Abstract
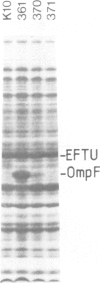
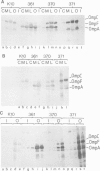
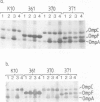
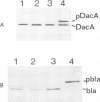
To test the importance of N-terminal pre-sequences in translocation of different classes of membrane proteins, we exchanged the normal signal sequence of an Escherichia coli outer membrane protein, OmpF, for the pre-sequence of the inner membrane protein, DacA. The DacA-OmpF hybrid was efficiently assembled into the outer membrane in a functionally active form. Thus the pre-sequence of DacA, despite its relatively low hydrophobicity compared with that of OmpF, contains all the essential information necessary to initiate the translocation of OmpF to the outer membrane. Since processing of DacA was also shown to be dependent upon SecA we conclude that the initiation of translocation of this inner membrane polypeptide across the envelope occurs by the same mechanism as outer membrane and periplasmic proteins. The N-terminal 11 amino acids of mature OmpF, which in the hybrid are replaced by the N-terminal nine amino acids of DacA, carry no essential assembly signals since the hybrid protein is apparently assembled with equal efficiency to OmpF.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austen B. M. Predicted secondary structures of amino-terminal extension sequences of secreted proteins. FEBS Lett. 1979 Jul 15;103(2):308–313. doi: 10.1016/0014-5793(79)81351-4. [DOI] [PubMed] [Google Scholar]
- Benson S. A., Bremer E., Silhavy T. J. Intragenic regions required for LamB export. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3830–3834. doi: 10.1073/pnas.81.12.3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson S. A., Silhavy T. J. Information within the mature LamB protein necessary for localization to the outer membrane of E coli K12. Cell. 1983 Apr;32(4):1325–1335. doi: 10.1016/0092-8674(83)90313-6. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Boyd A., Holland I. B. Regulation of the synthesis of surface protein in the cell cycle of E. coli B/r. Cell. 1979 Oct;18(2):287–296. doi: 10.1016/0092-8674(79)90048-5. [DOI] [PubMed] [Google Scholar]
- Chang S. H., Majumdar A., Dunn R., Makabe O., RajBhandary U. L., Khorana H. G., Ohtsuka E., Tanaka T., Taniyama Y. O., Ikehara M. Bacteriorhodopsin: partial sequence of mRNA provides amino acid sequence in the precursor region. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3398–3402. doi: 10.1073/pnas.78.6.3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehring R., Beyreuther K., Wright J. K., Overath P. In vitro and in vivo products of E. coli lactose permease gene are identical. Nature. 1980 Feb 7;283(5747):537–540. doi: 10.1038/283537a0. [DOI] [PubMed] [Google Scholar]
- Herrero E., Jackson M., Bassford P. J., Sinden D., Holland I. B. Insertion of a MalE beta-galactosidase fusion protein into the envelope of Escherichia coli disrupts biogenesis of outer membrane proteins and processing of inner membrane proteins. J Bacteriol. 1982 Oct;152(1):133–139. doi: 10.1128/jb.152.1.133-139.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst T. R., Randall L. L., Hardy S. J. Cellular location of heat-labile enterotoxin in Escherichia coli. J Bacteriol. 1984 Feb;157(2):637–642. doi: 10.1128/jb.157.2.637-642.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inokuchi K., Mutoh N., Matsuyama S., Mizushima S. Primary structure of the ompF gene that codes for a major outer membrane protein of Escherichia coli K-12. Nucleic Acids Res. 1982 Nov 11;10(21):6957–6968. doi: 10.1093/nar/10.21.6957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye M., Halegoua S. Secretion and membrane localization of proteins in Escherichia coli. CRC Crit Rev Biochem. 1980;7(4):339–371. doi: 10.3109/10409238009105465. [DOI] [PubMed] [Google Scholar]
- Josefsson L. G., Randall L. L. Different exported proteins in E. coli show differences in the temporal mode of processing in vivo. Cell. 1981 Jul;25(1):151–157. doi: 10.1016/0092-8674(81)90239-7. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Koyasu S., Fukuda A., Okada Y. The penicillin-binding proteins of Caulobacter crescentus. J Biochem. 1980 Jan;87(1):363–366. doi: 10.1093/oxfordjournals.jbchem.a132749. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lin J. J., Kanazawa H., Ozols J., Wu H. C. An Escherichia coli mutant with an amino acid alteration within the signal sequence of outer membrane prolipoprotein. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4891–4895. doi: 10.1073/pnas.75.10.4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg B., Van Alphen L. Molecular architecture and functioning of the outer membrane of Escherichia coli and other gram-negative bacteria. Biochim Biophys Acta. 1983 Mar 21;737(1):51–115. doi: 10.1016/0304-4157(83)90014-x. [DOI] [PubMed] [Google Scholar]
- Mock M., Pugsley A. P. The BtuB group col plasmids and homology between the colicins they encode. J Bacteriol. 1982 Jun;150(3):1069–1076. doi: 10.1128/jb.150.3.1069-1076.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H., Wu H. C. Amino acid sequence homology among the major outer membrane proteins of Escherichia coli. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1048–1052. doi: 10.1073/pnas.81.4.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver D. B., Beckwith J. E. coli mutant pleiotropically defective in the export of secreted proteins. Cell. 1981 Sep;25(3):765–772. doi: 10.1016/0092-8674(81)90184-7. [DOI] [PubMed] [Google Scholar]
- Oliver D. B., Beckwith J. Regulation of a membrane component required for protein secretion in Escherichia coli. Cell. 1982 Aug;30(1):311–319. doi: 10.1016/0092-8674(82)90037-x. [DOI] [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Site of synthesis of lipopolysaccharide. J Biol Chem. 1972 Jun 25;247(12):3973–3986. [PubMed] [Google Scholar]
- Pratt J. M., Holland I. B., Spratt B. G. Precursor forms of penicillin-binding proteins 5 and 6 of E. coli cytoplasmic membrane. Nature. 1981 Sep 24;293(5830):307–309. doi: 10.1038/293307a0. [DOI] [PubMed] [Google Scholar]
- Pugsley A. P. Genetic analysis of ColN plasmid determinants for colicin production, release, and immunity. J Bacteriol. 1984 May;158(2):523–529. doi: 10.1128/jb.158.2.523-529.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugsley A. P., Schnaitman C. A. Identification of three genes controlling production of new outer membrane pore proteins in Escherichia coli K-12. J Bacteriol. 1978 Sep;135(3):1118–1129. doi: 10.1128/jb.135.3.1118-1129.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reithmeier R. A., Bragg P. D. Proteolytic digestion and labelling studies of the organization of the proteins in the outer membrane of Escherichia coli. Can J Biochem. 1977 Oct;55(10):1082–1090. doi: 10.1139/o77-160. [DOI] [PubMed] [Google Scholar]
- Russel M., Model P. A mutation downstream from the signal peptidase cleavage site affects cleavage but not membrane insertion of phage coat protein. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1717–1721. doi: 10.1073/pnas.78.3.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russel M., Model P. Filamentous phage pre-coat is an integral membrane protein: analysis by a new method of membrane preparation. Cell. 1982 Jan;28(1):177–184. doi: 10.1016/0092-8674(82)90387-7. [DOI] [PubMed] [Google Scholar]
- Spratt B. G. Properties of the penicillin-binding proteins of Escherichia coli K12,. Eur J Biochem. 1977 Jan;72(2):341–352. doi: 10.1111/j.1432-1033.1977.tb11258.x. [DOI] [PubMed] [Google Scholar]
- Tautz D., Renz M. An optimized freeze-squeeze method for the recovery of DNA fragments from agarose gels. Anal Biochem. 1983 Jul 1;132(1):14–19. doi: 10.1016/0003-2697(83)90419-0. [DOI] [PubMed] [Google Scholar]
- Tommassen J., Lugtenberg B. Amino terminus of outer membrane PhoE protein: localization by use of a bla-phoE hybrid gene. J Bacteriol. 1984 Jan;157(1):327–329. doi: 10.1128/jb.157.1.327-329.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]