Abstract
Background
Recent clinical trials indicate that probiotic administration in critical illness has potential to reduce nosocomial infections and improve clinical outcome. However, the mechanism(s) of probiotic-mediated protection against infection and sepsis remain elusive. We evaluated the effects of Lactobacillus rhamnosus GG (LGG) and Bifidobacterium longum (BL) on mortality, bacterial translocation, intestinal epithelial homeostasis and inflammatory response in experimental model of septic peritonitis.
Methods
Cecal ligation and puncture (n = 14 per group) or sham laparotomy (n = 8 per group) were performed on 3 week-old FVB/N weanling mice treated concomitantly with LGG, BL or vehicle (orally gavaged). At 24 h, blood and colonic tissue were collected. In survival studies, mice were given probiotics every 24 h for 7 days (LGG; n = 14, BL; n = 10 or vehicle; n = 13, shams; n = 3 per group).
Results
Probiotics significantly improved mortality following sepsis (92% vs. 57% mortality for LGG and 92% vs. 50% mortality for BL; p = 0.003). Bacteremia was markedly reduced in septic mice treated with either probiotic compared to vehicle treatment (4.39 ± 0.56 vs. 1.07 ± 1.54; p = 0.0001 for LGG; vs. 2.70 ± 1.89; p = 0.016 for BL; data are expressed as mean ± SD). Sepsis in untreated mice increased colonic apoptosis and reduced colonic proliferation. Probiotics significantly reduced markers of colonic apoptosis and returned colonic proliferation to sham levels. Probiotics led to significant reductions in systemic and colonic inflammatory cytokine expression versus septic animals. Our data suggest involvement of the protein kinase B pathway (via AKT) and downregulation of Toll-like receptor 2/Toll-like receptor 4 via MyD88 in the colon may play mechanistic roles in the observed probiotic benefits.
Conclusions
Our data demonstrate that probiotic administration at initiation of sepsis can improve survival in pediatric experimental sepsis. The mechanism of this protection involves prevention of systemic bacteremia, perhaps via improved intestinal epithelial homeostasis, and attenuation of the local and systemic inflammatory response.
INTRODUCTION
Sepsis is a common cause of death in children and adults despite advances in the supportive care of the critically ill. Centers for Disease Control data shows death rates from sepsis have increased at a rate greater than any other common cause of mortality in the last year and sepsis is now one of the top 10 causes of death in the United States1. Annually, over 1 million deaths worldwide are associated with sepsis within the pediatric and neonatal population alone2,3. Even when pediatric and adult patients survive, they face substantial long-term adverse consequences following sepsis and critical illness4–6.
Critical Illness and sepsis are systemic syndromes that lead to a hostile environment in the gut, resulting in an imbalance of the intestinal microbiota in favor of pathogenic species7. The intestine plays a central role in the pathogenesis of sepsis and has been referred to as the “motor” of the systemic inflammatory response8,9. Perturbations of intestinal epithelial homeostasis in sepsis result in increased proinflammatory cytokine production10, barrier dysfunction11–13, and increased apoptosis14–17 which may lead to multiple organ failure.
Probiotic therapy represents a promising intervention for the treatment of nosocomial infections in the intensive care unit, which often lead to sepsis and multiple organ failure18. Probiotics are living nonpathogenic bacteria that colonize the intestine and provide a benefit to the host19. Results from recent randomized controlled trials in pediatric and adult populations suggest a benefit to the use of probiotics in the intensive care unit20–23. In an age of increasing antimicrobial resistance, the use of probiotics as a potential treatment for severe infections and subsequent septic shock may be a promising, cost-effective, preventative strategy.
The potential beneficial role(s) of probiotics on survival given in early systemic polymicrobial sepsis and the mechanisms by which probiotics may function to protect against experimental sepsis, particularly pediatric sepsis, are currently not well understood. Therefore, we sought to delineate the clinical utility and potential mechanistic targets of acutely administered probiotic therapy at initiation of experimental pediatric sepsis via a weanling mouse peritonitis model. We selected the two commonly clinically utilized probiotic strains, Lactobacillus rhamnosus GG (LGG) and Bifidobacterium longum (BL), given at clinically relevant doses, to evaluate possible strain-related differences in clinical outcome and mechanistic targets in the gut.
Lactobacillus spp. and Bifidobacterium spp., alone or in combination, are the most frequently used probiotic strains in the treatment of various gastrointestinal disorders24–26 or as therapy for clinical conditions including antibiotic-associated diarrhea27,28, ventilator-associated pneumonia21,22,29, sepsis and postoperative infections30,31. Although probiotics appear to be an effective treatment in various clinical conditions, the specific mechanisms responsible for their beneficial action are complex and not fully understood32. Based on the results from several in vivo and in vitro studies, we hypothesized that LGG and BL would prevent bacterial translocation33,34, reduce the overgrowth of pathogenic bacteria, decrease apoptosis in intestinal epithelial cells35–38 and reduce inflammation39–42.
MATERIALS AND METHODS
Probiotic treatment and septic peritonitis model
The animal protocol used in these studies was approved by the Institutional Animal Care and Use Committee of the University of Colorado Anschutz Medical Campus. Briefly, three week old FVB/N mice were orally gavaged with 200 μl of either LGG (1 × 109 colony forming unit (CFU)/ml), BL (1 × 107 CFU/ml), or sterile water (vehicle) immediately prior to initiation of the cecal ligation and puncture (CLP) procedure43. Briefly, a small midline abdominal incision was made, the cecum was ligated just distal to the ileocecal valve, and was then punctured twice with a 23-gauge needle. The cecum was squeezed to extrude a small amount of stool, replaced in the abdomen, and the peritoneum and skin were closed in layers. Sham mice were treated identically except the cecum was neither ligated nor punctured. All mice received 1.0 ml normal saline subcutaneously after the surgery to compensate for fluid loss. Animals were euthanized at either 24 h (for acute studies) or followed 7 days for survival (LGG; n = 14, BL; n = 10 or vehicle; n = 13, shams; n = 3 per group). For survival studies, mice were treated with probiotics daily for 7 days. For acute studies, mice received a single dose of probiotics prior to tissue collection.
Lactobacillus rhamnosus GG and Bifidobacterium longum culture
LGG (ATCC, Manassas, VA) was incubated in de Man, Rogosa and Sharpe broth (Becton Dickinson, Sparks, MD) for 24 h at 37°C and 5% CO2. BL (ATCC, Manassas, VA) was cultured in Trypticase soy broth (Becton Dickinson) for 72 h in an anaerobic chamber at 37°C. A600 was measured to determine the number of CFU per 1ml. BL and LGG were pelleted from the broth (10,000 rpm; 10 min) and resuspended in distilled water.
Bacteremia and bacterial analysis of the colon
Blood was collected at 24 h from the inferior vena cava of anesthetized mice, serially diluted in sterile 0.9% saline and cultured on Trypticase soy agar plates with 5% sheep blood (Becton Dickinson) for 24 h at 37°C/5% CO2. CFU were then enumerated for each animal (Shams n = 8 per group; Septic n = 10; LGG n = 7; BL n = 8).
DNA was extracted from collected frozen colon samples obtained from a separate study to ensure sufficient amount of tissue (Shams n = 4 per group; Septic n = 12; LGG n = 10; BL n = 9) (luminal and intestinal wall contents; 24-h time point) using UltraClean Fecal DNA kit (MO BIO Laboratories, Inc., Carlsbad, CA). The concentration, integrity and purity of DNA were determined using a NanoDrop (Thermo Fisher Scientific, Wilmington, DE). Quantification was performed by quantitative polymerase chain reaction (PCR) using standard curves derived from cloned 16S ribosomal ribonucleic acid genes44 using the following primers: Lactobacillus45: LactoF (5′ TGGAAACAGRTGCTAATACCC) and LactoR (5′ GYCCATTGTGGAAGATTCCC). Bifidobacterium46: Bif1F (5′ TCG CGT CYG GTG TGA AAG) and Bif1R (5′ CCA CAT CCA GCR TCC AC). The following cycling protocol was used: denaturation at 95°C (10 min) and 40 cycles of 95°C (15 s), 60°C (30 s), 65°C (1 min). Reporter dye emission (SYBR green) was detected by an automated sequence detector combined with ABI Prism 7300 Real Time PCR System (Applied Biosystems, Foster City, CA).
Gram staining was performed on tissue sections and evaluated by microscopy for presence of gram positive or gram negative bacteria.
Immunohistology
Colon was collected from each animal at 24 h and fixed overnight in 10% formalin, paraffin-embedded, and sectioned at 4–6 μm. Serial sections were stained (Shams n = 5 per group; Septic n = 5; LGG n = 4; BL n = 4). After deparaffinization and rehydration, sections were blocked with 1.5% rabbit or goat serum (Vector Laboratories, Burlingame, CA) in phosphate-buffered saline for 30 min, then incubated with either rabbit polyclonal cleaved caspase-3 (1:100; Cell Signaling, Danvers, MA) or mouse monoclonal proliferating cell nuclear antigen (1:100; Invitrogen, Camarillo, CA) antibody for 1 h, washed with phosphate-buffered saline, and incubated with either goat anti-rabbit or anti-mouse biotinylated secondary antibody (Vector Laboratories) for 30 min. Vectastain Elite ABC reagent (Vector Laboratories) was then applied, followed by diaminobenzidine as substrate. Sections were counterstained with hematoxylin, dehydrated and cover-slipped.
To determine apoptosis in the colonic epithelium, apoptotic epithelial cells were quantified in 100 consecutive crypts by using two complimentary methods: morphological analysis of hematoxylin & eosin stained sections where apoptotic cells were identified by characteristic morphology of nuclear fragmentation (karyorrhexis) and cell shrinkage with condensed nuclei (pyknosis)47; and second, by enumeration of cleaved caspase-3 positive cells. Colonic epithelial proliferation was determined by quantifying proliferating cell nuclear antigen-positive cells in 100 consecutive crypts. All counting was performed by a blinded evaluator.
RNA Preparation, RT, and Real-Time PCR
Total RNA was isolated from colonic tissue (snap frozen in liquid nitrogen, collected at 24 h) using the RNeasy Plus Mini Kit (Qiagen, Santa Clarita, CA) as described in the manufacturer’s protocol. RNA concentrations were quantified at 260 nm, and the purity and integrity were determined using a NanoDrop. Reverse transcription and real-time PCR assays were performed to quantify steady-state messenger RNA levels of interleukin (IL)-6, tumor necrosis factor-α (TNF-α), IL-1β, IL-10, MyD88, Toll-like receptor (TLR) -4, and TLR-2 (Shams n = 4 per group; Septic n = 7; LGG n = 6; BL n = 4). Complementary DNA was synthesized from 0.2 μg of total RNA. Predeveloped TaqMan primers and probes (Applied Biosystems) were used for detection. Reporter dye emission was detected by an automated sequence detector combined with ABI Prism 7300 Real Time PCR System (Applied Biosystems). Real-time PCR quantification was performed with TaqMan GAPDH controls.
Western Blot analysis
Individual frozen colon samples (Shams n = 3 per group; Septic n = 8; LGG n = 4; BL n = 4) (24-h time point) were homogenized with a hand-held homogenizer in a 5× volume of ice-cold homogenization buffer (50 mM Tris HCl, pH 7.4; 100 mM NaCl; 10 mM EDTA; 0.5% Triton X-100) with added protease inhibitors (Roche Diagnostics, Mannheim, Germany). The homogenates were centrifuged at 10,000 rpm for 5 min at 4°C and the supernatant was collected. Total protein concentration was quantified using the Bradford protein assay48. For protein analysis, 40 μg of protein was added to an equal volume of 2× Laemmli sample buffer and boiled for 5 min. The samples were run on 4–15% polyacrylamide gels (Bio-Rad Laboratories, Hercules, CA) at 200 V for 30 min. Protein was transferred to Immuno-Blot PVDF membranes (Bio-Rad Laboratories) at 65V for 4 hours. Membranes were blocked with 5% nonfat milk in Tris-buffered saline with 0.1% Tween 20 (Sigma-Aldrich, St. Louis, MO) for 1 h at room temperature and then incubated overnight at 4°C with one of the following rabbit polyclonal antibodies: Bax (Cell Signaling; 1:1,000), Bcl-w (Cell Signaling; 1:1,000), anti-Akt, (Cell Signaling; 1:1,000), anti-P-Akt (Cell Signaling; 1:1,000). After extensive washing, the membranes were incubated for 1 h at room temperature with horseradish peroxidase-conjugated anti-rabbit immunoglobulin G (Santa Cruz Biotechnology, Dallas, TX). Proteins were visualized with a chemiluminescent system (Pierce, Rockford, IL) by using Epi Chemi II Darkroom (UVP BioImaging System, Upland, CA).
Serum IL-6 and TNF-α analysis
Enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN) was used to determine the concentrations of TNF-α and IL-6 in serum (Shams n = 5 per group; Septic n = 7; LGG n = 5; BL n = 6) (24-h time point) according to the manufacturer’s instructions. Serum was collected after centrifuging blood for 10 min at 5,000 rpm and stored at −80°C until the assay was performed.
Statistics
Continuous data sets were tested for Gaussian distribution by using a Shapiro-Wilks Test for normality, and the Levene’s F-Test for equality of variances. Although results of these tests showed some departures from normality and equality of variances, it is well documented that the one-way ANOVA is robust with respect to violations of these assumptions. Thus, multiple group comparisons were performed with one-way analysis of variance followed by the Newman-Keuls post-hoc test. Survival studies were analyzed via the log-rank test. No measurements or animals were lost to observation or missing in the analysis. Data were analyzed using Prism 4.0 (GraphPad Software, San Diego, CA) and reported as means ± SD. A p value ≤ 0.05 was considered to be statistically significant.
RESULTS
Lactobacillus rhamnosus GG and Bifidobacterium longum improve mortality and prevent bacteremia in septic peritonitis
To determine if treatment with Lactobacillus rhamnosus GG or Bifidobacterium longum have an effect on mortality in peritonitis-induced sepsis, a separate cohort of mice (Shams n = 3 per group; Septic n = 13; LGG n = 14, BL n = 10) were subjected to CLP and followed 7 days for survival (fig. 1). Mice treated with either LGG or BL had significantly improved 7-day survival compared to untreated septic mice. All sham animals survived.
Figure 1. Effect of Lactobacillus rhamnosus GG and Bifidobacterium longum on mortality in sepsis.
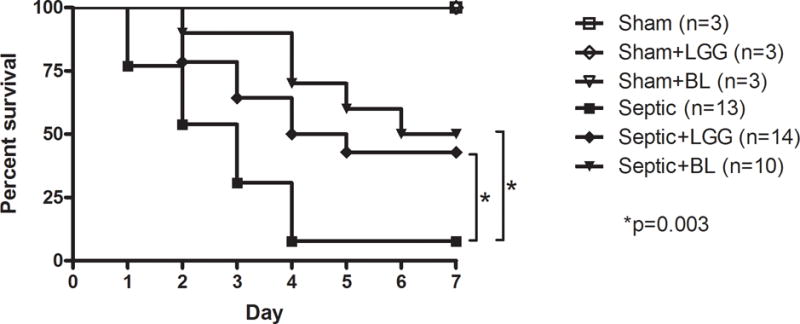
3 week old FVBN mice were subjected to 2 × 23-gauge cecal ligation and puncture. Control animals underwent sham laparotomy. All mice were followed for survival for 7 days. Septic mice treated with Lactobacillus rhamnosus GG (LGG) or Bifidobacterium longum (BL) had significantly decreased mortality compared to untreated septic mice (57% vs. 92% mortality and 50% vs. 92% mortality, respectively; p = 0.003). Shams n = 3 per group; Septic n = 13; LGG n = 14, BL n = 10. All sham mice survived.
To examine whether the probiotics LGG or BL could prevent bacteremia in sepsis, whole blood was cultured and bacteria were counted. The data are expressed as the Log of CFU per 1ml (fig. 2A–C). Septic animals had significantly increased bacteremia compared to shams. Although both probiotic strains reduced bacteremia, septic mice treated with LGG exhibited normalization to sham mouse blood bacterial counts while septic mice treated with BL exhibited significantly decreased bacteremia compared to untreated septic mice, but still had an increased bacterial load in the blood compared to shams.
Figure 2. Effect of Lactobacillus rhamnosus GG and Bifidobacterium longum treatment on bacteremia in sepsis.

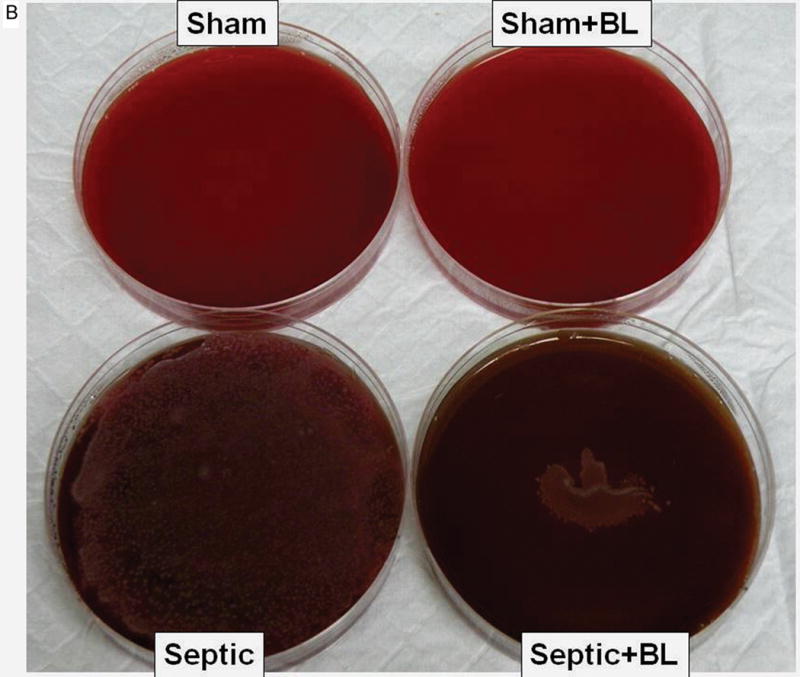
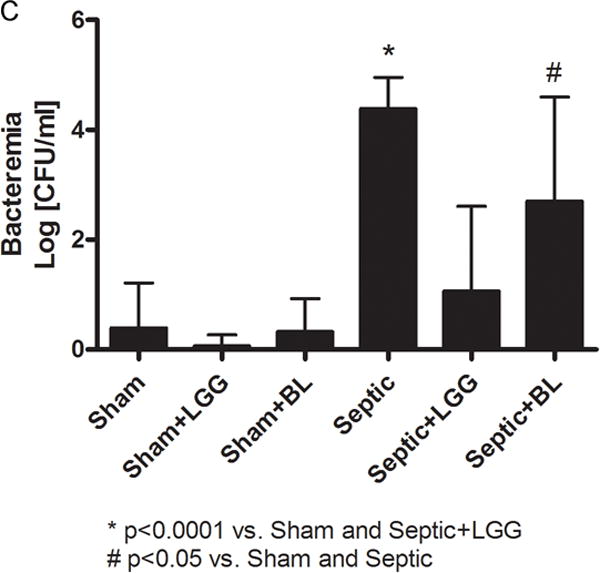
Bacterial growth on representative Trypticase soy agar plates with sheep blood (5%) are shown for each experimental group (A, B). Septic mice had increased bacteremia (C) compared to shams (4.39 ± 0.56 vs. 0.40 ± 0.81; p = 0.0001), while septic mice treated with Lactobacillus rhamnosus GG (LGG) or Bifidobacterium longum (BL) had reduced bacterial load in the blood (1.07 ± 1.54; p = 0.0001 and 2.70 ± 1.89; p = 0.016, respectively). Shams n = 8 per group; Septic n = 10; LGG n = 7; BL n = 8. Data are expressed as the mean ± SD.
Following oral administration, LGG and BL persist in the intestine for at least 24 h
DNA isolated from the colon, including luminal and intestinal wall content, was analyzed by PCR to quantify the presence of Lactobacillus spp. and Bifidobacterium spp. There was a significant increase of Lactobacilli in septic animals treated with LGG compared to untreated septic mice. Similarly, there was an increase of Bifidobacteria in septic mice treated with BL compared to untreated septic mice (table 1). These data demonstrate that LGG and BL are indeed able to survive and propagate in the gastrointestinal tract for at least 24 h following oral administration. Therefore, LGG and BL may help to prevent the overgrowth of pathogenic bacteria. This hypothesis was confirmed by Gram staining of intestinal wall specimens, which demonstrated an increased number of both Gram positive and Gram negative bacteria in the colon of septic mice compared to shams, and most importantly, less bacteria is seen in the colons of mice treated with either LGG or BL (fig. 3).
Table 1.
Lactobacillus and Bifidobacterium spp in the Colon of Septic Mice1
| Sham (n = 4) |
Sham + LGG (n = 4) |
Sham + BL (n = 4) |
Septic (n = 12) |
Septic + LGG (n = 10) |
Septic + BL (n = 9) |
|
|---|---|---|---|---|---|---|
| Lactobacillus spp. | 6.6×103±2.5×103 | 1.1×104±1.3×104 | – | 6.2×103±6.6×103 | 3.5×104±4.1×104 * | – |
| Bifidobacterium spp. | 4.2×105±1.1×105 | – | 1.7×106±2.4×106 | 2.0×106±1.5×106 | – | 2.4×107±2.9×107 * |
Polymerase chain reaction (PCR) quantification data are expressed as the mean of cells ± SD.
p < 0.05 versus septic.
Figure 3. Gram staining of colonic tissue.
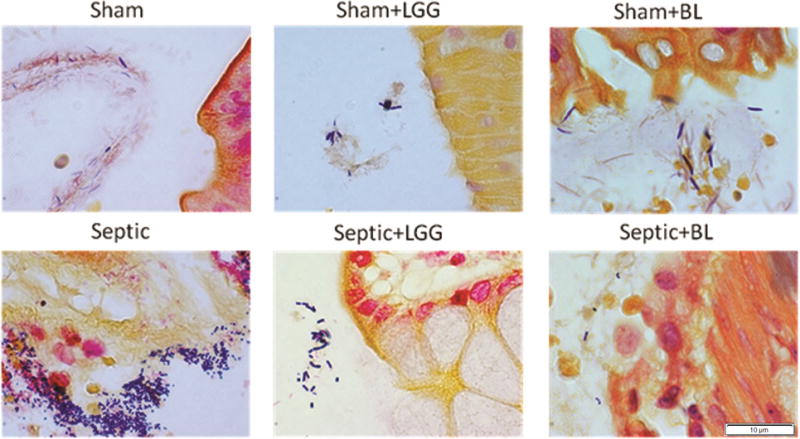
Increased numbers of Gram positive and Gram negative bacteria in the colon of septic mice compared to shams and mice treated with either Lactobacillus rhamnosus GG (LGG) or Bifidobacterium longum (BL).
Cell proliferation and apoptosis in colon are normalized in septic mice treated with probiotics
As measured by quantifying proliferating cell nuclear antigen positive cells in 100 consecutive crypts, septic mice exhibited a significant decrease in proliferation of the colonic epithelium compared to sham mice. In contrast, the proliferative response in septic animals treated with either probiotic strain was normalized to levels observed in sham mice (fig. 4).
Figure 4. Effect of Lactobacillus rhamnosus GG and Bifidobacterium longum treatment on colonic proliferation in sepsis.
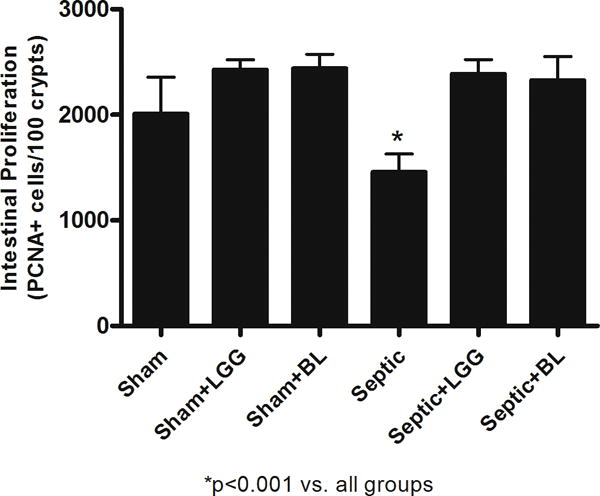
Colonic epithelial proliferation was determined by quantifying proliferating cell nuclear antigen-positive cells in 100 consecutive crypts. Septic mice had a significantly decreased number of proliferating cells compared to shams (1,456 ± 174 vs. 2,010 ± 347, p = 0.0128); whereas septic mice treated with Lactobacillus rhamnosus GG (LGG) or Bifidobacterium longum (BL) showed normalization to sham levels (LGG: 2387 ± 137, p = 0.0001; BL: 2,326 ± 224 vs. septic 1,456 ± 174, p = 0.0003). Shams n = 5 per group; Septic n = 5; LGG n = 4; BL n = 4. Data are expressed as the mean ± SD.
Colonic epithelial apoptosis was increased in untreated septic mice compared to shams, both when assayed by cleaved caspase-3 staining and also by morphological criteria in hematoxylin & eosin stained sections. In contrast, septic mice treated with either LGG or BL exhibited decreased colonic apoptosis, with levels similar to those seen in the colons of sham mice (fig. 5A–D). The ratio of proapoptotic to antiapoptotic molecules is often used as an indicator of sensitivity to apoptosis49. Untreated septic mice exhibited significantly increased ratio of Bax/Bcl-w protein expression compared to shams, suggesting a shift towards increased programmed cell death. In contrast, septic mice treated with either LGG or BL exhibited a decrease in the ratio of Bax/Bcl-w compared to untreated septic mice, suggesting a shift towards increased cell survival (fig. 5E).
Figure 5. Effect of Lactobacillus rhamnosus GG and Bifidobacterium longum treatment on colonic epithelial apoptosis.
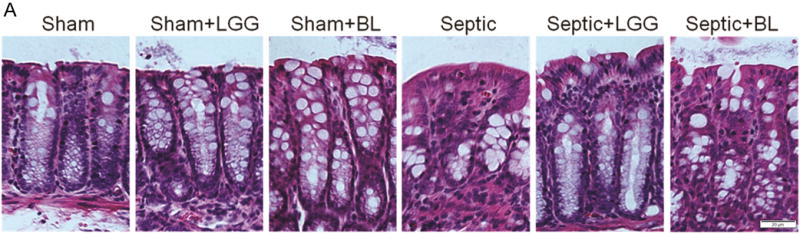

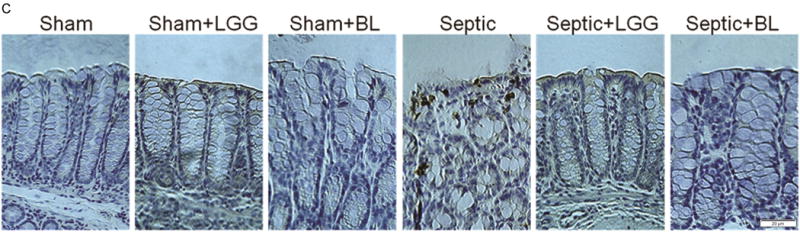
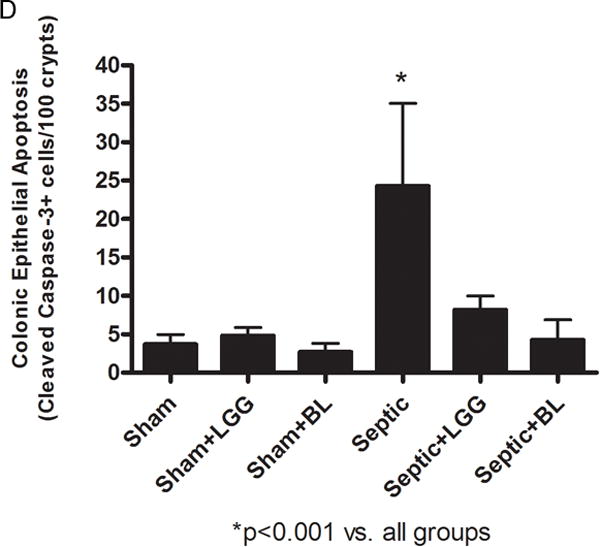

To determine apoptosis in the colonic epithelium, apoptotic epithelial cells were quantified in 100 consecutive crypts by using two complimentary methods: morphological analysis of hematoxylin & eosin (H&E) stained sections (A, B) and enumeration of cleaved caspase-3 (CC3) positive cells (C, D). Both methods showed significantly increased colonic apoptosis in untreated septic mice compared to controls (CC3: 24.30 ± 10.74 vs. shams 3.67 ± 1.32, p = 0.0001; H&E: 27.30 ± 8.86 vs. shams 4.78 ± 2.17, p = 0.0001). Lactobacillus rhamnosus GG (LGG) or Bifidobacterium longum (BL) treatment normalized apoptosis to levels seen in sham mice (for CC3, LGG: 8.20 ± 1.79 vs. septic 24.30 ± 10.74, p = 0.006; BL: 4.25 ± 2.63 vs. septic 24.30 ± 10.74, p = 0.0036 and for H&E; LGG: 7.11 ± 2.93 vs. septic 27.30 ± 8.86, p = 0.0001; BL: 8.25 ± 2.63 vs. septic 27.30 ± 8.86, p = 0.0014). Shams n = 5 per group; Septic n = 5; LGG n = 4; BL n = 4. (E) The ratio between proapoptotic Bax and antiapoptotic Bcl-w protein expression analyzed by Western blot showed shift towards cell death in untreated septic mice (septic 7.73 ± 6.84 vs. shams 1.00 ± 0.52, p = 0.036), whereas septic mice treated with LLG or BL exhibited a shift towards cell survival (LGG: 0.147 ± 0.049 vs. septic 7.73 ± 6.84, p = 0.048; BL: 0.602 ± 0.24 vs. septic 7.73 ± 6.84, p = 0.050). Shams n = 3 per group; Septic n = 8; LGG n = 4; BL n = 4. Data are expressed as the mean ± SD.
Increased p-Akt/Akt ratio in probiotic treated animals suggests involvement of protein kinase B pathway
Previously published data by Yan et al.38 showed that LGG promotes survival of intestinal epithelial cells through regulation of the anti-apoptotic Akt/protein kinase B signal transduction pathway. Likewise, our results show a significant increase of anti-apoptotic Akt in the colonic tissue of both probiotic groups, LGG and BL, compared to the untreated septic group (fig. 6A–B). This indicates a possible involvement of the pathway in the protective mechanism of both tested probiotic strains against sepsis.
Figure 6. Effect of Lactobacillus rhamnosus GG and Bifidobacterium longum treatment on expression of P-Akt/Akt.
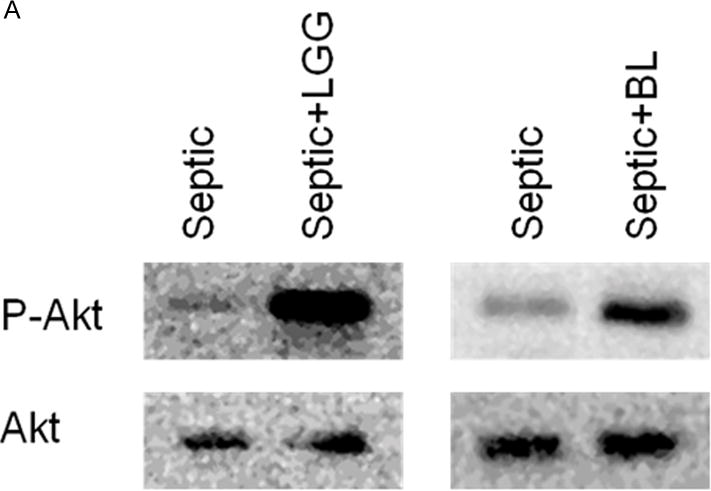
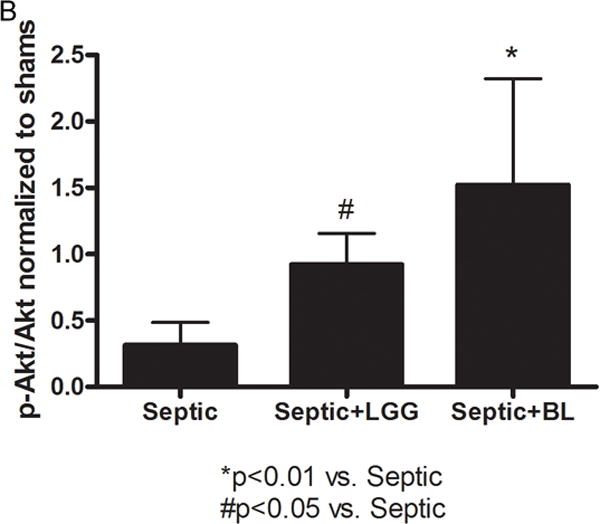
Representative Western blot for expression of p-AKT and Akt in the colon of all studied groups (A). P-Akt/Akt ratio for septic and probiotics treated septic mice normalized to shams (Lactobacillus rhamnosus GG (LGG) 0.922 ± 0.24 vs. septic 0.3159 ± 0.17, p = 0.0004 and Bifidobacterium longum (BL) 1.521 ± 0.80 vs. septic 0.3159 ± 0.17, p = 0.0017 (B). Shams n = 3 per group; Septic n = 8; LGG n = 4; BL n = 4. Data are expressed as the mean ± SD.
Lactobacillus rhamnosus GG and Bifidobacterium longum attenuate the systemic and local inflammatory response in the colon during sepsis
Reduction of inflammation as well as improvement of innate imunity are hypothesized to be protective mechanisms following probiotic administration39–42. To determine the effect of probiotics on these parameters, serum and colonic tissue levels of the proinflammatory cytokines IL-6 and TNF-α were measured by ELISA. In parallel, gene expression of IL-6, TNF-α, IL-1β and IL-10 in the colon was quantified using Real-Time PCR. All cytokine levels were measured 24 h after CLP.
Systemic levels of IL-6 signficantly increased in untreated septic mice compared to shams (fig. 7), while treatment with LGG or BL in septic mice led to significantly reduced systemic IL-6 levels compared to septic mice not treated with probiotics. Interestingly, systemic levels of TNF-α remained unchanged among all studied groups.
Figure 7. Effect of Lactobacillus rhamnosus GG and Bifidobacterium longum treatment on the systemic inflammatory response.
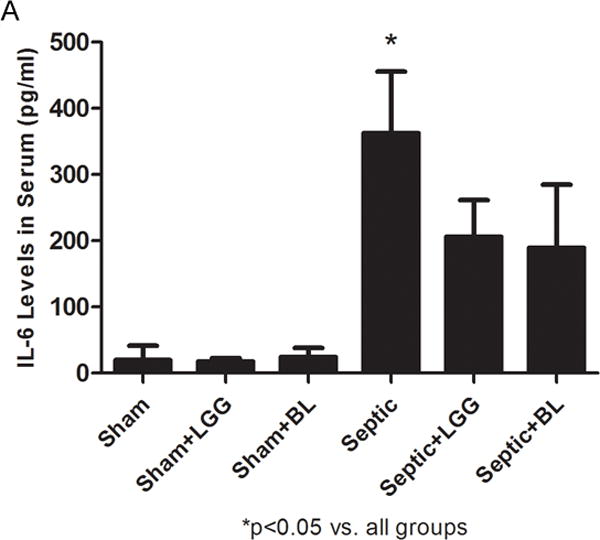
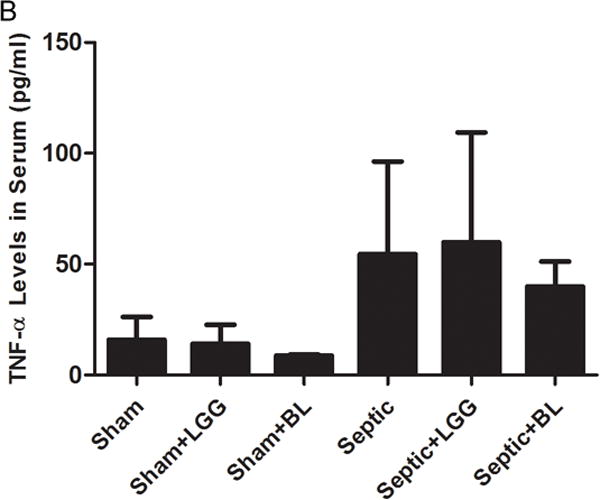
Enzyme-linked immunosorbent assay was used to determine the concentrations of TNF-α and IL-6 in serum. (A) IL-6 was significantly elevated in the serum of septic mice compared to shams (septic 383.5 ± 93.1 vs. shams 19.8 ± 21.4, p = 0.0001), while treatment with Lactobacillus rhamnosus GG (LGG) or Bifidobacterium longum (BL) in septic mice led to significantly reduced systemic IL-6 levels (LGG 206.1 ± 55.2 vs. septic 383.5 ± 93.1, p = 0.0075 and BL 189.4 ± 95.2 vs. septic 383.5 ± 93.1, p = 0.0069) compared to septic mice not treated with probiotics. (B) There were no differences between groups for TNF-α in the serum. Shams n = 5 per group; Septic n = 7; LGG n = 5; BL n = 6. Data are expressed as the mean ± SD.
Local, colonic gene expression of TNF-α, IL-1β and IL-10 (fig. 8A–C) was significantly increased in the colon of untreated septic animals, while levels of both were markedly decreased in colons of septic mice treated with LGG, or BL when compared to untreated septic mice. Colonic gene expression of IL-6 was increased in untreated septic mice and decreased in both probiotic treated groups although the differences did not reach statistical significance (data not shown).
Figure 8. Effect of Lactobacillus rhamnosus GG and Bifidobacterium longum treatment on the inflammatory response in the colon.
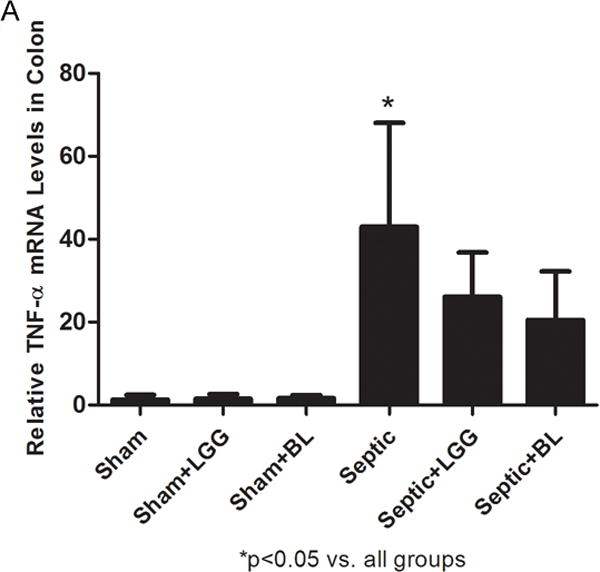


Reverse transcription and real-time polymerase chain reaction (PCR) assays were performed to quantify steady-state messenger RNA (mRNA) levels of TNF-α, IL-1β and IL-10. Gene expression of TNF-α (A), IL-1β (B) and IL-10 (C) was significantly increased in the colon of untreated septic animals (TNF-α: septic 47.02 ± 25.02 vs. shams 1.31 ± 1.11, p = 0.019; IL-1β: septic 125.82 ± 43.10 vs. shams 1.10 ± 0.57, p = 0.003; IL-10: septic 9303.45 ± 5926.29 vs. shams 1.31 ± 1.18, p = 0.024), while levels of both were markedly decreased in colons of septic mice treated with Lactobacillus rhamnosus GG (LGG) (TNF-α: 26.09 ± 10.68 vs. septic 47.02 ± 25.02, p = 0.049; IL-1β: 62.53 ± 41.46 vs. septic 125.82 ± 43.10, p = 0.050; IL-10: 2766.34 ± 1038.60 vs. septic 9303.45 ± 5926.29, p = 0.024), or Bifidobacterium longum (BL) (TNF-α: 20.50 ± 11.72 vs. septic 47.02 ± 25.02, p = 0.027; IL-1β: 49.67 ± 30.91 vs. septic 125.82 ± 43.10, p = 0.018; IL-10: 1689.26 ± 2200.26 vs. septic 9303.45 ± 5926.29, p = 0.008) when compared to untreated septic mice. Shams n = 4 per group; Septic n = 7; LGG n = 6; BL n = 4. Data are expressed as the mean ± SD.
Probiotics activate TLR pathways in the colon
TLRs signal through the MyD88 pathway that includes NFκB transcriptional factors activating various cytokines involved in the innate immunity response. MyD88 has an important role in early recruitment of inflammatory cells and in the control of bacterial infection50. Expression of the TLR-2, TLR-4 and MyD88 genes were markedly elevated in the colon of untreated septic mice compared to shams treatment significantly reduced these levels (fig. 9A–C).
Figure 9. Effect of Lactobacillus rhamnosus GG and Bifidobacterium longum treatment on Toll-like receptors and MyD88 gene expression in the colon.

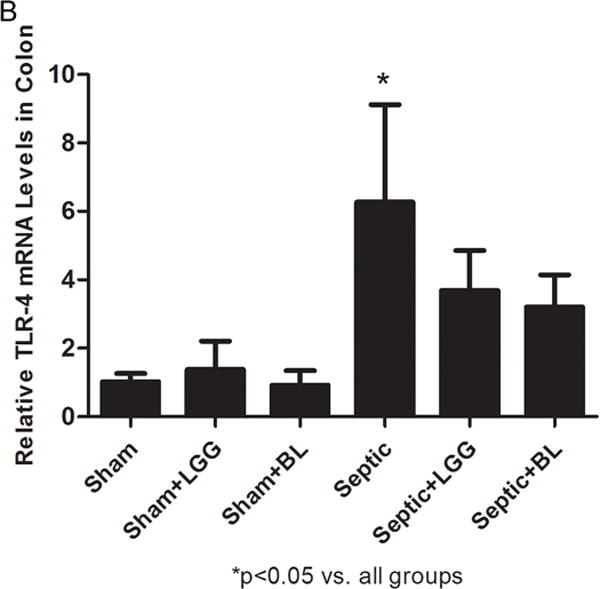
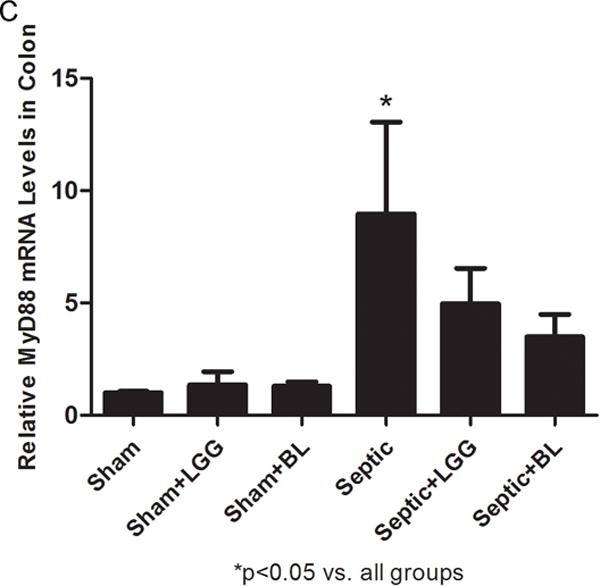
Reverse transcription and real-time polymerase chain reaction (PCR) assays were performed to quantify steady-state messenger RNA (mRNA) levels of toll-like receptor (TLR)-2 (A), TLR-4 (B) and MyD88 (C). Levels of TLR-2 (A), TLR-4 (B) and MyD88 (C) were markedly elevated in the colon of untreated septic mice compared to shams (TLR-2: 8.94 ± 7.11 vs. 1.04 ± 0.36, p = 0.041; TLR-4: 6.78 ± 2.85 vs. 1.02 ± 0.24, p = 0.015; MyD88: 7.85 ± 4.09 vs. 1.00 ± 0.07, p = 0.012), while Lactobacillus rhamnosus GG (LGG) (TLR-2: 4.37 ± 2.47 vs. 8.94 ± 7.11, p = 0.039; TLR-4: 3.69 ± 1.17 vs. 6.78 ± 2.85, p = 0.047; MyD88: 4.97 ± 1.57 vs. 7.85 ± 4.09, p = 0.046) or Bifidobacterium longum (BL) (TLR-2: 2.75 ± 1.11 vs. 8.94 ± 7.11, p = 0.023; TLR-4: 3.20 ± 0.94 vs. 6.78 ± 2.85, p = 0.029; MyD88: 3.50 ± 1.00 vs. 7.85 ± 4.09, p = 0.016) treatment significantly reduced these levels. Shams n = 4 per group; Septic n = 7; LGG n = 6; BL n = 4. Data are expressed as the mean ± SD.
DISCUSSION
This study demonstrates for the first time that two different probiotic strains, Lactobacillus rhamnosus GG and Bifidobacterium longum, confer a significant survival benefit in weanling mice subjected to septic peritonitis. To our knowledge, this is the first description of improved survival following live probiotic therapy in a pediatric or adult polymicrobial sepsis model. This survival advantage was associated with decreased bacteremia, decreased colonic apoptosis and increased colonic proliferation, decreased systemic and local expression of inflammatory cytokines, and reduced colonic expression of TLR-2/TLR-4 and MyD88.
Critical illness and its treatments (vasopressors, antibiotics, opiates, etc.) create a hostile environment in the gut, alter the microbiota, and thereby favor the growth of pathogens51. This is partially due to the loss of the beneficial lactic acid bacteria52, that can inhibit the overgrowth of pathogens by production of bacteriocins, hydrogen peroxide, organic acids, ammonia and by increased competition for adhesion sites on intestinal epithelia53,54. In critical illness, enhanced virulence gene expression in bacteria, called quorum sensing, leads to aggressive bacterial behavior, toxin expression and ultimately translocation into the gut wall and/or gut barrier dysfunction. This impairment of the gut barrier can lead to gut-derived sepsis, progression to organ failure and ultimately mortality in the critically ill51. Interestingly, the administration of beneficial probiotic organisms has been shown to enhance gut epithelial resistance to injury in in vitro models51. Consistent with these previous findings, our data demonstrate statistically significant reduction of bacteremia, improvement of colonic epithelial homeostasis, and enhancement of survival in animals treated with either probiotic strain compared to the untreated septic animals.
Existing clinical trials indicate that currently used probiotic strains may not be administered early enough to optimize benefits on prevention and therapeutic efficacy against severe infections and sepsis55–58. In our mouse model of sepsis, probiotics were administered early, at onset of infection, to better imitate the clinical setting of early treatment to prevent progressive infection and sepsis (i.e., prior to or at onset of nosocomial pneumonia or post-operative abdominal sepsis).
The observed overgrowth of potentially pathogenic bacteria has been shown to cause intestinal cell apoptosis and disruption of epithelial tight junction permeability59. As previously shown, intestinal proliferation and intestinal epithelial apoptosis are altered in the CLP sepsis model14. Our data are consistent with previous reports of reduced apoptosis with probiotics in intestinal injury35,37.
TLRs play a central role in the initiation of innate immune responses and in the development of subsequent adaptive immune responses to microbial pathogens60. In existing data from murine CLP models, TLR-2 and TLR-4 expression are significantly upregulated in multiple organs61,62 including the intestine63 when compared to sham mice. Our data are consistent with the hypothesis that downregulation of TLR-2/TLR-4 via MyD88 in the colon of Lactobacillus rhamnosus GG or Bifidobacterium longum treated mice may play a protective role in attenuating the local and systemic inflammatory response and ultimately the pathophysiology of polymicrobial sepsis.
Limitations of this research include that we did not administer antibiotics as part of this initial evaluation of early probiotics to prevent progression of infection and sepsis. This was to ensure the adequate colonization of our probiotic therapy and we plan to do future studies administering antibiotics following treatment with probiotics. Furthermore, time point studies are being planned currently to examine the effects of both probiotic strains more closely and to explore other possible pathways involved in their protective mechanisms in sepsis. It is important to note that previous clinical trials that have administered antibiotics with probiotic therapy have still noted significant reduction of nosocomial infections (such as ventilator pneumonia) despite antibiotic therapy concomitant with probiotic therapy64. Further, killed probiotic organisms have been shown to be as clinically effective in some studies as live probiotic therapy65. Finally, in our statistical analysis we did not adjust our analysis for multiplicity (i.e., multiple comparisons). Given our small sample sizes, and that these are the first experiments of this kind reported, such adjustments would decrease the statistical power of our tests. This would lead to an increase in the probability of making a type II error, i.e., the failure to reject a false null hypothesis. In any experimental setting, there is always a balance in type I and type II errors. Presently, for this line of research, we are more concerned with missing a potentially valuable finding that we hope will lead to further investigation.
In conclusion, our data show that early therapy with either Lactobacillus rhamnosus GG or Bifidobacterium longum can reduce mortality and systemic bacterial translocation in experimental sepsis in weanling mice. These results indicate that potential mechanistic explanations for this clinical benefit may include reduced intestinal epithelial apoptosis and restoration of colonic epithelial cell proliferation, which may be mediated by Akt/protein kinase B signal transduction pathway. Another potential mechanism may be probiotic-mediated attenuation of local and systemic inflammatory response, mediated by downregulation of TLR-2/TLR-4 signaling pathway via MyD88 in the colon.
We believe our data adds to existing clinical trial data supporting the potential efficacy of probiotics in critical illness. To this point, the design of larger scale clinical trials and/or more routine clinical administration of live probiotics to children and adults has recently been limited by concerns regarding safety and potential increased risk of infection from the administered probiotics. Although we did not specifically evaluate for bacteremia from our administered probiotic strains, we did observe a statistically significant reduction in overall bacteremia and mortality in our pediatric mice treated with live probiotic bacteria. Further, the results of the recent American Health Care Research and Quality report on the safety of probiotic therapy in over 600 published clinical trials and case reports are reassuring with regard to the safety of probiotic administration66, although isolated adverse effects of probiotic administration have been reported67. One recent clinical trial studying probiotics in severe pancreatitis (the PROPATRIA trial) found an unexpected increase in mortality in probiotic-treated patients68. This trial was unique as it administered multiple stains of probiotic bacteria and prebiotic-like fiber via a postpyloric feeding tube (placed in the small bowel). This postpyloric method of administration was associated with an increase in small bowel necrosis, which was subsequently associated with death in a number of patients receiving the prebiotic fiber/probiotic mixture. It is possible that the postpyloric administration of this fiber/multiple probiotic strain mixture in pancreatitis patients may carry significant risk and should likely be avoided69. In addition, methodological and safety concerns regarding the conduct of this trial have been raised69. In any case, careful and appropriate safety monitoring in all future probiotic clinical trials should be conducted. Perhaps the time has come to proceed with carefully designed, carefully monitored, multicenter randomized clinical trials of probiotic therapy to attempt to reduce the risk of infection, sepsis, and mortality in critically ill children and adults.
MS #201208046 – Final Boxed Summary Statement.
What is already known about the subject
-
*
Probiotic therapy appears to be useful in prevention of hospital-acquired and antibiotic-associated infections, but the mechanisms underlying this are not well defined
What this study documents that is novel
-
*
Probiotic administration in septic mice decreased mortality and systemic bacteremia and led to decreased apoptosis of colonic epithelium, decreased colonic epithelial proliferation and decreased systemic and colonic cytokine expression
Acknowledgments
DISCLOSURE OF FUNDING: Dr. Wischmeyer received support from the National Institute of Health, Bethesda, Maryland, (R01 GM078312) for this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Milbrandt EB, Kersten A, Rahim MT, Dremsizov TT, Clermont G, Cooper LM, Angus DC, Linde-Zwirble WT. Growth of intensive care unit resource use and its estimated cost in Medicare. Crit Care Med. 2008;36:2504–10. doi: 10.1097/CCM.0b013e318183ef84. [DOI] [PubMed] [Google Scholar]
- 2.Lukacs SL, Schoendorf KC, Schuchat A. Trends in sepsis-related neonatal mortality in the United States, 1985–1998. Pediatr Infect Dis J. 2004;23:599–603. doi: 10.1097/01.inf.0000131633.74921.90. [DOI] [PubMed] [Google Scholar]
- 3.Wynn JL, Scumpia PO, Delano MJ, O’Malley KA, Ungaro R, Abouhamze A, Moldawer KL. Increased mortality and altered immunity in neonatal sepsis produced by generalized peritonitis. Shock. 2007;28:675–83. doi: 10.1097/SHK.0b013e3180556d09. [DOI] [PubMed] [Google Scholar]
- 4.Herridge MS, Cheung AM, Tansey CM, Matte-Martyn A, Diaz-Granados N, Al-Saidi F, Cooper AB, Guest CB, Mazer CD, Mehta S, Stewart TE, Barr A, Cook D, Slutsky AS. One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med. 2003;348:683–93. doi: 10.1056/NEJMoa022450. [DOI] [PubMed] [Google Scholar]
- 5.Herridge MS, Tansey CM, Matte A, Tomlinson G, Diaz-Granados N, Cooper A, Guest CB, Mazer CD, Mehta S, Stewart TE, Kudlow P, Cook D, Slutsky AS, Cheung AM. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364:1293–304. doi: 10.1056/NEJMoa1011802. [DOI] [PubMed] [Google Scholar]
- 6.Adams-Chapman I, Stoll BJ. Neonatal infection and long-term neurodevelopmental outcome in the preterm infant. Curr Opin Infect Dis. 2006;19:290–7. doi: 10.1097/01.qco.0000224825.57976.87. [DOI] [PubMed] [Google Scholar]
- 7.Singhi SC, Baranwal A. Probiotic use in the critically ill. Indian J Pediatr. 2008;75:621–7. doi: 10.1007/s12098-008-0119-1. [DOI] [PubMed] [Google Scholar]
- 8.Clark JA, Coopersmith CM. Intestinal crosstalk: A new paradigm for understanding the gut as the “motor” of critical illness. Shock. 2007;28:384–93. doi: 10.1097/shk.0b013e31805569df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hassoun HT, Kone BC, Mercer DW, Moody FG, Weisbrodt NW, Moore FA. Post-injury multiple organ failure: The role of the gut. Shock. 2001;15:1–10. doi: 10.1097/00024382-200115010-00001. [DOI] [PubMed] [Google Scholar]
- 10.Mainous MR, Ertel W, Chaudry IH, Deitch EA. The gut: A cytokine-generating organ in systemic inflammation? Shock. 1995;4:193–9. [PubMed] [Google Scholar]
- 11.Clark JA, Gan H, Samocha AJ, Fox AC, Buchman TG, Coopersmith CM. Enterocyte-specific epidermal growth factor prevents barrier dysfunction and improves mortality in murine peritonitis. Am J Physiol Gastrointest Liver Physiol. 2009;297:G471–9. doi: 10.1152/ajpgi.00012.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De-Souza DA, Greene LJ. Intestinal permeability and systemic infections in critically ill patients: Effect of glutamine. Crit Care Med. 2005;33:1125–35. doi: 10.1097/01.ccm.0000162680.52397.97. [DOI] [PubMed] [Google Scholar]
- 13.Neal MD, Leaphart C, Levy R, Prince J, Billiar TR, Watkins S, Li J, Cetin S, Ford H, Schreiber A, Hackam DJ. Enterocyte TLR4 mediates phagocytosis and translocation of bacteria across the intestinal barrier. J Immunol. 2006;176:3070–9. doi: 10.4049/jimmunol.176.5.3070. [DOI] [PubMed] [Google Scholar]
- 14.Clark JA, Clark AT, Hotchkiss RS, Buchman TG, Coopersmith CM. Epidermal growth factor treatment decreases mortality and is associated with improved gut integrity in sepsis. Shock. 2008;30:36–42. doi: 10.1097/shk.0b013e31815D0820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coopersmith CM, Stromberg PE, Dunne WM, Davis CG, Amiot DM, 2nd, Buchman TG, Karl IE, Hotchkiss RS. Inhibition of intestinal epithelial apoptosis and survival in a murine model of pneumonia-induced sepsis. JAMA. 2002;287:1716–21. doi: 10.1001/jama.287.13.1716. [DOI] [PubMed] [Google Scholar]
- 16.Hotchkiss RS, Swanson PE, Freeman BD, Tinsley KW, Cobb JP, Matuschak GM, Buchman TG, Karl IE. Apoptotic cell death in patients with sepsis, shock, and multiple organ dysfunction. Crit Care Med. 1999;27:1230–51. doi: 10.1097/00003246-199907000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Osterberg J, Ljungdahl M, Haglund U. Influence of cyclooxygenase inhibitors on gut immune cell distribution and apoptosis rate in experimental sepsis. Shock. 2006;25:147–54. doi: 10.1097/01.shk.0000189843.78729.e2. [DOI] [PubMed] [Google Scholar]
- 18.Siempos II, Ntaidou TK, Falagas ME. Impact of the administration of probiotics on the incidence of ventilator-associated pneumonia: A meta-analysis of randomized controlled trials. Critical care medicine. 2010;38:954–62. doi: 10.1097/CCM.0b013e3181c8fe4b. [DOI] [PubMed] [Google Scholar]
- 19.Hammerman C, Bin-Nun A, Kaplan M. Germ warfare: Probiotics in defense of the premature gut. Clin Perinatol. 2004;31:489–500. doi: 10.1016/j.clp.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 20.Hojsak I, Abdovic S, Szajewska H, Milosevic M, Krznaric Z, Kolacek S. Lactobacillus GG in the prevention of nosocomial gastrointestinal and respiratory tract infections. Pediatrics. 2010;125:e1171–7. doi: 10.1542/peds.2009-2568. [DOI] [PubMed] [Google Scholar]
- 21.Morrow LE, Kollef MH, Casale TB. Probiotic prophylaxis of ventilator-associated pneumonia: A blinded, randomized, controlled trial. Am J Respir Crit Care Med. 2010;182:1058–64. doi: 10.1164/rccm.200912-1853OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Siempos II, Ntaidou TK, Falagas ME. Impact of the administration of probiotics on the incidence of ventilator-associated pneumonia: A meta-analysis of randomized controlled trials. Crit Care Med. 2010;38:954–62. doi: 10.1097/CCM.0b013e3181c8fe4b. [DOI] [PubMed] [Google Scholar]
- 23.Pitsouni E, Alexiou V, Saridakis V, Peppas G, Falagas ME. Does the use of probiotics/synbiotics prevent postoperative infections in patients undergoing abdominal surgery? A meta-analysis of randomized controlled trials. Eur J Clin Pharmacol. 2009;65:561–70. doi: 10.1007/s00228-009-0642-7. [DOI] [PubMed] [Google Scholar]
- 24.Bausserman M, Michail S. The use of Lactobacillus GG in irritable bowel syndrome in children: A double-blind randomized control trial. J Pediatr. 2005;147:197–201. doi: 10.1016/j.jpeds.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 25.Bin-Nun A, Bromiker R, Wilschanski M, Kaplan M, Rudensky B, Caplan M, Hammerman C. Oral probiotics prevent necrotizing enterocolitis in very low birth weight neonates. J Pediatr. 2005;147:192–6. doi: 10.1016/j.jpeds.2005.03.054. [DOI] [PubMed] [Google Scholar]
- 26.Drouault-Holowacz S, Bieuvelet S, Burckel A, Cazaubiel M, Dray X, Marteau P. A double blind randomized controlled trial of a probiotic combination in 100 patients with irritable bowel syndrome. Gastroenterol Clin Biol. 2008;32:147–52. doi: 10.1016/j.gcb.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 27.Arvola T, Laiho K, Torkkeli S, Mykkanen H, Salminen S, Maunula L, Isolauri E. Prophylactic Lactobacillus GG reduces antibiotic-associated diarrhea in children with respiratory infections: A randomized study. Pediatrics. 1999;104:e64. doi: 10.1542/peds.104.5.e64. [DOI] [PubMed] [Google Scholar]
- 28.Hempel S, Newberry SJ, Maher AR, Wang Z, Miles JN, Shanman R, Johnsen B, Shekelle PG. Probiotics for the prevention and treatment of antibiotic-associated diarrhea: A systematic review and meta-analysis. JAMA. 2012;307:1959–69. doi: 10.1001/jama.2012.3507. [DOI] [PubMed] [Google Scholar]
- 29.Schultz MJ. Symbiotics as a preventive measure against ventilator-associated pneumonia. Crit Care Med. 2010;38:1506–7. doi: 10.1097/CCM.0b013e3181d8c473. [DOI] [PubMed] [Google Scholar]
- 30.Giamarellos-Bourboulis EJ, Bengmark S, Kanellakopoulou K, Kotzampassi K. Pro- and synbiotics to control inflammation and infection in patients with multiple injuries. J Trauma. 2009;67:815–21. doi: 10.1097/TA.0b013e31819d979e. [DOI] [PubMed] [Google Scholar]
- 31.Kotzampassi K, Giamarellos-Bourboulis EJ, Voudouris A, Kazamias P, Eleftheriadis E. Benefits of a synbiotic formula (Synbiotic 2000Forte) in critically Ill trauma patients: Early results of a randomized controlled trial. World J Surg. 2006;30:1848–55. doi: 10.1007/s00268-005-0653-1. [DOI] [PubMed] [Google Scholar]
- 32.Shanahan F. Probiotics and inflammatory bowel disease: From fads and fantasy to facts and future. Br J Nutr. 2002;88(Suppl 1):S5–9. doi: 10.1079/BJN2002624. [DOI] [PubMed] [Google Scholar]
- 33.Luyer MD, Buurman WA, Hadfoune M, Speelmans G, Knol J, Jacobs JA, Dejong CH, Vriesema AJ, Greve JW. Strain-specific effects of probiotics on gut barrier integrity following hemorrhagic shock. Infect Immun. 2005;73:3686–92. doi: 10.1128/IAI.73.6.3686-3692.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zareie M, Johnson-Henry K, Jury J, Yang PC, Ngan BY, McKay DM, Soderholm JD, Perdue MH, Sherman PM. Probiotics prevent bacterial translocation and improve intestinal barrier function in rats following chronic psychological stress. Gut. 2006;55:1553–60. doi: 10.1136/gut.2005.080739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khailova L, Mount Patrick SK, Arganbright KM, Halpern MD, Kinouchi T, Dvorak B. Bifidobacterium bifidum reduces apoptosis in the intestinal epithelium in necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol. 2010;299:G1118–27. doi: 10.1152/ajpgi.00131.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tao Y, Drabik KA, Waypa TS, Musch MW, Alverdy JC, Schneewind O, Chang EB, Petrof EO. Soluble factors from Lactobacillus GG activate MAPKs and induce cytoprotective heat shock proteins in intestinal epithelial cells. Am J Physiol Cell Physiol. 2006;290:C1018–30. doi: 10.1152/ajpcell.00131.2005. [DOI] [PubMed] [Google Scholar]
- 37.Yan F, Cao H, Cover TL, Whitehead R, Washington MK, Polk DB. Soluble proteins produced by probiotic bacteria regulate intestinal epithelial cell survival and growth. Gastroenterology. 2007;132:562–75. doi: 10.1053/j.gastro.2006.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yan F, Polk DB. Probiotic bacterium prevents cytokine-induced apoptosis in intestinal epithelial cells. J Biol Chem. 2002;277:50959–65. doi: 10.1074/jbc.M207050200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aguero G, Villena J, Racedo S, Haro C, Alvarez S. Beneficial immunomodulatory activity of Lactobacillus casei in malnourished mice pneumonia: Effect on inflammation and coagulation. Nutrition. 2006;22:810–9. doi: 10.1016/j.nut.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 40.Arribas B, Rodriguez-Cabezas ME, Comalada M, Bailon E, Camuesco D, Olivares M, Xaus J, Zarzuelo A, Galvez J. Evaluation of the preventative effects exerted by Lactobacillus fermentum in an experimental model of septic shock induced in mice. Br J Nutr. 2009;101:51–8. doi: 10.1017/S0007114508986876. [DOI] [PubMed] [Google Scholar]
- 41.Matsumoto T, Ishikawa H, Tateda K, Yaeshima T, Ishibashi N, Yamaguchi K. Oral administration of Bifidobacterium longum prevents gut-derived Pseudomonas aeruginosa sepsis in mice. J Appl Microbiol. 2008;104:672–80. doi: 10.1111/j.1365-2672.2007.03593.x. [DOI] [PubMed] [Google Scholar]
- 42.Tok D, Ilkgul O, Bengmark S, Aydede H, Erhan Y, Taneli F, Ulman C, Vatansever S, Kose C, Ok G. Pretreatment with pro- and synbiotics reduces peritonitis-induced acute lung injury in rats. J Trauma. 2007;62:880–5. doi: 10.1097/01.ta.0000236019.00650.00. [DOI] [PubMed] [Google Scholar]
- 43.Baker CC, Chaudry IH, Gaines HO, Baue AE. Evaluation of factors affecting mortality rate after sepsis in a murine cecal ligation and puncture model. Surgery. 1983;94:331–5. [PubMed] [Google Scholar]
- 44.Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci U S A. 2007;104:13780–5. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Frank JA, Reich CI, Sharma S, Weisbaum JS, Wilson BA, Olsen GJ. Critical evaluation of two primers commonly used for amplification of bacterial 16S rRNA genes. Appl Environ Microbiol. 2008;74:2461–70. doi: 10.1128/AEM.02272-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rinttila T, Kassinen A, Malinen E, Krogius L, Palva A. Development of an extensive set of 16S rDNA-targeted primers for quantification of pathogenic and indigenous bacteria in faecal samples by real-time PCR. J Appl Microbiol. 2004;97:1166–77. doi: 10.1111/j.1365-2672.2004.02409.x. [DOI] [PubMed] [Google Scholar]
- 47.Vyas D, Robertson CM, Stromberg PE, Martin JR, Dunne WM, Houchen CW, Barrett TA, Ayala A, Perl M, Buchman TG, Coopersmith CM. Epithelial apoptosis in mechanistically distinct methods of injury in the murine small intestine. Histol Histopathol. 2007;22:623–30. doi: 10.14670/hh-22.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 49.Stern LE, Falcone RA, Jr, Kemp CJ, Stuart LA, Erwin CR, Warner BW. Effect of massive small bowel resection on the Bax/Bcl-w ratio and enterocyte apoptosis. J Gastrointest Surg. 2000;4:93–100. doi: 10.1016/s1091-255x(00)80038-4. [DOI] [PubMed] [Google Scholar]
- 50.Hajjar AM, Harowicz H, Liggitt HD, Fink PJ, Wilson CB, Skerrett SJ. An essential role for non-bone marrow-derived cells in control of Pseudomonas aeruginosa pneumonia. Am J Respir Cell Mol Biol. 2005;33:470–5. doi: 10.1165/rcmb.2005-0199OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alverdy JC, Chang EB. The re-emerging role of the intestinal microflora in critical illness and inflammation: Why the gut hypothesis of sepsis syndrome will not go away. J Leukoc Biol. 2008;83:461–6. doi: 10.1189/jlb.0607372. [DOI] [PubMed] [Google Scholar]
- 52.Wang X, Andersson R, Soltesz V, Leveau P, Ihse I. Gut origin sepsis, macrophage function, and oxygen extraction associated with acute pancreatitis in the rat. World J Surg. 1996;20:299–307. doi: 10.1007/s002689900048. [DOI] [PubMed] [Google Scholar]
- 53.Cleveland J, Montville TJ, Nes IF, Chikindas ML. Bacteriocins: Safe, natural antimicrobials for food preservation. Int J Food Microbiol. 2001;71:1–20. doi: 10.1016/s0168-1605(01)00560-8. [DOI] [PubMed] [Google Scholar]
- 54.Lee YK, Lim CY, Teng WL, Ouwehand AC, Tuomola EM, Salminen S. Quantitative approach in the study of adhesion of lactic acid bacteria to intestinal cells and their competition with enterobacteria. Appl Environ Microbiol. 2000;66:3692–7. doi: 10.1128/aem.66.9.3692-3697.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alberda C, Gramlich L, Meddings J, Field C, McCargar L, Kutsogiannis D, Fedorak R, Madsen K. Effects of probiotic therapy in critically ill patients: A randomized, double-blind, placebo-controlled trial. Am J Clin Nutr. 2007;85:816–23. doi: 10.1093/ajcn/85.3.816. [DOI] [PubMed] [Google Scholar]
- 56.Dani C, Biadaioli R, Bertini G, Martelli E, Rubaltelli FF. Probiotics feeding in prevention of urinary tract infection, bacterial sepsis and necrotizing enterocolitis in preterm infants. A prospective double-blind study Biol Neonate. 2002;82:103–8. doi: 10.1159/000063096. [DOI] [PubMed] [Google Scholar]
- 57.McNaught CE, Woodcock NP, Anderson AD, MacFie J. A prospective randomised trial of probiotics in critically ill patients. Clin Nutr. 2005;24:211–9. doi: 10.1016/j.clnu.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 58.Honeycutt TC, El Khashab M, Wardrop RM, 3rd, McNeal-Trice K, Honeycutt AL, Christy CG, Mistry K, Harris BD, Meliones JN, Kocis KC. Probiotic administration and the incidence of nosocomial infection in pediatric intensive care: A randomized placebo-controlled trial. Pediatr Crit Care Med. 2007;8:452–8. doi: 10.1097/01.PCC.0000282176.41134.E6. [DOI] [PubMed] [Google Scholar]
- 59.Alverdy JC, Laughlin RS, Wu L. Influence of the critically ill state on host-pathogen interactions within the intestine: Gut-derived sepsis redefined. Crit Care Med. 2003;31:598–607. doi: 10.1097/01.CCM.0000045576.55937.67. [DOI] [PubMed] [Google Scholar]
- 60.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 61.Williams DL, Ha T, Li C, Kalbfleisch JH, Schweitzer J, Vogt W, Browder IW. Modulation of tissue Toll-like receptor 2 and 4 during the early phases of polymicrobial sepsis correlates with mortality. Crit Care Med. 2003;31:1808–18. doi: 10.1097/01.CCM.0000069343.27691.F3. [DOI] [PubMed] [Google Scholar]
- 62.Edelman DA, Jiang Y, Tyburski J, Wilson RF, Steffes C. Toll-like receptor-4 message is up-regulated in lipopolysaccharide-exposed rat lung pericytes. J Surg Res. 2006;134:22–7. doi: 10.1016/j.jss.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 63.Yu M, Shao D, Liu J, Zhu J, Zhang Z, Xu J. Effects of ketamine on levels of cytokines, NF-kappaB and TLRs in rat intestine during CLP-induced sepsis. Int Immunopharmacol. 2007;7:1076–82. doi: 10.1016/j.intimp.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 64.Morrow LE, Kollef MH, Casale TB. Probiotic prophylaxis of ventilator-associated pneumonia: A blinded, randomized, controlled trial. Am J Respir Crit Care Med. 2010;182:1058–64. doi: 10.1164/rccm.200912-1853OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xiao SD, Zhang DZ, Lu H, Jiang SH, Liu HY, Wang GS, Xu GM, Zhang ZB, Lin GJ, Wang GL. Multicenter, randomized, controlled trial of heat-killed Lactobacillus acidophilus LB in patients with chronic diarrhea. Adv Ther. 2003;20:253–60. doi: 10.1007/BF02849854. [DOI] [PubMed] [Google Scholar]
- 66.Hempel S, S N, Ruelaz A, Wang Z, Miles JNV, Suttorp MJ, Johnsen B, Shanman R, Slusser W, Fu N, Smith A, Roth E, Polak J, Motala A, Perry T, Shekelle PG. (Evidence Report/Technology Assessment No. 200. AHRQ Publication No. 11-E007).Safety of Probiotics to Reduce Risk and Prevent or Treat Disease. 2011 Apr; [PMC free article] [PubMed] [Google Scholar]
- 67.De Groote MA, Frank DN, Dowell E, Glode MP, Pace NR. Lactobacillus rhamnosus GG bacteremia associated with probiotic use in a child with short gut syndrome. Pediatr Infect Dis J. 2005;24:278–80. doi: 10.1097/01.inf.0000154588.79356.e6. [DOI] [PubMed] [Google Scholar]
- 68.Besselink MG, van Santvoort HC, Buskens E, Boermeester MA, van Goor H, Timmerman HM, Nieuwenhuijs VB, Bollen TL, van Ramshorst B, Witteman BJ, Rosman C, Ploeg RJ, Brink MA, Schaapherder AF, Dejong CH, Wahab PJ, van Laarhoven CJ, van der Harst E, van Eijck CH, Cuesta MA, Akkermans LM, Gooszen HG. Probiotic prophylaxis in predicted severe acute pancreatitis: A randomised, double-blind, placebo-controlled trial. Lancet. 2008;371:651–9. doi: 10.1016/S0140-6736(08)60207-X. [DOI] [PubMed] [Google Scholar]
- 69.Morrow LE, Gogineni V, Malesker MA. Synbiotics and probiotics in the critically ill after the PROPATRIA trial. Current opinion in clinical nutrition and metabolic care. 2012;15:147–50. doi: 10.1097/MCO.0b013e32834fcea8. [DOI] [PubMed] [Google Scholar]


