probable factor for these incongruent phylogenies is the lack of adequate phylogenetic information within each individual gene, but other potential factors (e.g., phylogenetic inference methods, incomplete taxon sampling) should be considered. Two PCGs (nad4 and nad5) consistently supported the same relationshipsamong the five genera and the four Lygus species, as the two concatenated mitogenomic datasets. These two genes were also identified as good molecular markers for metazoan phylogenetic analyses (Havird & Santos, 2014). However, most previous studies focused on the phylogenetic performance of mitochondrial PCGs, whereas the phylogenetic potential of RNA genes were rarely assessed by comparing the performance of single and concatenated mitochondrial genes. In the present study, we found that rrnL and the combined 22 tRNAs performed well in phylogenetic analyses, as nad4 and nad5, suggesting their potential and importance in resolving the phylogeny of Miridae. However, we found that although cox1 probably was the most commonly used mitochondrial genes in studies of metazoans, this gene (especially cox1-barcode sequences) showed poor phylogenetic performance within Miridae, indicating that the cox1 sequences provided phylogenetic signals that may not be representative of the other mitochondrial genes in Miridae. Previous studies showed incongruent phylogenetic results of cox1 sequences, suggesting that the suitability of cox1 may be taxa-specific and relevant to species number used (Havird & Santos, 2014). Therefore, we should be cautious when the phylogenies are solely derived from sequence data of cox1, whereas additional mitochondrial genes could provide useful genetic information for phylogenetics and population genetics studies of mirid bugs.
Conclusions
In this study, we determined the mitogenomes of L. pratenszs and re-sequenced other four mirid mitogenomes, and provided a comparative analysis for all 15 sequenced mitogenomes at various taxonomic levels. The results showed that gene content, gene arrangement, codon usage and nucleotide composition were well conserved within Miridae. Four protein-coding genes (cox1, cox3, nad1 and nad3) had no length variability, where nad5 showed the most size variation; no intraspecific length variation was found in PCGs. Two genes (nad4 and nad5) showed relatively high substitution rates and informative sites in most taxonomic levels, where cox1 had the lowest in Miridae, Mirinae and Mirini. Taken sequence length, substitution rate and phylogenetic signal together, the individual genes (nad4, nad5 and rrnL) and the combined dataset of 22 tRNAs could be used as potential molecular markers for Miridae. Our results suggest that it is essential to evaluate and select suitable markers for different taxa groups when performing phylogenetic and population genetic studies.
Supplemental Information
Numbers on branches are Bayesian posterior probabilities.
Numbers on branches are bootstrap support values.
Numbers on branches are Bayesian posterior probabilities (left) and Bootstrap values (right).
The best partitioning schemes and substitution models were selected by PartitionFinder for the P123 and P123RT datasets, and each of 13 PCGs. The best substitution models for rrnL, rrnS, 22 tRNAs and cox1-barcode sequences were determined by jModelTest.
Alignment of protein-coding genes, rRNA genes and tRNAs used in this study.
Mitochondrial genome sequences of five mirids sequenced in this study.
Funding Statement
The study was funded by the Natural Science Foundation of Gansu Province (1506RJZA211), the Fundamental Research Funds for the Central Universities (lzujbky-2016-5) and the National Key Technology Support Program (2014B AD14B006). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Juan Wang performed the experiments, analyzed the data, wrote the paper, prepared figures and/or tables, collected the insect samples.
Li Zhang performed the experiments, analyzed the data, wrote the paper, prepared figures and/or tables.
Qi-Lin Zhang performed the experiments, analyzed the data, prepared figures and/or tables, reviewed drafts of the paper, collected the insect samples.
Min-Qiang Zhou, Xiao-Tong Wang and Xing-Zhuo Yang performed the experiments.
Ming-Long Yuan conceived and designed the experiments, analyzed the data, contributed reagents/materials/analysis tools, wrote the paper, prepared figures and/or tables, reviewed drafts of the paper, collected the insect samples.
DNA Deposition
Data Availability
The following information was supplied regarding data availability:
The raw data has been supplied as Supplementary Files.
References
- Abascal et al. (2006).Abascal F, Posada D, Knight RD, Zardoya R. Parallel evolution of the genetic code in arthropod mitochondrial genomes. PLOS Biology. 2006;4:e127. doi: 10.1371/journal.pbio.0040127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abascal, Zardoya & Telford (2010).Abascal F, Zardoya R, Telford MJ. TranslatorX: multiple alignment of nucleotide sequences guided by amino acid translations. Nucleic Acids Research. 2010;38:W7–W13. doi: 10.1093/nar/gkq291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd-Rabou et al. (2012).Abd-Rabou S, Shalaby H, Germain J-F, Ris N, Kreiter P, Malausa T. Identification of mealybug pest species (Hemiptera: Pseudococcidae) in Egypt and France, using a DNA barcoding approach. Bulletin of Entomological Research. 2012;102:515–523. doi: 10.1017/S0007485312000041. [DOI] [PubMed] [Google Scholar]
- Avise (2009).Avise JC. Phylogeography: retrospect and prospect. Journal of Biogeography. 2009;36:3–15. doi: 10.1111/j.1365-2699.2008.02032.x. [DOI] [Google Scholar]
- Boore (1999).Boore JL. Animal mitochondrial genomes. Nucleic Acids Research. 1999;27:1767–1780. doi: 10.1093/nar/27.8.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brabec et al. (2015).Brabec J, Kostadinova A, Scholz T, Littlewood DTJ. Complete mitochondrial genomes and nuclear ribosomal RNA operons of two species of Diplostomum (Platyhelminthes: Trematoda): a molecular resource for taxonomy and molecular epidemiology of important fish pathogens. Parasites & Vectors. 2015;8 doi: 10.1186/s13071-015-0949-4. Article 336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron (2014).Cameron SL. Insect mitochondrial genomics: implications for evolution and phylogeny. Annual Review of Entomology. 2014;59:95–117. doi: 10.1146/annurev-ento-011613-162007. [DOI] [PubMed] [Google Scholar]
- Cameron & Whiting (2008).Cameron SL, Whiting MF. The complete mitochondrial genome of the tobacco hornworm, Manduca sexta, (Insecta: Lepidoptera: Sphingidae), and an examination of mitochondrial gene variability within butterflies and moths. Gene. 2008;408:112–123. doi: 10.1016/j.gene.2007.10.023. [DOI] [PubMed] [Google Scholar]
- Cassis & Schuh (2012).Cassis G, Schuh RT. Systematics, biodiversity, biogeography, and host associations of the miridae (Insecta: Hemiptera: Heteroptera: Cimicomorpha) Annual Review of Entomology. 2012;57:377–404. doi: 10.1146/annurev-ento-121510-133533. [DOI] [PubMed] [Google Scholar]
- Chi et al. (2012).Chi MY, Han HL, Gao Q, Yang CH, Jin Q, Li J, Chen FQ, Wu CS, Zhang AB. Effects of different evolution models on DNA barcoding evaluated with the Noctuidae from Wuling Mountain, Hebei, northern China. Acta Entomologica Sinica. 2012;55:1193–1204. [Google Scholar]
- Dai et al. (2012).Dai X, Xun H, Chang J, Zhang J, Hu B, Li H, Yuan X, Cai W. The complete mitochondrial genome of the plant bug Nesidiocoris tenuis (Reuter)(Hemiptera: Miridae: Bryocorinae: Dicyphini) Zootaxa. 2012:30–44. [Google Scholar]
- DeWaard et al. (2010).DeWaard JR, Mitchell A, Keena MA, Gopurenko D, Boykin LM, Armstrong KF, Pogue MG, Lima J, Floyd R, Hanner RH, Humble LM. Towards a global barcode library for Lymantria (Lepidoptera: Lymantriinae) tussock moths of biosecurity concern. PLOS ONE. 2010;5:e14280. doi: 10.1371/journal.pone.0014280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchêne et al. (2011).Duchêne S, Archer FI, Vilstrup J, Caballero S, Morin PA. Mitogenome phylogenetics: the impact of using single regions and partitioning schemes on topology, substitution rate and divergence time estimation. PLOS ONE. 2011;6:e27138. doi: 10.1371/journal.pone.0027138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foottit et al. (2008).Foottit R, Maw H, Von Dohlen C, Hebert P. Species identification of aphids (Insecta: Hemiptera: Aphididae) through DNA barcodes. Molecular Ecology Resources. 2008;8:1189–1201. doi: 10.1111/j.1755-0998.2008.02297.x. [DOI] [PubMed] [Google Scholar]
- Hall (1999).Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for windows 95/98/NT. Nucleic Acids Symposium Series. 1999;41:95–98. [Google Scholar]
- Hassanin, Leger & Deutsch (2005).Hassanin A, Leger N, Deutsch J. Evidence for multiple reversals of asymmetric mutational constraints during the evolution of the mitochondrial genome of Metazoa, and consequences for phylogenetic inferences. Systematic Biology. 2005;54:277–298. doi: 10.1080/10635150590947843. [DOI] [PubMed] [Google Scholar]
- Havird & Santos (2014).Havird JC, Santos SR. Performance of single and concatenated sets of mitochondrial genes at inferring metazoan relationships relative to full mitogenome data. PLOS ONE. 2014;9:e84080. doi: 10.1371/journal.pone.0084080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert, Ratnasingham & Waard (2003).Hebert PD, Ratnasingham S, De Waard JR. Barcoding animal life: cytochrome c oxidase subunit 1 divergences among closely related species. Proceedings of the Royal Society of London B: Biological Sciences. 2003;270:S96–S99. doi: 10.1098/rsbl.2003.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinbo, Kato & Ito (2011).Jinbo U, Kato T, Ito M. Current progress in DNA barcoding and future implications for Entomology. Entomological Science. 2011;14:107–124. doi: 10.1111/j.1479-8298.2011.00449.x. [DOI] [Google Scholar]
- Jung, Duwal & Lee (2011).Jung S, Duwal RK, Lee S. COI barcoding of true bugs (Insecta, Heteroptera) Molecular Ecology Resources. 2011;11:266–270. doi: 10.1111/j.1755-0998.2010.02945.x. [DOI] [PubMed] [Google Scholar]
- Jung & Lee (2012).Jung S, Lee S. Molecular phylogeny of the plant bugs (Heteroptera: Miridae) and the evolution of feeding habits. Cladistics. 2012;28:50–79. doi: 10.1111/j.1096-0031.2011.00365.x. [DOI] [PubMed] [Google Scholar]
- Katoh & Standley (2013).Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution. 2013;30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubisz et al. (2012).Kubisz D, Kajtoch Ł, Mazur M, Rizun V. Molecular barcoding for central-eastern European Crioceris leaf-beetles (Coleoptera: Chrysomelidae) Open Life Sciences. 2012;7:69–76. [Google Scholar]
- Lanfear et al. (2012).Lanfear R, Calcott B, Ho SY, Guindon S. Partitionfinder: combined selection of partitioning schemes and substitution models for phylogenetic analyses. Molecular Biology and Evolution. 2012;29:1695–1701. doi: 10.1093/molbev/mss020. [DOI] [PubMed] [Google Scholar]
- Lee et al. (2013).Lee W, Park J, Lee GS, Lee S, Akimoto S. Taxonomic status of the Bemisia tabaci complex (Hemiptera: Aleyrodidae) and reassessment of the number of its constituent species. PLOS ONE. 2013;8:e63817. doi: 10.1371/journal.pone.0063817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levkanicova & Bocak (2009).Levkanicova Z, Bocak L. Identification of net-winged beetle larvae (Coleoptera: Lycidae) using three mtDNA fragments: a comparison of their utility. Systematic Entomology. 2009;34:210–221. doi: 10.1111/j.1365-3113.2008.00457.x. [DOI] [Google Scholar]
- Li et al. (2016).Li F, Zhang H, Wang W, Weng H, Meng Z. Complete mitochondrial genome of the Japanese pine sawyer, Monochamus alternatus (Coleoptera: Cerambycidae) Mitochondrial DNA Part A. 2016;27:1144–1145. doi: 10.3109/19401736.2014.936321. [DOI] [PubMed] [Google Scholar]
- Lu et al. (2010).Lu Y, Wu K, Jiang Y, Xia B, Li P, Feng H, Wyckhuys KA, Guo Y. Mirid bug outbreaks in multiple crops correlated with wide-scale adoption of Bt cotton in China. Science. 2010;328:1151–1154. doi: 10.1126/science.1187881. [DOI] [PubMed] [Google Scholar]
- Miller, Pfeiffer & Schwartz (2010).Miller MA, Pfeiffer W, Schwartz T. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. Gateway computing environments workshop (GCE), 2010; 2010. pp. 1–8. [Google Scholar]
- Monaghan et al. (2005).Monaghan MT, Balke M, Gregory TR, Vogler AP. DNA-based species delineation in tropical beetles using mitochondrial and nuclear markers. Philosophical Transactions of the Royal Society of London B: Biological Sciences. 2005;360:1925–1933. doi: 10.1098/rstb.2005.1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadimi, Daubois & Hijri (2016).Nadimi M, Daubois L, Hijri M. Mitochondrial comparative genomics and phylogenetic signal assessment of mtDNA among arbuscular mycorrhizal fungi. Molecular Phylogenetics and Evolution. 2016;98:74–83. doi: 10.1016/j.ympev.2016.01.009. [DOI] [PubMed] [Google Scholar]
- Okimoto et al. (1992).Okimoto R, Macfarlane J, Clary D, Wolstenholme D. The mitochondrial genomes of two nematodes, Caenorhabditis elegans and Ascaris suum. Genetics. 1992;130:471–498. doi: 10.1093/genetics/130.3.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park et al. (2011).Park DS, Foottit R, Maw E, Hebert PD. Barcoding bugs: DNA-based identification of the true bugs (Insecta: Hemiptera: Heteroptera) PLOS ONE. 2011;6:e18749. doi: 10.1371/journal.pone.0018749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park et al. (2010).Park DS, Leem YJ, Hahn K-W, Suh SJ, Hong KJ, Oh HW. Molecular identification of mealybugs (Hemiptera: Pseudococcidae) found on Korean pears. Journal of Economic Entomology. 2010;103:25–33. doi: 10.1603/EC09144. [DOI] [PubMed] [Google Scholar]
- Perna & Kocher (1995).Perna NT, Kocher TD. Patterns of nucleotide composition at fourfold degenerate sites of animal mitochondrial genomes. Journal of Molecular Evolution. 1995;41:353–358. doi: 10.1007/BF01215182. [DOI] [PubMed] [Google Scholar]
- Posada (2008).Posada D. jModelTest: phylogenetic model averaging. Molecular Biology and Evolution. 2008;25:1253–1256. doi: 10.1093/molbev/msn083. [DOI] [PubMed] [Google Scholar]
- Raupach et al. (2010).Raupach MJ, Astrin JJ, Hannig K, Peters MK, Stoeckle MY, Wägele JW. Molecular species identification of Central European ground beetles(Coleoptera: Carabidae) using nuclear rDNA expansion segments and DNA barcodes. Frontiers in Zoology. 2010;7:1–15. doi: 10.1186/1742-9994-7-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raupach et al. (2014).Raupach MJ, Hendrich L, Küchler SM, Deister F, Morinière J, Gossner MM. Building-up of a DNA barcode library for true bugs (insecta: hemiptera: heteroptera) of Germany reveals taxonomic uncertainties and surprises. PLOS ONE. 2014;9:e106940. doi: 10.1371/journal.pone.0106940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roehrdanz et al. (2016).Roehrdanz R, Cameron SL, Toutges M, Wichmann SS. The complete mitochondrial genome of the tarnished plant bug, Lygus lineolaris (Heteroptera: Miridae) Mitochondrial DNA Part A. 2016;27:48–49. doi: 10.3109/19401736.2013.869689. [DOI] [PubMed] [Google Scholar]
- Ronquist et al. (2012).Ronquist F, Teslenko M, Van der Mark P, Ayres DL, Darling A, Hohna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP. MrBayes 32: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology. 2012;61:539–542. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt & Sperling (2008).Schmidt BC, Sperling FA. Widespread decoupling of mtDNA variation and species integrity in Grammia tiger moths (Lepidoptera: Noctuidae) Systematic Entomology. 2008;33:613–634. doi: 10.1111/j.1365-3113.2008.00433.x. [DOI] [Google Scholar]
- Seixas, Paiva & Russo (2016).Seixas VC, Paiva PC, Russo CAM. Complete mitochondrial genomes are not necessarily more informative than individual mitochondrial genes to recover a well-established annelid phylogeny. Gene Reports. 2016;5:10–17. doi: 10.1016/j.genrep.2016.07.011. [DOI] [Google Scholar]
- Sheffield et al. (2008).Sheffield NC, Song H, Cameron SL, Whiting MF. A comparative analysis of mitochondrial genomes in Coleoptera (Arthropoda: Insecta) and genome descriptions of six new beetles. Molecular Biology and Evolution. 2008;25:2499–2509. doi: 10.1093/molbev/msn198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon et al. (2006).Simon C, Buckley TR, Frati F, Stewart JB, Beckenbach AT. Incorporating molecular evolution into phylogenetic analysis, and a new compilation of conserved polymerase chain reaction primers for animal mitochondrial DNA. Annual Review of Ecology, Evolution, and Systematics. 2006;37:545–579. doi: 10.1146/annurev.ecolsys.37.091305.110018. [DOI] [Google Scholar]
- Stamatakis (2014).Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura et al. (2013).Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Molecular Biology and Evolution. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al. (2015).Wang Y, Chen J, Jiang L-Y, Qiao GX. Hemipteran mitochondrial genomes: features, structures and implications for phylogeny. International Journal of Molecular Sciences. 2015;16:12382–12404. doi: 10.3390/ijms160612382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al. (2014a).Wang P, Li H, Wang Y, Zhang JH, Dai X, Chang J, Hu BW, Cai WZ. The mitochondrial genome of the plant bug Apolygus lucorum (Hemiptera: Miridae): presently known as the smallest in Heteroptera. Insect Science. 2014a;21:159–173. doi: 10.1111/1744-7917.12029. [DOI] [PubMed] [Google Scholar]
- Wang et al. (2014b).Wang Y, Li H, Wang P, Song F, Cai WZ. Comparative mitogenomics of plant bugs (Hemiptera: Miridae): identifying the AGG codon reassignments between Serine and Lysine. PLOS ONE. 2014b;9:e101375. doi: 10.1371/journal.pone.0101375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al. (2016a).Wang Y, Li H, Xun H, Cai W. Complete mitochondrial genome sequence of the plant bug Adelphocoris fasciaticollis (Hemiptera: Heteroptera: Miridae) Mitochondrial DNA Part A. 2016a;27:222–223. doi: 10.3109/19401736.2014.880898. [DOI] [PubMed] [Google Scholar]
- Wang et al. (2016b).Wang YT, Liu YX, Tong XL, Ren QP, Jiang GF. The complete mitochondrial genome of the longhorn beetle, Massicus raddei. Mitochondrial DNA Part A. 2016b;27:209–211. doi: 10.3109/19401736.2014.880892. [DOI] [PubMed] [Google Scholar]
- Wheeler (2001).Wheeler AG. Biology of the plant bugs (Hemiptera: Miridae): pests, predators, opportunists. Cornell University Press; New York: 2001. [Google Scholar]
- Wiemers & Fiedler (2007).Wiemers M, Fiedler K. Does the DNA barcoding gap exist?—a case study in blue butterflies (Lepidoptera: Lycaenidae) Frontiers in Zoology. 2007;4 doi: 10.1186/1742-9994-4-8. Article 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff et al. (2016).Wolff JN, Camus MF, Clancy DJ, Dowling DK. Complete mitochondrial genome sequences of thirteen globally sourced strains of fruit fly (Drosophila melanogaster) form a powerful model for mitochondrial research. Mitochondrial DNA Part A. 2016;27:4672–4674. doi: 10.3109/19401736.2015.1106496. [DOI] [PubMed] [Google Scholar]
- Xia (2013).Xia X. DAMBE5: a comprehensive software package for data analysis in molecular biology and evolution. Molecular Biology and Evolution. 2013;30:1720–1728. doi: 10.1093/molbev/mst064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye et al. (2015).Ye F, Liu T, Zhu W, You P. Complete mitochondrial genome of Whitmania laevis (Annelida, Hirudinea) and comparative analyses within Whitmania mitochondrial genomes. Belgian Journal of Zoology. 2015;145:115–129. [Google Scholar]
- Yu, Kong & Li (2016).Yu H, Kong L, Li Q. Evaluation of the efficacy of twelve mitochondrial protein-coding genes as barcodes for mollusk DNA barcoding. Mitochondrial DNA Part A. 2016;27:1336–1339. doi: 10.3109/19401736.2014.945579. [DOI] [PubMed] [Google Scholar]
- Yuan et al. (2015a).Yuan ML, Zhang QL, Guo ZL, Wang J, Shen YY. Comparative mitogenomic analysis of the superfamily Pentatomoidea (Insecta: Hemiptera: Heteroptera) and phylogenetic implications. BMC Genomics. 2015a;16:460. doi: 10.1186/s12864-015-1679-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan et al. (2015b).Yuan ML, Zhang QL, Guo ZL, Wang J, Shen YY. The complete mitochondrial genome of Corizus tetraspilus (Hemiptera: Rhopalidae) and phylogenetic analysis of Pentatomomorpha. PLOS ONE. 2015b;10:e0129003. doi: 10.1371/journal.pone.0129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan et al. (2016).Yuan ML, Zhang QL, Zhang L, Guo ZL, Liu YJ, Shen YY, Shao R. High-level phylogeny of the Coleoptera inferred with mitochondrial genome sequences. Molecular Phylogenetics and Evolution. 2016;104:99–111. doi: 10.1016/j.ympev.2016.08.002. [DOI] [PubMed] [Google Scholar]
- Zhang et al. (2016).Zhang B, Zhou MQ, Wang J, Pu Y, Zhang L, Yuan ML. Species checklist and research status of alfalfa insect pests reported in China. Pratacultural Science. 2016;33:785–812. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Numbers on branches are Bayesian posterior probabilities.
Numbers on branches are bootstrap support values.
Numbers on branches are Bayesian posterior probabilities (left) and Bootstrap values (right).
The best partitioning schemes and substitution models were selected by PartitionFinder for the P123 and P123RT datasets, and each of 13 PCGs. The best substitution models for rrnL, rrnS, 22 tRNAs and cox1-barcode sequences were determined by jModelTest.
Alignment of protein-coding genes, rRNA genes and tRNAs used in this study.
Mitochondrial genome sequences of five mirids sequenced in this study.
Data Availability Statement
The following information was supplied regarding data availability:
The raw data has been supplied as Supplementary Files.