INTRODUCTION
Breast cancer is a heterogeneous disease in which tumor differentiation within lesions and in primary versus metastatic disease can be the basis of a poor prognosis and clinical outcomes.1 Initial staging of breast cancer integrates pathologic analysis of receptor status in biopsied tissues. The three main biomarkers of interest in breast cancer include estrogen receptor (ER), progesterone receptor, and human epidermal growth factor receptor 2 (HER2).2 These receptors are assessed using immunohistochemistry, fluorescence in situ hybridization, or chromogen in situ hybridization to direct therapy.3
HER2 overexpression is a hallmark of aggressive breast cancer, and its expression in a primary tumor directly influences treatment.4 Because of this, HER2 has become a critical target for molecular imaging and therapy.4,5 Approximately 20% of invasive ductal breast malignancies are classified as HER2 positive as a result of gene amplification and/or the subsequent overexpression of the HER2 protein on the surface of tumor cells.6 Patients with HER2-positive breast cancer receive specific, targeted HER2 therapies to reduce the risk of death.7 However, the current methods in the clinic to assess receptor status have issues when it comes to staging breast cancer, and consequently assigning therapy. Inherent errors include bias in and misinterpretation of immunohistochemistry or in situ hybridization results, sampling issues, and discordance between primary metastatic sites of disease.8–10 Although not typically within the standard course of treatment, there have been reports of clinical benefit of HER2-targeted therapy even in patients with a primary HER2-negative tumor.11 The hypothesis behind this phenomenon is owing to the heterogeneity of not just the primary tumors, but of metastatic disease as well.12
One approach to address these issues with current staging methods in HER2-positive breast cancer is to use targeted molecular imaging. Imaging modalities that target HER2 have the potential to not only diagnose HER2-positive breast cancer, but also detect distant metastases via a single, noninvasive procedure.5,13,14 Molecular imaging, particularly with tracers used in PET, can address issues of heterogeneity and discordance between primary and metastatic disease in breast cancer. PET is especially useful when paired with methods for anatomic imaging (eg, MR imaging or computed tomography [CT]). These modalities used in tandem can detect exact location of disease, and allow more accurate surgical resection. PET imaging can also be used to select patients for targeted therapies and monitor treatment effects.15
PET is a highly sensitive, quantitative molecular imaging technique with high spatial resolution. PET is the select modality for imaging in the clinic owing to its favorable properties. Several groups have developed antibody-based radioligands over the past 15 years for PET imaging preclinically and clinically.16–19 From this body of work, many of these targeted probes have been used to image HER2-positive breast cancer.5,13 The clinical translation of antibodies has its drawbacks owing to often low tumor penetration because of high molecular weight (approximately 150 kDa) and slow clearance.16 Also, conventional positron emitters (eg, 11C, 13N, 15O, and 18F) have half-lives that may be too short to permit adequate biodistribution of labeled antibodies before imaging. Pairing these targeting moieties with longer-lived radioisotopes such at zirconium-89 (89Zr) for PET and indium-111 (111In) for single-photon emission CT (SPECT) (89Zr, t1/2 = 78 hours; 111In, t1/2 = 67 hours) can be used to match the biological half-life of antibodies in vivo (t1/2 approximately 72 hours), and allow radioactivity accumulation in tumors for up to 6 days.20 Antibody fragments and affibody molecules (small proteins engineered to bind to a large number of target proteins or peptides with high affinity) can be labeled with shorter-lived isotopes such as gallium-68 (68Ga) or fluorine-18 (18F) (68Ga, t1/2 = 68 minutes; 18F, t1/2 = 110 minutes) for a faster turnaround and more transient detection of disease.21–24 Isotopes with “medium” half-lives such as copper-64 (64Cu, t1/2 = 12 hours) are also used for both full-length and antibody fragments for PET imaging.25–27
Many of the PET and SPECT imaging tracers that have made it to the clinic (either in current clinical use, or have made it to phase I/II trials) to detect HER2-positive breast cancer and metastases, are summarized in Table 1. These agents are used for a more accurate diagnosis and patient selection for therapy in breast cancer.
Table 1.
Summary of clinical trials with PET and SPECT radiotracers targeting HER2-positive breast cancer
| Radiotracer | Description of Trial | Clinical Trial or Reference | Sponsor | Status |
|---|---|---|---|---|
| 89Zr-DFO-trastuzumab | HER2+ BC | NCT01832051 | University Medical Center Groningen | Completed |
| Imaging the effect of HSP90 inhibition | NCT01081600 | University Medical Center Groningen | Completed | |
| HER2+ MBC | NCT01420146 | Jules Bordet Institute | Completed | |
| HER2+ BC | NCT02065609 | Washington University School of Medicine | Recruiting | |
| HER2+ MBC | NCT02286843 | Memorial Sloan Kettering Cancer Center | Recruiting | |
| IMPACT-MBC | NCT01957332 | University Medical Center Groningen | Recruiting | |
|
| ||||
| 64Cu-DOTA-trastuzumab | HER2+ MBC | NCT00605397 | Memorial Sloan Kettering Cancer Center | Completed |
| Predicting treatment response in HER2+ BC | NCT02827877 | City of Hope Medical Center | Verified | |
|
| ||||
| 111In-CHX-A DTPA- trastuzumab | HER2+ BC | NCT01445054 | National Cancer Institute | Completed |
|
| ||||
| 111In-MxDTPA- trastuzumab | HER2+ BC | Wong et al,49 2010 | City of Hope Medical Center | Completed |
| HER2+ MBC | Perik et al,47 2006 | University Medical Center Groningen | Completed | |
| HER2+ MBC | Gaykema et al,48 2014 | University Medical Center Groningen | Completed | |
|
| ||||
| 68Ga-F(ab′)2- trastuzumab | HER2+ BC | NCT00613847 | Memorial Sloan Kettering Cancer Center | Completed |
|
| ||||
| 68Ga-ABY-025 | HER2+ BC | NCT01858116 | Biomedical Radiation Sciences | Completed |
| HER2+ BC | NCT02095210 | Dorte Nielsen | Recruiting | |
|
| ||||
| 111In-ABY-025 | HER2+ MBC | NCT01216033 | Biomedical Radiation Sciences | Completed |
|
| ||||
| 64Cu-MM-302 | HER2+ MBC | NCT02735798 | University of California, San Francisco | Verified |
|
| ||||
| [131I]-SGMIB anti- HER2 VHH1 | Healthy Patients and HER2+ BC | NCT02683083 | Camel-IDS NV | Recruiting |
|
| ||||
| 68Ga-HER2-Nanobody | HER2+ BC | EudraCT 2012- 001135-31 | Vrije Universiteit Brussels | Completed |
Abbreviations: BC, breast cancer; HER2, human epidermal growth factor 2; IMPACT, IMaging PAtients for Cancer Drug selecTion; MBC, metastatic breast cancer; SGMIB, N-succinimidyl 4-guanidinomethyl 3-iodobenzoate; SPECT, single photon emission computed tomography.
Inaccurate knowledge of receptor status in metastases owing to tumor heterogeneity and differentiation throughout cancer spreading may lead to suboptimal selection of patients for HER2-targeted therapy.12,28,29 Data show that more than 10% of patients with HER2-negative primary breast cancer may still benefit from HER2-targeted treatment.11 This observation indicates that HER2-negative patients may have distant disease that is in fact HER2 positive. Targeting these metastases can reduce the risk of death in this aggressive subtype of breast cancer. Current treatments targeted to HER2-positive breast lesions, including hormone therapy, are listed in Table 2.
Table 2.
Drugs approved for anti-HER2 therapy in HER2-positive breast cancer
| Drug (Chemical Name) | Classification | Mechanism of Action | Company | FDA Approved |
|---|---|---|---|---|
| Trastuzumab | Antibody | Binds to extracellular segment of the HER2/neu receptor | Genentech | 1998 |
| Pertuzumab | Antibody | Inhibits the dimerization of HER2 with other HER receptors | Genentech | 2012 |
| Ado-trastuzumab emtansine | Antibody–drug conjugate | Binds at plus ends of cellular microtubules and inhibits cell division in tumor cells | Genentech | 2013 |
| Lapatinib | Tyrosine kinase inhibitor | Interrupts HER2/neu and EGFR pathway | GlaxoSmithKline | 2007 |
Abbreviations: EGFR, epidermal growth factor receptor; FDA, Food and Drug Administration; HER2, human epidermal growth factor 2.
Trastuzumab is a monoclonal antibody that targets HER2 and is the first-line therapy for HER2-positive breast tumors.29–32 The history of trastuzumab dates back to 1998 when it was approved by the Food and Drug Administration, and has been used regularly in the clinic ever since.31 Imaging modalities have been constructed using a functionalized version of this antibody to detect HER2-positive disease and metastasis.
PET AND SINGLE PHOTON EMISSION COMPUTED TOMOGRAPHY IMAGING WITH MONOCLONAL ANTIBODIES IN HUMAN EPIDERMAL GROWTH FACTOR RECEPTOR 2-POSITIVE BREAST CANCER
Zirconium-89-DFO-Trastuzumab
89Zr radiopharmaceuticals are an attractive option for antibody-based imaging agents owing to their compatible half-life (approximately 78 hours) to monoclonal antibodies (approximately 72 hours).20 Dijkers and colleagues33 were the first to label clinical-grade trastuzumab with 89Zr and use it for immunoPET imaging in animals. This radiotracer was later implemented to gauge HER2 status in not just primary tumor, but in patients with nonaccessible metastases.33 With this product, Oude and colleagues34 used 89Zr-DFO-trastuzumab to annotate response of HER2 to HSP90 therapy preclinically. From here, there have been numerous reports of preclinical and clinical studies with this tracer that have evolved both imaging and therapy within HER2-positive breast cancer.
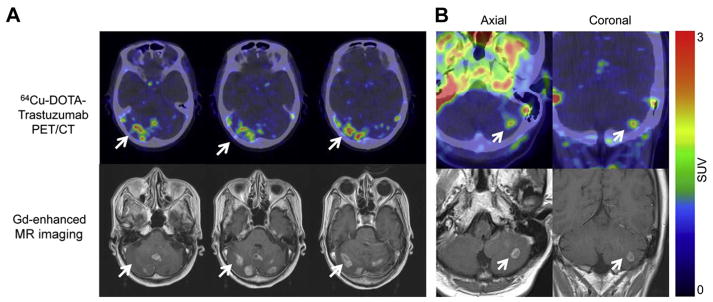
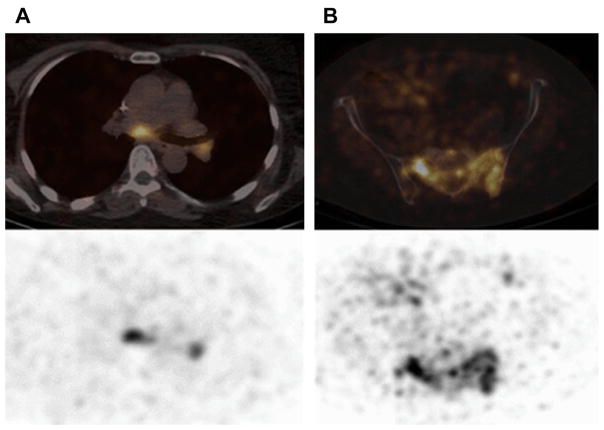
89Zr-DFO-trastuzumab has been favored owing to a higher spatial resolution and a better signal-to-noise ratio than 111In-trastuzumab, and ease of data quantification with PET versus SPECT. Preliminary results (Dijkers and colleagues35) with 89Zr-DFO-trastuzumab immunoPET in HER2-positive breast cancer patients indicated exceptional tumor uptake and spatial resolution. Fourteen patients with HER2-positive metastatic breast cancer received 37 MBq of 89Zr-trastuzumab at 1 of 3 doses (10 or 50 mg for those who had not received trastuzumab and 10 mg for those who were undergoing trastuzumab treatment). The patients underwent serial PET scans between days 2 and 5. The results of the study showed that the best time for assessment of the uptake of 89Zr-trastuzumab was 4 to 5 days after injection. Fig. 1 represents the biodistribution of 89Zr-DFO-trastuzumab, including detection of metastatic brain lesions that were not observed by fludeoxyglucose F 18 (18F-FDG). Fusion PET/MR imaging scans in Fig. 2 further highlight this observation, revealing the true usefulness of this radiotracer in patients. This pilot study showed promise in the ability of 89Zr-DFO-trastuzumab to detect not only primary tumor, but also metastatic disease that conventional PET was unable to accomplish.36
Fig. 1.
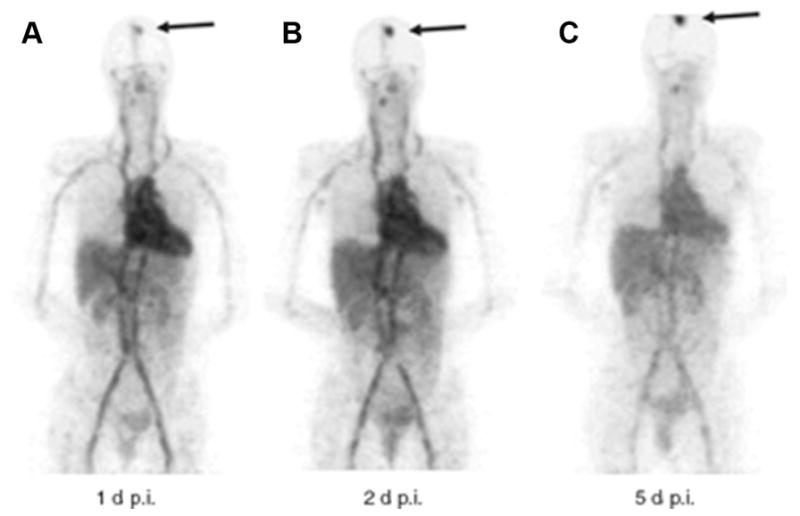
89Zr-trastuzumab biodistribution in time. (A–C) Three 89Zr-trastuzumab scans of a patient already on tras-tuzumab treatment (cohort 3) show the increase over time in the tumor/nontumor ratio with regard to uptake of the tracer. Arrow indicates 89Zr-trastuzumab uptake in the only lesion. p.i., postinjection; 89Zr, zirconium-89. (From Dijkers EC, Oude Munnink TH, Kosterink JG, et al. Biodistribution of 89Zr-trastuzumab and PET imaging of HER2-positive lesions in patients with metastatic breast cancer. Clin Pharmacol Ther 2010;87(5):588; with permission.)
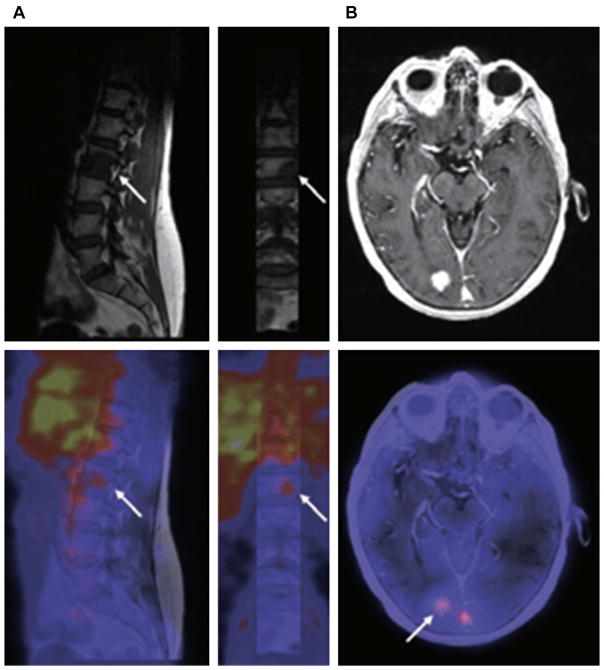
Fig. 2.
Examples of fusion images from human epidermal growth factor receptor 2 (HER2) PET and MR imaging scans. (A) In a vertebral metastasis seen on MR imaging but unapproachable for biopsy, HER2 status was revealed by 89Zr-trastuzumab uptake on PET imaging. (B) Example of HER2-positive brain lesion undetected by conventional scans, revealed by 89Zr-trastuzumab PET imaging, and subsequently confirmed by MR imaging. Arrows indicate lesions. HER2, human epidermal growth factor receptor 2; 89Zr, zirconium-89. (From Dijkers EC, Oude Munnink TH, Kosterink JG, et al. Biodistribution of 89Zr-trastuzumab and PET imaging of HER2-positive lesions in patients with metastatic breast cancer. Clin Pharmacol Ther 2010;87(5):589; with permission.)
Zirconium-89-DFO-Trastuzumab to Detect Unsuspected Human Epidermal Growth Factor Receptor 2-Positive Metastatic Disease
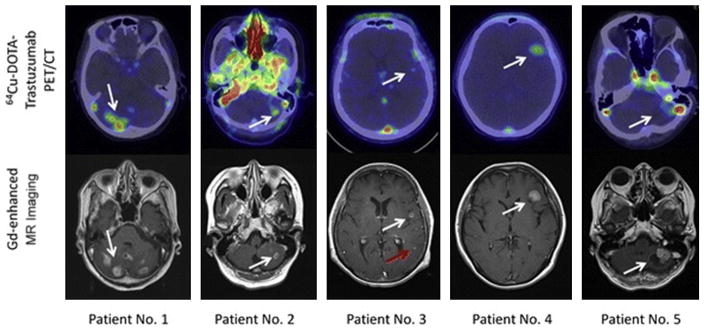
Ulaner and colleagues12 explored the potential of 89Zr-DFO-trastuzumab to detect HER2-positive metastases in HER2-negative primary tumors. Nine patients with HER2-negative primary breast cancer were enrolled in this study, and imaged with 89Zr-DFO-trastuzumab PET/CT to detect for 89Zr-DFO-trastuzumab–avid metastases. All patients enrolled were confirmed pathologically as HER2 negative. Metastases that were observed via 89Zr-trastuzumab PET/CT were biopsied and pathologically examined to define HER2 status. Based on these findings, patients with HER2-positive metastases received HER2-targeted therapy to evaluate treatment response. An example of one such patient is represented in Fig. 3.
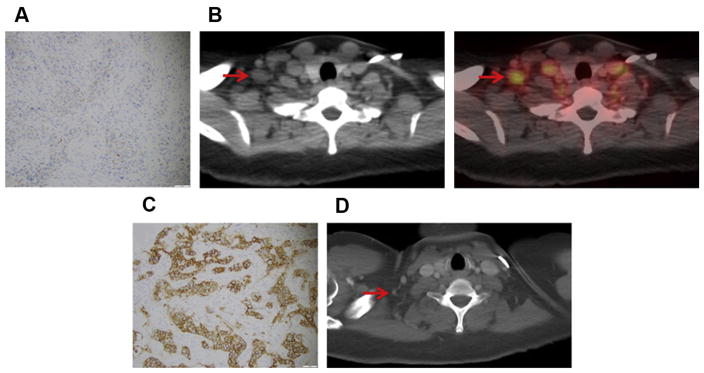
Fig. 3.
A 41-year-old woman with primary ER-positive, human epidermal growth factor receptor 2 (HER2)-negative invasive ductal breast carcinoma and recurrence in the thoracic nodes. (A) Immunohistochemistry score of primary breast malignancy was 11 (at 20 × magnification), consistent with HER2-negative malignancy. (B) Axial computed tomography (CT) and 89Zr-trastuzumab PET/CT demonstrated 89Zr-trastuzumab avidity in enlarged right supraclavicular nodes (arrows, maximum standardized uptake value of 4.6) and left internal mammary nodes (not shown). (C) Biopsy of right supraclavicular node demonstrated metastatic breast carcinoma with immunohistochemistry score of 31 (at 20 × magnification), consistent with HER2-positive disease. The patient began systemic treatment including trastuzumab and pertuzumab. (D) Follow-up axial CT after 2 months of treatment demonstrated resolution of nodes on CT examination (arrow). (From Ulaner GA, Hyman DM, Ross DS, et al. Detection of HER2-positive metastases in patients with HER2-negative primary breast cancer using 89Zr-trastuzumab PET/CT. J Nuc Med 2016;57(10):1526; with permission.)
Of the 9 patients, 5 exhibited metastatic uptake with 89Zr-DFO-trastuzumab PET/CT. Of these 5 patients, 2 had biopsies taken of potential metastatic sites based from their PET scans. These 2 patients exhibited HER2-positive metastases (as confirmed via pathologic workup of the biopsied tissue) and subsequently benefitted from HER2-targeted therapy. The remaining 3 patients with suspicious uptake were also biopsied, and did not present evidence of HER2-positive disease at metastatic lesions, and were considered false positive for 89Zr-DFO-trastuzumab PET. An example of a false-positive scan is presented in Fig. 4.12
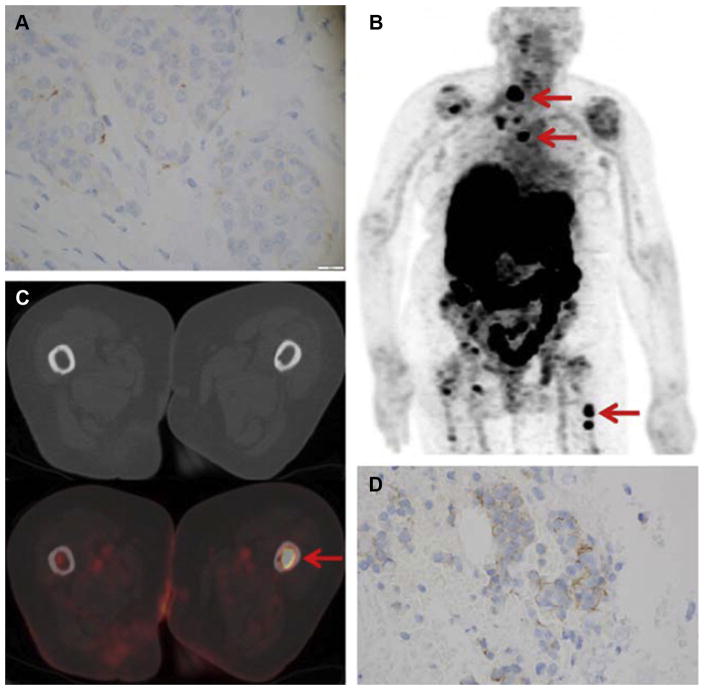
Fig. 4.
An 83-year-old woman with primary ER-positive/human epidermal growth factor receptor 2 (HER2)-negative invasive ductal breast carcinoma. (A) Immunohistochemistry score of primary breast malignancy was 11 (at 400 × magnification), consistent with HER2-negative malignancy. (B) 89Zr-trastuzumab maximum intensity projection demonstrates several foci of 89Zr-trastuzumab avidity that localize to osseous structures (arrows). Avidity in liver and bowel is considered physiologic. (C) Axial computed tomography (CT) and 89Zr-trastuzumab PET/CT demonstrate 89Zr-trastuzumab avidity in proximal left femur (arrow; maximum standardized uptake value of 7.7). (D) Biopsy of proximal left femur demonstrated metastatic breast carcinoma with immunohistochemistry score of 11 (at 400 × magnification), consistent with HER2-negative disease. (From Ulaner GA, Hyman DM, Ross DS, et al. Detection of HER2-positive metastases in patients with HER2-negative primary breast cancer using 89Zr-trastuzumab PET/CT. J Nuc Med 2016;57(10):1527; with permission.)
The significant result of this study is that 89Zr-DFO-trastuzumab PET/CT can detect unsuspected HER2-positive metastases in patients with a HER2-negative primary breast cancer. Although these preliminary results encompass a small sample size, it still gives strong evidence that HER2-targeted imaging can identify candidates for HER2-targeted therapy. These results provide a possible explanation for the hypothesis that a minority of patients with HER2-negative tumors benefit from HER2-targeted therapy because of the heterogeneity within distant disease. More specific HER2-targeting agents are necessary in the clinic to elucidate fully this relationship via imaging, and decrease the potential for false-positive uptake.
Annotating Treatment Response with Zirconium-89-DFO-Trastuzumab
Heath shock protein 90 (HSP90) is an ATP-dependent molecular chaperone protein that is involved in several oncogenic pathways, and has been a target for many cancer therapies.37 HSP90 inhibitors have emerged as a promising anticancer strategy in many cancers, including HER2-positive breast cancer.38 HSP90 inhibitors have been combined with trastuzumab (either as treatment or assessing treatment response) in HER2-positive metastatic breast cancer.39
The primary aim of a clinical trial led by Gaykema and colleagues34 was to evaluate the usefulness of 89Zr-DFO-trastuzumab PET (for HER2-positive breast cancer) or 89Zr-DFO-bevaci-zumab PET (for ER-positive breast cancer) to determine the in vivo degradation of proteins owing to HSP90 inhibitor NVP-AUY922. Sixteen patients (10 HER2-positive and 6 ER-positive tumors) were included in this study. During this study, NVP-AUY922 (70 mg/m2) was administered intravenously weekly to patients with advanced HER2- or ER-positive breast cancer. Serial PET imaging with 18F-FDG, 89Zr-DFO-trastuzumab, or 89Zr-DFO-bevacizumab was performed to assess biomarkers of interest (HER2, ER, and 18F-FDG used as a metabolic marker). Blood samples were collected to detect HSP90 levels and the extracellular form of HER2. One partial response was observed from NVP-AUY922 treatment, and 7 patients showed no response. Changes in the maximum standardized uptake value in patients with 89Zr-trastuzumab PET before and after treatment was heterogeneous and correlated with size change on CT. Treatment with HSP90 inhibitor NVP-AUY922 showed a clinical response in HER2-positive metastatic breast cancer. Changes in 89Zr-DFO-trastuzumab PET were concomitant with change in size of lesions, as measured by CT.
Another ongoing study to assess the usefulness of 89Zr-trastuzumab to image therapy response is the ZEPHIR trial (Phase II Prospective Imaging Study Evaluating the Utility of Pre-treatment Zr89 Labelled Trastuzumab PET/CT and an Early FDG-PET/CT Response to Identify Patients With Advanced HER2+ BC Unlikely to Benefit From a Novel anti-HER2 Therapy: TDM1), led by the Jules Bordet Institute. This clinical trial is currently enrolling patients from Belgium and the Netherlands eligible to receive that antibody–drug conjugate T-DM1 for HER2-positive advanced disease. HER2-positive metastatic breast cancer patients with an immunohistochemistry 3+ or a fluorescence in situ hybridization score of greater than 2.2 eligible for T-DM1 treatment underwent a pretreatment PET/CT scan with 89Zr-trastuzumab. 18F-FDG PET/CT was performed at baseline and before T-DM1 cycle 2. Patients were grouped into 4 HER2–PET/CT patterns according to the proportion of 18F-FDG–avid tumor concurrent with 89Zr-trastuzumab uptake. In the 56 patients analyzed, 29% had negative 89Zr-trastuzumab PET/CT and intrapatient heterogeneity was found in 46% of patients.40 89Zr-trastuzumab imaging, combined with early metabolic response assessment with 18F-FDG accurately predicted morphologic response after 3 treatment cycles with T-DM1. This methodology has the potential for improving the understanding of tumor heterogeneity in metastatic breast cancer and for selecting patients who may or may not benefit from T-DM1.
Currently, patients are still being recruited for many 89Zr-DFO-trastuzumab PET imaging trials. The IMPACT trial (IMaging PAtients for Cancer Drug selecTion) for metastatic breast cancer, led by the University Medical Center Groningen, is using 89Zr-DFO-trastuzumab for baseline scans to recruit patients for antibody therapy.41 Another trial has been completed at the Jules Bordet Institute to assess 89Zr-DFO-trastuzumab uptake in metastatic breast cancer,42 and the ZEPHIR trial is still recruiting patients to increase the number of patients that may potentially benefit from T-DM1 therapy.43 Patients are still being recruited at Memorial Sloan Kettering Cancer Center to extend the usefulness of 89Zr-DFO-trastuzumab to detect metastatic disease in HER2-negative primary breast cancer.44 The Washington University School of Medicine is recruiting patients headlining their own clinical trial to assess uptake and dosimetry of 89Zr-DFO-trastuzumab in patients.45 Overall, 89Zr-DFO-trastuzumab is an extremely useful clinical tool for assessing HER2-positive breast cancer, and has proven useful as a method to detect heterogeneity within both primary and metastatic lesions. The future of this radiotracer lies in its capacity for patient selection, which is what the IMPACT trial and the Memorial Sloan Kettering Cancer Center study aim to accomplish.
Copper-64-DOTA-Trastuzumab
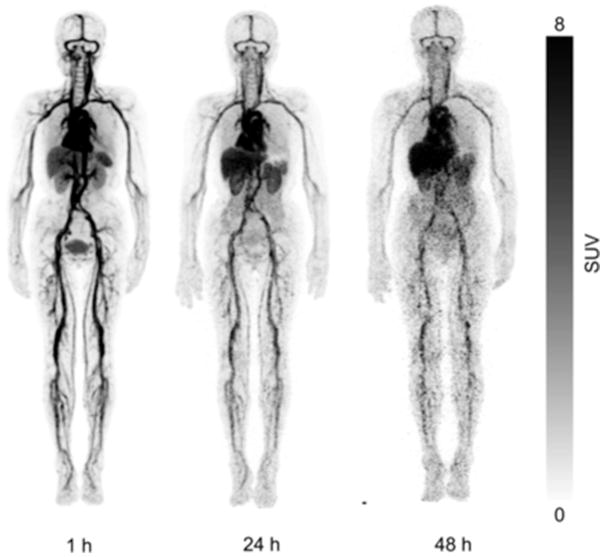
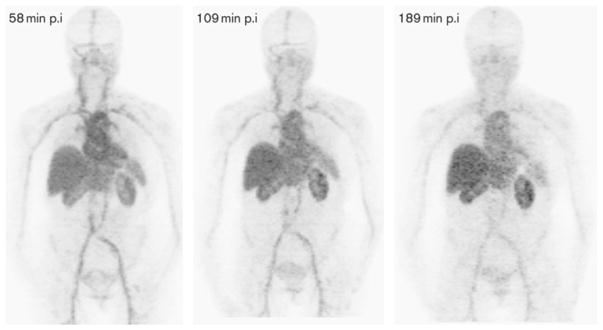
The transition to shorter-lived PET radioisotopes started with 64Cu-labeling of trastuzumab. The first study was to determine the safety, distribution, internal dosimetry, and initial HER2-positive tumor images of 64Cu-DOTA-trastuzumab in humans by Tamura and colleagues.26 PET imaging was performed on 6 patients with primary or metastatic HER2-positive breast cancer at 1, 24, and 48 hours after injection of approximately 130 MBq of the tracer 64Cu-DOTA-trastuzumab. Samples were collected from the blood, urine, and nontarget tissue samples to assess the biodistribution and internal dosimetry of the probe.26 The results from this study identified 48 hours as the best time point for 64Cu-DOTA-trastuzumab after injection (Fig. 5).
Fig. 5.
Whole-body copper-64-DOTA-trastuzumab PET images at 1, 24, and 48 hours after injection (patient 4). (From Tamura K, Kurihara H, Yonemori K, et al. 64Cu-DOTA-trastuzumab PET imaging in patients with HER2-positive breast cancer. J Nucl Med 2013;54(11):1871; with permission.)
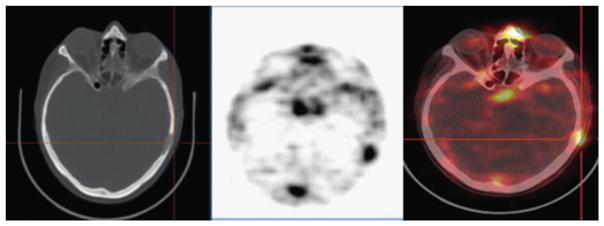
An important outcome from this study was identifying that the radiation exposure during 64Cu-DOTA-trastuzumab PET was comparable with that during 18F-FDG PET, which is the conventional tracer for PET imaging in the clinic. Although the radioactivity in the blood was high, the uptake of 64Cu-DOTA-trastuzumab in nontarget tissues was low, which is critical for improving detection. Brain metastases were observed in 2 patients that were administered 64Cu-DOTA-trastuzumab PET, indicative of blood–brain barrier passage of the antibody tracer (Fig. 6). In 3 patients, 64Cu-DOTA-trastuzumab PET imaging also revealed primary breast tumors in areas that were initially identified by CT. The findings of this study indicated that 64Cu-DOTA-trastuzumab PET could detect HER2-positive lesions in patients with both primary and metastatic breast cancer.
Fig. 6.
Copper-64 (64Cu)-DOTA-trastuzumab PET images of human epidermal growth factor receptor 2 (HER2)-positive metastatic brain lesions (arrows). (A) Brain metastases were clearly visualized by 64Cu-DOTA-trastuzumab PET in patient 1. Significant uptake values were found in areas corresponding to brain metastatic lesions that were detected by MR imaging. Some brain metastases could not be detected on conventional CT. (B) In patient 4, 64Cu-DOTA-trastuzumab PET imaging could detect solitary brain metastasis that had also been identified in similar location by MR imaging. Gd, gadolinium. (From Tamura K, Kurihara H, Yonemori K, et al. 64Cu-DOTA-trastuzumab PET imaging in patients with HER2-positive breast cancer. J Nucl Med 2013;54(11):1874; with permission.)
Copper-64-DOTA-Trastuzumab in Metastatic Disease
Owing to successful identification of brain metastasis from HER2-positive primary breast tumors, a more focused study was done by Kurihara and colleagues25 with 64Cu-DOTA-trastuzumab PET in HER2-positive metastatic breast cancer. PET imaging was performed on 5 patients with confirmed brain metastases from HER2-positive breast cancer, at 24 or 48 hours after the injection of approximately 130 MBq of 64Cu-DOTA-trastuzumab. 64Cu-DOTA-trastuzumab PET visualized metasta-tic brain lesions in all 5 patients (Fig. 7). This study gave further evidence that trastuzumab may pass through the blood–brain barrier and is able to detect HER2-positive metastases. From these studies, 64Cu-DOTA-trastuzumab PET could be a potential noninvasive procedure for serial identification of metastatic brain lesions in patients with HER2-positive breast cancer.25
Fig. 7.
Copper-64 (64Cu)-DOTA-trastuzumab PET images of metastatic brain tumors in patients with human epidermal growth factor receptor 2 (HER2)-positive primary breast tumors. The white arrows show the metastatic brain tumors. Upper panels: 64Cu-DOTA-trastuzumab PET images. Lower panels: gadolinium (Gd)-DTPA-enhanced T1-weighted MR imaging images. White arrows indicate metastatic brain lesions detectable by both MR imaging and 64Cu-DOTA-trastuzumab PET, and the red arrow indicates a lesion detectable by MR imaging but not by 64Cu-DOTA-trastuzumab PET. In the PET image from patient 2, nonspecific high uptake in the blood was noted. (From Kurihara H, Hamada A, Yoshida M, et al. 64Cu-DOTA-trastuzumab PET imaging and HER2 specificity of brain metastases in HER2-positive breast cancer patients. EJNMMI Res 2015;5(8):4; with permission.)
In summary, 64Cu-DOTA-trastuzumab has been successful in detecting both primary and metastatic disease in patients. Currently, patients are being recruited at the City of Hope Medical Center to use 64Cu-DOTA-trastuzumab to predict treatment response with trastuzumab and pertuzumab before surgery in HER2-positive breast cancer.46 The future of this tracer will be assessed by this trial and its involvement in predicting treatment response and facilitating patient selection for antibody therapy in HER2-positive breast cancer.
Indium-111-Trastuzumab
Trastuzumab functionalized as an SPECT molecular imaging probe began with the pairing of the antibody to the SPECT isotope, indium-111 (111In). 111In-MxDTPA-trastuzumab imaging was done by Perik and colleagues47 to assess effects and clinical benefit of trastuzumab therapy. The ultimate result from this study was to confirm that HER2 imaging is feasible during trastuzumab treatment, and can successfully annotate response. It was concluded that the same antibody could be used for both treatment and therapy benefit in the same patient. This was further confirmed in a follow-up study also done by Gay-kema and colleagues,48 who showed that treatment with trastuzumab in patients resulted in decreased tumor 111In-MxDTPA-trastuzumab uptake of about 20%.
Wong and colleagues49 evaluated the organ bio-distribution, pharmacokinetics, immunogenicity, and tumor uptake of 111In-MxDTPA-trastuzumab in patients with HER2-overexpressing breast cancers and to determine whether 90Y-trastuzumab should be evaluated in subsequent clinical therapy trials. Patients with HER2-overexpressing breast cancers that were scheduled to receive trastuzumab therapy first underwent (unlabeled) trastuzumab therapy (4–8 mg/kg), followed by 185 MBq 111In-MxDTPA-trastuzumab (10 mg, 4 hours after therapy). Blood and urine samples (24 hours) were obtained for further analysis. Nuclear scans were performed at defined time points for 7 days. Eight patients received 111In-MxDTPA-trastuzumab, which was well-tolerated with no adverse side effects. Three of 7 patients with known lesions showed positive tracer uptake in PET imaging. No immunogenic responses were observed for 2 months after injection, indicating the safety of the tracer.
The outcome of this study indicates that 90Y-trastuzumab may be an appropriate agent to evaluate further in the clinic. No immunogenic response to 111In-MxDTPA-trastuzumab was observed, and the pharmacokinetics and organ biodistribution were comparable with other 90Y-labeled monoclonal antibodies. Cardiac uptake was a slight concern/difference to other radioimmunotherapy antibodies in the clinic, but was found to be similar to hepatic uptake and not expected to cause dose-limiting cardiotoxicity. HER2-targeted therapy and subsequent interrogation of these treatment effects through imaging could be a useful tool for predicting patient response. This would allow physicians to identify patients that do and do not respond to targeted therapies, opening educated options of pursuing different avenues of treatment.
The National Cancer Institute also currently sponsors a clinical trial to assess the effects of 111In-CHX-A DTPA-trastuzumab imaging in HER2-positive breast cancer. Fluorescence in situ hybridization at both primary and metastatic sites of breast cancer is used to assess HER2 status. The results of these studies have yet to be published.
Indium-111-DTPA-Pertuzumab
Pertuzumab is another monoclonal antibody marketed by Genentech for treatment for HER2-positive breast cancer.50 This therapy is often used in tandem with trastuzumab and docetaxel.51 Pertuzumab inhibits dimerization of HER2 with other HER2 receptors, which is thought to slow tumor growth.50 A clinical trial was attempted to recruit patients with imaging with 111In-DTPA-per-tuzumab to predict response to trastuzumab in HER2-positive metastatic breast cancer, but this trial was terminated owing to poor accrual of patients.52 89Zr-DFO-pertuzumab has also been explored preclinically,53 but has not yet reached clinical trials, likely owing to the high interest and success with 89Zr-DFO-trastuzumab.
Iodine-131-SGMID Anti-Her2 VHH1
A monoclonal antibody directed against HER2 (VHH1) labeled with iodine-131 (131I) with the radio-iodinating reagent N-succinimidyl 4-guanidinomethyl 3-iodobenzoate (SGMIB), is also being explored in the clinic for potential imaging of HER2-positive breast cancer.54 SGMIB improves internalization and subsequent tumor retention of radioactivity, which has potential to improve on the current state of antibody imaging. The primary goal of the aforementioned clinical trial is to evaluate the safety, biodistribution, and dosimetry of [131I]-SGMIB anti-HER2 VHH1 in both healthy volunteers and patients with HER2-positive breast cancer. Tumor targeting potential of [131I]-SGMIB anti-HER2 VHH1 in patients with HER2-positive breast cancer will also be explored in this clinical study.
Copper-64-MM-302
MM-302 is an antibody–drug conjugate of HER2-targeted liposomal doxorubicin, as a single therapy or in combination with trastuzumab or trastuzumab and cyclophosphamide, and is currently being explored in clinical trials.55,56 Initial results showed that the drug was safe and efficacious in a group of pretreated women with HER2-positive metastatic breast cancer. MM-302 labeled with 64Cu is currently being used as an imaging agent for patient selection for combination therapy with trastuzumab and MM-302 in HER2-positive metastatic cancer, including breast. Patients will receive standard imaging at baseline (18F-FDG PET/CT), along with MR brain imaging. Patients will then receive 64Cu-MM-302 after MM-302 administration, and continue to receive subsequent doses of unlabeled MM-302 plus trastuzumab every 3 weeks and to be assessed for disease progression.57
ANTIBODY FRAGMENTS IN CLINICAL PET IMAGING
68Ga-DOTA-F(ab′)2-trastuzumab has been developed at Memorial Sloan Kettering Cancer Center as a PET imaging reagent for assessing HER2 expression status by in vivo imaging.58 Preclinical studies demonstrated promising results in the monitoring of treatment response to HSP90-targeted drugs that inhibit HER2.59 Initial clinical data were collected to assess the toxicity, pharmacokinetics, biodistribution, and dosimetry profile of 68Ga-DOTA-F(ab′)2-trastuzumab with PET/CT. Beylergil and colleagues58 led this study, where 16 women with breast cancer were enrolled in this clinical trial, with 1 patient who did not receive 68Ga-DOTA-F(ab′)2-trastuzumab and was excluded from analysis. HER2-negative (n = 7) and HER2-positive (n = 8) cases were studied in this initial study. In the HER2-positive group, 7 had received trastuzumab treatment previously and 1 had not. It was determined that 68Ga-DOTA-F(ab′)2-trastuzumab was well-tolerated, and tumor targeting was seen in 4 of the 8 patients with HER2-positive disease. Serial imaging of a single patient is represented in Fig. 8. 68Ga-DOTA-F(ab′)2-trastuzumab was also successful in detecting metastatic disease (Figs. 9 and 10). It was hypothesized that high circulating levels of trastuzumab inhibiting with tumor targeting by 68Ga-DOTA-F(ab′)2-trastuzumab, and could explain only 50% of targeting of known (18F-FDG confirmed) lesions.
Fig. 8.
Serial MIP images (patient 6) displayed at the same relative intensity. Although a slight decrease in blood pool is present, blood pool activity dominates the distribution. At 58, 109, and 189 minutes, the blood pool has a maximum standardized uptake value of 16.9, 13.6, and 9.8, respectively. There is slight increased uptake over time in the liver and kidneys. The patient had human epidermal growth factor 2 (HER2)-positive disease and was receiving lapatinib. Tumors in the left adrenal, hilar nodes, and bones were not visualized on Ga-PET. HER2, human epidermal growth factor receptor 2; MIP, maximum intensity projection; p.i, postinjection. (From Beyergil V, Morris PG, Smith-Jones PM, et al. Pilot study of 68Ga-DOTA-F(ab′)2-trastuzumab in patients with breast cancer. Nucl Med Commun 2013;34(12):1162; with permission.)
Fig. 9.
Lytic lesion in the left calvarium with gallium-68 (68Ga)-DOTA-F(ab′)2-trastuzumab uptake (patient 1). 68Ga-DOTA-F(ab′)2-trastuzumab, 68Ga-1,4,7,10-tetraazacyclododecane- N,N′,N′,N‴-tetraacetic acid (DOTA)-F(ab′) 2-trastuzumab. (From Beyergil V, Morris PG, Smith-Jones PM, et al. Pilot study of 68Ga-DOTA-F(ab′)2-trastuzumab in patients with breast cancer. Nucl Med Commun 2013;34(12):1163; with permission.)
Fig. 10.
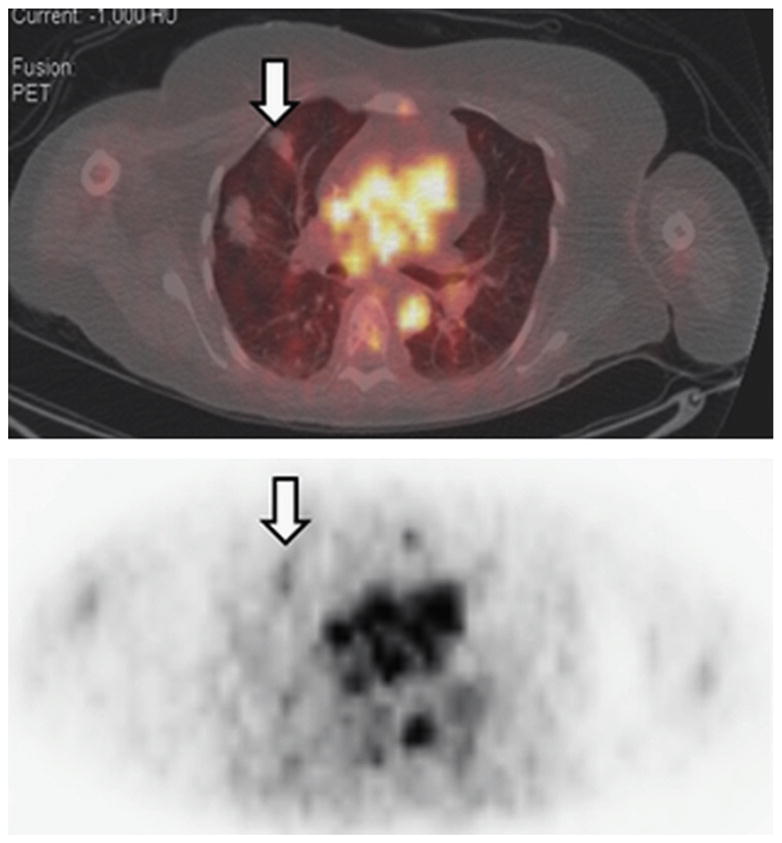
In patient 2, fused images obtained at B1 hours show mild 68Ga-DOTAF(ab′)2-trastuzumab uptake in a lung metastasis (arrows). 68Ga-DOTA-F(ab′)2- trastuzumab, 68Ga-1,4,7,10-tetraazacyclododecane-N,N′,N′,N‴-tetraacetic acid (DOTA)-F(ab′′)2-trastuzumab. (From Beyergil V, Morris PG, Smith-Jones PM, et al. Pilot study of 68Ga-DOTA-F(ab′)2-trastuzumab in patients with breast cancer. Nucl Med Commun 2013; 34(12):1163; with permission.)
CLINICAL PET IMAGING WITH RADIOLABELED AFFIBODIES
Indium-111-ABY-025 and Gallium-68-ABY-025
Affibody molecules are small affinity ligands that are designed to mimic antibody-binding properties.60 Affibodies are capable of binding to a wide range of protein targets and are used for diagnostic imaging and therapy applications. The first clinical studies using radiolabeled affibodies were performed by Baum and colleagues,23 with 111In- or 68Ga-labeled ABY-002 for imaging HER2-expressing malignant breast tumors. Three patients with metastatic breast cancer and known lesions (as identified by CT and/or 18F-FDG PET/ CT) received approximately 80 to 90 mg of radiola-beled ABY-002. Two patients received both 111In-ABY-002 and 68Ga-ABY-002, and 1 patient received 68Ga-ABY-002 only. Rapid blood clearance of radiolabeled ABY-002 was observed and allowed for quality images with both SPECT and PET radionuclides as quickly as 2 hours after injection. Radiolabeled ABY-002 was detected in metastatic lesions, along with background uptake in the kidneys and the liver. The majority of identified lesions (via 18F-FDG PET) were successfully observed using ABY-002 imaging. However, 2 sites of metastatic lesions (near the kidney and liver) were positive on 18F-FDG PET/CT scans, but not observed with ABY-002 imaging, owing to high background accumulation in these organs.61 Therefore, these particular tracers were not pursued further, but the affibodies were modified to improve background radioactivity and clearance of the ligand. The benefit of affibodies in molecular imaging is the fast pharmacokinetics and overall turnaround, but further clinical studies are required to optimize the dose, time, sensitivity, and specificity of these ligands.
Another version of affibody molecule for HER2-targeted imaging (ABY-025) was developed by Sörensen and colleagues22 ABY-025 is a reengineered affibody molecule that targets an exclusive epitope of the HER2 receptor that is not targeted by other HER2-targeted therapeutics. The advance of ABY-025 (vs ABY-002) lies in the improved tumor/background ratios, allowing for successful detection metastatic disease (most importantly in the liver) to be observed. A preliminary study evaluated the biodistribution, safety, dosimetry, and tumor-targeting potential of 111In-ABY-025 for assessing HER2 status in metastatic breast cancer. Seven patients with metastatic breast cancer and HER2-positive or negative primary tumors received approximately 100 mg (approximately 140 MBq) of 111In-ABY-025. SPECT/CT imaging was performed after 4, 24, and 48 hours. Blood samples were taken to measure radioactivity, antibodies, serum HER2, and toxicity markers. HER2 status was verified by biopsies, and the metastases were detected by 18F-FDG PET/CT 5 days before 111In-ABY-025 imaging.22
111In-ABY-025 produced high-contrast SPECT images within 4 to 24 hours. No anti-ABY-025 antibodies were observed ex vivo, indicating a non-immunogenic radiotracer, which is optimal for clinical translation. Background tissue uptake was highest in the kidneys, then the liver and spleen. 111In-ABY-025 shows promise as a noninvasive tool for detecting HER2 in metastatic breast cancer, even during the occurrence of HER2-targeted antibody treatment.
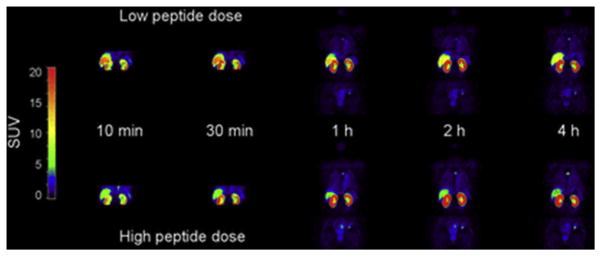
Sandström and colleagues21 produced a 68Ga-labeled ABY-025 for diagnosis of HER2-positive breast cancer tumors with PET. The aim of the preliminary study with 68Ga-ABY-025 was to measure the biodistribution and estimate the radiation dosimetry of 68Ga-ABY-025 for 2 different peptide mass doses in the same group of patients (Fig. 11). Eight patients with metastatic breast cancer were included in this study. Each patient received a 45-minute dynamic (abdominal) and 3 whole-body PET/CT scans at 1, 2, and 4 hours after injection of a low peptide dose and a high peptide dose. The highest background uptake was observed in the kidneys and liver at all imaging time points.
Fig. 11.
Representative whole-body images at 10, 30, 60, 120, and 240 minutes after injection of a low and high peptide dose in the same patient. (From Sandström M, Lindskog K, Velikyan I, et al. Bio-distribution and radiation dosimetry of the anti-HER2 affibody molecule 68Ga-ABY-025 in breast cancer patients. J Nuc Med 2016; 57(6):868; with permission.)
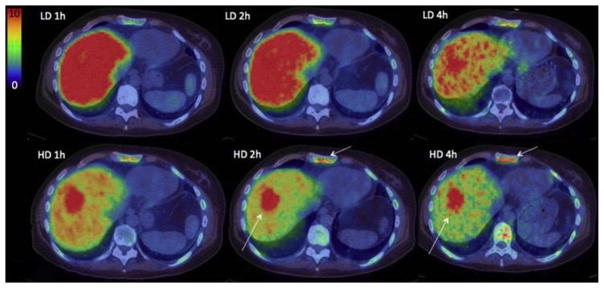
In a phase I/II study, 68Ga-ABY-025 to study effect of peptide mass and the effects on quantified uptake in tumors to pathologic analysis.24 Sixteen women with metastatic breast cancer (confirmed by 18F-FDG PET/CT) and receiving treatment were included. PET imaging was performed at 1, 2, and 4 hours after injection of 212 ± 46 MBq 68Ga-ABY-025 (Fig. 12 for example).
Fig. 12.
Uptake images of gallium-68 (68Ga)-BY-025 with low (LD) and high administered peptide dose (HD) at 1, 2 and 4 hours after injection in patient 2. All images are normalized to the same scale of standardized uptake value 10. A rainbow color scale is used: red color, standardized uptake value of greater than 10. Normal soft tissue uptake was higher with LD. Note the gradually higher contrast of metastases in liver and bone (arrows), owing to both the disappearance of normal liver uptake and tumor uptake accumulation. (From Sörensen J, Velikyan I, Sandberg D, et al. Measuring HER2-receptor expression in metastatic breast cancer using [68Ga]ABY-025 affibody PET/CT. Theranostics 2016;6(2):266; with permission.)
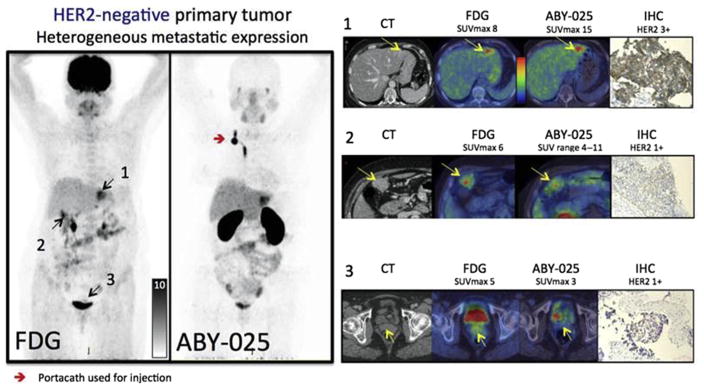
In the cohort of patients (10 total, 6 with HER2-positive, and 4 with HER2-negative primary tumors), 68Ga-ABY-025 PET/CT with 2 different doses of injected peptide was performed. The optimal time point for 68Ga-ABY-025 PET was found to be 4 hours, and accurately quantifies whole-body HER2-receptor status in metastatic breast cancer, and showed a change in uptake among patients receiving targeted treatment in 3 of the 16 patients. Patients with widespread metastatic disease were used as an example of 18F-FDG versus 68Ga-ABY-025 PET (Fig. 13) to gauge the ability of the radiotracer to detect distant disease.
Fig. 13.
Based on the results from gallium-68-ABY-025 PET/computed tomography (CT), mixed expression of human epidermal growth factor receptor 2 (HER2) in metastatic breast cancer was seen in several patients (arrows) and confirmed by biopsies in 2. Patient 9 had HER2-negative primary tumor and was enrolled as negative control. Fluorodeoxyglucose (FDG)-PET/CT showed metastases in left liver lobe, peritoneal lymph nodes and cervix of uterus (arrows). ABY-025 uptake was high in the liver metastasis, low in peritoneal metastases and absent in the cervical region (not shown). According to immunohistochemistry (IHC), the liver finding was true positive and both other sites were true negative. SUVmax, maximum standardized uptake volume. (From Sörensen J, Velikyan I, Sandberg D, et al. Measuring HER2-receptor expression in metastatic breast cancer using [68Ga] ABY-025 affibody PET/CT. Theranostics 2016;6(2):268; with permission.)
CLINICAL PET IMAGING WITH RADIOLABELED NANOBODIES
Gallium-68-Human Epidermal Growth Factor Receptor 2-Nanobody
Nanobodies directed against HER2 have also been developed as probes for molecular imaging. Nanobodies are produced from a heavy chain component of antibodies, are the smallest antigen-binding antibody fragments, and have ideal characteristics for PET imaging.61
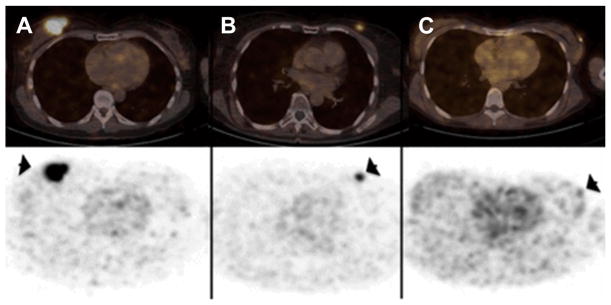
Initial studies by Keyaerts and colleagues62 assessed the safety, biodistribution, and dosimetry of a 68Ga-HER2-Nanobody. In this study, 20 women with primary or metastatic breast carcinoma were injected with 68Ga-HER2-Nanobody (average dose, 107 MBq) and dosimetry calculations were based from images obtained at 10, 60, and 90 minutes after tracer administration. Representative images of this study are shown in Fig. 14.
Fig. 14.
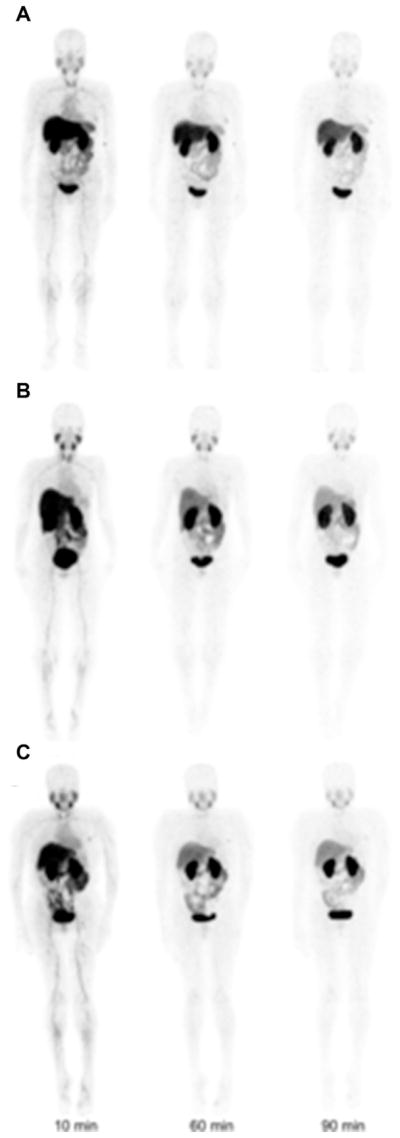
Representative maximum intensity projection images at 10, 60, and 90 minutes after injection of gallium-68 (68Ga)-human epidermal growth factor receptor 2 (HER2)-nanobody for different mass subgroups. (A) Patient 4, injected with 0.01 mg of 68Ga-HER2-nanobody. (B) Patient 12, injected with 0.1 mg. (C) Patient 17, injected with 1.0 mg. (From Keyaerts M, Xavier C, Heemskerk J, et al. Phase I study of 68Ga-HER2-nanobody for PET/CT assessment of HER2 expression in breast carcinoma. J Nuc Med 2016;57(1):30; with permission.)
The tumor-targeting potential was assessed in primary and metastatic lesions. No adverse reactions to the tracer were recorded, and only 10% of injected dose remaining in the blood at 1 hour after injection. Background uptake was observed in the kidneys, liver, and intestines. Primary tumors were more variable in tracer accumulation (Fig. 15), whereas metastatic lesions showed specific, easily delineated tracer accumulation (Fig. 16).62
Fig. 15.
Uptake of gallium-68 (68Ga)-human epidermal growth factor receptor 2-nanobody in primary breast carcinoma lesions (arrows) on PET/computed tomography (CT) images (top) and PET images (bottom). (A) Patient 14 showed highest tracer uptake (mean standardized uptake value, 11.8). (B) Patient 15 showed moderate tracer uptake, which was easily discernable from background (mean standardized uptake value, 4.9). (C) Patient 6 showed no uptake (mean standardized uptake value, 0.9), with CT showing marker clip at tumor region. (From Keyaerts M, Xavier C, Heemskerk J, et al. Phase I study of 68Ga-HER2-nanobody for PET/CT assessment of HER2 expression in breast carcinoma. J Nuc Med 2016;57(1):31; with permission.)
Fig. 16.
Uptake of gallium-68 (68Ga)-human epidermal growth factor receptor 2-nanobody in metastatic lesions on PET/computed tomography images (top) and PET images (bottom). (A) Patient 18, with invaded lymph nodes in mediastinum and left hilar region. (B) Patient 20, with bone metastasis in pelvis. (From Keyaerts M, Xavier C, Heemskerk J, et al. Phase I study of 68Ga-HER2-nanobody for PET/CT assessment of HER2 expression in breast carcinoma. J Nuc Med 2016;57(1):32; with permission.)
Overall, 68Ga-HER2-Nanobody PET/CT is a well-tolerated procedure with a comparable radiation dose to other routinely used PET tracers. The biodistribution of this radiotracer is favorable, with the highest uptake in the kidneys, liver, and intestines but low background levels in all other organs, including where primary or metastatic disease is most often found. Tracer accumulation in HER2-positive metastases is high, can be easily distinguished from background tissues, and warrants further assessment in a phase II trial.
18F-Human Epidermal Growth Factor Receptor 2-Nanobody
Xavier and colleagues63 have recently labeled the GMP-produced HER2-nanobody with 18F with and shown success with tumor targeting preclinically. Specific uptake for HER2-positive xenografts (5.94 ± 1.17% IA/g, 1 hour after injection) was observed with high tumor-to-tissue ratios. [18F]-anti-HER2-nanobody showed rapid clearance through the kidneys (4% IA/g at 3 hours post-injection). The probe was also able to image HER2-positive tumors when coadministered with trastuzumab, indicating the potential use of this tracer for patient selection and monitor patients undergoing therapy. With the success of this 18F-labeled probe and the positive results from phase I/II 68Ga-HER2-nanobody trials, this probe should enter the clinic with ease.
SUMMARY
HER2 receptor status is an important biomarker in patients with breast cancer, integral to treatment decisions and prognosis. HER2-targeted therapies have demonstrated clear survival advantages in patients with HER2-positive breast carcinoma. The possibility of heterogeneity of HER2 expression both within primary tumors and between primary and metastatic disease sites may limit the value of individual tumor biopsies and demonstrates a need for accurate, whole body assessment of HER2 status. Molecular imaging, most importantly PET, has been identified as a noninvasive tool to assess HER2-positive primary lesions and metastases. Radiolabeled antibodies, antibody fragments, and affibody molecules enable PET imaging to be a reliable and quantitative method for detecting HER2-positive cancer. HER2-targeted PET imaging has the potential to become a clinically valuable tool in the care of patients with breast cancer.
KEY POINTS.
Knowledge of the receptor status of breast cancer is integral to patient treatment and prognosis.
Expression of estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 (HER2) is assessed using immunohistochemistry, fluorescence in situ hybridization, or chromogen in situ hybridization.
HER2 is expressed in low levels on the surface of normal cells but overexpressed in invasive breast cancers, a biomarker for aggressive breast cancer and response to HER2-targeted therapies.
Monoclonal antibodies are used as HER2-targeted therapies, which can be labeled with positron-, gamma-emitting radionuclides for PET/computed tomography and single-photon emission computed tomography molecular imaging agents.
PET imaging allows for noninvasive whole body analysis of tumors and may detect previously unsuspected HER2-positive metastases in patients with a HER2-negative primary breast tumor.
Footnotes
Disclosure Statement: The authors have nothing to disclose.
References
- 1.Weigelt B, Peterse JL, van’t Veer LJ. Breast cancer metastasis: markers and models. Nat Rev Cancer. 2005;5(8):591–602. doi: 10.1038/nrc1670. [DOI] [PubMed] [Google Scholar]
- 2.Dunnwald LK, Rossing MA, Li CI. Hormone receptor status, tumor characteristics, and prognosis: a prospective cohort of breast cancer patients. Breast Cancer Res. 2007;9(1):1–10. doi: 10.1186/bcr1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van de Vijver M, Bilous M, Hanna W, et al. Chromogenic in situ hybridisation for the assessment of HER2 status in breast cancer: an international validation ring study. Breast Cancer Res. 2007;9(5):R68–77. doi: 10.1186/bcr1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arteaga CL, Sliwkowski MX, Osborne CK, et al. Treatment of HER2-positive breast cancer: current status and future perspectives. Nat Rev Clin Oncol. 2012;9(1):16–32. doi: 10.1038/nrclinonc.2011.177. [DOI] [PubMed] [Google Scholar]
- 5.Capala J, Bouchelouche K. Molecular imaging of HER2-positive breast cancer - a step toward an individualized “Image and Treat” strategy. Curr Opin Oncol. 2010;22(6):559–66. doi: 10.1097/CCO.0b013e32833f8c3a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elias SG, Adams A, Wisner DJ, et al. Imaging features of HER2 overexpression in breast cancer: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2014;23(8):1464–83. doi: 10.1158/1055-9965.EPI-13-1170. [DOI] [PubMed] [Google Scholar]
- 7.O’Sullivan CC, Bradbury I, Campbell C, et al. Efficacy of adjuvant trastuzumab for patients with human epidermal growth factor receptor 2–positive early breast cancer and tumors ≤ 2 cm: a meta-analysis of the randomized trastuzumab trials. J Clin Oncol. 2015;33(24):2600–8. doi: 10.1200/JCO.2015.60.8620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paik S, Bryant J, Tan-Chiu E, et al. Real-world performance of HER2 testing—national surgical adjuvant breast and bowel project experience. J Natl Cancer Inst. 2002;94(11):852–4. doi: 10.1093/jnci/94.11.852. [DOI] [PubMed] [Google Scholar]
- 9.Becker S, Becker-Pergola G, Fehm T, et al. Her2 Expression on disseminated tumor cells from bone marrow of breast cancer patients. Anticancer Res. 2005;25(3B):2171–5. [PubMed] [Google Scholar]
- 10.Perez EA, Suman VJ, Davidson NE, et al. HER2 testing by local, central, and reference laboratories in specimens from the north central cancer treatment group N9831 intergroup adjuvant trial. J Clin Oncol. 2006;24(19):3032–8. doi: 10.1200/JCO.2005.03.4744. [DOI] [PubMed] [Google Scholar]
- 11.Paik S, Kim C, Wolmark N. HER2 status and benefit from adjuvant trastuzumab in breast cancer. N Engl J Med. 2008;358(13):1409–11. doi: 10.1056/NEJMc0801440. [DOI] [PubMed] [Google Scholar]
- 12.Ulaner GA, Hyman D, Ross D, et al. Detection of HER2-positive metastases in patients with HER2-negative primary breast cancer using the 89Zr-DFO-trastuzumab PET/CT. J Nuc Med. 2016;57(10):1523–8. doi: 10.2967/jnumed.115.172031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tolmachev V. Imaging of HER-2 overexpression in tumors for guiding therapy. Curr Pharm Des. 2008;14(28):2999–3019. doi: 10.2174/138161208786404290. [DOI] [PubMed] [Google Scholar]
- 14.Weissleder R. Molecular imaging in cancer. Science. 2006;312(5777):1168–71. doi: 10.1126/science.1125949. [DOI] [PubMed] [Google Scholar]
- 15.Ulaner GA, Riedl CC, Dickler MN, et al. Molecular imaging of biomarkers in breast cancer. J Nuc Med. 2016;57(Suppl 1):53S–9S. doi: 10.2967/jnumed.115.157909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Warram JM, de Boer E, Sorace AG, et al. Antibody based imaging strategies of cancer. Cancer Metastasis Rev. 2014;33(0):809–22. doi: 10.1007/s10555-014-9505-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lamberts LE, Williams SP, Terwisscha van Scheltinga AGT, et al. Antibody positron emission tomography imaging in anticancer drug development. J Clin Oncol. 2015;33(13):1491–504. doi: 10.1200/JCO.2014.57.8278. [DOI] [PubMed] [Google Scholar]
- 18.Boerman OC, Oyen WJG. Immuno-PET of cancer: a revival of antibody imaging. J Nuc Med. 2011;52(8):1171–2. doi: 10.2967/jnumed.111.089771. [DOI] [PubMed] [Google Scholar]
- 19.van Dongen GA, Visser GW, Lub-de Hooge MN, et al. Immuno-PET: a navigator in monoclonal antibody development and applications. Oncologist. 2007;12(12):1379–89. doi: 10.1634/theoncologist.12-12-1379. [DOI] [PubMed] [Google Scholar]
- 20.Deri MA, Zeglis BM, Francesconi LC, et al. PET imaging with (89)Zr: from radiochemistry to the clinic. Nucl Med Biol. 2013;40(1):3–14. doi: 10.1016/j.nucmedbio.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sandström M, Lindskog K, Velikyan I, et al. Bio-distribution and radiation dosimetry of the anti-HER2 affibody molecule 68Ga-ABY-025 in breast cancer patients. J Nuc Med. 2016;57(6):867–71. doi: 10.2967/jnumed.115.169342. [DOI] [PubMed] [Google Scholar]
- 22.Sörensen J, Sandberg D, Sandström M, et al. First-in-human molecular imaging of HER2 expression in breast cancer metastases using the 111In-ABY-025 affibody molecule. J Nuc Med. 2014;55(5):730–5. doi: 10.2967/jnumed.113.131243. [DOI] [PubMed] [Google Scholar]
- 23.Baum RP, Prasad V, Müller D, et al. Molecular imaging of HER2-expressing malignant tumors in breast cancer patients using synthetic 111In- or 68Ga-labeled affibody molecules. J Nuc Med. 2010;51(6):892–7. doi: 10.2967/jnumed.109.073239. [DOI] [PubMed] [Google Scholar]
- 24.Sörensen J, Velikyan I, Sandberg D, et al. Measuring HER2-receptor expression in metastatic breast cancer using [(68)Ga]ABY-025 affibody PET/CT. Theranostics. 2016;6(2):262–71. doi: 10.7150/thno.13502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kurihara H, Hamada A, Yoshida M, et al. 64Cu-DOTA-trastuzumab PET imaging and HER2 specificity of brain metastases in HER2-positive breast cancer patients. EJNMMI Res. 2015;5(1):1–8. doi: 10.1186/s13550-015-0082-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tamura K, Kurihara H, Yonemori K, et al. 64Cu-DOTA-trastuzumab PET imaging in patients with HER2-positive breast cancer. J Nucl Med. 2013;54(11):1869–75. doi: 10.2967/jnumed.112.118612. [DOI] [PubMed] [Google Scholar]
- 27.Mortimer JE, Bading JR, Colcher DM, et al. Functional imaging of human epidermal growth factor receptor 2–positive metastatic breast cancer using 64Cu-DOTA-trastuzumab PET. J Nucl Med. 2014;55(1):23–9. doi: 10.2967/jnumed.113.122630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344(11):783–92. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 29.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353(16):1673–84. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 30.Breast cancer: trastuzumab therapy for small, HER2-positive breast tumours. Nat Rev Clin Oncol. 2015;12(3):126. [Google Scholar]
- 31.Baselga J. Phase I and II clinical trials of trastuzumab. Ann Oncol. 2001;12(Suppl 1):S49–55. doi: 10.1093/annonc/12.suppl_1.s49. [DOI] [PubMed] [Google Scholar]
- 32.Buzdar AU, Ibrahim NK, Francis D, et al. Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2–positive operable breast cancer. J Clin Oncol. 2005;23(16):3676–85. doi: 10.1200/JCO.2005.07.032. [DOI] [PubMed] [Google Scholar]
- 33.Dijkers ECF, Kosterink JGW, Rademaker AP, et al. Development and characterization of clinical-grade 89Zr-trastuzumab for HER2/neu immunoPET imaging. J Nuc Med. 2009;50(6):974–81. doi: 10.2967/jnumed.108.060392. [DOI] [PubMed] [Google Scholar]
- 34.Gaykema SB, Schröder CP, Vitfell-Rasmussen J, et al. 89Zr-trastuzumab and 89Zr-bevacizumab PET to evaluate the effect of the HSP90 inhibitor NVP-AUY922 in metastatic breast cancer patients. Clin Cancer Res. 2014;20(15):3945–54. doi: 10.1158/1078-0432.CCR-14-0491. [DOI] [PubMed] [Google Scholar]
- 35.Dijkers EC, Oude Munnink TH, Kosterink JG, et al. Biodistribution of 89Zr-trastuzumab and PET imaging of HER2-positive lesions in patients with metastatic breast cancer. Clin Pharmacol Ther. 2010;87(5):586–92. doi: 10.1038/clpt.2010.12. [DOI] [PubMed] [Google Scholar]
- 36.Gaykema SB, Brouwers AH, Hovenga S, et al. Zirconium-89-trastuzumab positron emission tomography as a tool to solve a clinical dilemma in a patient with breast cancer: a case report. J Clin Oncol. 2012;30(6):e74–5. doi: 10.1200/JCO.2011.38.0204. [DOI] [PubMed] [Google Scholar]
- 37.Jhaveri K, Taldone T, Modi S, et al. Advances in the clinical development of heat shock protein 90 (Hsp90) inhibitors in cancers. Biochim Biophys Acta. 2012;1823(3):742–55. doi: 10.1016/j.bbamcr.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Friedland JC, Smith DL, Sang J, et al. Targeted inhibition of Hsp90 by ganetespib is effective across a broad spectrum of breast cancer subtypes. Invest New Drugs. 2014;32(1):14–24. doi: 10.1007/s10637-013-9971-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lavaud P, Andre F. Strategies to overcome trastuzumab resistance in HER2-overexpressing breast cancers: focus on new data from clinical trials. BMC Med. 2014;12:132. doi: 10.1186/s12916-014-0132-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gebhart G, Lamberts LE, Wimana Z, et al. Molecular imaging as a tool to investigate heterogeneity of advanced HER2-positive breast cancer and to predict patient outcome under trastuzumab emtansine (T-DM1): the ZEPHIR trial. Ann Oncol. 2016;27(4):619–24. doi: 10.1093/annonc/mdv577. [DOI] [PubMed] [Google Scholar]
- 41.University Medical Center Groningen, VU University Medical Center, University Medical Center Nijmegen. Clinical-Trials.gov [Internet] Groningen (The Netherlands): University Medical Center Groningen; 2016. [Accessed March 14, 2017]. IMaging patients for cancer drug selecTion - meta-static breast cancer (IMPACT-MBC) NLM Identifier: NCT01957332. Available at: https://clinicaltrials.gov/ct2/show/NCT01957332. [Google Scholar]
- 42.Jules Bordet Institute. ClinicalTrials.-gov [Internet] Brussels (Belgium): Institut Jules Bordet; 2016. [Accessed March 14, 2017]. Pilot imaging study with 89Zr-trastuzumab in HER2-positive metastatic breast cancer patients (IJBMNZrT003) NLM Identifier: NCT01420146. Available at: https://clinicaltrials.gov/ct2/show/NCT01420146. [Google Scholar]
- 43.Jules Bordet Institute. Clinical Trials.gov [Internet] Brussels (Belgium): Institut Jules Bordet; 2016. [Accessed March 14, 2017]. HER2 Imaging Study to Identify HER2 positive metastatic breast cancer patient unlikely to benefit from T-DM1 (ZEPHIR) NLM Identifier: NCT01565200. Available at: https://clinicaltrials.gov/ct2/show/NCT01565200. [Google Scholar]
- 44.Memorial Sloan Kettering Cancer Center. United States Department of Defense, Genentech, Inc. ClinicalTrials.gov [Internet] New York: Memorial Sloan Kettering Cancer Center (US); 2016. [Accessed March 14, 2017]. Can HER2 targeted 89Zr-trastuzumab PET/CT identify unsuspected HER2 positive breast cancer metastases, which are amenable to HER2 targeted therapy? NLM Identifier: NCT02286843. Available at: https://clinicaltrials.gov/ct2/show/NCT02286843. [Google Scholar]
- 45.Washington University School of Medicine, National Cancer Institute (NCI) ClinicalTrials.gov [Internet] St Louis (MO): Washington University School of Medicine (US); 2016. [Accessed March 14, 2017]. 89ZrTrastuzumab breast imaging with positron emission tomography. NLM Identifier: NCT02065609. Available at: https://clinicaltrials.gov/ct2/show/NCT02065609. [Google Scholar]
- 46.City of Hope Medical Center, National Cancer Institute (NCI) ClinicalTrials.gov [Internet] Duarte (CA): City of Hope Medical Center, (US); 2016. [Accessed March 14, 2017]. Copper (Cu) 64-DOTA-trastuzumab PET in predicting response to treatment with trastuzumab and pertuzumab before surgery in patients with HER2 positive breast cancer. NLM Identifier: NCT02827877. Available at: https://clinicaltrials.gov/ct2/show/NCT02827877. [Google Scholar]
- 47.Perik PJ, Lub-De Hooge MN, Gietema JA, et al. Indium-111–labeled trastuzumab scintigraphy in patients with human epidermal growth factor receptor 2–positive metastatic breast cancer. J Clin Oncol. 2006;24(15):2276–82. doi: 10.1200/JCO.2005.03.8448. [DOI] [PubMed] [Google Scholar]
- 48.Gaykema S, de Jong J, Perik P, et al. (111)In-trastuzumab scintigraphy in HER2-positive metastatic breast cancer patients remains feasible during trastuzumab treatment. Mol Imaging. 2014;13:1–6. doi: 10.2310/7290.2014.00011. [DOI] [PubMed] [Google Scholar]
- 49.Wong JYC, Raubitschek A, Yamauchi D, et al. A pretherapy biodistribution and dosimetry study of indium-111-radiolabeled trastuzumab in patients with human epidermal growth factor receptor 2-over-expressing breast cancer. Cancer Biother Radiopharm. 2010;25(4):387–94. doi: 10.1089/cbr.2010.0783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harbeck N, Beckmann MW, Rody A, et al. HER2 dimerization inhibitor pertuzumab – mode of action and clinical data in breast cancer. Breast Care (Basel) 2013;8(1):49–55. doi: 10.1159/000346837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Swain SM, Baselga J, Kim S-B, et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med. 2015;372(8):724–34. doi: 10.1056/NEJMoa1413513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ontario Clinical Oncology Group (OCOG) ClinicalTrials.gov [Internet] Hamilton (Canada): McMaster University, Ontario Clinical Oncology Group (Canada); 2016. [Accessed March 14, 2017]. Imaging with 111 indium (111In)-pertuzumab (PmAb) to predict response to trastuzumab (TmAb) in human epidermal growth factor-2 (HER2) positive metastatic breast cancer (MBC) or locally advanced breast cancer (LABC) (PETRA) NLM Identifier: NCT01805908. Available at: https://clinicaltrials.gov/ct2/show/NCT01805908. [Google Scholar]
- 53.Marquez BV, Ikotun OF, Zheleznyak A, et al. Evaluation of (89)Zr-pertuzumab in breast cancer xenografts. Mol Pharm. 2014;11(11):3988–95. doi: 10.1021/mp500323d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Camel-IDS NV. ClinicalTrials.gov [Internet] Brussels (Belgium): Camel-IDS NV; 2016. [Accessed March 14, 2017]. Study to evaluate the safety, biodistribution, radiation dosimetry and tumor imaging potential of [131I]-SGMIB anti-HER2 VHH1 in healthy volunteers and breast cancer patients (CAM-VHH1) NLM Identifier: NCT02683083. Available at: https://clinicaltrials.gov/ct2/show/NCT02683083. [Google Scholar]
- 55.Merrimack Pharmaceuticals. ClinicalTrials.gov [Internet] Cambridge (MA): Merrimack Pharmaceuticals (US); 2016. [Accessed March 14, 2017]. MM-302 plus trastuzumab vs. chemotherapy of physician’s choice plus trastuzumab in HER2-positive locally advanced/metastatic breast cancer patients (HERMIONE) NLM Identifier: NCT02213744. Available at: https://clinicaltrials.gov/ct2/show/NCT02213744. [Google Scholar]
- 56.Merrimack Pharmaceuticals. ClinicalTrials.gov [Internet] Cambridge (MA): Merrimack Pharmaceuticals (US); 2016. [Accessed March 14, 2017]. Safety and pharmacokinetic study of MM-302 in patients with advanced breast cancer. NLM Identifier: NCT01304797. Available at: https://clinicaltrials.gov/ct2/show/NCT01304797. [Google Scholar]
- 57.University of California, San Francisco. ClinicalTrials.gov [Internet] San Francisco (CA): University of California, San Francisco (US); 2016. [Accessed March 14, 2017]. 64-Cu labeled brain PET/MRI for MM-302 in advanced HER2+ cancers with brain mets. NLM Identifier: NCT02735798. Available at: https://clinicaltrials.gov/ct2/show/NCT02735798. [Google Scholar]
- 58.Beylergil V, Morris PG, Smith-Jones PM, et al. Pilot study of (68)Ga-DOTA-F(ab′)(2)-trastuzumab in patients with breast cancer. Nucl Med Commun. 2013;34(12):1157–65. doi: 10.1097/MNM.0b013e328365d99b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weber WA. Chaperoning drug development with PET. J Nuc Med. 2006;47(5):735–7. [PubMed] [Google Scholar]
- 60.Feldwisch J, Tolmachev V. Engineering of affibody molecules for therapy and diagnostics. Methods Mol Biol. 2012;899:103–26. doi: 10.1007/978-1-61779-921-1_7. [DOI] [PubMed] [Google Scholar]
- 61.Chakravarty R, Goel S, Cai W. Nanobody: the “magic bullet” for molecular imaging? Theranostics. 2014;4(4):386–98. doi: 10.7150/thno.8006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Keyaerts M, Xavier C, Heemskerk J, et al. Phase I study of 68Ga-HER2-Nanobody for PET/CT assessment of HER2 expression in breast carcinoma. J Nuc Med. 2016;57(1):27–33. doi: 10.2967/jnumed.115.162024. [DOI] [PubMed] [Google Scholar]
- 63.Xavier C, Blykers A, Vaneycken I, et al. 18F-nano-body for PET imaging of HER2 overexpressing tumors. Nucl Med Biol. 2016;43(4):247–52. doi: 10.1016/j.nucmedbio.2016.01.002. [DOI] [PubMed] [Google Scholar]