Abstract
We report here, for the first time, the primary structure of uncoupling protein as established by amino acid sequencing. Like the ADP/ATP carrier, this protein has a tripartite structure comprising three similar sequences of approximately 100 residues each. These six 'repeats' exhibit striking conservation of several residues, in particular glycine and proline, at possible structurally strategic positions. Although the two proteins differ strongly in their amino acid composition, their sequences are distantly homologous. Three membrane-spanning alpha-helices can be deduced from hydropathy plots. A modified plot accounting for amphiphilic helices indicates 5-6 such alpha-segments. In addition an amphiphilic beta-strand of membrane-spanning length can be discerned. The tripartite sequence structure is also distinctly reflected in the hydropathy distribution. Based on the membrane disposition of the segments of the ADP/ATP carrier, a model for the transmembrane folding path of the polypeptide chain of the uncoupling protein is proposed.
Full text
PDF

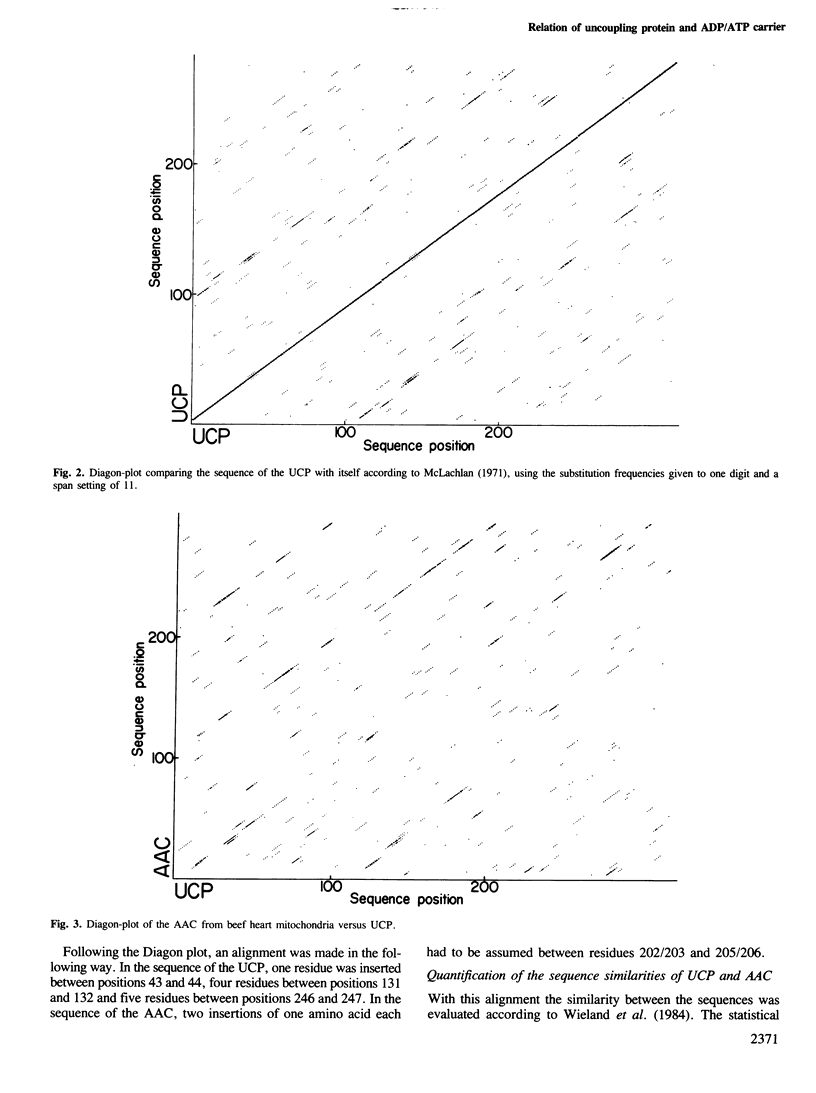
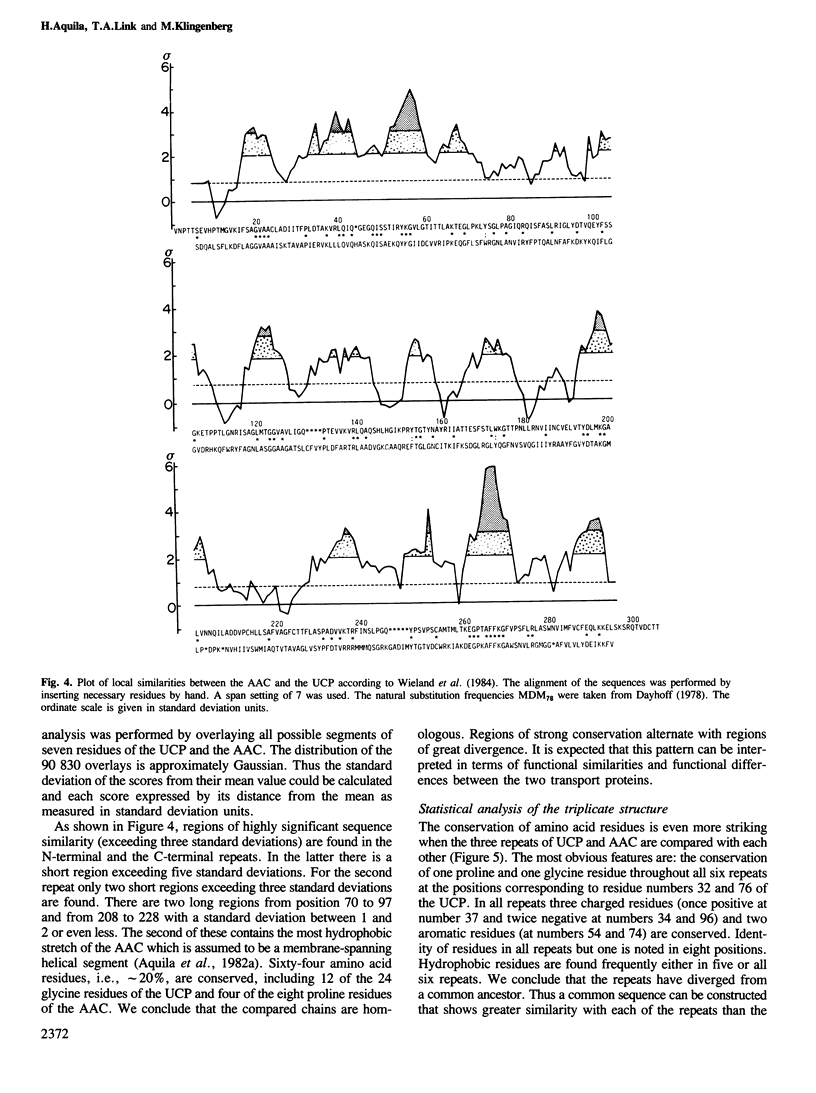

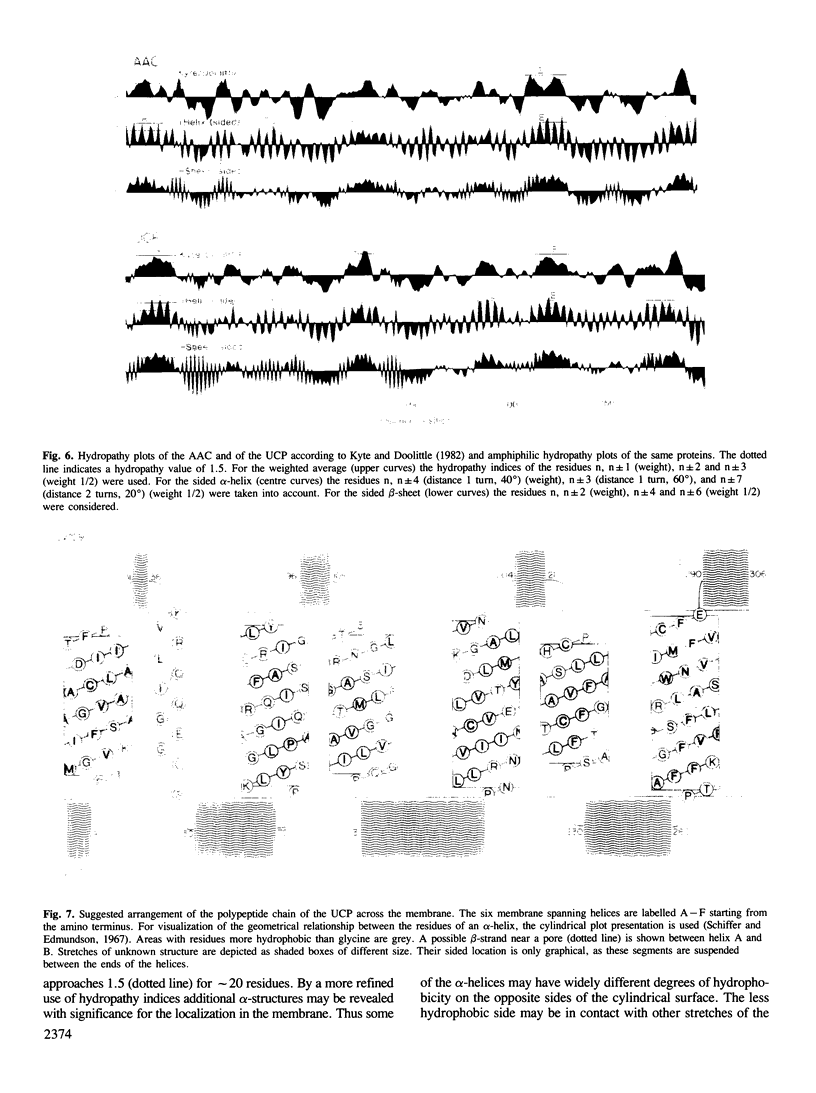


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aquila H., Misra D., Eulitz M., Klingenberg M. Complete amino acid sequence of the ADP/ATP carrier from beef heart mitochondria. Hoppe Seylers Z Physiol Chem. 1982 Mar;363(3):345–349. [PubMed] [Google Scholar]
- Chang J. Y. Manual micro-sequence analysis of polypeptides using dimethylaminoazobenzene isothiocyanate. Methods Enzymol. 1983;91:455–466. doi: 10.1016/s0076-6879(83)91043-1. [DOI] [PubMed] [Google Scholar]
- Engelman D. M., Henderson R., McLachlan A. D., Wallace B. A. Path of the polypeptide in bacteriorhodopsin. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2023–2027. doi: 10.1073/pnas.77.4.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavito R. M., Jenkins J., Jansonius J. N., Karlsson R., Rosenbusch J. P. X-ray diffraction analysis of matrix porin, an integral membrane protein from Escherichia coli outer membranes. J Mol Biol. 1983 Feb 25;164(2):313–327. doi: 10.1016/0022-2836(83)90079-7. [DOI] [PubMed] [Google Scholar]
- Klingenberg M., Aquila H., Riccio P. Isolation of functional membrane proteins related to or identical with the ADP, ATP carrier of mitochondria. Methods Enzymol. 1979;56:407–414. doi: 10.1016/0076-6879(79)56039-x. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lin C. S., Hackenberg H., Klingenberg E. M. The uncoupling protein from brown adipose tissue mitochondria is a dimer. A hydrodynamic study. FEBS Lett. 1980 May 5;113(2):304–306. doi: 10.1016/0014-5793(80)80614-4. [DOI] [PubMed] [Google Scholar]
- Lin C. S., Klingenberg M. Characteristics of the isolated purine nucleotide binding protein from brown fat mitochondria. Biochemistry. 1982 Jun 8;21(12):2950–2956. doi: 10.1021/bi00541a023. [DOI] [PubMed] [Google Scholar]
- Lin C. S., Klingenberg M. Isolation of the uncoupling protein from brown adipose tissue mitochondria. FEBS Lett. 1980 May 5;113(2):299–303. doi: 10.1016/0014-5793(80)80613-2. [DOI] [PubMed] [Google Scholar]
- McLachlan A. D. Tests for comparing related amino-acid sequences. Cytochrome c and cytochrome c 551 . J Mol Biol. 1971 Oct 28;61(2):409–424. doi: 10.1016/0022-2836(71)90390-1. [DOI] [PubMed] [Google Scholar]
- Nicholls D. G. The bioenergetics of brown adipose tissue mitochondria. FEBS Lett. 1976 Jan 15;61(2):103–110. doi: 10.1016/0014-5793(76)81014-9. [DOI] [PubMed] [Google Scholar]
- Riccio P., Aquila H., Klingenberg M. Purification of the carboxy-atractylate binding protein from mitochondria. FEBS Lett. 1975 Aug 1;56(1):133–138. doi: 10.1016/0014-5793(75)80127-x. [DOI] [PubMed] [Google Scholar]
- Richardson J. S. The anatomy and taxonomy of protein structure. Adv Protein Chem. 1981;34:167–339. doi: 10.1016/s0065-3233(08)60520-3. [DOI] [PubMed] [Google Scholar]
- Rosenblatt M., Beaudette N. V., Fasman G. D. Conformational studies of the synthetic precursor-specific region of preproparathyroid hormone. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3983–3987. doi: 10.1073/pnas.77.7.3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraste M., Walker J. E. Internal sequence repeats and the path of polypeptide in mitochondrial ADP/ATP translocase. FEBS Lett. 1982 Aug 2;144(2):250–254. doi: 10.1016/0014-5793(82)80648-0. [DOI] [PubMed] [Google Scholar]
- Schiffer M., Edmundson A. B. Use of helical wheels to represent the structures of proteins and to identify segments with helical potential. Biophys J. 1967 Mar;7(2):121–135. doi: 10.1016/S0006-3495(67)86579-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland B., Tomasselli A. G., Noda L. H., Frank R., Schulz G. E. The amino acid sequence of GTP:AMP phosphotransferase from beef-heart mitochondria. Extensive homology with cytosolic adenylate kinase. Eur J Biochem. 1984 Sep 3;143(2):331–339. doi: 10.1111/j.1432-1033.1984.tb08376.x. [DOI] [PubMed] [Google Scholar]
- Zimmerman C. L., Pisano J. J. High-performance liquid chromatography of amino acid derivatives. Methods Enzymol. 1977;47:45–51. doi: 10.1016/0076-6879(77)47007-1. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Membrane proteins: the amino acid composition of membrane-penetrating segments. Eur J Biochem. 1981 Nov;120(2):275–278. doi: 10.1111/j.1432-1033.1981.tb05700.x. [DOI] [PubMed] [Google Scholar]





