Abstract
Objective
Mucociliary clearance sustains a baseline functionality and an “on demand” capability to upregulate clearance upon irritant exposure involving mucus hypersecretion and accelerated ciliary beat frequency (CBF) modulated by nitric oxide (NO). This study characterized these elements as well as cellular and exogenous NO concentrations subsequent to a single exposure to tobacco smoke (TS) or e-cigarette vapor (EV) on cultured human airway epithelium.
Materials and methods
Air-liquid interface (ALI) airway epithelial cultures per nonsmoking human subjects were subjected to single TS or EV exposures. Measures of ciliary function and secretion were performed and cellular and exogenous NO concentrations under control and experimental conditions were assessed.
Results
Both TS and EV exposures resulted similar patterns of decline in CBF within 1 min of the completion of exposure followed by a gradual return often exceeding baseline within 1 h. Post-exposure examination of exposed cultures suggested morphologic differences in secretory function relative to controls. The relative NO concentrations of TS and EV chamber air were sharply different with EV NO being only slightly elevated relative to cellular NO production.
Discussion and conclusions
Epithelial remodeling and mucociliary dysfunction have been clearly associated with TS exposure. However, information contrasting epithelial structure/function following a single acute TS or EV exposure is limited. This study demonstrates a similar pattern of epithelial response to acute TS or EV exposure. Inasmuch as NO may contribute to an inflammatory milieu and generation of toxic metabolites, it is plausible that recurrent exposures over time may be contributory to chronic pathologies.
Keywords: Cilia, ciliary beat frequency, airway epithelium, tobacco cigarette smoke, e-cigarette
Introduction
Mucociliary clearance effected by the coordinated action of secretory and ciliated cells of the respiratory airways is a fundamental primary defense mechanism. Numerous studies have pointed to the adverse effects of tobacco smoke on structure/function relationships in ciliary organization and mucociliary clearance (Aufderheide et al., 2015; Carson et al., 2013; Cohen et al., 2009; Wang et al., 2012; Zhou et al., 2009). Typically, these studies, even of acute exposure, address outcomes associated with multiple exposures thus precluding evaluation of early smoke/mucosa interactions that may provide insights into initiating responses leading to adverse health effects. Moreover, the advent of e-cigarettes and other nicotine “vaping” devices has led to the commercial promotion of these products as having reduced risk for adverse health effects associated with tobacco products. In addition to published studies pointing to dysmorphology and dysfunction of mucociliary clearance mechanisms associated with tobacco smoke exposure, there is a growing awareness of the role of the regulation of ciliary function modulation by nitric oxide (NO) (Jain et al., 1993; Jiao et al., 2011; Maniscalco et al., 2016; Ostrowski et al., 2012; Sisson et al., 2009; Vleeming et al., 2002; Wyatt, 2015; Zhou et al., 2009, 2011). While a variety of agents such as alcohol, tobacco smoke and microbiological products have been shown to affect this mechanism, there is little data to inform how NO modulation of ciliary function might be associated with e-cigarette use (Hariri et al., 2016; Sisson et al., 2009; Zhou et al., 2009). Given the evidence from previous studies (Cao et al., 2013; Cueto & Pryor, 1994; Ferrer-Sueta & Radi, 2009) that NO may contribute to an inflammatory milieu and the generation of toxic metabolites, it is plausible that recurrent exposures over time may be contributory to pathologies such as chronic obstructive respiratory disease (COPD) and chronic rhinosinusitis. This study seeks to contrast ciliary and secretory function responses to tobacco cigarette smoke (TS) and e-cigarette vapor (EV) in the context of differences in NO concentrations associated with the two types of exposures.
Materials and methods
Human subjects and nasal biopsies
Healthy nonsmoking human research subjects were recruited and consented for this study using a protocol approved by the UNC institutional review board. Each subject volunteering for inclusion in the study completed a respiratory health initial screening questionnaire in which the subject self-reported history of tobacco use and exposure including secondhand environmental smoke exposure and related symptoms if not a nonsmoker. Subjects also self-reported physician-based diagnoses of asthma and allergies, use of medications or treatments delivered through the nose, and any conditions that might create risk of injury or medically impact performance of the nasal biopsy procedure. Absence of tobacco smoke exposure was confirmed by urine cotinine testing. On the day of the nasal biopsy procedure, each subject also provided a supplemental questionnaire self-reporting any recent respiratory symptoms, tobacco smoke exposure, swimming activity and recent exposure to odors/fumes/air pollution other than tobacco smoke. For this study, only tissue from nonsmokers was used and the responses of subjects to the second questionnaire administered on the day of the biopsy were used as indicated to exclude a subject from sampling on that particular day. Differentiated nasal epithelium was obtained non-invasively by gentle curettage of the inferior nasal turbinates. The tissue was placed in culture medium and transported to the laboratory for culture.
Air–liquid interface cell culturing
Procedures for propagating air–liquid interface (ALI) cultures of human airway epithelium have been comprehensively described (Müller et al., 2013). Briefly, biopsied cell clumps were dispersed into small clusters and transitioned to a culture environment to facilitate mitotic expansion over a period of several days. Upon achieving optimal expansion, cells were subsequently seeded onto clear transwell culture inserts and maintained in a submerged condition under medium for additional days. Once the cells were confluent, ALI conditions were established with the addition of 50 nM retinoic acid and with nutritional maintenance only through the basal porous membrane of the transwell. The cultures were maintained for at least 1 month under standard conditions in order to achieve optimal differentiation before undertaking exposure protocols.
Scanning electron microscopy
Representative control, TS-exposed, and EV-exposed cultures from this study were processed by standard techniques. Transwell wafers were fixed immediately upon completion of exposure in 2% glutaraldehyde/2% paraformaldehyde in 0.1 M phosphate buffer. The specimens were post-fixed in 1% buffered osmium tetroxide, dehydrated through a graded ethanol series, and critical point dried under CO2. The specimens were mounted on standard specimen mounts, sputter coated with gold/palladium, and examined in a LEO scanning electron microscope at an accelerating voltage of 10 kV.
Determination of baseline cellular NO production and assessment of exogenous NO concentration in TS and EV
Cellular NO production was determined by positioning three ALI cultures in a vessel (Cytometric Sciences LLC, Chapel Hill, NC) designed for cellular NO measurement (Ostrowski et al., 2012). Transwells seeded with cells and transwells without cells to serve as controls were incubated in vessels for 6h at 35 °C after which NO concentration was determined using a Sievers 270B NO analyzer at a sampling flow rate of 500 ml/min. Twenty measurements were made in cultures derived from 12 different nonsmoking volunteers. Following NO measurements, total protein was determined in the cultures used for each experiment (Bio-Rad, Atlanta, GA).
Exogenous NO also was assessed in fresh TS and EV using the same 3 L exposure chamber as for exposure of cell cultures. For TS, the exposure chamber was flushed at 3 lpm (liters per minute) with the combustion products of a single Kentucky Research Cigarette 3R4F in the same fashion as that used for cell culture exposure. The atmosphere inside the chamber was sampled for NO immediately upon completion of combustion. For EV, the exposure chamber was flushed at 3 lpm with 20, 5 s “puffs” simulating a single “vaping” event in the same fashion as that used for cell culture exposure. The atmosphere inside the chamber was sampled for NO concentration immediately upon completion of the event. Four determinations each of NO concentration were made for TS and EV.
Exposure of cultures to TS or EV
For TS exposure, a single filtered University of Kentucky Research Cigarette 3R4F was positioned and lit in the holder of a 3l plastic exposure chamber and smoke drawn through the chamber at 3 lpm until the cigarette was consumed requiring ∼2-3 min. For EV exposure, a commonly distributed commercially available e-cigarette brand was used. The product was manufactured in China with ingredients identified as having “classic tobacco” flavor and a nicotine concentration of 2.4%. Natural and artificial flavorings were stated as made in USA with domestic and imported ingredients. Other identified ingredients included propylene glycol, vegetable glycerin, and distilled water. A single typical “vaping” event was simulated by initiating 20 consecutive vapor “puffs” into the chamber. Each “puff was followed by a brief lag time of several seconds.
Ciliary beat frequency measurement and analysis
Two culture isolates from each individual were independently exposed to either TS or EV. Following exposure to TS or EV, the cultures were transferred immediately from the exposure chamber at the conclusion of the exposure to the stage of the microscope and a single field of cilia identified and positioned optimally for viewing and measurement. The Sisson-Ammons Video Analysis System (SAVA) was then initialized to capture ciliary beat frequency (CBF) of the single field each minute for the next hour (Sisson et al., 2003). The high speed camera was interfaced to a Nikon Diaphot inverted microscope and measurements were made using a 20 × phase contrast lens. Prior to exposure, a mean baseline CBF was determined in all cultures by taking 20 random measurements throughout the culture. All data were captured on a Dell PC running the SAVA software. The captured CBF data were exported to an Excel spreadsheet and the difference in CBF for each minute relative to the pre-exposure baseline control was calculated.
This observed data structure yielded 120 CBF point observations per individual, 60 points for exposure to TS and 60 points for exposure to EV. The significance of the difference in CBF from baseline mean 1-min post-exposure was detected with a paired t-test within each exposure group. Additionally, a paired t-test was used to detect a difference in the early drop in CBF 1-min post-exposure between the two exposure groups. To optimize the information provided by the repeated CBF point data, a mixed effect model approach to detect the difference in change of CBF between the two exposures was used for measurements from post-1-min to post-1-h. Specifically, a mixed linear regression model with an individual-specific random intercept modeled the change in CBF as well as adjusting for smoke exposure type, functions of time, and time by smoke exposure type interaction. The final model is defined in the footnote of Table 1. The random intercept allowed adjustment for the potential correlation of the repeated measurements among same individuals. Since the individual curves were not linear (see Figure 1), we used a cube root transformation on time in the model. Furthermore, a quadratic form of time was included in the model to account for a possible change in the effect of time on change of CBF. All analyzes were carried out using SAS version 9.4 (SAS Institute Inc., Cary, NC).
Table 1.
Regression parameter estimates for the percent change in CBF.
| Effect | Estimate ± SE | p value |
|---|---|---|
| β0 | −91.766 ±5.150 | <.0001 |
| β1 | 11.556 ±3.212 | .0003 |
| β2 | 52.995 ± 3.628 | <.0001 |
| β3 | −6.612 ±0.661 | <.0001 |
| β4 | −2.630 ± 1.052 | .0123 |
Model: Y = β0 + β1 EV + β2Time + β3Time2 + β4EV × Time2 + ε, Where, Y = percent change from mean CBF frequency, EV = 1 if exposure is EV; 0 otherwise; and .
Figure 1.
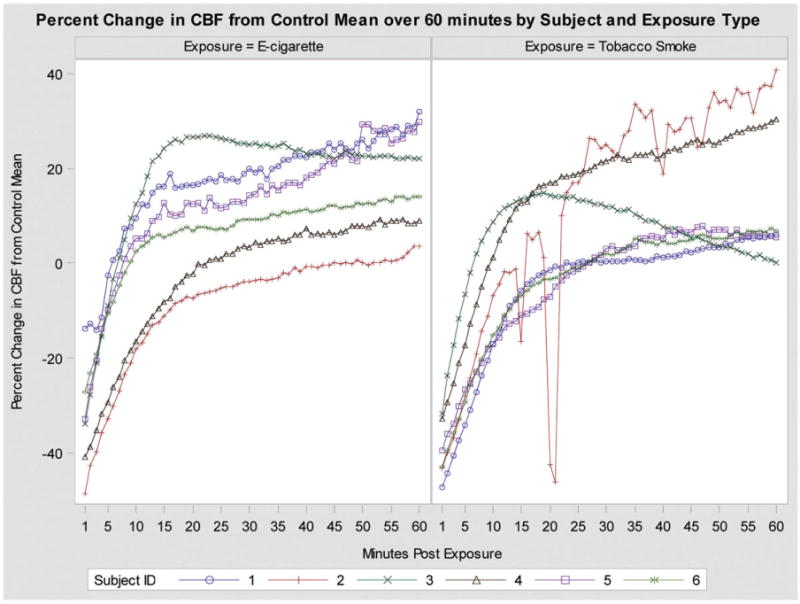
Graphic illustration of change in CBF over 1-h post-exposure among six subjects exposed to both TS and EV.
Results
In the present study, ALI airway epithelial cultures derived from nasal epithelium obtained from healthy nonsmokers were used to evaluate temporal changes in CBF subsequent to a single acute exposure to TS or EV. These studies using matched pairs of genetically homogeneous cell cultures demonstrated that within 1 min of completion of a simulated smoking or vaping event, CBF declines significantly but is restored to levels, often above baseline over the course of 1-h post-exposure.
Determination of cellular production of NO in unexposed cells and assessment of exogenous NO in TS and EV
Using the customized vessel in concert with an NO analyzer we were able to calculate the mean rate of NO production by control unexposed cells to be on the order of 3.7 ppb/mg total protein/h based on 20 measurements and accounting for a measured environmental background of 0.7 ppb NO. NO concentration sampled directly from the exposure chamber after a single simulated tobacco smoking event ranged from 398 to 1444ppb with a mean of 1072 ppb. NO concentration sampled directly from the exposure chamber following a simulated vaping event was measured to range from 0.6 to 4.7 ppb with a mean of 2.8 ppb.
Morphology and ultrastructure studies
Vigorous ciliary motility was readily evident at the light microscopic level in control, unexposed ALI cultures of human nasal epithelium with only slight variations in mean CBF among different subjects. In contrast, 1 min following exposure to either TS or EV, ciliary motility was both visually and quantifiably reduced. However, over the following hour, CBF was gradually restored in both TS and EV exposures.
The pattern of initial suppression of CBF is reflected in scanning electron micrograph (SEM) studies of the control and post-exposure cultures. In control unexposed cultures, the ALI culture conditions promoted the organization of the nasal cells into a configuration of ciliated and secretory cells emulating the pseudostratified columnar organization characteristic of the large airways. Patches of secretions were observed on the luminal borders primarily associated with secretory cells (Figure 2(A)). In contrast, cell cultures exposed to TS demonstrated matting and coalescence of cilia as a function of marked hypersecretion (Figure 2(B)). Secretory material uniformly permeated ciliary beds and also appeared in globular form on the luminal surfaces of cells. Although, secretory material appeared associated with ciliary beds in EV-exposed cells (Figure 2(C)), its presence was not as permeating as the pattern seen with TS. In addition, a fibrillar mesh-like material was observed associated with EV-exposed cilia (Figure 2(D)). This material was not observed in TS-exposed cells but this may have been due to the more extensive hypersecretions present in TS exposures.
Figure 2.
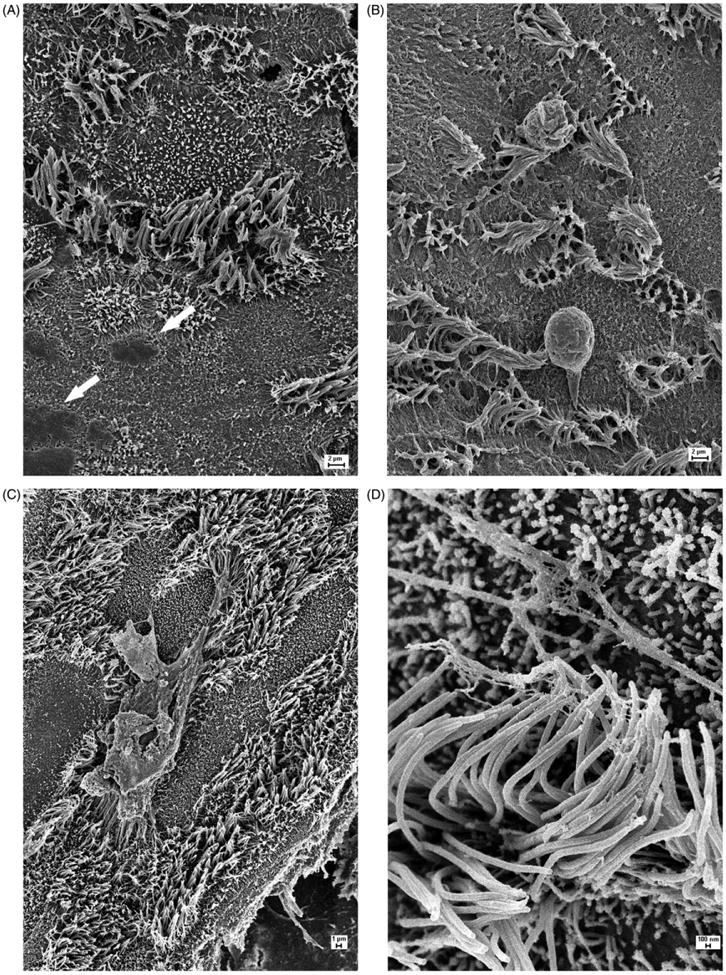
(A) SEM image of a fully differentiated control ALI culture of human nasal epithelium. Ciliary beds are well-organized and accumulated secretory products can be seen at the luminal borders of secretory cells adjacent to ciliated cells (arrows). (B) SEM image of an ALI culture of human nasal epithelium exposed to combustion products of one standard research cigarette. Note clumping of cilia and pervasive overlying secretory material as well as globular components of secretions. (C) SEM image of an ALI culture of human nasal epithelium exposed to a simulated e-cigarette “vaping” event. Note the presence of secretory materials overlying ciliary beds but to a more limited extent than in TS-exposed cells. (D) High-magnification SEM image of a ciliary bed from an ALI culture exposed to a simulated e-cigarette “vaping” event illustrating fibrillar material adhering to ciliary shafts. This feature was not evident in TS-exposed cells perhaps because of the more excessive secretions present associated with TS exposure.
Temporal analysis of CBF in acute TS and EV exposure
Under the acute exposure protocol described, both TS and EV elicited a marked suppression of CBF in cultures from all six subjects with a mean decline of 40 and 33% under baseline respectively within the first minute following exposure and initiation of acquisition of CBF (p < .0001 and p = .001, respectively). However, there was no significant difference between the drops of the two exposures within the first minute (p = .344). Equally noteworthy was a gradual recovery of CBF with an average increase over baseline of 15% for TS and 19% for EV during the subsequent post-exposure hour of CBF capture. On average, the curves returned to the control mean around minute 20 post-exposure.
The data from post-1-min to post-1-h were modeled using a mixed model approach with adjustment for exposure type (using TS as the reference group), time, time2, and smoke exposure by time interaction. The final model, presented in Table 1, has a significant difference in change in CBF for type of smoke exposure, time, time2 and interaction between smoke exposure type and time. The change in CBF from control mean is higher for epithelial cultures exposed to EV than for cultures exposed to TS (p = .0003). The estimated coefficient and estimated standard error (SE) for the EV exposure group is 11.556 percent change in CBF (SE = 3.212). There is also a significant effect of time on change in CBF, for both time and time2 (both have p < .0001). The estimated coefficients and SEs for time and time are 52.995 percent change in CBF from control mean (SE = 3.628) and —6.612 percent change in CBF from control mean (SE = 0.661). The estimated coefficient and SE for the interaction between type of smoke exposure and time is —2.630 (SE= 1.052) with a p = .013 (Table 1). The fitted models for EV and TS groups are depicted in Figure 3.
Figure 3.
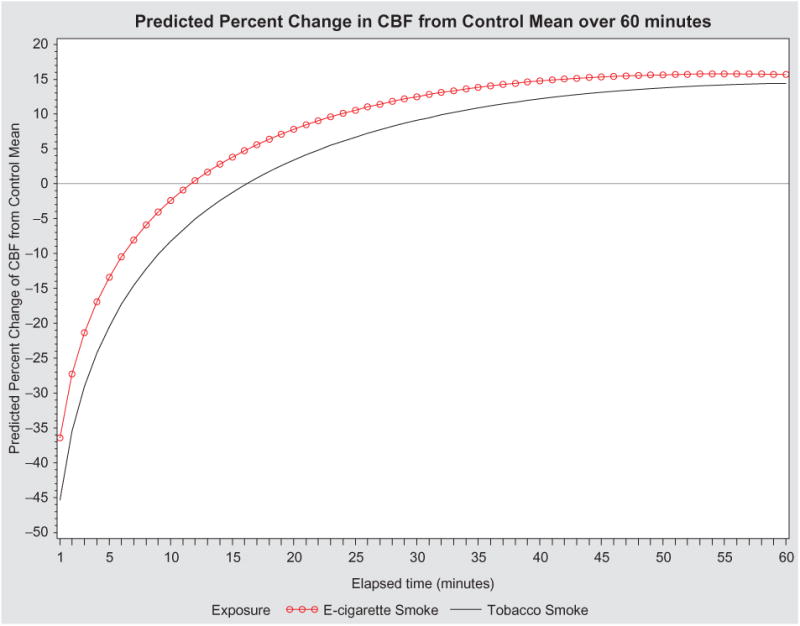
Graphic illustration of predicted percent change in CBF of all subjects from control mean over 1-h post-exposure to both TS and EV.
Figure 1 depicts the raw data for all subjects from 1-min to 1-h post-exposure. The change in CBF over time for tissues exposed to TS or EV fitted by the mixed regression model is depicted in Figure 3. The inclusion of time in the model is reflected in curvature of the lines as time increases from the initial smoke exposure. The interaction between exposure type and time is depicted through the difference in the two curves changing over time. This model suggests that the initial drop in CBF from the control mean for cells exposed to TS is much greater than the initial drop in CBF for cells exposed to EV. For recovery, in both exposure types, change in CBF from control mean recovers above the mean. However the recovery in tissues exposed to EV slows over time, allowing the recovery for tissues exposed to TS to eventually catch up.
Discussion
These experiments document the side-by-side temporal response of well-differentiated nasal airway epithelial cells maintained in ALI culture to acute, single exposures of TS or EV intended to simulate a single smoking event. Two cultures derived from each of six individual subjects were used in order to facilitate paired comparisons of TS and EV using genetically homogeneous samples. Every culture isolate whether exposed to TS or EV demonstrated a similar pattern of marked initial suppression of CBF in the immediate aftermath of exposure. CBF determinations over the subsequent post-exposure hour showed a return to or above average baseline CBF with both types of exposure. Previous studies (Jain et al., 1993) appropriating ciliary agonists and NOS inhibitors have clearly demonstrated that baseline CBF likely is not modulated by NO but that CBF can be significantly accelerated through the NO pathway and that agonist stimulated ciliary acceleration can be suppressed by NOS inhibitors.
Different approaches to the chemical analysis of TS and EV and to the assessment of nicotine delivery concentration (Famele et al., 2017; Margham et al., 2016; Oh & Kacker, 2014; Peace et al., 2016) have confounded simple comparisons. While nicotine delivery appears to be comparable or higher in TS (Famele et al., 2017; Margham et al., 2016), other studies point to poor quality control in e-cigarette production such that reported nicotine levels range from 45 to 131% of stated label concentration (Peace et al., 2016). While the suppression of CBF immediately post-exposure from both types of sources is suggestive of epithelial patho-physiology, it is noteworthy that although the advertised chemical constituents contained in e-cigarettes are substan-tively limited relative to the many well-documented toxic components present in TS, the post-exposure pattern of suppression of CBF by EV exposure was similar to that of TS. Moreover, CBF steadily increased in both types of exposure over the hour following exposure. This increase was noteworthy inasmuch as CBF returned to baseline and often was significantly above baseline after 1 h. Comparing the overall 1 h trend in both types of exposures, it appeared that TS exhibited a slightly more suppressive effect on CBF in the minutes after exposure although EV-exposed cultures exhibited a higher return to above baseline CBF. Morphologic examination by SEM of control cells compared to cells exposed to TS and EV may provide some insight into this pattern. While secretions may be observed in controls, they appear most closely associated with the surfaces of cells from which they are secreted (Figure 2(A)). In contrast, TS-exposed cells exhibit a pervasive and permeating thick layer of secretions overlying the entire culture surface. Individual cilia appeared matted together suggestive of the imposition of significant mechanical stress and consequent limited motility. In contrast, cells exposed to EV exhibited some secretory materials over the culture surface but clearly not at the level of TS-exposed cells thus perhaps accounting for the more rapid recovery and percentage change in CBF. EV-exposed cells also exhibited some fibrillar material distributed between cilia and microvilli, a finding not observed in TS-exposed cells, perhaps because the more overwhelming volume of secretions in TS exposure obscured this feature. The similarity of effects induced by TS and EV on CBF suggests that the response is due to a common dominant component, nicotine. However, it is plausible that factors such as mechanical stress conferred by mucus hypersecretion and physicochemical differences in EV relative to TS are in play. Clearly, EV exposures were characterized by an evident foglike vapor in the exposure chamber in contrast to a less translucent smoke combustion product observed in TS exposures. The potential for NO outgassing from particulates in TS exposures plausibly contributed to the increased NO levels sampled from the chamber at the times of TS exposure. The post-exposure morphologic differences are most likely due to other components of smoke like particulate matter in TS.
Previous studies have demonstrated that the molecular mechanism responsible for upregulation of ciliary activity in the wake of agonist challenge is likely driven by modulation of NO production (Jain et al., 1993). These studies and others have shown that NO production is a highly regulated signaling mechanism (Cueto & Pryor, 1994). A key component of NO function in human physiology is the delicate balance existing between normal function and pathology which can come from both over- and under-production. Although NO in the respiratory system can be routinely quantified, we know less about its production at the cell molecular level. While appropriate NO levels are important to normal function, overproduction may have adverse pulmonary health consequences in the short term and may contribute to the evolution of chronic respiratory disease processes (He & Frost, 2016). In the respiratory tract, the excretion of NO into airways lumina has well-documented relevance to such diverse processes as airways inflammation, irritant response, neurotransmission, innate immunity, and endothelial cell function as well as the regulation of ciliary motility (Maniscalco et al, 2016). Previous studies from this laboratory have demonstrated that active smokers exhibit increased nasal NO levels relative to nonsmokers and non-smokers exposed to second hand smoke (Zhou et al, 2011) and that both smokers and nonsmokers exposed to secondhand tobacco smoke exhibit chronically elevated CBF in fresh biopsies of nasal mucosa regardless of the recent history of smoking events prior to sampling (Zhou et al., 2009). These studies also demonstrated the generation of NO by cultured nasal epithelial cells in the presence of cigarette smoke condensate.
Cellular NO is produced by cells through the action of three nitric oxide synthases (NOS). In airway epithelium, the dominant NOS is inducible NOS that can be activated by external stimuli, like smoke. In the present study, we have been able to demonstrate a baseline of ∼3.7 ppb NO/mg total protein/h in unstimulated, unexposed cells, a vanishingly small complement compared to the level of NO in TS determined in our experiments and reported elsewhere (Guerin, 1980). The present study as well as others (Yakovlev, 2015) also point to substantially higher levels of NO in TS than is produced endogenously by cells. Inasmuch as NO readily diffuses across cell membranes (Ferrer-Sueta & Radi, 2009; Yakovlev, 2015) it is not possible to distinguish the relative contribution of endogenous and exogenous NO complements to modulation of ciliary function in response to TS or EV. Previous studies have suggested that NO appears to have limited influence on CBF in the absence of stimulation (Jain et al., 1993). A plausible physiologic basis for these changes may reside in acute injury provoked by tobacco or vaping components early in the exposure followed by acceleration of CBF through a molecular mechanism physiologically modulated by NO.
Our studies have shown similar patterns of CBF acceleration in both EV and TS exposure recovery where NO concentration is only slightly elevated relative to background in the EV environment and substantially increased in the TS environment. Of added concern is the potential of TS and EV constituents including NO and reactive nitrogen species to alter not only CBF but also membrane integrity (Miersch et al., 2008) in ways that impact NO diffusion as well as the potential pathologic consequences of remodeling (Cao et al., 2013) through modifications of growth and differentiation (Vallette et al., 1998).
In summary, this study has documented a biphasic response of CBF in genetically homogeneous cultures of differentiated human airway epithelium exposed briefly to either TS or EV which involves initial, rapid suppression of CBF followed by a rapid recovery to frequencies typically above baseline. This pattern poses physiologic and adverse health implications not only for single, acute exposures but the nature of ciliary modulation itself by NO and other associated reactive nitrogen species may pose additional routes for a variety of pathologies. Moreover, based on the physiologic responses of ciliated epithelium to both TS and EV in these experiments, the CBF endpoints evaluated are not so dissimilar as to support a consideration that e-cigarettes pose a more limited risk to adverse health effects than conventional tobacco cigarettes. Although e-cigarette products are presently marketed as a safe alternative to tobacco use, the similarity of response of airway mucosal cells to TS and EV exposures in this study calls into question the validity of such statements.
Acknowledgments
The authors thank Ms. Sally Ivins for coordinating subject recruitment and informed consent of research volunteers.
Funding: This study was supported by Clinical Innovator Awards to J.L.C. and I.J. from the Flight Attendant Medical Research Institute.
Footnotes
Disclosure statement: J.L.C. and M.J.H. are majority owners of Cytometric Sciences LLC.
References
- Aufderheide M, Scheffler S, Ito S, et al. Ciliatoxicity in human primary bronchiolar epithelial cells after repeated exposure at the air-liquid interface with native mainstream smoke of K3R4F cigarettes with and without charcoal filter. Exp Toxicol Pathol. 2015;67:407–11. doi: 10.1016/j.etp.2015.04.006. [DOI] [PubMed] [Google Scholar]
- Cao Y, Fujii M, Ishihara K, et al. Effect of a peroxynitrite scavenger, a manganese-porphyrin compound on airway remodeling in a murine asthma. Biol Pharm Bull. 2013;36:850–5. doi: 10.1248/bpb.b12-00805. [DOI] [PubMed] [Google Scholar]
- Carson JL, Brighton LE, Collier AM, Bromberg PA. Correlative ultrastructural investigations of airway epithelium following experimental exposure to defined air pollutants and lifestyle exposure to tobacco smoke. Inhal Toxicol. 2013;25:134–40. doi: 10.3109/08958378.2013.763314. [DOI] [PubMed] [Google Scholar]
- Cohen NA, Zhang S, Sharp DB, et al. Cigarette smoke condensate inhibits transepithelial chloride transport and ciliary beat frequency. Laryngoscope. 2009;119:2269–74. doi: 10.1002/lary.20223. [DOI] [PubMed] [Google Scholar]
- Cueto R, Pryor WA. Cigarette smoke chemistry: conversion of nitric oxide to nitrogen dioxide and reactions of nitrogen oxides with other smoke components as studied by Fourier transform infrared spectroscopy. Vib Spectrosc. 1994;7:97–111. [Google Scholar]
- Famele M, Palmisani J, Ferranti C, et al. Liquid chromatog-raphy with tandem mass spectrometry method for the determination of nicotine and minor tobacco alkaloids in electronic cigarette refill liquids and second-hand generated aerosol. J Sep Sci. 2017;40:1049–56. doi: 10.1002/jssc.201601076. [DOI] [PubMed] [Google Scholar]
- Ferrer-Sueta G, Radi R. Chemical biology of peroxynitrite: kinetics, diffusion, and radicals. ACS Chem Biol. 2009;4:161–77. doi: 10.1021/cb800279q. [DOI] [PubMed] [Google Scholar]
- Guerin MR. Chemical composition of cigarette smoke. In: Gori GB, Bock FG, editors. The Banbury report 3; A safe cigarette? Cold Spring Harbor NY: Cold Spring Harbor Laboratory; 1980. p. 191. [Google Scholar]
- Hariri BM, Payne SJ, Chen B, et al. In vitro effects of anthocya-nidins on sinonasal epithelial nitric oxide production and bacterial physiology. Am J Rhinol Allergy. 2016;4:261–8. doi: 10.2500/ajra.2016.30.4331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W, Frost MC. CellNO trap: novel device for quantitative, real-time, direct measurement of nitric oxide from cultured RAW 267.4 macrophages. Redox Biol. 2016;8:383–97. doi: 10.1016/j.redox.2016.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain B, Rubinstein I, Robbins RA, et al. Modulation of airway epithelial cell ciliary beat frequency by nitric oxide. Biochem Biophys Res Commun. 1993;191:83–8. doi: 10.1006/bbrc.1993.1187. [DOI] [PubMed] [Google Scholar]
- Jiao J, Wang H, Lou W, et al. Regulation of ciliary beat frequency by the nitric oxide signaling pathway in mouse nasal and tracheal epithelial cells. Exp Cell Res. 2011;317:2548–53. doi: 10.1016/j.yexcr.2011.07.007. [DOI] [PubMed] [Google Scholar]
- Maniscalco M, Bianco A, Mazzarella G, Motta A. Recent advances on nitric oxide in the upper airways. Curr Med Chem. 2016;23:2736–45. doi: 10.2174/0929867323666160627115335. [DOI] [PubMed] [Google Scholar]
- Margham J, McAdam K, Forster M, et al. Chemical composition of aerosol from an E-cigarette: a quantitative comparison with cigarette smoke. Chem Res Toxicol. 2016;29:1662–78. doi: 10.1021/acs.chemrestox.6b00188. [DOI] [PubMed] [Google Scholar]
- Miersch S, Espey MG, Chaube R, et al. Plasma membrane cholesterol content affects nitric oxide diffusion dynamics and signaling. J Biol Chem. 2008;283:18513–21. doi: 10.1074/jbc.M800440200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller L, Brighton LE, Carson JL, et al. Culturing of human nasal epithelial cells at the air liquid interface. J Vis Exp. 2013;80 doi: 10.3791/50646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh AY, Kacker A. Do electronic cigarettes impart a lower potential disease burden than conventional tobacco cigarettes? Review on E-cigarette vapor versus tobacco smoke. Laryngoscope. 2014;124:2702–6. doi: 10.1002/lary.24750. [DOI] [PubMed] [Google Scholar]
- Ostrowski LE, Stewart D, Hazucha M. Interferon c stimulates accumulation of gas phase nitric oxide in differentiated cultures of normal and cystic fibrosis airway epithelial cells. Lung. 2012;190:563–71. doi: 10.1007/s00408-012-9395-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peace MR, Baird TR, Smith N, et al. Concentration of nicotine and glycols in 27 electronic cigarette formulations. J Anal Toxicol. 2016;40:403–7. doi: 10.1093/jat/bkw037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisson JH, Pavlik JA, Wyatt TA. Alcohol stimulates ciliary motility of isolated airway axonemes through a nitric oxide, cyclase, and cyclic nucleotide-dependent kinase mechanism. Alcohol Clin Exp Res. 2009;33:610–6. doi: 10.1111/j.1530-0277.2008.00875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisson JH, Stoner JA, Ammons BA, Wyatt TA. All-digital image capture and whole-field analysis of ciliary beat frequency. J Microsc. 2003;211:103–11. doi: 10.1046/j.1365-2818.2003.01209.x. [DOI] [PubMed] [Google Scholar]
- Vallette G, Tenaud I, Branka JE, et al. Control of growth and differentiation of normal human epithelial cells through the manipulation of reactive nitrogen species. Biochem J. 1998;331:713–7. doi: 10.1042/bj3310713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vleeming W, Rambali B, Opperhuizen A. The role of nitric oxide in cigarette smoking and nicotine addiction. Nicotine Tob Res. 2002;4:341–8. doi: 10.1080/14622200210142724. [DOI] [PubMed] [Google Scholar]
- Wang LF, White DR, Andreoli SM, et al. Cigarette smoke inhibits dynamic ciliary beat frequency in pediatric adenoid explants. Otolaryngol Head Neck Surg. 2012;146:659–63. doi: 10.1177/0194599811431414. [DOI] [PubMed] [Google Scholar]
- Wyatt TA. Cyclic GMP and cilia motility. Cells. 2015;4:315–30. doi: 10.3390/cells4030315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakovlev VA. Role of nitric oxide in the radiation-induced bystander effect. Redox Biol. 2015;6:396–400. doi: 10.1016/j.redox.2015.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Wang X, Brighton L, et al. Increased nasal epithelial ciliary beat frequency associated with lifestyle tobacco smoke exposure. Inhal Toxicol. 2009;21:875–81. doi: 10.1080/08958370802555898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Zou B, Hazucha M, Carson JL. Nasal nitric oxide and lifestyle exposure to tobacco smoke. Ann Otol Rhinol Laryngol. 2011;120:455–9. doi: 10.1177/000348941112000706. [DOI] [PMC free article] [PubMed] [Google Scholar]


