Abstract
Vaccines for many infectious diseases are poorly developed or simply unavailable. There are significant technological and practical design issues that contribute to this problem; thus, a solution to the vaccine problem will require a systematic approach to test the multiple variables that are required to address each of the design challenges. Nanoparticle technology is an attractive methodology for optimizing vaccine development because design variables can be tested individually or in combination. The biology of individual components that constitute an effective vaccine is often well understood and may be integrated into particle design, affording optimal immune responses to specific pathogens. Here, we review technological variables and design parameters associated with creating modular nanoparticle vaccine systems that can be used as vectors to protect against disease. Variables, such as the material and size of the core matrix, surface modification for attaching targeting ligands and routes of administration, are discussed. Optimization of these variables is important for the development of nanoparticle-based vaccine systems against infectious diseases and cancer.
Keywords: DC targeting, nanoparticle, oral vaccination, PEG, PLGA, vaccine delivery
Vaccine design: what are the issues?
Vaccine development for many infectious diseases, such as HIV, malaria and even cancer, is not well advanced or is simply unavailable. There are a number of significant scientific challenges that have limited this development. First, most vaccines offer protection by eliciting neutralizing-antibody titers. For many conditions involving intracellular pathogens, such as HIV and malaria, humoral immunity is not sufficient protection and cellular immunity is critical for complete immunity [1]. Unfortunately, few if any approaches are available that prime cell-mediated immunity efficiently by direct intracellular delivery of antigen. Second, we have yet to develop ‘tunable’ adjuvants that can be engineered to optimize the magnitude and direction of an immune response [2,3]. Third, standard immunization protocols, involving parenteral (subcutaneous or intramuscular) injection, are not practiced easily in underdeveloped nations, where refrigeration and medical-support resources are often limited. Finally, there is a lack of a general approach to the design of oral vaccines that yield both systemic and mucosal immunity. Oral vaccines are generally preferable because they are painless, yield better patient compliance and can be administered without trained personnel. In light of these issues, there is a critical need for safe and stable vaccine systems that would address these factors [4–7].
Variables affecting vaccine design
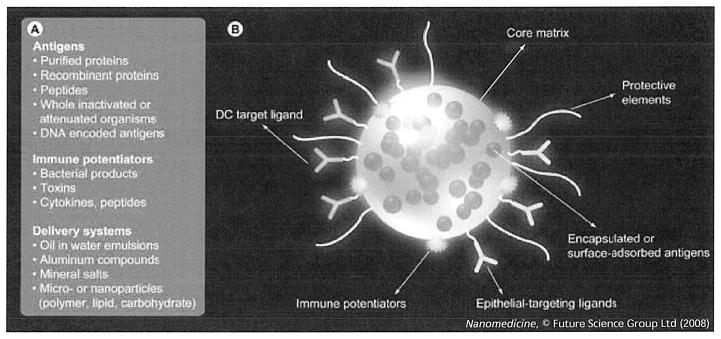
Several key variables are assembled and integrated in the design of vaccines (Figure 1) [8,9]. The first variable is the form of the antigen itself, which can be whole inactivated or attenuated pathogens, purified recombinant proteins and peptides or DNA-encoded antigens. Pathogens are emerging and changing continually (e.g., SARS and avian influenza), meaning that new potential immunogens are appearing constantly. Thus, there is a clear need to design vaccine systems that can test the efficacy of vaccines for emerging pathogens rapidly and efficiently [10]. Unfortunately, large-scale and safe production of stable vaccine products typically involves the purification of natural or recombinant forms of antigenic subunits. Once purified, however, individual antigens often become less immunogenic compared with whole pathogens or crude extracts, necessitating a means to amplify the immune response against the purified subunit antigen. Thus, a second necessary component of a vaccine involves potentiating or stimulating the innate and adaptive arms of the immune system to the antigen subunit with an adjuvant [8,9]. Adjuvants can be defined broadly as systems (molecules, particles, synthetic or natural) that act nonspecifically (e.g., through the induction of proinflammatory cytokines and/or stimulation of Toll-like receptors [TLRs]). For simplicity, we will define adjuvants as substances that can enhance an immune response and these could be either immune potentiators or delivery systems that stabilize antigen and increase the cellular uptake, trafficking and presentation of the antigen. Immune potentiators may include bacterial products, toxins or other molecules that augment specific immunity. Potentiators have various benefits but also many attendant risks (e.g., triggering deleterious inflammatory responses). Vaccine-delivery systems present a defined amount of bulk antigen in a repetitive or sustained fashion to the appropriate immune cells and to the appropriate compartments within those cells. Although a number of indirect delivery systems have been studied, such as the ‘gene gun’ [11], a growing trend in vaccine-delivery systems is to target and deliver both antigen and immune potentiator molecules to target cells of the immune system. This is highly reminiscent of the strategy of viruses that use specific components of the immune system to their advantage for delivery of their payload.
Figure 1. Nanopaticulate-based vaccines may address several of the key variables associated with constructing effective vaccines.
(A) Several key variables need to be incorporated in the design of optimal vaccines. Variables, such as the nature of the antigen, immunepotentiation and the delivery vehicle, can be integrated simultaneously into the design of particulates for vaccination. (B) A modular vaccine particulate system. A core matrix supports the encapsulation or direct conjugation of antigens. Targeting ligands, such as DC-specific antibodies or epithelial cell-specific ligands, facilitate intracellular transport. Negotiation of the particulate through physiological barriers (low pH in the gastrointestinal tract, elimination from bloodstream) can be achieved with the addition of protective elements to the particle surface. DC: Dendritic cell.
Vaccines in present use consist of live, attenuated or inactivated pathogens; the only US FDA-approved adjuvant is colloidal alum (aluminum phosphate or aluminum hydroxide). Live or attenuated formulations can be administered but are associated with many risks. Alum, which is used to increase the effectiveness of whole inactivated or component vaccines, has greatly limited immunostimulatory properties [12,13] and associated allergic side effects [13,14]. In addition, because of the historical emphasis on eliciting humoral immune responses, most adjuvants, including alum, are optimized for the effective induction of high antibody serum titers but are ineffective at eliciting a strong cellular, T-cell-mediated immune response or mucosal immune response. T-cell responses are essential for eliminating intracellular pathogens; mucosal immunity is an important consideration for complete protection against cellular and viral pathogens transmitted through mucosal surfaces [15] (e.g., herpes simplex virus and enteric pathogens). These factors, coupled with the difficulties of manufacture, storage and transport, have together limited the utility of current approaches in the clinic and in the field [16–18].
Vaccine development with nanoparticle systems
Viruses and pathogens that elicit or subvert immune responses are, in essence, small particles endowed with the ability to interact with or avoid cells of the immune system in a variety of ways. Much has been learned about their individual strategies and this new biological information is instructive for the design of new, rational vaccine technologies. Nanoparticles offer a new modality for the design of vaccine systems. The attractiveness of these systems derives from their size and the flexible addition and subtraction of antigen, adjuvant, immune potentiators, molecular recognition and transport-mediation elements, as well as intracellular uptake mediators (Figure 1B). Owing to the size of these materials, they can be administered through several routes, such as oral, subcutaneous and nasal passages. All these variables are important in optimizing an effective vaccine-delivery system and several approaches have been proposed and demonstrated for this purpose, as shown in Table 1. Detailed discussion of these systems is beyond the scope of this review and the reader is recommended to consult excellent reviews on the subject [5,9,17,19–21]. Here, we highlight a number of variables and design challenges that have a role in optimizing biodegradable nanoparticle-based vaccines. These variables include: the core material of the particle itself, surface modification, targeting to dendritic cells (DCs) for improved antigen presentation and transport through the body for effective priming of the immune system.
Table 1.
Examples of targeted and untargeted particulate systems.
| Particle system | Targeting ligands | Antigen* | Size | Route of administration‡ | Type of immunity |
|---|---|---|---|---|---|
| Cross-linked monomers [62,99] | DEC-205 [62], CpG oligonucleotides [99] | OVA | 200–500 nm | sc. | Cellular and humoral [62,99] |
| PLGA | Monophosphoryl lipid A [100], RGD [92] | MUC1 peptide [100] HA peptide [101] |
200–310 nm | sc. [100, 101] in. [101] oral [92] |
Cellular [100] and humoral [92,101] |
| Magnetic [102] | BSA | 100–150 nm | ip. | Humoral [102] | |
| PEG-PPS [40,41] | OVA | 20–100 nm | id. | Cellular and humoral | |
| Liposomes | LPS, IFN-G, CD11C and anti-DEC205 [103], CpG [104] | OVA [103], TAA [104] | 100–500 nm | iv. [103], sc. [104] | Cellular and humoral |
| Polystyrene [105] | OVA | 40–50 nm | id. | Cellular and humoral | |
| Chitosan | Mannose [106] | OVA [107], HA [108], BSA [109], HBV [106] | 400 nm-3 μm | in. [107,108], ip., oral [109], im. [106] | Cellular [106] and humoral |
| Dendriplexes-PLGA [110] | Anthrax | 200–400 nm | im. | Humoral | |
| Poly(glutamic acid) [111–113] | HIV epitopes | 200–450 nm | in. | Cellular |
MUC1, OVA, TAA, HA, BSA, HBV.
sc., id., im., in., ip., iv.
BSA: Bovine serum albumin; HA: Hemagglutinin antigen; HBV: Hepatitis B viral peptide; id.: Intradermal; im.: Intramuscular; in.: Intranasal; ip.: Intraperitoneal; iv.: Intravenous; MUC1: Mucin 1 peptide; OVA: Ovalbumin; PEG-PPS: Poly(ethylene glycol) polypropylene sulfide); PLGA: Poly(lactic-co-glycolic acid); sc.: Subcutaneous; TAA: Tumor-associated antigens.
The core matrix
Synthetic or natural biomaterials have been used as drug-delivery carriers and as vaccine-adjuvant agents. However, advanced formulation of these materials into modular systems for targeting different cell types and administration through various biological routes is a new application. Considering the fact that these systems are normally perceived by the body as foreign agents, induction of inflammatory reaction or immune responses is a natural result after administration. For vaccines, this is a favorable outcome because it replaces the role of immune potentiators and primes the immune response against associated antigen. For this reason, it is not a surprise that nano- and microparticulates have gained attention in their role as potent vaccine carriers.
Biodegradable nanoparticulates have been reported as promising antigen-delivery systems for different vaccine applications. One of the most widely studied nanoparticle systems is that fabricated from polylactides (PLA) and copolymers of lactide and glycolide (PLGA). These polymers have established use in humans and have a long safety record [2,7,10] and, as a result, have gained increasing attention as core substances for encapsulation and delivery of antigens. Other polymeric and lipid-based systems can also be considered [22–24], but several factors should be considered in the selection of the core material. First, the system should offer control over the size range of fabrication, down to 100 nm and potentially even lower – an important feature for passing through biological barriers. Second, the particle system should demonstrate reproducible biodegradability or efficient clearance from the body. Third, a capability for sustained release of an encapsulated or incorporated, protected antigen can potentially abrogate the booster requirement. The PLGA system is a good example of such a material in which the release properties of encapsulated antigen can be varied by adjusting factors, such as the PLA to polyglycolic acid copolymer ratios [6,25,26]. Fourth, the system should be amenable to scale-up for production of the necessary quantities of material for mass vaccination. Finally, and most importantly, is the ability to control surface properties of the system because surface properties enable the introduction of modular functionalities, such as targeting ligands to specific cells or protective elements and transport mediator elements providing for efficient transit of the particles into the appropriate body compartments after administration.
PLGA has adjuvant effects comparable with that of Freund’s complete adjuvant [27,28]. Animals challenged either orally [29] or subcutaneously [30] with particles encapsulating ovalbumin (OVA; as a model antigen) show elevation in cellular and humoral immune responses as well as the induction of immunological memory. The greatest asset of this system may be that slow release from these polymers may induce long-term effects. For example, one oral dose of hepatitis B antigen entrapped in PLGA yielded a long-term protection equivalent to three doses of injected antigen [31]. This single dose effect has also been reported for tetanus [32] and diphtheria toxoid [33,34].
Particle size is an important consideration for particle trafficking into the body as well as uptake by antigen-presenting cells (APCs). For uptake into cells, particles with an average diameter below 1 μm are most suitable for the induction of systemic immunity because they are internalized efficiently by DCs as well as macrophages. The influence of size on the induction of immunity, however, is not clear and may depend on the route of administration. For example, micron-sized particles administered either orally or intranasally can illicit effective immune responses. The increased size of the particles may facilitate their trapping in gut-associated lymphoid tissue or nasal-associated lymphoid tissue, thus inducing efficient mucosal responses [35]. Micron-sized particles injected subcutaneously or intramuscularly can also elicit greater humoral and cellular immune responses compared with conventional vaccine adjuvants [36,37]. For transport through the lymphatic vasculature, however, some recent studies suggest that nanomaterials (<100 nm) provide enhanced immunogenicity compared with larger systems. For optimal transport through lymphatic vessels after intradermal injection, the design of these agents needs to take into account our current understanding of interstitial transport mechanics and the function of lymphatic vessels [38,39]. Particles injected intradermally can interact with immature DCs residing in tissues nearest to the external environment, such as underneath the epidermis and the intestinal and nasal epithelia. Once DCs encounter an antigen, the cells ‘mature’ and migrate to lymphatic organs, such as the spleen and lymph nodes, to interact with lymphocytes. This physiological targeting is attractive because it harnesses local immune responses directly. To facilitate transport through lymphatic vessels and lymph nodes, an ideal nanoparticle must be sufficiently large to prevent leakage into blood vessels. At the same time, the particle needs to be sufficiently small for rapid transport through the lymphatics. Particles in the size range 20–50 nm can satisfy both criteria [40,41]. Further, it was discovered that, if such particles are surface modified with hydroxyl groups rather than methoxy groups, they can recruit the complement pathway, effectively activating DCs and initiating a potent vaccine response [40].
Nanoparticle surface modification
The ability to modify and functionalize the surface of particles should provide a powerful basis for targeting to specific cells and/or coating with protective polymers for systemic transport. For PLA and its copolymers, particles can be fabricated by a number of techniques, including solvent evaporation, double emulsion, phase-inversion nanoencapsulation, polymer precipitation, polycondensation and electrospray methods. To introduce functionality into PLGA surfaces, several approaches have been studied, including:
Fabricating particles with PLGA copolymers with amine [42,43] or acid [43] end groups;
Blending or adsorbing functional polymers, such as polylysine [44,45], poly(ethylene-altmaleic acid) (PEMA) [46] or poly (ethylene glycol) (PEG) [47], into PLGA and forming particles and matrices from these blends [45,46,48–50];
Plasma treatment of the PLGA matrix for the purpose of modifying its surface properties and introducing hydrophilic functional groups into the polymer [51.52].
Our group has introduced functional fatty acids that can be incorporated into the particle during the formation process. This strategy facilitated a high density of incorporated ligands and prolonged presentation while maintaining sustained delivery of the encapsulated agent at the target site [53]. Targeting modules can be added to the particle surface easily using conventional or recombinant monoclonal antibodies. In this way, the particles can be used not only as a means to encapsulate antigens but also as a means to target antigens to APCs in conjunction with other adjuvants required for eliciting the desired type of immune response.
An important consideration is that an immune response can be generated against the nanoparticles themselves and/or against the surface ligands [54]. This complication may induce rapid clearance of the particles by neutralizing antibodies but may also lead to adverse side effects associated with the generation of an immune response against the targeting vehicle. Thus, an important design criterion needs to account for this possibility by either masking the vehicle or modifying the surface and associated ligands to minimize recognition by the immune system while maintaining potential for targeting to APCs.
Targeting professional APCs
Recent work has established that targeting antigen to DCs is a powerful and novel strategy for vaccination [19,55–57]. Of the main types of APC (B cell, macrophages and DCs), the DC is the most potent and is responsible for initiating all antigen-specific immune responses [58,59]. Therefore, the priming or loading of DCs with antigen controls whether subsequent immunity will develop and whether effective vaccination can be achieved (Figure 2). Any vaccine design strategy should, if possible, take this principle into account. One biological feature of DCs is their ability to sense conditions under which antigen is encountered, initiating a process of ‘DC maturation’. Using receptors for microbial and inflammatory products, DCs respond to antigen exposure in different ways depending on the nature of the pathogen (virus, bacteria, protozoan) encountered. This information is transmitted to T cells by altered patterns of cytokine release at the time of antigen presentation in lymph nodes, altering the type of T-cell response elicited [60]. Thus, by targeting DCs, the ability to enhance the delivery of antigen and antigen responses in general can be coupled to controlling the nature of the immune response depending on the desired vaccination outcome. The elucidation of these basic features of DC biology has provided important tools for engineering vaccines with potent therapeutic outcomes [61,62].
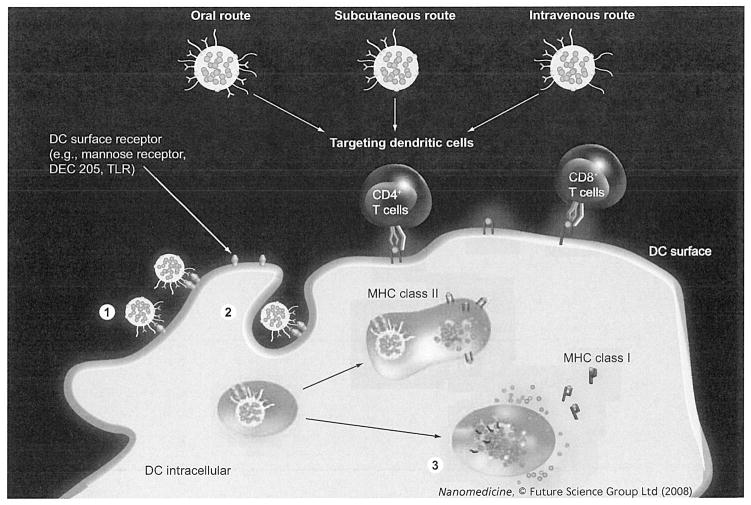
Figure 2. Efficient homing to professional antigen-presenting cells dictates particle-design considerations.
The route of administration will dictate the particular design features of nanoparticle vaccines. Irrespective of the route of administration, an optimal vaccine particle should be able to (1) bind professional antigen-presenting cells, such as DCs, effectively; (2) internalize and (3) break endosomal barriers for efficient antigen presentation to CD8+ T cells.
DC: Dendritic cell; TLR: Toll-like receptor.
Several approaches are being explored to enhance the delivery of antigens to DCs. These approaches make use of the fact that DCs express cell-surface receptors that can mediate the endocytosis of bound antigen. One such receptor, the lectin DEC-205, which is expressed on lymphoid, interstitial, epidermal Langerhans DCs and thymic endothelial cells, has been used in vitro and in mice to boost both humoral and cellular responses [63–65]. In these experiments, antigens were fused to an anti-DEC205 heavy chain and a recombinant antibody molecule was used for immunization. In a separate set of experiments, microparticles surface-modified with anti-DEC205, when injected subcutaneously into a mouse, targeted DCs and induced efficient humoral and cellular responses to model encapsulated antigens [62]. A variety of other endocytic receptors, including a mannose-specific lectin (mannose receptor) and IgG Fc receptors, have also been targeted in this way with similar enhancement of antigen-presentation efficiency [66]. Similarly, TLR ligands (e.g., monophosphoryl lipid A [67] or CpG DNA [68]), which target TLR4 or TLR9, respectively, have been incorporated into biodegradable particles to target APCs. Thus, targeting exogenous antigens to internalizing surface molecules on systemically distributed APCs overcomes a major rate-limiting step in immunization and thus in vaccination: uptake of antigen by DCs.
An open question is which ligands should be used for DC targeting and at what density they should be arrayed on the particles to interact with DCs most efficiently and how these parameters affect antigen uptake and presentation by DCs. These variables may also change with the route of administration. Thus, it is possible that systemically administered particles may have different requirements for efficient antigen presentation and targeting to DCs to elicit immunity compared with orally, intradermally or nasally administered particles.
Other studies show that direct administration of nanoparticles into the lymphatic system may not require attachment of ligands to DC surface markers. This strategy takes advantage of the physiology of lymphatic drainage and the phagocytic nature of DCs; particulate matter is internalized into the vessels from the interstitial space and internalized nonspecifically by DCs. As mentioned previously, size has a critical role in this transport-mediated process.
Surface modification also has a role in extending nanoparticle-trafficking time in vessels and enhancing transport. Increasing residence time in the lymphatics and bloodstream increases the chance of nanoparticle encounters with APCs. Thus, surface modification with steric-stabilizing groups, such as PEG and block copolymers of PEG and poly (propylene glycol) (Pluronics), may have a key role in enhancing vaccine efficacy after intradermal or subcutaneous injections [19]. PEG or poloxamer modification of the nanoparticle surface reduces nonspecific interactions in the interstitium, extending convective transport times and improving nanoparticle systemic trafficking, thus increasing chance encounters with APCs [41,69–72].
Enhancing antigen presentation: disrupting DC endosomal compartments & cross-presentation
Another important feature of antigen presentation is the intracellular compartments to which internalized antigens are delivered. Receptors used for targeting, such as DEC-205, have the ability to deliver antigens to late endosomal elements that serve as efficient sites for the formation of immunogenic peptides and their loading onto MHC class II molecules (which are needed for CD4 T-cell and antibody responses) [58,59]. Effective vaccination, however, will also often require the production of CD8 cytotoxic T-cell responses, which occur only when antigen is present in the cytoplasm. DCs are adept at this function through ‘cross-presentation’, whereby exogenous antigens escape endocytic vesicles and enter the cytoplasm where they are cleaved into peptides by the proteasome, imported into the endoplasmic reticulum and loaded onto newly synthesized MHC class I molecules (which are required for the stimulation of CD8 T cells). It is possible to enhance the efficiency of cross-presentation by artificially causing the limited disruption of endosome-lysosome membranes during antigen uptake. This has been accomplished in vitro and in vivo using antigen-loaded, pH-responsive particles [55,73,74]. Such particles are degradable at lysosomal pH and, indeed, particle composition (degree of acid-induced degradability) may affect the magnitude and pathway of antigen presentation [62]. Thus, engineering features in the nanoparticle through direct selection of the polymer material or co-encapsulation of endosomal disruption elements may enable the antigen to be delivered to the appropriate intracellular compartment. This approach is analogous to that taken by many pathogens, including viruses, such as poliovirus and adenovirus, which effect endosome disruption in order to gain access to the cytosol for purposes of infection [75–78].
Oral administration: enhancing mucosal immunity
The potential efficacy of nanoparticle vaccine systems will be determined in part by their route of administration into the body. Although parenteral injection (i.e., intradermal, intramuscular or intravenous) is an acceptable solution, in many cases, a vaccine product that is available orally would find extended ease of use and applicability on a global scale. Oral immunization generates IgA and CD8 T cells in the mucosa, thereby increasing the immune system’s first line of defense against many pathogens. For orally administered vaccines, epithelial cells constitute the principal barrier that separates an organism’s interior from the outside world [79–81]. Epithelial cells, such as those that line the gastrointestinal (GI) tract, form continuous monolayers that simultaneously confront the extracellular fluid compartment.
It is epithelial cells that will first confront and respond to nanoparticles administered to an intact organism. The permeability and transport properties of epithelia will determine the extent to which nanoparticles can gain access to all of die other cell types in the body. To understand the potential therapeutic and pathophysiological consequences of nanoparticle challenge, it is critical to define the ways in which nanoparticles interact with and influence the functions of a variety of epithelial-cell types. Remarkably little is known concerning the mechanisms through which epithelial cells interact with nanoparticles. As might be expected, the capacity of epithelial cells to internalize particles by endocytosis falls dramatically as the radius of the particle increases [82]. Unexpectedly, the transit of at least certain types of nanoparticles across epithelial tight junctions can increase substantially with particle size [83]. In addition, physical properties of nanoparticles, including their electrical charges and chemical compositions, profoundly influence the nature of their interactions with epithelia. Finally, different epithelial cell types exhibit markedly distinct reactions to the same nanoparticle populations [84]. Considerably more research is required, therefore, to define a meaningful set of rules that can be used to predict the effectiveness and consequences of epithelia–particle interactions.
The intestinal epithelium provides a barrier function, preventing easy passage of solutes into the underlying tissue. However, through the process of ‘antigen sampling’ [85,86], underlying mucosal-associated lymphoid tissue sample the environment for the presence of pathogens. This sampling is carried out by an apical to basolateral transcytotic event and is mediated by M cells located in lymphoid follicle-associated epithelium, which is present throughout the GI tract. Indeed, many studies have focused on identifying apical-membrane receptors as potential targets for vaccine delivery [87,88]. In addition, absorptive enterocytes may transport microorganisms or other nanoparticulates to intraepithelial lymphocytes. Finally, recent evidence has suggested that DCs may perform this function directly, with a population of DCs being intercalated between epithelial cells and extending processes into the gut lumen to sample the microorganisms present [89]. Because adherence to cells is an essential first step in crossing the epithelial barrier by any of these mechanisms, design of nanoparticulates administered orally should include, in addition to DC-targeting ligands, a second set of recognition elements: those that target the epithelium and mediate transcytosis to underlying APCs [90].
The importance of targeting the intestinal epithelium is highlighted in studies that involved feeding mice with polystyrene particles coated with the M-cell target ligand, the lectin Ulex europaeus 1 (UEA1) and a model antigen (OVA). These mice showed systemic immune responses that were elevated tenfold in comparison with untargeted particles [90]. Similarly, UEAl-modified liposomes also targeted murine M cells in vivo [91]. Recently, OVA-loaded PLGA-based nanoparticles modified with RGD peptides that interact preferentially with β1 integrins on the apical surface of M cells concentrated in intestinal M cells and produced significant IgG responses [92]. A significant challenge in this regard is that any system involving epithelial-cell targeting would have to insure that the intestinal epithelia are not the target of cytotoxic T cells. It is feasible that some of the antigen will be processed by these cells and presented on MHC class I, instigating undesirable immune effects.
The possibility of antigen tolerance with oral immunizations should also be considered. The immune system has developed a method of partially suppressing systemic responses to agents that pass through the GI tract in large amounts, such as food and commensal bacteria [93]. This tolerance induction presents a potential challenge for orally administered particles, which often need to be administered at larger doses compared with parenteral routes of administration to overcome degradation in the GI tract. Thus, in addition to protection, particle administration by the oral route needs to account for the possibility of immune tolerance.
Protection during transit: nanoparticle transport through the GI tract
Vaccine particles administered orally will encounter a corrosive environment in the GI tract with areas of low and high pH, as well as resident degradative enzymes and solubilizing agents. One of the reasons that biodegradable particulates have gained attention as oral vaccines is because of their ability to protect antigens en route to immune sites across the intestinal epithelium [17,94–96]. But, although the antigen is protected from environmental elements in transit, little protection is offered to elements coupled to the surface of the particle during the journey to immune-effector sites. This protection may be necessary to insure proper particle function and targeting. For this reason, a ‘shielding’ that protects the nanoparticulate and its immune-recognition elements may be required for transit to the GI epithelium. Ideally, this shielding should be environmentally sensitive, pH responsive or simply protective. Subsequently, on reaching its destination in the higher pH intestinal site, particles will need to expose the recognition elements to enable specific interactions with target epithelial cells followed by transcytosis through the epithelium and subsequent interactions with subepithelial DCs. In our own work, we have demonstrated that a coating of a pH-responsive surfactant, deoxycholic acid, can enhance oral bioavailability of agents encapsulated in biodegradable particles through a mechanism that involves both protection of the particle in the harsh environments of the stomach as well as enhancing intestinal transport [97].
Conclusion
Both quantitative as well as qualitative characteristics of the elements listed here will be equally important in assessing the success of the nanoparticle approach as vaccination agents. Thus, future approaches for the rational synthesis of vaccine systems should be based on an understanding of how individual components may enhance vaccine efficacy for the particular route of delivery. The components of such systems should be driven by biological mechanisms and, like biological systems, their qualitative and quantitative effects need to be subjected to a thorough analysis before full synthesis. Only after this understanding is achieved can integration proceed to assemble novel vaccine systems amenable to clinical translation.
Future perspective
The development of nanoparticle-based vaccine therapeutics is progressing rapidly owing to a better understanding of the biology of antigen processing and presentation combined with new innovative ways to produce nanoparticulate systems. These systems are not only capable of encapsulating a wide range of antigen species but also support the addition of modules that may enable selective targeting to and uptake by desired cell types, the introduction of specific immunomodulators to guide the immune response and the incorporation of elements that will deliver internalized antigens to appropriate intracellular compartments. In addition, these particles can be derivatized with elements that can aid in penetration across epithelial barriers and survival in the GI tract on oral administration. A special advantage is that they can be assembled and modified in a modular fashion, enabling the systematic evaluation of each element or combination of elements in terms of their vaccine efficacy.
Although biodegradable polymeric nano- and microparticles have been tested as vaccine-delivery systems for over 15 years [35,98], rarely did a product enter clinical trials and none have been approved yet for use in humans. Compared with small-molecule drugs, relatively little is known about how these nanomaterials interact with the body; before advanced clinical trials can proceed, an intensive study is needed into the in vivo trafficking patterns of these systems as a function of the different components that may be added to the surface to enhance vaccine efficacy. In addition, more information is needed regarding how these systems may interact with different immune system cells beyond APCs. Recently, standard guidelines purveying how to properly assess for toxicity of nano-based therapeutics have been proposed [54], however, some major questions still remain regarding the pharmacokinetics and bio-distribution of nanoparticle vaccine-based systems by different routes of administration in various animals models.
Executive summary.
Current vaccines: what are the issues?
Failure to stimulate the cellular arm of the immune response effectively.
Lack of flexibility in vaccine design that enables immune responses to a variety of antigens.
Effective worldwide use of vaccines is limited by the robustness of current formulations and the availability in regions where trained staff and resources are scarce.
Challenges associated with the formulation of oral vaccines for many antigens have limited their number as well as their efficacy in protection.
Biodegradable vaccine delivery-vehicle design: what are the design variables?
Core matrix considerations – size, biodegradability, clearance, antigen release profile and control over surface properties.
Surface modification with ligands – to enhance circulation time of particles, to protect particle integrity during transit, to target specific cells, to enhance uptake and to induce better intracellular delivery of antigen.
Acknowledgments
We wish to thank Michael Look for careful review of this manuscript.
Footnotes
For reprint orders, please contact: reprints@futuremedicine.com
Financial & competing interests disclosure
This work was supported by a NIRT grant (NSF award number: CTS-0609326) to TM Fahmy. Support for this work was also provided by NIH grant EB000487 to WM Saltzman. TM Fahmy is a consultant for L2 Diagnositics and Carigent Therapeutics. I Mellman is a consultant for Genetech. Inc. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Zinkernagel RM. Immunity, immunopathology and vaccines against HIV? Vaccine. 2002;20:1913–1917. doi: 10.1016/s0264-410x(02)00066-x. [DOI] [PubMed] [Google Scholar]
- 2.Jiang W, Gupta RK, Deshpande MC, Schwendeman SP. Biodegradable poly(lactic-co-glycolic acid) microparticles for injectable delivery of vaccine antigens. Adv Drug Deliv Rev. 2005;57:391–410. doi: 10.1016/j.addr.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 3.Sesardic D, Dobbelaer R. European Union regulatory developments for new vaccine adjuvants and delivery systems. Vaccine. 2004;22:2452–2456. doi: 10.1016/j.vaccine.2003.11.071. [DOI] [PubMed] [Google Scholar]
- 4.Friede M, Aguado MT. Need for new vaccine formulations and potential of particulate antigen and DNA delivery systems. Adv Drug Deliv Rev. 2005;57:325–331. doi: 10.1016/j.addr.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 5.Storni T, Kundig TM, Senti G, Johansen P. Immunity in response to particulate antigen-delivery systems. Adv Drug Deliv Rev. 2005;57:333–355. doi: 10.1016/j.addr.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 6.Gupta RK, Singh M, O’Hagan DT. Poly(lactide-co-glycolide) microparticles for the development of single-dose controlled-release vaccines. Adv Drug Deliv Rev. 1998;32:225–246. [PubMed] [Google Scholar]
- 7.Aguado MT, Lambert PH. Controlled-release vaccines – biodegradable polylactide/polyglycolide (PL/PG) microspheres as antigen vehicles. Immunobiology. 1992;184:113–125. doi: 10.1016/S0171-2985(11)80470-5. [DOI] [PubMed] [Google Scholar]
- 8.Pashine A, Valiante NM, Ulmer JB. Targeting the innate immune response with improved vaccine adjuvants. Nat Med. 2005;11:S63–S68. doi: 10.1038/nm1210. [DOI] [PubMed] [Google Scholar]
- 9.Bramwell VW, Perrie Y. The rational design of vaccines. Drug Discov Today. 2005;10:1527–1534. doi: 10.1016/S1359-6446(05)03600-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bramwell VW, Eyles JE, Oya Alpar H. Particulate delivery systems for biodefense subunit vaccines. Adv Drug Deli v Rev. 2005;57:1247–1265. doi: 10.1016/j.addr.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 11.Tang DC, DeVit M, Johnston SA. Genetic immunization is a simple method for eliciting an immune response. Nature. 1992;356:152–154. doi: 10.1038/356152a0. [DOI] [PubMed] [Google Scholar]
- 12.Gupta RK, Siber GR. Adjuvants for human vaccines – current status, problems and future prospects. Vaccine. 1995;13:1263–1276. doi: 10.1016/0264-410x(95)00011-o. [DOI] [PubMed] [Google Scholar]
- 13.Lindblad EB. Aluminium adjuvants – in retrospect and prospect. Vaccine. 2004;22:3658–3668. doi: 10.1016/j.vaccine.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 14.Gupta RK, Relyveld EH, Lindblad EB, Bizzini B, Ben-Efraim S, Gupta CK. Adjuvants – a balance between toxicity and adjuvanticity. Vaccine. 1993;11:293–306. doi: 10.1016/0264-410x(93)90190-9. [DOI] [PubMed] [Google Scholar]
- 15•.Berzofsky JA, Ahlers JD, Belyakov IM. Strategies for designing and optimizing new generation vaccines. Nat Rev Immunol. 2001;1:209–219. doi: 10.1038/35105075. These are excellent reviews that consider general vaccine design. [DOI] [PubMed] [Google Scholar]
- 16.Singh M, Srivastava I. Advances in vaccine adjuvants for infectious diseases. Curr HIV Res. 2003;1:309–320. doi: 10.2174/1570162033485195. [DOI] [PubMed] [Google Scholar]
- 17.O’Hagan DT, Valiante NM. Recent advances in the discovery and delivery of vaccine adjuvants. Nat Rev Drug Discov. 2003;2:727–735. doi: 10.1038/nrd1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh M, O’Hagan D. Advances in vaccine adjuvants. Nat Biotechnol. 1999;17:1075–1081. doi: 10.1038/15058. [DOI] [PubMed] [Google Scholar]
- 19.Reddy ST, Swartz MA, Hubbell JA. Targeting dendritic cells with biomaterials: developing the next generation of vaccines. Trends Immunol. 2006;27:573–579. doi: 10.1016/j.it.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 20.Schijns VE, Degen WG. Vaccine immunopotentiators of the future. Clin Pharmacol Ther. 2007;82:750–755. doi: 10.1038/sj.clpt.6100394. [DOI] [PubMed] [Google Scholar]
- 21.Singh M, Chakrapani A, O’Hagan D. Nanoparticles and microparticles as vaccine-delivery systems. Expert Rev Vaccines. 2007;6:797–808. doi: 10.1586/14760584.6.5.797. [DOI] [PubMed] [Google Scholar]
- 22.Migunov AI, Kuznetsov OK, Kiselev OI. Use of liposomes for vaccines design. Vopr Virusol. 2001;46:4–7. [PubMed] [Google Scholar]
- 23.Wassef NM, Alving CR, Richards RL. Liposomes as carriers for vaccines. Immunomethods. 1994;4:217–222. doi: 10.1006/immu.1994.1023. [DOI] [PubMed] [Google Scholar]
- 24.Calvo P, Remunan-Lopez C, Vila-Jato JL, Alonso MJ. Chitosan and chitosan/ethylene oxide-propylene oxide block copolymer nanoparticles as novel carriers for proteins and vaccines. Pharm Res. 1997;14:1431–1436. doi: 10.1023/a:1012128907225. [DOI] [PubMed] [Google Scholar]
- 25.Kohn J, Niemi SM, Albert EC, Murphy JC, Langer R, Fox JG. Single-step immunization using a controlled release, biodegradable polymer with sustained adjuvant activity. J Immunol Methods. 1986;95:31–38. doi: 10.1016/0022-1759(86)90314-5. [DOI] [PubMed] [Google Scholar]
- 26.Langer R, Cleland JL, Hanes J. New advances in microsphere-based single-dose vaccines. Adv Drug Deliv Rev. 1997;28:97–119. doi: 10.1016/s0169-409x(97)00053-7. [DOI] [PubMed] [Google Scholar]
- 27.Eldridge JH, Staas JK, Meulbroek JA, Tice TR, Gilley RM. Biodegradable and biocompatible poly(DL-lactide-co-glycolide) microspheres as an adjuvant for staphylococcal enterotoxin-B toxoid which enhances the level of toxin-neutralizing antibodies. Infect Immun. 1991;59:2978–2986. doi: 10.1128/iai.59.9.2978-2986.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosas JE, Hernandez RM, Gascon AR, et al. Biodegradable PLGA microspheres as a delivery system for malaria synthetic peptide SPf66. Vaccine. 2001;19:4445–4451. doi: 10.1016/s0264-410x(01)00192-x. [DOI] [PubMed] [Google Scholar]
- 29.Maloy KJ, Donachie AM, O’Hagan DT, Mowat AM. Induction of mucosal and systemic immune responses by immunization with ovalbumin entrapped in poly(lactide-co-glycolide) microparticles. Immunology. 1994;81:661–667. [PMC free article] [PubMed] [Google Scholar]
- 30.O’Hagan DT, Jeffery H, Davis SS. Long-term antibody-responses in mice following subcutaneous immunization with ovalbumin entrapped in biodegradable microparticles. Vaccine. 1993;11:965–969. doi: 10.1016/0264-410x(93)90387-d. [DOI] [PubMed] [Google Scholar]
- 31.Nellore RV, Pande PG, Young D, Bhagat HR. Evaluation of biodegradable microspheres as vaccine adjuvant for hepatitis B surface antigen. J Parenter Sci Technol. 1992;46:176–180. [PubMed] [Google Scholar]
- 32.Singh M, Li XM, Wang H, et al. Immunogenicity and protection in small-animal models with controlled-release tetanus toxoid microparticles as a single-dose vaccine. Infect Immun. 1997;65:1716–1721. doi: 10.1128/iai.65.5.1716-1721.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singh M, Singh O, Singh A, Talwar GP. Immunogenicity studies on diphtheria toxoid loaded biodegradable microspheres. Int J Pharm. 1992;85:R5–R8. [Google Scholar]
- 34.Singh M, Li XM, Wang H, et al. Controlled release microparticles as a single dose diphtheria toxoid vaccine: immunogenicity in small animal models. Vaccine. 1998;16:346–352. doi: 10.1016/s0264-410x(97)80912-7. [DOI] [PubMed] [Google Scholar]
- 35.Eldridge JH, Meulbroek JA, Staas JK, Tice TR, Gilley RM. Vaccine-containing biodegradable microspheres specifically enter the gut-associated lymphoid tissue following oral administration and induce a disseminated mucosal immune response. Adv Exp Med Biol. 1989;251:191–202. doi: 10.1007/978-1-4757-2046-4_18. [DOI] [PubMed] [Google Scholar]
- 36.Cui C, Stevens VC, Schwendeman SP. Injectable polymer microspheres enhance immunogenicity of a contraceptive peptide vaccine. Vaccine. 2007;25:500–509. doi: 10.1016/j.vaccine.2006.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mossman SP, Evans LS, Fang H, et al. Development of a CTL vaccine for Her-2/neu using peptide-microspheres and adjuvants. Vaccine. 2005;23:3545–3554. doi: 10.1016/j.vaccine.2005.01.149. [DOI] [PubMed] [Google Scholar]
- 38.Skobe M, Detmar M. Structure, function, and molecular control of the skin lymphatic system. J Investig Dermatol Symp Proc. 2000;5:14–19. doi: 10.1046/j.1087-0024.2000.00001.x. [DOI] [PubMed] [Google Scholar]
- 39.Swartz MA. The physiology of the lymphatic system. Adv Drug Deliv Rev. 2001;50:3–20. doi: 10.1016/s0169-409x(01)00150-8. [DOI] [PubMed] [Google Scholar]
- 40•.Reddy ST, van der Vlies AJ, Simeoni E, et al. Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nat Biotechnol. 2007;25:1159–1164. doi: 10.1038/nbt1332. Discusses trafficking of nanoparticles in lymphatic vessels and innovative methods for augmenting the adaptive immune response with particles that trigger complement activation. [DOI] [PubMed] [Google Scholar]
- 41.Reddy ST, Rehor A, Schmoekel HG, Hubbell JA, Swartz MA. In vivo targeting of dendritic cells in lymph nodes with poly(propylene sulfide) nanoparticles. J Control Release. 2006;112:26–34. doi: 10.1016/j.jconrel.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 42.Lavik EB, Hrkach JS, Lotan N, Nazarov R, Langer R. A simple synthetic route to the formation of a block copolymer of poly(lactic-co-glycolic acid) and polylysine for the fabrication of functionalized, degradable structures for biomedical applications. J Biomed Mater Res. 2001;58:291–294. doi: 10.1002/1097-4636(2001)58:3<291::aid-jbm1019>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 43.Caponetti G, Hrkach JS, Kriwet B, et al. Microparticles of novel branched copolymers of lactic acid and amino acids: preparation and characterization. J Pharm Sci. 1999;88:136–141. doi: 10.1021/js970457f. [DOI] [PubMed] [Google Scholar]
- 44.Faraasen S, Voros J, Csucs G, Textor M, Merkle HP, Walter E. Ligand-specific targeting of microspheres to phagocytes by surface modification with poly(L-lysine)-grafted poly(ethylene glycol) conjugate. Pharm Res. 2003;20:237–246. doi: 10.1023/a:1022366921298. [DOI] [PubMed] [Google Scholar]
- 45.Zheng J, Hornsby PJ. Production of microspheres with surface amino groups from blends of poly(lactide-co-glycolide) and poly(epsilon-CBZ-L-lysine) and use for encapsulation. Biotechnol Prog. 1999;15:763–767. doi: 10.1021/bp9900817. [DOI] [PubMed] [Google Scholar]
- 46.Keegan ME, Falcone JL, Leung TC, Saltzman WM. Biodegradable microspheres with enhanced capacity for covalently bound surface ligands. Macromolecules. 2004;37(26):9779–9784. [Google Scholar]
- 47.Muller M, Voros J, Csucs G, et al. Surface modification of PLGA microspheres. J Biomed Mater Res. 2003;66A:55–61. doi: 10.1002/jbm.a.10502. [DOI] [PubMed] [Google Scholar]
- 48.Park A, Wu B, Griffith LG. Integration of surface modification and 3D fabrication techniques to prepare patterned poly(L-lactide) substrates allowing regionally selective cell adhesion. J Biomater Sci Polym Ed. 1998;9:89–110. doi: 10.1163/156856298x00451. [DOI] [PubMed] [Google Scholar]
- 49.Croll TI, O’Connor AJ, Stevens GW, Cooper-White JJ. Controllable surface modification of poly(lactic-co-glycolic acid) (PLGA) by hydrolysis or aminolysis I: physical, chemical, and theoretical aspects. Biomacromolecules. 2004;5:463–473. doi: 10.1021/bm0343040. [DOI] [PubMed] [Google Scholar]
- 50.Cao Y, Croll TI, Cooper-White JJ, O’Connor AJ, Stevens GW. Production and surface modification of polylactide-based polymeric scaffolds for soft-tissue engineering. Methods Mol Biol. 2004;238:87–112. doi: 10.1385/1-59259-428-x:87. [DOI] [PubMed] [Google Scholar]
- 51.Yang J, Wan Y, Bei J, Wang S. Plasma-treated, collagen-anchored polylactone: its cell affinity evaluation under shear or shear-free conditions. J Biomed Mater Res. 2003;67A:1139–1147. doi: 10.1002/jbm.a.10034. [DOI] [PubMed] [Google Scholar]
- 52.Wan Y, Qu X, Lu J, et al. Characterization of surface property of poly(lactide-co-glycolide) after oxygen plasma treatment. Biomaterials. 2004;25:4777–4783. doi: 10.1016/j.biomaterials.2003.11.051. [DOI] [PubMed] [Google Scholar]
- 53.Fahmy TM, Samstein RM, Harness CC, Saltzman MW. Surface modification of biodegradable polyesters with fatty acid conjugates for improved drug targeting. Biomaterials. 2005;26:5727–5736. doi: 10.1016/j.biomaterials.2005.02.025. [DOI] [PubMed] [Google Scholar]
- 54•.Dobrovolskaia MA, McNeil SE. Immunological properties of engineered nanomaterials. Nat Nanotechnol. 2007;2:469–477. doi: 10.1038/nnano.2007.223. Reviews toxicology considerations for nanomaterials and their interaction with the immune system. [DOI] [PubMed] [Google Scholar]
- 55.Kwon YJ, Standley SM, Goh SL, Frechet JM. Enhanced antigen presentation and immunostimulation of dendritic cells using acid-degradable cationic nanoparticles. J Control Release. 2005;105:199–212. doi: 10.1016/j.jconrel.2005.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pitaksuteepong T, Davies NM, Baird M, Rades T. Uptake of antigen encapsulated in polyethylcyanoacrylate nanoparticles by D1-dendritic cells. Pharmazie. 2004;59:134–142. [PubMed] [Google Scholar]
- 57.Cui Z, Baizer L, Mumper RJ. Intradermal immunization with novel plasmid DNA-coated nanoparticles via a needle-free injection device. J Biotechnol. 2003;102:105–115. doi: 10.1016/s0168-1656(03)00029-4. [DOI] [PubMed] [Google Scholar]
- 58.Mellman I. Antigen processing and presentation by dendritic cells: cell biological mechanisms. Adv Exp Med Biol. 2005;560:63–67. doi: 10.1007/0-387-24180-9_9. [DOI] [PubMed] [Google Scholar]
- 59.Mellman I, Steinman RM. Dendritic cells: specialized and regulated antigen processing machines. Cell. 2001;106:255–258. doi: 10.1016/s0092-8674(01)00449-4. [DOI] [PubMed] [Google Scholar]
- 60.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 61.Pardoll DM. Spinning molecular immunology into successful immunotherapy. Nat Rev Immunol. 2002;2:227–238. doi: 10.1038/nri774. [DOI] [PubMed] [Google Scholar]
- 62.Kwon YJ, James E, Shastri N, Frechet JM. In vivo targeting of dendritic cells for activation of cellular immunity using vaccine carriers based on pH-responsive microparticles. Proc Natl Acad Sci USA. 2005;102:18264–18268. doi: 10.1073/pnas.0509541102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hawiger D, Inaba K, Dorsett Y, et al. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J Exp Med. 2001;194:769–779. doi: 10.1084/jem.194.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bonifaz L, Bonnyay D, Mahnke K, Rivera M, Nussenzweig MC, Steinman RM. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J Exp Med. 2002;196:1627–1638. doi: 10.1084/jem.20021598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bonifaz LC, Bonnyay DP, Charalambous A, et al. In vivo targeting of antigens to maturing dendritic cells via the DEC-205 receptor improves T cell vaccination. J Exp Med. 2004;199:815–824. doi: 10.1084/jem.20032220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shen H, Ackerman AL, Cody V, et al. Enhanced and prolonged cross-presentation following endosomal escape of exogenous antigens encapsulated in biodegradable nanoparticles. Immunology. 2006;117:78–88. doi: 10.1111/j.1365-2567.2005.02268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Elamanchili P, Lutsiak CM, Hamdy S, Diwan M, Samuel J. ‘Pathogen-mimicking’ nanoparticles for vaccine delivery to dendritic cells. J Immunother. 2007;30:378–395. doi: 10.1097/CJI.0b013e31802cf3e3. [DOI] [PubMed] [Google Scholar]
- 68.Hunter SK, Andracki ME, Krieg AM. Biodegradable microspheres containing group B Streptococcus vaccine: immune response in mice. Am J Obstet Gynecol. 2001;185:1174–1179. doi: 10.1067/mob.2001.117658. [DOI] [PubMed] [Google Scholar]
- 69.Hawley AE, Illum L, Davis SS. Preparation of biodegradable, surface engineered PLGA nanospheres with enhanced lymphatic drainage and lymph node uptake. Pharm Res. 1997;14:657–661. doi: 10.1023/a:1012117531448. [DOI] [PubMed] [Google Scholar]
- 70.Moghimi SM. Modulation of lymphatic distribution of subcutaneously injected poloxamer 407-coated nanospheres: the effect of the ethylene oxide chain configuration. FEBS Lett. 2003;540:241–244. doi: 10.1016/s0014-5793(03)00273-4. [DOI] [PubMed] [Google Scholar]
- 71.Oussoren CG. Storm: Lymphatic uptake and biodistribution of liposomes after subcutaneous injection: III. Influence of surface modification with poly(ethyleneglycol) Pharm Res. 1997;14:1479–1484. doi: 10.1023/a:1012145410859. [DOI] [PubMed] [Google Scholar]
- 72.Vandorpe J, Schacht E, Dunn S, et al. Long circulating biodegradable poly(phosphazene) nanoparticles surface modified with poly(phosphazene)-poly(ethylene oxide) copolymer. Biomaterials. 1997;18:1147–1152. doi: 10.1016/s0142-9612(97)00052-5. [DOI] [PubMed] [Google Scholar]
- 73.Kwon YJ, Standley SM, Goodwin AP, Gillies ER, Frechet JM. Directed antigen presentation using polymeric microparticulate carriers degradabie at lysosomal pH for controlled immune responses. Mol Pharm. 2005;2:83–91. doi: 10.1021/mp0498953. [DOI] [PubMed] [Google Scholar]
- 74.Standley SM, Kwon YJ, Murthy N, et al. Acid-degradable particles for protein-based vaccines: enhanced survival rate for tumor-challenged mice using ovalbumin model. Bioconjug Chem. 2004;15:1281–1288. doi: 10.1021/bc049956f. [DOI] [PubMed] [Google Scholar]
- 75.Basta S, Stoessel R, Basler M, van den Broek M, Groettrup M. Cross-presentation of the long-lived lymphocytic choriomeningitis virus nucleoprotein does not require neosynthesis and is enhanced via heat shock proteins. J Immunol. 2005;175:796–805. doi: 10.4049/jimmunol.175.2.796. [DOI] [PubMed] [Google Scholar]
- 76.Bosnjak L, Miranda-Saksena M, Koelle DM, Boadle RA, Jones CA, Cunningham AL. Herpes simplex virus infection of human dendritic cells induces apoptosis and allows cross-presentation via uninfected dendritic cells. J Immunol. 2005;174:2220–2227. doi: 10.4049/jimmunol.174.4.2220. [DOI] [PubMed] [Google Scholar]
- 77.Fausch SC, Da Silva DM, Kast WM. Differential uptake and cross-presentation of human papillomavirus virus-like particles by dendritic cells and Langerhans cells. Cancer Res. 2003;63:3478–3482. [PubMed] [Google Scholar]
- 78.Larsson M, Fonteneau JF, Somersan S, et al. Efficiency of cross presentation of vaccinia virus-derived antigens by human dendritic cells. Eur J Immunol. 2001;31:3432–3442. doi: 10.1002/1521-4141(200112)31:12<3432::aid-immu3432>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 79.Caplan MJ. Membrane polarity in epithelial cells: protein sorting and establishment of polarized domains. Am J Physiol. 1997;272:F425–F429. doi: 10.1152/ajprenal.1997.272.4.F425. [DOI] [PubMed] [Google Scholar]
- 80.Frey A, Giannasca KT, Weltzin R, et al. Role of the glycocalyx in regulating access of microparticles to apical plasma membranes of intestinal epithelial cells: implications for microbial attachment and oral vaccine targeting. J Exp Med. 1996;184:1045–1059. doi: 10.1084/jem.184.3.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Madara JL. Maintenance of the macromolecular barrier at cell extrusion sites in intestinal epithelium: physiological rearrangement of tight junctions. J Membr Biol. 1990;116:177–184. doi: 10.1007/BF01868675. [DOI] [PubMed] [Google Scholar]
- 82.Foster KA, Yazdanian M, Audus KL. Microparticulate uptake mechanisms of in-vitro cell culture models of the respiratory epithelium. J Pharm Pharmacol. 2001;53:57–66. doi: 10.1211/0022357011775190. [DOI] [PubMed] [Google Scholar]
- 83.Turley SJ, Inaba K, Garrett WS, et al. Transport of peptide-MHC class II complexes in developing dendritic cells. Science. 2000;288:522–527. doi: 10.1126/science.288.5465.522. [DOI] [PubMed] [Google Scholar]
- 84.Akerman ME, Chan WC, Laakkonen P, Bhatia SN, Ruoslahti E. Nanocrystal targeting in vivo. Proc Natl Acad Sci USA. 2002;99:12617–12621. doi: 10.1073/pnas.152463399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tuma PL, Hubbard AL. Transcytosis: crossing cellular barriers. Physiol Rev. 2003;83:871–932. doi: 10.1152/physrev.00001.2003. [DOI] [PubMed] [Google Scholar]
- 86.Kaiserlian D. Epithelial cells in antigen. Sampling and presentation in mucosal tissues. Curr Top Microbiol Immunol. 1999;236:55–78. doi: 10.1007/978-3-642-59951-4_4. [DOI] [PubMed] [Google Scholar]
- 87.Brayden DJ, Baird AW. Apical membrane receptors on intestinal M cells: potential targets for vaccine delivery. Adv Drug Deliv Rev. 2004;56:721–726. doi: 10.1016/j.addr.2003.10.036. [DOI] [PubMed] [Google Scholar]
- 88.Higgins LM, Lambkin I, Donnelly G, et al. In vivo phage display to identify M cell-targeting ligands. Pharm Res. 2004;21:695–705. doi: 10.1023/b:pham.0000022418.80506.9a. [DOI] [PubMed] [Google Scholar]
- 89.Rescigno M, Urbano M, Valzasina B, et al. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol. 2001;2:361–367. doi: 10.1038/86373. [DOI] [PubMed] [Google Scholar]
- 90.Foster N, Hirst BH. Exploiting receptor biology for oral vaccination with biodegradable particulates. Adv Drug Deliv Rev. 2005;57:431–450. doi: 10.1016/j.addr.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 91.Clark MA, Blair H, Liang L, Brey RN, Brayden D, Hirst BH. Targeting polymerised liposome vaccine carriers to intestinal M cells. Vaccine. 2001;20:208–217. doi: 10.1016/s0264-410x(01)00258-4. [DOI] [PubMed] [Google Scholar]
- 92.Garinot M, Fievez V, Pourcelle V, et al. PEGylated PLGA-based nanoparticles targeting M cells for oral vaccination. J Control Release. 2007;120:195–204. doi: 10.1016/j.jconrel.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 93.Mestecky J, Russell MW, Elson CO. Perspectives on mucosal vaccines: is mucosal tolerance a barrier? J Immunol. 2007;179:5633–5638. doi: 10.4049/jimmunol.179.9.5633. [DOI] [PubMed] [Google Scholar]
- 94.van der Lubben IM, Verhoef JC, Borchard G, Junginger HE. Chitosan for mucosal vaccination. Adv Drug Deliv Rev. 2001;52:139–44. doi: 10.1016/s0169-409x(01)00197-1. [DOI] [PubMed] [Google Scholar]
- 95.Wikingsson L, Sjoholm I. Polyacryl starch microparticles as adjuvant in oral immunisation, inducing mucosal and systemic immune responses in mice. Vaccine. 2002;20:3355–3363. doi: 10.1016/s0264-410x(02)00288-8. [DOI] [PubMed] [Google Scholar]
- 96.Moser C, Metcalfe IC, Viret JF. Virosomal adjuvanted antigen delivery systems. Expert Rev Vaccines. 2003;2:189–196. doi: 10.1586/14760584.2.2.189. [DOI] [PubMed] [Google Scholar]
- 97.Samstein RM, Perica K, Balderrama F, Look M, Fahmy TM. The use of deoxycholic acid to enhance the oral bioavailability of biodegradable nanoparticles. Biomaterials. 2007;29(6):703–708. doi: 10.1016/j.biomaterials.2007.10.026. [DOI] [PubMed] [Google Scholar]
- 98.O’Hagan DT, Jeffery H, Roberts MJ, McGee JP, Davis SS. Controlled release microparticles for vaccine development. Vaccine. 1991;9:768–771. doi: 10.1016/0264-410x(91)90295-h. [DOI] [PubMed] [Google Scholar]
- 99.Standley SM, Mende I, Goh SL, et al. Incorporation of CpG oligonucleotide ligand into protein-loaded particle vaccines promotes antigen-specific CD8 T-cell immunity. Bioconjug Chem. 2007;18:77–83. doi: 10.1021/bc060165i. [DOI] [PubMed] [Google Scholar]
- 100.Newman KD, Sosnowski DL, Kwon GS, Samuel J. Delivery of MUC1 mucin peptide by Poly(D,L-lactic-co-glycolic acid) microspheres induces type 1 T helper immune responses. J Pharm Sci. 1998;87:1421–1427. doi: 10.1021/js980070s. [DOI] [PubMed] [Google Scholar]
- 101.Lemoine D, Preat V. Polymeric nanoparticles as delivery system for influenza virus glycoproteins. J Control Release. 1998;54:15–27. doi: 10.1016/s0168-3659(97)00241-1. [DOI] [PubMed] [Google Scholar]
- 102.Sinyakov MS, Dror M, Lublin-Tennenbaum T, Salzberg S, Margel S, Avtalion RR. Nano- and microparticles as adjuvants in vaccine design: success and failure is related to host natural antibodies. Vaccine. 2006;24:6534–6541. doi: 10.1016/j.vaccine.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 103.van Broekhoven CL, Parish CR, Demangel C, Britton WJ, Altin JG. Targeting dendritic cells with antigen-containing liposomes: a highly effective procedure for induction of antitumor immunity and for tumor immunotherapy. Cancer Res. 2004;64:4357–4365. doi: 10.1158/0008-5472.CAN-04-0138. [DOI] [PubMed] [Google Scholar]
- 104.de Jong S, Chikh G, Sekirov L, et al. Encapsulation in liposomal nanoparticles enhances the immunostimulatory, adjuvant and anti-tumor activity of subcutaneously administered CpG ODN. Cancer Immunol Immunother. 2007;56:1251–1264. doi: 10.1007/s00262-006-0276-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Xiang SD, Scholzen A, Minigo G, et al. Pathogen recognition and development of particulate vaccines: does size matter? Methods. 2006;40:1–9. doi: 10.1016/j.ymeth.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 106.Zhou X, Liu B, Yu X, et al. Controlled release of PEI/DNA complexes from mannose-bearing chitosan microspheres as a potent delivery system to enhance immune response to HBV DNA vaccine. J Control Release. 2007;121:200–207. doi: 10.1016/j.jconrel.2007.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nagamoto T, Hattori Y, Takayama K, Maitani Y. Novel chitosan particles and chitosan-coated emulsions inducing immune response via intranasal vaccine delivery. Pharm Res. 2004;21:671–674. doi: 10.1023/b:pham.0000022414.17183.58. [DOI] [PubMed] [Google Scholar]
- 108.Amidi M, Romeijn SG, Verhoef JC, et al. N-trimethyl chitosan (TMC) nanoparticles loaded with influenza subunit antigen for intranasal vaccination: biological properties and immunogenicity in a mouse model. Vaccine. 2007;25:144–153. doi: 10.1016/j.vaccine.2006.06.086. [DOI] [PubMed] [Google Scholar]
- 109.Jain S, Sharma RK, Vyas SP. Chitosan nanoparticles encapsulated vesicular systems for oral immunization: preparation, in-vitro and in-vivo characterization. J Pharm Pharmacol. 2006;58:303–310. doi: 10.1211/jpp.58.3.0003. [DOI] [PubMed] [Google Scholar]
- 110.Ribeiro S, Rijpkema SG, Durrani Z, Florence AT. PLGA-dendron nanoparticles enhance immunogenicity but not lethal antibody production of a DNA vaccine against anthrax in mice. Int J Pharm. 2007;331:228–232. doi: 10.1016/j.ijpharm.2006.11.063. [DOI] [PubMed] [Google Scholar]
- 111.Wang X, Uto T, Akagi T, Akashi M, Baba M. Induction of potent CD8+ T-cell responses by novel biodegradable nanoparticles carrying human immunodeficiency virus type 1 gp120. J Virol. 2007;81:10009–10016. doi: 10.1128/JVI.00489-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Akagi T, Wang X, Uto T, Baba M, Akashi M. Protein direct delivery to dendritic cells using nanoparticles based on amphiphilic poly(amino acid) derivatives. Biomaterials. 2007;28:3427–3436. doi: 10.1016/j.biomaterials.2007.04.023. [DOI] [PubMed] [Google Scholar]
- 113.Uto T, Wang X, Sato K, et al. Targeting of antigen to dendritic cells with poly(γ-glutamic acid) nanoparticles induces antigen-specific humoral and cellular immunity. J Immunol. 2007;178:2979–2986. doi: 10.4049/jimmunol.178.5.2979. [DOI] [PubMed] [Google Scholar]