Abstract
AIM
To analyze the incidence, risk factors, prevention, treatment and outcome of small for size syndrome (SFSS) after living donor liver transplantation (LDLT).
METHODS
Through-out more than 10 years: During the period from April 2003 to the end of 2013, 174 adult-to-adults LDLT (A-ALDLT) had been performed at National Liver Institute, Menoufiya University, Shibin Elkoom, Egypt. We collected the data of those patients to do this cohort study that is a single-institution retrospective analysis of a prospectively collected database analyzing the incidence, risk factors, prevention, treatment and outcome of SFSS in a period started from the end of 2013 to the end of 2015. The median period of follow-up reached 40.50 m, range (0-144 m).
RESULTS
SFSS was diagnosed in 20 (11.5%) of our recipients. While extra-small graft [small for size graft (SFSG)], portal hypertension, steatosis and left lobe graft were significant predictors of SFSS in univariate analysis (P = 0.00, 0.04, 0.03, and 0.00 respectively); graft size was the only independent predictor of SFSS on multivariate analysis (P = 0.03). On the other hand, there was lower incidence of SFSS in patients with SFSG who underwent splenectomy [4/10 (40%) SFSS vs 3/7 (42.9%) no SFSS] but without statistical significance, However, there was none significant lower incidence of the syndrome in patients with right lobe (RL) graft when drainage of the right anterior and/or posterior liver sectors by middle hepatic vein, V5, V8, and/or right inferior vein was done [4/10 (28.6%) SFSS vs 52/152 (34.2%) no SFSS]. The 6-mo, 1-, 3-, 5-, 7- and 10-year survival in patients with SFSS were 30%, 30%, 25%, 25%, 25% and 25% respectively, while, the 6-mo, 1-, 3-, 5-, 7- and 10-year survival in patients without SFSS were 70.1%, 65.6%, 61.7%, 61%, 59.7%, and 59.7% respectively, with statistical significant difference (P = 0.00).
CONCLUSION
SFSG is the independent and main factor for occurrence of SFSS after A-ALDLT leading to poor outcome. However, the management of this catastrophe depends upon its prevention (i.e., selecting graft with proper size, splenectomy to decrease portal venous inflow, and improving hepatic vein outflow by reconstructing large draining veins of the graft).
Keywords: Living donor liver transplantation, Outcome after living donor liver transplantation, Small for size syndrome, Small for size graft, Portal inflow, Venous outflow
Core tip: Small for size syndrome (SFSS) was diagnosed in 20 (11.5%) of our recipients where, small for size dysfunction affected 16 of patients (80%) and small for size non function was present in four patients (20%). Regarding graft size in patients with SFSS; 10, 5 and 5 of patients had extra-small graft [small for size graft (SFSG), graft recipient weight ratio (GRWR) < 0.8], small graft (GRWR ≥ 0.8 and < 1) and medium sized graft (GRWR ≥ 1) respectively. Extra small graft (SFSG), portal hyper-perfusion, severe portal hypertension (PHTN), and venous outflow obstruction were the main direct causes of SFSS in 10 (50%), 3 (15%), 4 (20%), and 3 (15%) of patients respectively. While extra-small graft, PHTN, steatosis and left lobe graft were significant predictors of SFSS in univariate analysis, only graft size was independent predictor of SFSS on multivariate analysis. On the other hand, there was non-significant lower incidence of SFSS in patients with SFSG when splenectomy was done, furthermore, there was non-significant lower incidence of the syndrome in patients with right lobe graft when drainage of the right anterior and/or posterior liver sectors by middle hepatic vein, V5, V8, and/or right inferior vein was done. The SFSS related mortalities were recorded in 13/20 of patients (65%). The 6-mo, 1-, 3-, 5-, 7- and 10-year survival in patients with SFSS were 30%, 30%, 25%, 25%, 25% and 25% respectively, while, the 6-mo, 1-, 3-, 5-, 7- and 10-year survival in patients without SFSS were 70.1%, 65.6%, 61.7%, 61%, 59.7%, and 59.7% respectively, with statistical significant difference.
INTRODUCTION
Living donor liver transplantation (LDLT) is acceptable management option for end-stage liver disease (ESLD) patients to overcome organ shortage and waiting list death. On the other hand, adult-to-adults LDLT (A-ALDLT) is affected by the so-called SFSG[1]. Until now, there is debate about the least volume of the graft required for A-ALDLT[2,3]. The volume of liver graft is determined by either graft recipient weight ratio (GRWR)[4], or the ratio of graft volume relative to standard liver volume of the recipient (GV/SLV); SFSG are those with a GRWR < 0.8% and/or those with a GV/SLV < 35%[2,3]. So, if GRWR < 0.8 % or a GV/SLV < 35%, the graft should be regarded as SFSG[5-8]. As SFSS occurrence depends upon the liver graft volume as well as other different negative factors, SFSG and SFSS definitions differ in different institutes and at different times[9,10].
SFSS diagnosis is determined by persistent elevation of bilirubin and large volume of ascites during the early period post liver transplantation (LT) with absence of other possible causes[2,3,11]. Generally, it is characterized by occurrence of the followings at the end of the 1st week post LT: Persistent cholestasis, coagulopathy, ascites, encephalopathy and/or bleeding from gastrointestinal tract and/or renal failure in some severe conditions[4,11-19]. Moreover, SFSS can be defined as Total bilirubin > 10 mg/dL and/or output of ascites > 1 L/d on the 14th day after LT[7].
The loss of balance between the rapid liver regeneration and the increased demand of liver to do his function is the principal pathogenesis of SFSS[3,20], moreover, it has become evident that SFSS is not just caused by SFSG, but by multiple factors. These factors are divided into graft-related factors and recipient related ones[19,21-23].
The graft related factors include: (1) high portal inflow[17,20,24]; (2) low venous outflow[25,26]; (3) Preexisting steatosis in the donor[27,28]; (4) advanced donor age[29]; and (5) both warm and cold ischemia times[16,30,31]. However, recipient-related causes include severe preoperative ESLD and poor health status[7,16,32,33].
As occurrence of SFSS is determined by the balance between the functional mass of the liver, inflow of portal venous (PV), and outflow of hepatic vein (HV), Strategies to prevent it depend upon increasing the volume of liver graft and controlling adequate PV inflow and HV outflow by the surgical and the non-surgical techniques[22,34]. For increasing graft volume, a larger-sized graft, such as the right lobe (RL) graft, is used as the standard strategy for A-A LDLT to fulfill the required metabolic demands of adult recipients[35-38]. There are different techniques for control of graft inflow (i.e., splenectomy, splenic artery embolization, splenic artery ligation, mesocaval - or portocaval shunts)[39,40]. For outflow modulation; any short HV (especially RIV, V5, V8) larger than 0.5 cm are preserved, to be anastomosed with the recipient inferior vena cava (IVC)[3].
Splanchnic vasoconstrictors, intravenous octreotides, and oral propranolol may improve the persistent hyperbilirubinemia and coagulopathy in SFSS adult recipients[40,41]. The purpose of this work was to analyze the incidence, risk factors, prevention, treatment and outcome of SFSS after LDLT.
MATERIALS AND METHODS
Patients
Two hundred ten LDLT operations were done between April 2003 and December 2013 in our surgical department, National Liver Institute, Menoufiya university, our study included 174 adult patients after exclusion of cases with data loss and pediatrics, after taking the approval of our institutional reviewers (IRB); we did this cohort study which is a single-institution retrospective analysis of a prospectively collected database that analyzed the incidence, risk factors, prevention, treatment and outcome of SFSS in a period started from the end of 2013 to end of 2015, with patients observation from the 1st post-operative day (POD 1) until December 2015 or until patient death. The median period of follow-up reached 40.50 m, range (0-144 m).
The characteristics of recipients and their donors (including operative parameters): Regarding recipient gender, males were 154 (88.5%) while females were 20 (11.5%); furthermore, the mean age of them reached 46.5 ± 8.1 years. As regard donor gender, male donors were 118 (67.8%) and females were 56 (32.2%); the donors mean age reached 27.2 ± 6.7 years. According to Child-Pugh score, child A, B, and C were 9 (5.2%) 53 (30.5%) and 112 (64.4%) respectively, on the other hand, the mean model for end stage liver disease score(MELD) was 16.09 ± 4.3, moreover, MELD < 18, MELD 18-24, and MELD > 24 were 114 (65.5%), 50 (28.7%) and 10 (5.7%) respectively. Pre LT portal hypertension (PHTN) affected 144 (82.8%) of them.
Steatosis affected nine (5.2%) of grafts. The RL graft was given to 166 (95.4%) and the LL was given to 8 (4.6%) of them. The MHV was reconstructed in 17 (9.8%) of patients, furthermore, there were single, double, three and four HV anastomoses in 110 (63.2%), 53 (30.5%), 10 (5.7%) and 1 (0.6%) of them respectively. However, drainage of right anterior and/or posterior sectors by MHV, V5, V8, and/or RIV in RL grafts occurred in 56/166 (33.7%) of patients. The mean actual graft weight and actual GRWR were 820.9 ± 174.2 g and 1.04 ± 0.2 g respectively, moreover, SFSG (GRWR < 0.8) was found in 17 (9.8%) of patients, where splenectomy was done in seven (41.2%) of them to decrease portal hyper-flow. The decision to do intra-operative splenectomy was as follow: 4 cases due to severe pre transplant PHTN and SFSG (GRWR = 0.7, 0.73, 0.74, and 0.75) and the other 3 cases due to extra SFSG (GRWR = 0.57, 0.65, and 0.66).
Regarding cold ischemia and warm ischemia times, their mean reached 74.9 ± 51.2 min and 52.9 ± 15.2 min respectively. On the other hand, the mean intra-operative plasma and blood transfusion reached 8.2 ± 8.9 units and 7.05 ± 7.4 units respectively. Lastly, operative time mean was 13.1 ± 3.2 h while the in-hospital stay mean after LT was 22.4 ± 15.9 d (Table 1).
Table 1.
Characteristics of patients and their donors
| Character | n (%) 174 (100%) (mean ± SD) |
| Donor age (yr) (mean ± SD) | 27.2 ± 6.7 |
| Recipient age (yr) (mean ± SD) | 46.5 ± 8.1 |
| Donor gender | |
| Males | 118 (67.8) |
| Females | 56 (32.2) |
| Recipient gender | |
| Males | 154 (88.5) |
| Females | 20 (11.5) |
| Child class | |
| A | 9 (5.2) |
| B | 53 (30.5) |
| C | 112 (64.4) |
| MELD score | |
| < 18 | 114 (65.5) |
| 18-24 | 50 (28.7) |
| > 24 | 10 (5.7) |
| MELD score (mean ± SD) | 16.09 ± 4.3 |
| Pre LT PHTN | 144 (82.8) |
| Steatosis | 9 (5.2) |
| Graft type | |
| Right lobe | 166 (95.4) |
| Left lobe | 8 (4.6) |
| MHV with the graft | |
| RL graft | 10 (5.7) |
| LL graft | 7 (4.1) |
| No of HV anastomoses | |
| 1 | 110 (63.2) |
| 2 | 53 (30.5) |
| 3 | 10 (5.7) |
| 4 | 1 (0.6) |
| Drainage of RT anterior and/or posterior sectors by MHV, V5, V8, and/or RIV in RT lobe grafts (n = 166) | 56/166 (33.7) |
| Actual graft weight (g) (mean ± SD) | 820.9 ± 174.2 |
| Actual GRWR | 1.04 ± 0.2 |
| SFSG (GRWR < 0.8) | 17 (9.8) |
| Splenectomy in SFSG (n = 17) | 7/17 (41.2) |
| Cold ischemia time (min) (mean ± SD) | 74.9 ± 51.2 |
| Warm ischemia time (min) (mean ± SD) | 52.9 ± 15.2 |
| Intraoperative blood transfusion (units) (mean ± SD) | 7.05 ± 7.4 |
| Intraoperative plasma transfusion (units) (mean ± SD) | 8.2 ± 8.9 |
| Operative time (h) (mean ± SD) | 13.1 ± 3.2 |
| Hospital stay post LT (d) (mean ± SD) | 22.4 ± 15.9 |
MELD: Model for end stage liver disease; PHTN: Portal hypertension; MHV: Middle hepatic vein; RIV: RT inferior vein; GRWR: Graft recipient weight ratio; SFSG: Small for size graft.
Methods
We collected our data from the unit of LT of our Institute after obtaining written informed consents for operations and researches from recipients and their donors. Our donor’s age was > 19 years, furthermore, they underwent the followings: Liver function tests, abdominal ultrasound, liver biopsy, CT angiography, CT volumetric study and psychological assessment. We studied the following.
Preoperative data: Age of donors and recipients, their gender, donors body mass index and liver biopsy, recipient Child Pugh, MELD scores and PHTN. For pre-operative prevention of SFSS; the following strategies were done: (1) appropriate donor selection: (2) steatosis < 10%; (3) donor diet program and/or daily exercise for controlling steatosis in donors; (4) younger donors; (5) in the early cases, estimated (by volumetric study) GRWR < 0.8 were refused, and then in late cases we refused estimated GRWR < 1 for obtaining actual GRWR < 0.8; and (6) appropriate recipient selection by refusing MELD scores < 30.
Intra-operative data: RL or LL grafts, graft with or without MHV, No of HV anastomoses, HV drainage of the RT anterior and/or posterior liver sectors, actual graft weight, and GRWR, performing splenectomy or not, cold ischemia and worm ischemia times per minutes, plasma and blood transfusion per units and operative time per hours.
For intra-operative prevention of SFSS, the following strategies were done: (1) in the donor operation, with RL graft without middle hepatic vein (MHV) (our standard technique), any short hepatic vein (specially RIV, V5, V8) > 0.5 cm was preserved for possible anastomosis with recipient veins, while MHV was taken with the graft in some cases (dominant MHV and/or SFSG), on the other hand, MHV was taken with all LL grafts except one of them[42]; (2) during back table preparation, the required interposition vein grafts (patch, pantaloon or jumping grafts) that were obtained mainly from the native PV or PUV were reconstructed with the graft veins and prepared for reconstruction with the recipient veins to maximize the liver graft outflow; (3) in the recipient operation, IVC was preserved during explantation of the native liver, the RL graft HV drainage pathways consisted of the RHV without MHV or with it in some cases, furthermore, the RIV, V5 and/or V8 veins were reconstructed in some cases when indicated (Figures 1, 2, 3 and 4). The standard technique used in reconstruction of the RHV was an end-to side anastomosis between RHV of the graft and the RHV of the recipient with caudal extension to the IVC[43]. However, the LL graft HV drainage pathways consisted of the MHV with the LHV in one stump or separately (N.B the standard technique of HV reconstruction was performing a wide end-to-side anastomosis, between the graft and recipient veins avoiding rotation with extended incision to the vena cava)[3]. It was fundamental to perform complete reconstruction of these pathways of HV outflow to avoid HV congestion of the RL or LL grafts.
Figure 1.
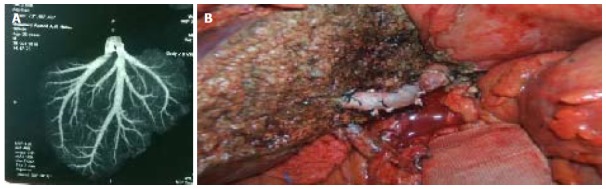
Graft with V5 to be anastomosed with recipient liver transplantation hepatic vein. A: Computed tomography venography showing large V5; B: A jumping graft between the V5 vein of liver graft and liver transplantation hepatic vein of recipient.
Figure 2.
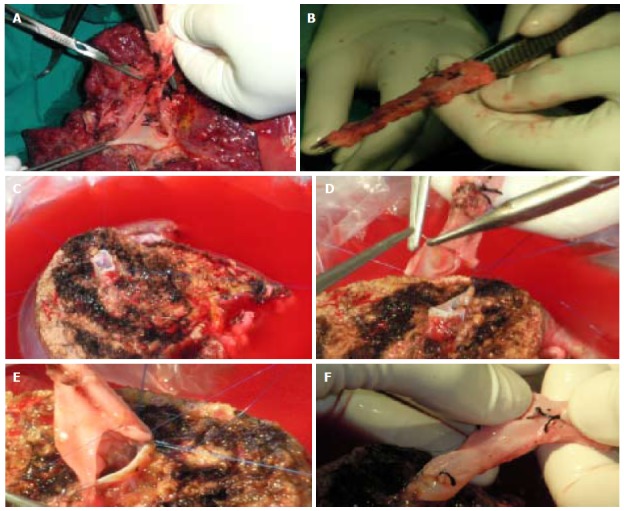
Graft with V8 to be anastomosed with recipient inferior vena cava by jumping graft. A: Obtaining the venous graft from native PV; B: The venous graft; C: V8 vein during back table preparation; D and E: Anastomosing the venous graft to V8; F: Preparation for anastomosing the venous graft to IVC. IVC: Inferior vena cava.
Figure 3.
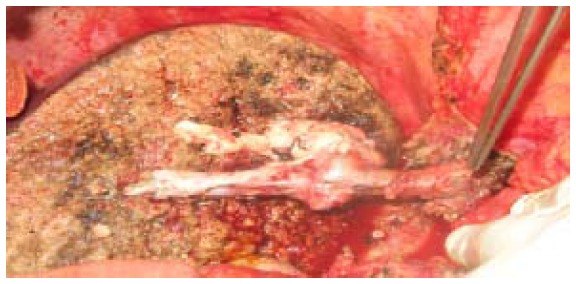
Venous graft obtained from native PUV, portal venous and hepatic vein to communicate 2 V5, 1V8 and right hepatic vein of liver graft with recipient inferior vena cava.
Figure 4.
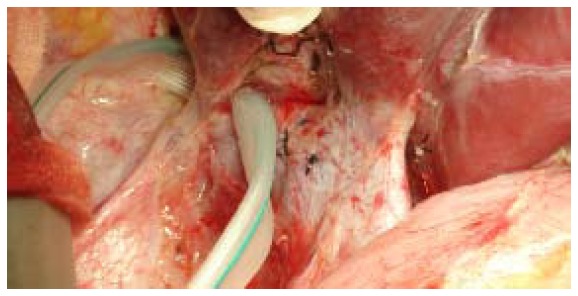
Small right hepatic vein (encircled) and large right inferior vein harvested and anastomosed with recipient inferior vena cava.
The portal vein (PV) reconstruction was then performed in an end-to-end fashion using 3 loupe magnification and by using 6/0 prolene continuous stitches with the routine use of about 1 cm growth factor during tying[44]. After PV reconstruction, doppler ultrasonography (US) was done to assess PV flow (PVF).
Postoperative management: (1) based on our institutional policy and similar to other schools like Japanese school; immunosuppression protocol was as follow: Triple-drug regimen that included calcineurin Inhibitors (CNIs) as FK-506 or cyclosporin, mycophenolate mofetil (MMF), and steroids. Three months after LT, steroids were withdrawn while we performed withdrawal of MMF 6 mo after operation. In late cases, for minimizing the dose of CNI, we administered an interleukin-2 receptor blocker on the day of LT and on the 4th day postoperative; (2) Doppler ultrasonography (PV and HV patency, flow and velocities) was performed routinely just after vascular reconstruction and after closure of the abdomen and then twice daily until the 7th day after operation (POD7), and once per day until hospital discharge; (3) Diagnosis of SFSS: The patients laboratory and clinical parameters (i.e., Serum bilirubin, INR, volume of ascites, and encephalopathy) were followed up to detect the occurrence of SFSS that was classified into small for size dysfunction (SFSD) and small for size non function (SFSNF) (N.B, SFSD is dysfunction of the graft (the presence of persistent hyperbilirubinemia, ascites and coagulopathy) during the early post LT period with absence of other possible causes like Immunological (e.g., graft rejection), technical (e.g., HA or PV obstruction, HV outflow occlusion or biliary leak), infection (e.g., cholangitis). However, SFSS is SFSD or failure of the graft (SFSNF) (loss of graft function leading to patient loss or necessity of retransplantation) during the early post LT period with absence of those previously mentioned causes[11]; and (4) management of SFSS: Strategies for prevention were mentioned in the pre- and intra-operative data; furthermore, meticulous post-transplant care was taken in cases with SFSG; Treatment: Right now, very little literature payed attention on how to manage the SFSS after its development; however, oral propranolol (2 × 40 mg/d) and a somatostatin infusion (250-μg bolus followed by perfusion at a rate of 250-50 μg/h for 5 d were given to some of our patients with SFSS to decrease PVF[23,41,45]. Moreover, liver symptomatic support was taken by all patients with the syndrome[15].
Follow-up and outcome of patients: They were followed-up daily until hospital discharge, then weekly until the end of the 1st month then monthly until the end of the follow-up period to detect SFSS and its outcome regarding survival, mortalities, causes of deaths as well as the outcome of SFSG.
Statistical techniques
We used SPSS software (version 21, Chicago, IL, United States) for data processing. Categorical variables were analyzed with the χ2 or Fisher exact tests. Continuous variables were compared using the student T or Mann whitney tests. The pre-operative, intra-operative and post-operative variables were descriptively studied. We did comparison between patients with and without SFSS regarding the pre- and intra-operative variables using univariate analysis and then multivariate analysis. Furthermore, their outcome as well as cause of death was compared by univariate analyses. On the other hand, Kaplan-Meier curve was applied and plotted for survival analysis (patient and graft survival) and the log-rank tests were used for comparing patient and graft survival according to SFSS and for comparing patient survival according to SFSG. In the previous tests, if P value was < 0.05, it was considered significant.
RESULTS
Some characteristics of patients with SFSS
SFSS was diagnosed in 20 (11.5%) of our recipients where, SFSD affected 16 of patients (80%) and SFSNF was present in four patients (20%). Persistent hyperbillirubinaemia, ascitis, and coagulopathy affected 100%, 90%, and 85% of our SFSS cases respectively, where; all the 16 patients with SFSD had persistent hyperbilirubinemia, ascites and coagulopathy during the early post-LT period; however, all the 4 cases with SFSNF had persistent hyperbilirubinaemia, 2 of them had massive ascites and one of them had coagulopsthy; furthermore, they developed graft failure and died from SFSS complications (e.g., Sepsis, MOF, ARDS, DIC) during the 1st week post-transplant. Regarding graft size in patients with SFSS, 10, 5, and 5 of patients had extra-small graft (SFSG, GRWR < 0.8), small graft (GRWR ≥ 0.8 and < 1) and medium sized graft (GRWR ≥ 1) respectively. Extra small graft (SFSG), portal hyperperfusion, severe PHTN, and venous outflow obstruction were the main direct causes of SFSS in 10 (50%), 3 (15%), 4 (20%), and 3 (15%) of patients respectively. Moreover; Portal hyper-perfusion was assessed by doppler US post operatively, severe PHTN was the persistent pre transplant severe PHTN that was assessed by complete history, clinical examination laboratory and imaging, lastly, venous outflow obstruction was known by post-transplant doppler ultrasonography US (Table 2).
Table 2.
Some characteristics of patients with small for size syndrome
| Character | n (%) |
| SFSS | 20 (100) |
| Type of SFSS | |
| SFSD | 16 (80) |
| SFSNF | 4 (20) |
| Main presentation | |
| Hyperbilirubinaemia | 20 (100) |
| Large volume of ascites | 18 (90) |
| Coagulopathy | 17 (85) |
| Graft size | |
| GRWR < 0.8 (SFSG) | 10 (50) |
| GRWR ≥ 0.8 and < 1 | 5 (25) |
| GRWR ≥ 1 | 5 (25) |
| Main aetiology of SFSS | |
| Extra small graft (SFSG) | 10 (50) |
| Portal hyperperfusion | 3 (15) |
| Severe PHTN | 4 (20) |
| Outflow obstruction | 3 (15) |
SFSS: Small for size syndrome; SFSD: Small for size dysfunction; SFSNF: Small for size non function; PHTN: Portal hypertension.
Comparison between patients with and without SFSS
The following variables were statistically significant predictors of SFSS on univariate analysis, Pre LT PHTN, graft steatosis, LL graft, SFSG, mean actual graft weight 640.50 ± 211.049 g, mean actual GRWR 0.862 ± 0.2158 g and mean intra-operative plasma transfusion 11.40 ± 7.816 units. On the other hand, there was lower incidence of SFSS in patients with SFSG who underwent splenectomy [4/10 (40%) SFSS vs 3/7 (42.9%) no SFSS] but without statistical significance, However, there was none significant lower incidence of the syndrome in patients with RL graft when drainage of the RT anterior and/or posterior sectors by MHV, V5, V8, and/or RIV was done [4/10 (28.6%) SFSS vs 52/152 (34.2%) no SFSS], furthermore, there was lower incidence of the syndrome in patients with RL graft without MHV who underwent reconstruction of V5, V8 and/or RIV [3/13 (23.1%) SFSS vs 43/143 (30.1%) no SFSS] but without statistical significance. On the other hand, Child score, MELD score, cold and worm ischemia times had no effect on occurrence of the syndrome (Table 3).
Table 3.
Comparison between patients with and without small for size syndrome (Univariate analysis)
| Character | SFSS, n (%) 20 (100) (mean ± SD) | No SFSS, n (%) 154 (100) (mean ± SD) | P value |
| Child class | < 0.05 | ||
| A | 1 (5) | 8 (5.2) | |
| B | 7 (35) | 46 (29.9) | |
| C | 12 (60) | 100 (64.9) | |
| MELD score | < 0.05 | ||
| < 18 | 16 (80) | 98 (63.6) | |
| 18-24 | 4 (20) | 46 (29.9) | |
| > 24 | 0 (0) | 10 (6.5) | |
| Pre LT PHTN | 0.046 | ||
| Yes | 20 (100%) | 128 (83.1%) | |
| No | 0 (0) | 26 (16.9%) | |
| Steatosis | 0.035 | ||
| Yes | 3 (15) | 6 (3.9) | |
| No | 17 (85) | 148 (96.1) | |
| Graft type | 0 | ||
| RL | 14 (70) | 152 (98.7) | |
| LL | 6 (30) | 2 (1.3) | |
| SFSG (GRWR < 0.8) | 0 | ||
| Yes | 10 (50) | 7 (4.5) | |
| No | 10 (50) | 147 (95.5) | |
| Actual graft weight (g) (mean ± SD) | 640.50 ± 211.049 | 844.39 ± 154.888 | 0 |
| Actual GRWR (g) (mean ± SD) | 0.862 ± 0.2158 | 1.065 ± 0.1922 | 0.001 |
| Cold ischemia time (min) (mean ± SD) | 73.95 ± 55.350 | 75.13 ± 50.923 | < 0.05 |
| Warm ischemia time (min) (mean ± SD) | 50.95 ± 14.248 | 52.08 ± 16.336 | < 0.05 |
| Intraoperative plasma transfusion (units) (mean ± SD) | 11.40 ± 7.816 | 7.81 ± 8.943 | 0.021 |
| No. of HV anastomoses | < 0.05 | ||
| 1 | 11 (55) | 99 (64.3) | |
| 2 | 8 (40) | 45 (29.2) | |
| 3 | 1 (5) | 9 (5.8) | |
| 4 | 0 (0) | 1 (0.6) | |
| Splenectomy in patients with SFSG (n = 17) | < 0.05 | ||
| Yes | 4 (40) | 3 (42.9) | |
| No | 6 (60) | 4 (57.1) | |
| Drainage of RT anterior and/or posterior sectors (MHV, V5, V8, RIV) in RL graft with or without MHV (n = 166) | < 0.05 | ||
| Yes | 4 (28.6) | 52 (34.2) | |
| No | 10 (71.4) | 100 (65.8) | |
| MHV reconstruction in patients with RL graft (n = 166) | < 0.05 | ||
| Yes | 1 (7.1) | 9 (5.9) | |
| No | 13 (92.9) | 143 (94.1) | |
| Drainage of RT anterior and/or posterior sectors (V5, V8, RIV) in RL graft without MHV(n = 156) | < 0.05 | ||
| Yes | 3 (23.1) | 43 (30.1) | |
| No | 10 (76.9) | 100 (69.9) |
MELD: Model for end stage liver disease; Pre LT PHTN: Pre liver transplant portal hypertension; RL: Right lobe; LL: Left lobe; SFSG: Small for size graft; GRWR: Graft recipient weight ratio; MHV: Middle hepatic vein; RIV: Right inferior vein.
On multivariate analysis, mean actual graft weight 640.50 ± 211.049 g, and mean actual GRWR 0.862 ± 0.2158 g were the only independent predictors of SFSS, however, graft steatosis had trend towards independence (P = 0.06) (Table 4).
Table 4.
Multivariate analysis of predictors of small for size syndrome (Binary logistic regression)
| P value | Exp(B) |
95%CI for EXP (B) |
||
| Upper | Lower | |||
| Pre LT PHTN | 0.998 | 0.000 | 0.000 | |
| Steatosis | 0.060 | 0.145 | 0.020 | 1.074 |
| Graft type | 0.166 | 6.407 | 0.463 | 88.717 |
| Actual GRWR < 0.8 | 0.050 | 4.303 | 1.024 | 18.082 |
| Actual graft WT | 0.030 | 1.004 | 1.000 | 1.008 |
| Intraoperative plasma transfusion (units) | 0.235 | 0.963 | 0.905 | 1.025 |
Pre LT PHTN: Pre liver transplant portal hypertension; GRWR: Graft recipient weight ratio.
Outcome of patients
Patients with SFSG had statistically significant higher mortality than those without SFSG (76.5% vs 40.8%, P = 0.005), furthermore, mortality was significantly higher in SFSS patients than those without SFSS (75% vs 40.3%, P = 0.003), On the other hand, the most frequent cause of death in patients with the syndrome was the syndrome itself and its complications (i.e., Sepsis, graft failure, DIC, renal failure, ARDS, and MOF), furthermore, the 4 cases with SFSNF died during the 1st week post LT due to the syndrome complications (e.g., sepsis, MOF, ARDS, DIC) and the other 16 cases with SFSD were classified into: Five a live patients, 2 patients died from post LT bleeding, and 9 patients died from the syndrome complications (i.e., Sepsis, graft failure, DIC, renal failure, ARDS, MOF). However, sepsis was the most frequent reason for mortality in non SFSS patients 19 (30.6%); moreover, MOF from causes other than SFSS, post-operative bleeding, intra-operative bleeding, PVT, renal impairment from causes other than SFSS, metastatic cholangiocarcinoma, early graft dysfunction from causes other than SFSS, HCC recurrence, ischemic reperfusion injury, HAT were the other causes of death in 11 (17.7%), 10 (16.1%), 8 (12.9%), 4 (6.4%), 2 (3.2%), 2 (3.2%), 2 (3.2%), 2 (3.2%), 1 (1.6%), and 1 (1.6%) of them respectively. Regarding clavien grading, all the previous causes of death in both groups were grade V. The 6-mo, 1-, 3-, 5-, 7- and 10-year survival in patients with SFSS were 30%, 30%, 25%, 25%, 25% and 25% respectively, while, the 6-mo, 1-, 3-, 5-, 7- and 10-year survival in patients without SFSS were 70.1%, 65.6%, 61.7%, 61%, 59.7%, and 59.7% respectively, with statistical significant difference. Lastly, graft survival in patients with SFSS was 20%, however it was 57.8% in patients without the syndrome with statistical significant difference (P = 0.001) (Table 5 and Figure 5).
Table 5.
Outcome of patients
| Total number | SFSS n (%) 20 (100) | - | No SFSS n (%) 154 (100) | - | P value |
| Overall mortality | 15 (75) | Grade | 62 (40.3) | Grade | 0.003 |
| Cause of mortality and their Dindo-Clavien score | |||||
| Sepsis from causes other than SFSS | 0 | - | 19 (30.6) | V | 0 |
| SFSS (sepsis, graft failure, DIC, renal failure, ARDS, MOF) | 13 (86.7) | V | 0 | - | |
| MOF from causes other than SFSS | 0 | - | 11 (17.7) | V | |
| Post-operative bleeding | 2 (13.3) | V | 10 (16.1) | V | |
| Intra-operative bleeding | 0 | - | 8 (12.9) | V | |
| PVT | 0 | - | 4 (6.4) | V | |
| Renal impairment from causes other than SFSS | 0 | - | 2 (3.2) | V | |
| Metastatic cholangiocarcinoma | 0 | - | 2 (3.2) | V | |
| Early graft dysfunction from causes other than SFSS | 0 | - | 2 (3.2) | V | |
| HCC recurrence | 0 | - | 2 (3.2) | V | |
| Ischemic reperfusion injury | 0 | - | 1 (1.6) | V | |
| HAT | 0 | - | 1 (1.6) | V | |
| 6-mo survival | 6 (30) | - | 108 (70.1) | - | 0.000 |
| 1-yr survival | 6 (30) | - | 101 (65.6) | - | 0.002 |
| 3-yr survival | 5 (25) | - | 95 (61.7) | - | 0.002 |
| 5-yr survival | 5 (25) | - | 94 (61) | - | 0.002 |
| 7-yr survival | 5 (25) | - | 92 (59.7) | - | 0.003 |
| 10-yr survival | 5 (25) | - | 92 (59.7) | - | 0.003 |
| Survival per months (mean ± SD) | 16.3 ± 28.9 | - | 39.9 ± 34.3 | - | 0.002 |
| Graft survival | 4 (20) | 89 (57.8) | 0.001 | ||
| Graft survival per months (mean ± SD) | 16.2 ± 28.9 | 39.7 ± 34.3 | 0.003 |
SFSS: Small for size syndrome; DIC: Disseminated intravascular coagulation; ARDS: Adult respiratory distress syndrome; MOF: Multi organ failure; PVT: Portal vein thrombosis; HCC: Hepatocellular carcinoma; HAT: Hepatic artery thrombosis.
Figure 5.
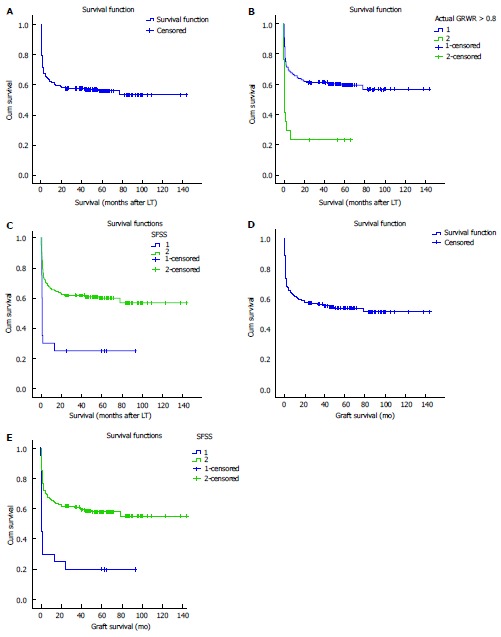
Kaplan-Meier survival curves (1, 2, 3). A: KM survival curve; B: SFSG and survival [SFSG (GRWR < 0.8) = 2, Log-Rank = 0.00]; C: SFSS and survival (SFSS = 1, Log-Rank = 0.00); D: KM graft survival curve; E: SFSS and graft survival (SFSS = 1, Log-Rank = 0.00). SFSG: Small for size graft; GRWR: Graft recipient weight ratio; SFSS: Small for size syndrome.
DISCUSSION
SFSS limits LT expansion; furthermore, it is the major cause of worse short-term prognosis after LDLT[17]. Therefore, better understanding of its pathogenesis, risk factors, strategies for prevention and treatment may improve outcomes after LDLT.
The incidence of SFSS in LL LDLT is higher than RL LDLT (20% vs 10%)[46]; as the LL graft gives only about 40 % of the needed liver mass that affect the metabolic demands of adult recipients leading to SFSS[1,18,47]. Similarly, the syndrome rate was significantly higher in our LL LDLT than RL LDLT (75% vs 8.4%, P = 0.000), and this was due to small NO of our LL LDLT (eight cases), where six of them (75%) had SFSG. On the other hand, LL SFSG was the only independent predictor of graft dysfunction in Yi et al[48] (2008) study. However, SFSS rate was 22.2%, and 19.5% in LL LDLT of Soejima et al[7] (2003), and Soejima et al[49] (2012) studies respectively, and 11.5% in our study that included mainly RL LDLT(166 cases). On the other hand, it was 9.6% and 12.5% in LDLT of Gruttadauria et al[50] (2015) and Ben-Haim et al[32] (2001) studies respectively. In contrast, it was higher (22.7%, 50% and 37.5%) in RL LDLT of Goralczyk et al[15] (2011), LL LDLT of Katsuragawa et al[51] (2009) and LL LDLT of Lauro et al[52] (2007) studies respectively, and obviously lower (6.3%) in Botha et al[53] (2010) study.
SFSS is a disease related to partial liver grafts denoting its inability to perform the functional requirements of the adult recipients resulting in hepatic dysfunction and/or failure and usually manifests as hyperbilirubinemia, ascites, coagulopathy, and encephalopathy[15,19,23,40,46,47]. Furthermore, it is characterized microscopically by cholestasis, hemorrhagic necrosis around the central veins and ballooning of hepatocytes due to microcirculatory disturbances[54]. Similarly, the syndrome was presented by hyperbilirubinemia, ascites, and coagulopathy in 100%, 90%, and 85% of our patients respectively. Moreover, we had 4 (20%) cases with SFSNF and 16 (80%) patients with SFSD.
The principal pathogenesis of SFSS is the unbalance between regeneration of the liver and the increased liver function demand, resulting in graft dysfunction[1]. Furthermore, it is clear that the syndrome is not just caused by SFSG, but also by multiple factors including technical issues, quality of the graft, and recipient factors[3,18,22,32,55,56] (where, the balance between PV inflow, outflow of HV, and functional mass of liver determines its development)[17]. So, for preventing SFSS, it is important to increase the graft volume, and to control adequate PV inflow and HV outflow by the surgical and the none surgical techniques[34]. On the other hand we divided our strategies for preventing the occurrence of the syndrome into pre-operative and intra-operative ones.
The required graft size for successful LT is 30%-40% of the expected liver volume for the recipient (GV/SLV) or 0.8%-1.0% of the body weight (GRWR)[19]; as the insufficient graft size is the primary cause of SFSS due to the relative shortage of hepatic parenchymal cells[2,3,12,16,17,55]; furthermore, SFSG suffers from a transient PHTN early after reperfusion, that is associated with up-regulation of endothelin-1 in the graft and ultra-structural evidence of sinusoidal damage[8], so, the incidence of SFSS increases when the graft is SFSG[11,23,52,57]. In Similar, SFSG was independent predictor of SFSS in Lei et al[58] (2012) study, similarly, SFSG was the most frequent cause of SFSS (50%) in our series, and the only independent predictor of it in our multivariate analysis despite our efforts to decrease SFSG by selecting larger-sized RL graft and by selecting donors with estimated GRWR > 1(in our late cases) as a pre-operative strategy for preventing SFSS. In contrast, Graft size had no impact on SFSS in Shimazu et al[59] (2004), and Ikegami et al[60] (2009) studies.
Although, SFSS is frequently encountered in SFSG (GRWR < 0.8), it may also be found in recipients of larger grafts (GRW > 0.8)[9,10,61-64]. Similarly, in our work the incidence of SFSS in normal size graft (GRWR > 0.8) was 6.4% (10/157); and this was due to the effect of other negative factors.
Steatotic liver grafts should not be used if the graft volume is small to avoid SFSS[16,17]; furthermore, graft steatosis is an exclusion criterion for donation in LDLT[65]. The mechanisms of poor steatotic graft function after reperfusion include defective anaerobic metabolism of the fatty hepatocytes, decreased lumen of sinusoids by the fat droplets, and higher free radicals caused by lipid peroxidation[3,27]. In similar, severe steatosis was significantly associated with poor function post LDLT in Hayashi et al[66] (1999) study. In addition, despite our refusal of grafts with steatosis > 10% to avoid the occurrence of SFSS, steatosis was significant predictor of SFSS in our univariate analysis; moreover it had a trend towards being independent predictor in multivariate analysis. In contrast, graft steatosis had no impact on graft dysfunction in Yi et al[48] (2008) study. Similarly, Sterneck et al[67] (1995) reported that grafts with mild to moderate steatosis had good function, and Soejima et al[56] (2003) found that a graft with 20%-50% macrovesicular steatosis (moderate grade) was accepted for transplantation.
Because LDLT is a scheduled procedure, daily exercise and diet control are required for steatosis control in donors[1,4]. In similar, donor diet programs and/or daily exercise for controlling steatosis in our donors were parts of our preoperative strategies for avoiding SFSS.
The principal mechanism in SFSS seems to be sinusoidal shear stress secondary to increased PV pressure (PVP) and/or PVF which cause graft over-perfusion leading to hepatic microcirculatory disturbance, hepatocyte functional insufficiency, over-regeneration of the hepatocytes, hepatocellular damage and death[3,16,17,19,20,23,46,51,52,68]; furthermore, Portal hyper-perfusion and insufficient venous outflow decrease the arterial perfusion (the so-called hepatic arterial buffer response), with a reduced capacity for regeneration, resulting in impaired liver function[18,19,23,69]; Similarly, portal inflow volume was independent predictor of SFSS in Lei et al[58] (2012) study. In similar, in our work, pre LT PHTN was significant predictor of SFSS in univariate analysis; furthermore, severe pre LT PHTN that persisted post LT was the etiology of the syndrome in 4 (20%) of our cases of SFSS, however, portal hyper-perfusion (identified by doppler US) was the cause of it in 3 (15%) of them.
One of the ways to get portal decompression is depriving the splenic part of portal flow by splenectomy[3,17,19,39,46,51,52,68,70]. Furthermore, splenectomy increases the HA blood flow leading to increased oxygen supply[18]. Similarly, we did splenectomy in 7/17 of our patients with SFSG to decrease portal overflow that lead to non-significant lower incidence of the syndrome (40% SFSS vs 42.9% no SFSS).
Theoretically, a RL graft including MHV is the best graft for LDLT regarding the recipients; but, this type of graft is not performed in most major transplant centers due to increased donor risk by decreasing the residual volume of the liver[3,23]. So, the RL graft without MHV is the standard technique in A-ALDLT[1,15,71]; however deprivation of the anterior segment venous drainage cause graft congestion, leading to graft dysfunction in spite of the increased volume of the graft[1,25,26,36]. Therefore, reconstruction of the anterior segments drainage veins (V5/V8)[15,17,23,72,73] with or without the reconstruction of the RIV is frequently necessary to prevent this[3]. Similarly, in our series, RL graft without MHV was our standard technique of LT, moreover, we did reconstruction of V5, V8 and/or RIV in 46/156 of our patients with RL graft without MHV that lead to non-significant lower rate of the syndrome (23.1% SFSS vs 30.1% no SFSS). Nevertheless, venous outflow obstruction (Known by doppler US) was the reason for the syndrome in 3 (15%) of our SFSS cases. In addition, venous outflow capacity was independent predictor of SFSS in Lei et al[58] (2012) study.
Early graft function is better when the graft is given by a younger donor[74,75]; as, the grafts from older donors have diminished regenerative capacity[75,76], lower blood flow and poor function due to aging[18]. Similarly, Ikegami et al[77] (2000) in their LL LDLT, found that regeneration of grafts from older donors of LDLT were inferior to those of grafts from younger ones and Tanemura et al[78] (2012), in their RL LDLT reported that donor age equal or more than 50 years was independent predictor of impaired regeneration of remnant liver at 6 mo post LT, furthermore, donor age was significant predictor of graft dysfunction and poor graft survival in Yi et al[48] (2008) and Moon et al[79] (2010) studies, and was independent predictor of SFSS in Sanefuji et al[80] (2010) study, while Ikegami et al[29] (2008) found that grafts from younger donors had lower bilirubin levels and ascites production post LDLT. On the other hand, in their RL LDLT, the Kyoto group reported that the functional recovery of recipients from older donors was comparable to that of those from younger ones[81]. Similarly, donor age was not significant predictor of SFSS in our series where our donors had younger age (mean = 27.2 ± 6.7 years).
Both warm[30] and cold ischemia times[31] impair regeneration after LDLT. Conversely, in our series, there was no significant correlation between cold or worm ischemia times and SFSS occurrence. Similarly, ischemia time did not affect graft function in Yi et al[48] (2008) study.
A higher MELD score has negative insult on graft function that may cause its dysfunction or failure especially in SFSG; due to its inability to meet the increased metabolic and synthetic demands of those high-risk recipients with severely damaged liver function[1,16,17]. In similar, MELD score was independent predictive of SFSS in Lei et al[58] (2012) study. However, Yoshizumi et al[75] (2008) reported that a larger liver graft is necessary with older donors (> 50 years) and higher MELD score (> 20), and Emiroglu et al[82] (2007) mentioned that recipients with high MELD scores should be given grafts only when their GRWR is > 1 to improve graft survival also, Ikegami et al[77] (2000) recommended that patients with high-risk should be given a younger and larger grafts to minimize the risk of SFSS. On the other hand, pre-operative MELD score did not affect SFSS rate in our work.
The preoperative Child Pugh score is mostly associated the portal hyper-perfusion state after LT leading to SFSS[1,18]. Similarly, Ben-Haim et al[32] (2001) reported that patients with severe decompensation (Child B, C) require larger grafts to prevent occurrence of SFSS, while, Soejima et al[7] (2003) found that the rate of SFSS after A-A LDLT was higher in cirrhotic patients (43.8%) in comparison with non-cirrhotics (5%). Conversely, there was no significant correlation between Child score and SFSS in our work.
Most literature mentions how to prevent SFSS occurrence. However, very few literatures discuss the treatment of this syndrome after its occurrence. In Goralczyk et al[15] (2011) study, most SFSS cases were treated with successful symptomatic therapy. Furthermore, intravenous octreotide, and oral propranolol were found to decrease the hyperbilirubinemia and coagulopathy seen in patients with SFSS in Ozden et al[41] (2007) study. On the other hand, symptomatic liver support was given to all our patients with SFSS but with poor outcome; moreover, oral propranolol and a somatostatin infusion were given to some of our patients with SFSS to decrease portal flow and improve the syndrome outcome but also with poor outcome.
Approximately 50% of recipients with SFSG die of sepsis 4 to 6 wk after LT[83]; moreover survival rates of patients with SFSG are worse than those with adequate graft size[2,12]. In similar, SFSG was significant predictor of poor survival in our work (P = 0.005), also, it had negative impact on survival in Lo et al[84] (1999), Sugawara et al[55] (2001), and Lee et al[8] (2004) studies. Furthermore, it was independent predictor of graft loss in Katsuragawa et al[51] (2009) study. Conversely, SFSG did not affect survival in Shimazu and Kitajima[59] (2004), Shimada et al[85] (2004), Ikegami et al[60] (2009), Selzner et al[86] (2009), Moon et al[79] (2010), Kaido et al[47] (2011), and Li and Li[87] (2013) studies.
SFSS results in higher incidence of septic complications, pulmonary failure, renal failure, and increased mortality[22,23,46], furthermore, it causes prolonged hospitalization, graft and patient loss[15]. Similarly, in our series, SFSS lead to significant higher mortality rate (P = 0.003), and the most frequent cause of death was the syndrome itself and its complications (i.e., sepsis, graft failure…). In similar, recipients who developed SFSS had inferior patient survival in Soejima et al[7] (2003), and Lauro et al[52] (2007) studies. In addition, it was the direct cause of 3 mortalities in Soejima et al[43] (2006) study. In conclusion: SFSG is the independent and main factor for occurrence of SFSS after A-ALDLT leading to poor outcome. However, the management of this catastrophe depends upon its prevention (i.e., selecting graft with proper size, splenectomy to decrease portal venous (PV) inflow, and improving HV outflow by reconstructing large draining veins of the graft).
COMMENTS
Background
Small for size syndrome (SFSS) is dysfunction of the graft (the presence of persistent hyperbilirubinemia, ascites and coagulopathy) during the early post liver transplantation (LT) period with absence of other possible causes like technical, immunological or infection causes, or failure of the graft (loss of its function leading to patient loss or necessity of retransplantation) during the early post LT period with absence of the previously mentioned causes. Small for size graft (SFSG) is the independent and main factor for occurrence of this syndrome that limits LT expansion and leads to worse short-term prognosis after living donor liver transplantation (LDLT). Therefore, better understanding of SFSS pathogenesis, risk factors, strategies for prevention and treatment may improve outcomes after LDLT. Moreover, the management of this catastrophe depends mainly on its prevention by pre-, intra- and post- operative measures like selecting graft with proper size, proper control of portal vein (PV) inflow and hepatic vein (HV) outflow.
Research frontiers
SFSG is the independent and main factor for occurrence of SFSS after A-ALDLT leading to poor outcome; so it is crucial to select graft with proper size to avoid this catastrophic complication. Furthermore, proper control of PV inflow by splenectomy and HV outflow by reconstruction of large tributaries of graft HV may prevent occurrence of this syndrome, however, these conclusions need further studies.
Innovations and breakthroughs
The study goes with other literature studies that mentioned the correlation between SFSG and SFSS and their negative insult on outcome after A-A LDLT, however, the innovation and breakthroughs in the work is that the authors gave an idea about the important rule of intra-operative splenectomy (specially in SFSG) as well as the meticulous reconstruction of HV tributaries of liver graft in preventing the occurrence of this syndrome (despite the non-statistical significance), as the literature data is very few regarding these points.
Applications
The study emphasizes the rule of pre-, intra- and post-operative strategies for prevention of SFSS as selection of graft with proper size. Furthermore, the authors encourage performing further studies to emphasize the rule of intra-operative splenectomy as well as the rule of reconstructing large HV tributaries of the transplanted liver graft in preventing the occurrence of SFSS.
Terminology
SFSG: Is the graft where graft recipient weight ratio (GRWR) < 0.8; SFSD: It is dysfunction of the graft (the presence of persistent hyperbilirubinemia, ascites and coagulopathy) during the early post LT period with absence of other possible causes like Immunological (e.g., graft rejection), technical (e.g., HA or PV obstruction, HV outflow occlusion or biliary leak), infection (e.g., cholangitis); SFSNF: Failure of the graft (loss of its function leading to patient loss or necessity of retransplantation) during the early post LT period with absence of those previously mentioned causes; SFSS: SFSD and/or SFSNF.
Peer-review
It is an interesting quite large series.
Footnotes
Institutional review board statement: The study was reviewed and approved for publication by our institutional reviewers.
Informed consent statement: The data were collected from our records in the LT unit and written informed consents were obtained from both donors and recipients regarding operations and researches.
Conflict-of-interest statement: All the authors have no conflict of interest related to the manuscript.
Data sharing statement: The technical appendix and original anonymous dataset is available on request from the corresponding author at emadgadsalemaa@yahoo.com.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Egypt
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): E
Peer-review started: December 19, 2016
First decision: March 28, 2017
Article in press: June 20, 2017
P- Reviewer: Chuang WL, Kute VB, Pulitanò C S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
References
- 1.Imura S, Shimada M, Ikegami T, Morine Y, Kanemura H. Strategies for improving the outcomes of small-for-size grafts in adult-to-adult living-donor liver transplantation. J Hepatobiliary Pancreat Surg. 2008;15:102–110. doi: 10.1007/s00534-007-1297-3. [DOI] [PubMed] [Google Scholar]
- 2.Kiuchi T, Tanaka K, Ito T, Oike F, Ogura Y, Fujimoto Y, Ogawa K. Small-for-size graft in living donor liver transplantation: how far should we go? Liver Transpl. 2003;9:S29–S35. doi: 10.1053/jlts.2003.50198. [DOI] [PubMed] [Google Scholar]
- 3.Ikegami T, Shimada M, Imura S, Arakawa Y, Nii A, Morine Y, Kanemura H. Current concept of small-for-size grafts in living donor liver transplantation. Surg Today. 2008;38:971–982. doi: 10.1007/s00595-008-3771-1. [DOI] [PubMed] [Google Scholar]
- 4.Shimada M, Fujii M, Morine Y, Imura S, Ikemoto T, Ishibashi H. Living-donor liver transplantation: present status and future perspective. J Med Invest. 2005;52:22–32. doi: 10.2152/jmi.52.22. [DOI] [PubMed] [Google Scholar]
- 5.Nishizaki T, Ikegami T, Hiroshige S, Hashimoto K, Uchiyama H, Yoshizumi T, Kishikawa K, Shimada M, Sugimachi K. Small graft for living donor liver transplantation. Ann Surg. 2001;233:575–580. doi: 10.1097/00000658-200104000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ikegami T, Nishizaki T, Yanaga K, Shimada M, Kakizoe S, Nomoto K, Hiroshige S, Sugimachi K. Changes in the caudate lobe that is transplanted with extended left lobe liver graft from living donors. Surgery. 2001;129:86–90. doi: 10.1067/msy.2001.109499. [DOI] [PubMed] [Google Scholar]
- 7.Soejima Y, Shimada M, Suehiro T, Hiroshige S, Ninomiya M, Shiotani S, Harada N, Hideki I, Yonemura Y, Maehara Y. Outcome analysis in adult-to-adult living donor liver transplantation using the left lobe. Liver Transpl. 2003;9:581–586. doi: 10.1053/jlts.2003.50114. [DOI] [PubMed] [Google Scholar]
- 8.Lee HH, Joh JW, Lee KW, Kim SJ, Lee DS, Park JH, Choi SH, Heo JS, Hyon WS, Kwak MS, et al. Small-for-size graft in adult living-donor liver transplantation. Transplant Proc. 2004;36:2274–2276. doi: 10.1016/j.transproceed.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 9.Ito T, Kiuchi T, Yamamoto H, Maetani Y, Oike F, Kaihara S, Itoh K, Tanaka K. Efficacy of anterior segment drainage reconstruction in right-lobe liver grafts from living donors. Transplantation. 2004;77:865–868. doi: 10.1097/01.tp.0000116415.21371.f9. [DOI] [PubMed] [Google Scholar]
- 10.Shirouzu Y, Ohya Y, Suda H, Asonuma K, Inomata Y. Massive ascites after living donor liver transplantation with a right lobe graft larger than 0.8% of the recipient’s body weight. Clin Transplant. 2010;24:520–527. doi: 10.1111/j.1399-0012.2009.01117.x. [DOI] [PubMed] [Google Scholar]
- 11.Dahm F, Georgiev P, Clavien PA. Small-for-size syndrome after partial liver transplantation: definition, mechanisms of disease and clinical implications. Am J Transplant. 2005;5:2605–2610. doi: 10.1111/j.1600-6143.2005.01081.x. [DOI] [PubMed] [Google Scholar]
- 12.Kiuchi T, Kasahara M, Uryuhara K, Inomata Y, Uemoto S, Asonuma K, Egawa H, Fujita S, Hayashi M, Tanaka K. Impact of graft size mismatching on graft prognosis in liver transplantation from living donors. Transplantation. 1999;67:321–327. doi: 10.1097/00007890-199901270-00024. [DOI] [PubMed] [Google Scholar]
- 13.Sugawara Y, Makuuchi M. Small-for-size graft problems in adult-to-adult living-donor liver transplantation. Transplantation. 2003;75:S20–S22. doi: 10.1097/01.TP.0000046616.76542.DF. [DOI] [PubMed] [Google Scholar]
- 14.Liu C, Lee RC, Loong CC, Hsia CY, Yeh YC, Chiou SY. Increasing donor body weight to prevent small-for-size syndrome in living donor liver transplantation. World J Surg. 2010;34:2401–2408. doi: 10.1007/s00268-010-0656-4. [DOI] [PubMed] [Google Scholar]
- 15.Goralczyk AD, Obed A, Beham A, Tsui TY, Lorf T. Posterior cavoplasty: a new approach to avoid venous outflow obstruction and symptoms for small-for-size syndrome in right lobe living donor liver transplantation. Langenbecks Arch Surg. 2011;396:389–395. doi: 10.1007/s00423-010-0736-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yagi S, Uemoto S. Small-for-size syndrome in living donor liver transplantation. Hepatobiliary Pancreat Dis Int. 2012;11:570–576. doi: 10.1016/s1499-3872(12)60227-6. [DOI] [PubMed] [Google Scholar]
- 17.Rajekar H. Small-for-size syndrome in adult liver transplantation: A review. Indian Journal of Transplantation. 2013;7:53–58. [Google Scholar]
- 18.Raut V, Alikhanov R, Belghiti J, Uemoto S. Review of the surgical approach to prevent small-for-size syndrome in recipients after left lobe adult LDLT. Surg Today. 2014;44:1189–1196. doi: 10.1007/s00595-013-0658-6. [DOI] [PubMed] [Google Scholar]
- 19.Taniguchi M, Shimamura T, Todo S, Furukawa H. Small-for-size syndrome in living-donor liver transplantation using a left lobe graft. Surg Today. 2015;45:663–671. doi: 10.1007/s00595-014-0945-x. [DOI] [PubMed] [Google Scholar]
- 20.Troisi R, Ricciardi S, Smeets P, Petrovic M, Van Maele G, Colle I, Van Vlierberghe H, de Hemptinne B. Effects of hemi-portocaval shunts for inflow modulation on the outcome of small-for-size grafts in living donor liver transplantation. Am J Transplant. 2005;5:1397–1404. doi: 10.1111/j.1600-6143.2005.00850.x. [DOI] [PubMed] [Google Scholar]
- 21.Schindl MJ, Redhead DN, Fearon KC, Garden OJ, Wigmore SJ; Edinburgh Liver Surgery and Transplantation Experimental Research Group (eLISTER) The value of residual liver volume as a predictor of hepatic dysfunction and infection after major liver resection. Gut. 2005;54:289–296. doi: 10.1136/gut.2004.046524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morioka D, Egawa H, Kasahara M, Ito T, Haga H, Takada Y, Shimada H, Tanaka K. Outcomes of adult-to-adult living donor liver transplantation: a single institution’s experience with 335 consecutive cases. Ann Surg. 2007;245:315–325. doi: 10.1097/01.sla.0000236600.24667.a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Graham JA, Samstein B, Emond JC. Early Graft Dysfunction in Living Donor Liver Transplantation and the Small for Size Syndrome. Curr Transplant Rep. 2014;1:43–52. doi: 10.1007/s40472-013-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hickman R, Stapleton GN, Mets B, Hlatshwayo S, Janicki P. Hepatic blood flow during reduced liver grafting in pigs. A comparison of controls and recipients of intact allografts. Dig Dis Sci. 1995;40:1246–1251. doi: 10.1007/BF02065532. [DOI] [PubMed] [Google Scholar]
- 25.Lee S, Park K, Hwang S, Lee Y, Choi D, Kim K, Koh K, Han S, Choi K, Hwang K, et al. Congestion of right liver graft in living donor liver transplantation. Transplantation. 2001;71:812–814. doi: 10.1097/00007890-200103270-00021. [DOI] [PubMed] [Google Scholar]
- 26.Yamamoto H, Maetani Y, Kiuchi T, Ito T, Kaihara S, Egawa H, Itoh K, Kamiyama Y, Tanaka K. Background and clinical impact of tissue congestion in right-lobe living-donor liver grafts: a magnetic resonance imaging study. Transplantation. 2003;76:164–169. doi: 10.1097/01.TP.0000072340.87482.17. [DOI] [PubMed] [Google Scholar]
- 27.Nocito A, El-Badry AM, Clavien PA. When is steatosis too much for transplantation? J Hepatol. 2006;45:494–499. doi: 10.1016/j.jhep.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 28.Lo CM. Nonalcoholic steatohepatitis in donors for living donor liver transplantation. Transplantation. 2007;83:265–266. doi: 10.1097/01.tp.0000250675.55779.dd. [DOI] [PubMed] [Google Scholar]
- 29.Ikegami T, Taketomi A, Ohta R, Soejima Y, Yoshizumi T, Shimada M, Maehara Y. Donor age in living donor liver transplantation. Transplant Proc. 2008;40:1471–1475. doi: 10.1016/j.transproceed.2008.02.084. [DOI] [PubMed] [Google Scholar]
- 30.Selzner M, Camargo CA, Clavien PA. Ischemia impairs liver regeneration after major tissue loss in rodents: protective effects of interleukin-6. Hepatology. 1999;30:469–475. doi: 10.1002/hep.510300215. [DOI] [PubMed] [Google Scholar]
- 31.Selzner N, Selzner M, Tian Y, Kadry Z, Clavien PA. Cold ischemia decreases liver regeneration after partial liver transplantation in the rat: A TNF-alpha/IL-6-dependent mechanism. Hepatology. 2002;36:812–818. doi: 10.1053/jhep.2002.35535. [DOI] [PubMed] [Google Scholar]
- 32.Ben-Haim M, Emre S, Fishbein TM, Sheiner PA, Bodian CA, Kim-Schluger L, Schwartz ME, Miller CM. Critical graft size in adult-to-adult living donor liver transplantation: impact of the recipient’s disease. Liver Transpl. 2001;7:948–953. doi: 10.1053/jlts.2001.29033. [DOI] [PubMed] [Google Scholar]
- 33.Shih KC, Man K. Small-for-size liver graft injury--impact on tumor behavior. Transplant Rev (Orlando) 2010;24:1–10. doi: 10.1016/j.trre.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 34.Tanaka K, Yamada T. Living donor liver transplantation in Japan and Kyoto University: what can we learn? J Hepatol. 2005;42:25–28. doi: 10.1016/j.jhep.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 35.Fan ST, Lo CM, Liu CL, Wang WX, Wong J. Safety and necessity of including the middle hepatic vein in the right lobe graft in adult-to-adult live donor liver transplantation. Ann Surg. 2003;238:137–148. doi: 10.1097/01.sla.0000077921.38307.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kasahara M, Takada Y, Fujimoto Y, Ogura Y, Ogawa K, Uryuhara K, Yonekawa Y, Ueda M, Egawa H, Tanaka K. Impact of right lobe with middle hepatic vein graft in living-donor liver transplantation. Am J Transplant. 2005;5:1339–1346. doi: 10.1111/j.1600-6143.2005.00817.x. [DOI] [PubMed] [Google Scholar]
- 37.Asakuma M, Fujimoto Y, Bourquain H, Uryuhara K, Hayashi M, Tanigawa N, Peitgen HO, Tanaka K. Graft selection algorithm based on congestion volume for adult living donor liver transplantation. Am J Transplant. 2007;7:1788–1796. doi: 10.1111/j.1600-6143.2007.01849.x. [DOI] [PubMed] [Google Scholar]
- 38.Yu Y, Lu L, Qian X, Chen N, Yao A, Pu L, Zhang F, Li X, Kong L, Sun B, et al. Antifibrotic effect of hepatocyte growth factor-expressing mesenchymal stem cells in small-for-size liver transplant rats. Stem Cells Dev. 2010;19:903–914. doi: 10.1089/scd.2009.0254. [DOI] [PubMed] [Google Scholar]
- 39.Ikegami T, Soejima Y, Taketomi A, Sanefuji K, Kayashima H, Harada N, Yamashita Y, Maehara Y. Living donor liver transplantation with extra-small graft; inflow modulation using splenectomy and temporary portocaval shunt. Hepatogastroenterology. 2008;55:670–672. [PubMed] [Google Scholar]
- 40.Famularo S, Nefotyou K, Fotiadis N, Khan N, Foxton M, Khan AZ. Small-for-Size Liver Syndrome: a Case Series with a Proposal for Management Based on Portal Flow Modulation. J Gastrointest Cancer. 2015;46:185–189. doi: 10.1007/s12029-015-9701-8. [DOI] [PubMed] [Google Scholar]
- 41.Ozden I, Kara M, Pinarbasi B, Salmaslioglu A, Yavru A, Kaymakoglu S, Emre A, Bilge O, Alper A. Somatostatin and propranolol to treat small-for-size syndrome that occurred despite splenic artery ligation. Exp Clin Transplant. 2007;5:686–689. [PubMed] [Google Scholar]
- 42.Li C, Wen TF, Yan LN, Li B, Yang JY, Wang WT, Xu MQ, Wei YG. Predictors of patient survival following living donor liver transplantation. Hepatobiliary Pancreat Dis Int. 2011;10:248–253. doi: 10.1016/s1499-3872(11)60041-6. [DOI] [PubMed] [Google Scholar]
- 43.Egawa H, Inomata Y, Uemoto S, Asonuma K, Kiuchi T, Okajima H, Yamaoka Y, Tanaka K. Hepatic vein reconstruction in 152 living-related donor liver transplantation patients. Surgery. 1997;121:250–257. doi: 10.1016/s0039-6060(97)90353-6. [DOI] [PubMed] [Google Scholar]
- 44.Khalaf H. Vascular complications after deceased and living donor liver transplantation: a single-center experience. Transplant Proc. 2010;42:865–870. doi: 10.1016/j.transproceed.2010.02.037. [DOI] [PubMed] [Google Scholar]
- 45.Xu X, Man K, Zheng SS, Liang TB, Lee TK, Ng KT, Fan ST, Lo CM. Attenuation of acute phase shear stress by somatostatin improves small-for-size liver graft survival. Liver Transpl. 2006;12:621–627. doi: 10.1002/lt.20630. [DOI] [PubMed] [Google Scholar]
- 46.Sudhindran S, Menon RN, Balakrishnan D. Challenges and Outcome of Left-lobe Liver Transplants in Adult Living Donor Liver Transplants. J Clin Exp Hepatol. 2012;2:181–187. doi: 10.1016/S0973-6883(12)60106-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Emond JC, Renz JF, Ferrell LD, Rosenthal P, Lim RC, Roberts JP, Lake JR, Ascher NL. Functional analysis of grafts from living donors. Implications for the treatment of older recipients. Ann Surg. 1996;224:544–552; discussion 552-554. doi: 10.1097/00000658-199610000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yi NJ, Suh KS, Cho YB, Lee HW, Cho EH, Cho JY, Shin WY, Kim J, Lee KU. The right small-for-size graft results in better outcomes than the left small-for-size graft in adult-to-adult living donor liver transplantation. World J Surg. 2008;32:1722–1730. doi: 10.1007/s00268-008-9641-6. [DOI] [PubMed] [Google Scholar]
- 49.Soejima Y, Shirabe K, Taketomi A, Yoshizumi T, Uchiyama H, Ikegami T, Ninomiya M, Harada N, Ijichi H, Maehara Y. Left lobe living donor liver transplantation in adults. Am J Transplant. 2012;12:1877–1885. doi: 10.1111/j.1600-6143.2012.04022.x. [DOI] [PubMed] [Google Scholar]
- 50.Gruttadauria S, Pagano D, Liotta R, Tropea A, Tuzzolino F, Marrone G, Mamone G, Marsh JW, Miraglia R, Luca A, et al. Liver Volume Restoration and Hepatic Microarchitecture in Small-for-Size Syndrome. Ann Transplant. 2015;20:381–389. doi: 10.12659/AOT.894082. [DOI] [PubMed] [Google Scholar]
- 51.Katsuragawa H, Yamamoto M, Katagiri S, Yoshitoshi K, Ariizumi S, Kotera Y, Takahashi Y, Takasaki K. Graft size and donor age are independent factors for graft loss in adult-to-adult living-donor liver transplantation using the left liver. J Hepatobiliary Pancreat Surg. 2009;16:178–183. doi: 10.1007/s00534-008-0026-x. [DOI] [PubMed] [Google Scholar]
- 52.Lauro A, Diago Uso T, Quintini C, Di Benedetto F, Dazzi A, De Ruvo N, Masetti M, Cautero N, Risaliti A, Zanfi C, et al. Adult-to-adult living donor liver transplantation using left lobes: the importance of surgical modulations on portal graft inflow. Transplant Proc. 2007;39:1874–1876. doi: 10.1016/j.transproceed.2007.05.052. [DOI] [PubMed] [Google Scholar]
- 53.Botha JF, Langnas AN, Campos BD, Grant WJ, Freise CE, Ascher NL, Mercer DF, Roberts JP. Left lobe adult-to-adult living donor liver transplantation: small grafts and hemiportocaval shunts in the prevention of small-for-size syndrome. Liver Transpl. 2010;16:649–657. doi: 10.1002/lt.22043. [DOI] [PubMed] [Google Scholar]
- 54.Demetris AJ, Kelly DM, Eghtesad B, Fontes P, Wallis Marsh J, Tom K, Tan HP, Shaw-Stiffel T, Boig L, Novelli P, et al. Pathophysiologic observations and histopathologic recognition of the portal hyperperfusion or small-for-size syndrome. Am J Surg Pathol. 2006;30:986–993. doi: 10.1097/00000478-200608000-00009. [DOI] [PubMed] [Google Scholar]
- 55.Sugawara Y, Makuuchi M, Takayama T, Imamura H, Dowaki S, Mizuta K, Kawarasaki H, Hashizume K. Small-for-size grafts in living-related liver transplantation. J Am Coll Surg. 2001;192:510–513. doi: 10.1016/s1072-7515(01)00800-6. [DOI] [PubMed] [Google Scholar]
- 56.Soejima Y, Shimada M, Suehiro T, Kishikawa K, Yoshizumi T, Hashimoto K, Minagawa R, Hiroshige S, Terashi T, Ninomiya M, et al. Use of steatotic graft in living-donor liver transplantation. Transplantation. 2003;76:344–348. doi: 10.1097/01.TP.0000071205.52835.A4. [DOI] [PubMed] [Google Scholar]
- 57.Chui AK, Rao AR, Island ER, Lau WY. Critical graft size and functional recovery in living donor liver transplantation. Transplant Proc. 2004;36:2277–2278. doi: 10.1016/j.transproceed.2004.08.036. [DOI] [PubMed] [Google Scholar]
- 58.Lei JY, Wang WT, Yan LN. Risk factors of SFSS in adult-to-adult living donor liver transplantation using the right liver: a single-center analysis of 217 cases. Hepatogastroenterology. 2012;59:1491–1497. doi: 10.5754/hge11634. [DOI] [PubMed] [Google Scholar]
- 59.Shimazu M, Kitajima M. Living donor liver transplantation with special reference to ABO-incompatible grafts and small-for-size grafts. World J Surg. 2004;28:2–7. doi: 10.1007/s00268-003-7263-6. [DOI] [PubMed] [Google Scholar]
- 60.Ikegami T, Masuda Y, Ohno Y, Mita A, Kobayashi A, Urata K, Nakazawa Y, Miwa S, Hashikura Y, Miyagawa S. Prognosis of adult patients transplanted with liver grafts < 35% of their standard liver volume. Liver Transpl. 2009;15:1622–1630. doi: 10.1002/lt.21716. [DOI] [PubMed] [Google Scholar]
- 61.Gruttadauria S, Mandala’ L, Miraglia R, Caruso S, Minervini MI, Biondo D, Volpes R, Vizzini G, Marsh JW, Luca A, et al. Successful treatment of small-for-size syndrome in adult-to-adult living-related liver transplantation: single center series. Clin Transplant. 2007;21:761–766. doi: 10.1111/j.1399-0012.2007.00735.x. [DOI] [PubMed] [Google Scholar]
- 62.Taniguchi M, Shimamura T, Suzuki T, Yamashita K, Oura T, Watanabe M, Kamiyama T, Matsushita M, Furukawa H, Todo S. Transient portacaval shunt for a small-for-size graft in living donor liver transplantation. Liver Transpl. 2007;13:932–934. doi: 10.1002/lt.21080. [DOI] [PubMed] [Google Scholar]
- 63.Perkins JD. Treatment of small-for-size syndrome. Liver Transpl. 2008;14:571–572. doi: 10.1002/lt.21446. [DOI] [PubMed] [Google Scholar]
- 64.Kiuchi T, Onishi Y, Nakamura T. Small-for-size graft: not defined solely by being small for size. Liver Transpl. 2010;16:815–817. doi: 10.1002/lt.22113. [DOI] [PubMed] [Google Scholar]
- 65.Monsour HP Jr, Wood RP, Ozaki C, Katz S, Clark J, Dyer C, Camel S. Utility of preoperative liver biopsy in live-related donor patients for liver transplantation. Transplant Proc. 1994;26:138–139. [PubMed] [Google Scholar]
- 66.Hayashi M, Fujii K, Kiuchi T, Uryuhara K, Kasahara M, Takatsuki M, Takeichi T, Kitade H, Sugimoto T, Uemoto S, et al. Effects of fatty infiltration of the graft on the outcome of living-related liver transplantation. Transplant Proc. 1999;31:403. doi: 10.1016/s0041-1345(98)01679-0. [DOI] [PubMed] [Google Scholar]
- 67.Sterneck MR, Fischer L, Nischwitz U, Burdelski M, Kjer S, Latta A, Malago M, Petersen J, Pothmann W, Rogiers X. Selection of the living liver donor. Transplantation. 1995;60:667–671. doi: 10.1097/00007890-199510150-00009. [DOI] [PubMed] [Google Scholar]
- 68.Ogura Y, Hori T, El Moghazy WM, Yoshizawa A, Oike F, Mori A, Kaido T, Takada Y, Uemoto S. Portal pressure <15 mm Hg is a key for successful adult living donor liver transplantation utilizing smaller grafts than before. Liver Transpl. 2010;16:718–728. doi: 10.1002/lt.22059. [DOI] [PubMed] [Google Scholar]
- 69.Marcos A, Olzinski AT, Ham JM, Fisher RA, Posner MP. The interrelationship between portal and arterial blood flow after adult to adult living donor liver transplantation. Transplantation. 2000;70:1697–1703. doi: 10.1097/00007890-200012270-00006. [DOI] [PubMed] [Google Scholar]
- 70.Sato Y, Yamamoto S, Oya H, Nakatsuka H, Tsukahara A, Kobayashi T, Watanabe T, Hatakeyama K. Splenectomy for reduction of excessive portal hypertension after adult living-related donor liver transplantation. Hepatogastroenterology. 2002;49:1652–1655. [PubMed] [Google Scholar]
- 71.Inomata Y, Uemoto S, Asonuma K, Egawa H. Right lobe graft in living donor liver transplantation. Transplantation. 2000;69:258–264. doi: 10.1097/00007890-200001270-00011. [DOI] [PubMed] [Google Scholar]
- 72.Sano K, Makuuchi M, Miki K, Maema A, Sugawara Y, Imamura H, Matsunami H, Takayama T. Evaluation of hepatic venous congestion: proposed indication criteria for hepatic vein reconstruction. Ann Surg. 2002;236:241–247. doi: 10.1097/00000658-200208000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kornberg A, Heyne J, Schotte U, Hommann M, Scheele J. Hepatic venous outflow reconstruction in right lobe living-donor liver graft using recipient’s superficial femoral vein. Am J Transplant. 2003;3:1444–1447. doi: 10.1046/j.1600-6135.2003.00239.x. [DOI] [PubMed] [Google Scholar]
- 74.Olthoff KM, Merion RM, Ghobrial RM, Abecassis MM, Fair JH, Fisher RA, Freise CE, Kam I, Pruett TL, Everhart JE, et al. Outcomes of 385 adult-to-adult living donor liver transplant recipients: a report from the A2ALL Consortium. Ann Surg. 2005;242:314–323, discussion 323-discussion 325. doi: 10.1097/01.sla.0000179646.37145.ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yoshizumi T, Taketomi A, Uchiyama H, Harada N, Kayashima H, Yamashita Y, Soejima Y, Shimada M, Maehara Y. Graft size, donor age, and patient status are the indicators of early graft function after living donor liver transplantation. Liver Transpl. 2008;14:1007–1013. doi: 10.1002/lt.21462. [DOI] [PubMed] [Google Scholar]
- 76.Nadalin S, Malagò M, Radtke A, Erim Y, Saner F, Valentin-Gamazo C, Schröder T, Schaffer R, Sotiropoulos GC, Li J, et al. Current trends in live liver donation. Transpl Int. 2007;20:312–330. doi: 10.1111/j.1432-2277.2006.00424.x. [DOI] [PubMed] [Google Scholar]
- 77.Ikegami T, Nishizaki T, Yanaga K, Shimada M, Kishikawa K, Nomoto K, Uchiyama H, Sugimachi K. The impact of donor age on living donor liver transplantation. Transplantation. 2000;70:1703–1707. doi: 10.1097/00007890-200012270-00007. [DOI] [PubMed] [Google Scholar]
- 78.Tanemura A, Mizuno S, Wada H, Yamada T, Nobori T, Isaji S. Donor age affects liver regeneration during early period in the graft liver and late period in the remnant liver after living donor liver transplantation. World J Surg. 2012;36:1102–1111. doi: 10.1007/s00268-012-1496-1. [DOI] [PubMed] [Google Scholar]
- 79.Moon JI, Kwon CH, Joh JW, Jung GO, Choi GS, Park JB, Kim JM, Shin M, Kim SJ, Lee SK. Safety of small-for-size grafts in adult-to-adult living donor liver transplantation using the right lobe. Liver Transpl. 2010;16:864–869. doi: 10.1002/lt.22094. [DOI] [PubMed] [Google Scholar]
- 80.Sanefuji K, Iguchi T, Ueda S, Nagata S, Sugimachi K, Ikegami T, Gion T, Soejima Y, Taketomi A, Maehara Y. New prediction factors of small-for-size syndrome in living donor adult liver transplantation for chronic liver disease. Transpl Int. 2010;23:350–357. doi: 10.1111/j.1432-2277.2009.00985.x. [DOI] [PubMed] [Google Scholar]
- 81.Kuramitsu K, Egawa H, Keeffe EB, Kasahara M, Ito T, Sakamoto S, Ogawa K, Oike F, Takada Y, Uemoto S. Impact of age older than 60 years in living donor liver transplantation. Transplantation. 2007;84:166–172. doi: 10.1097/01.tp.0000269103.87633.06. [DOI] [PubMed] [Google Scholar]
- 82.Emiroglu R, Yilmaz U, Coskun M, Karakayali H, Haberal M. Higher graft-to-host ratio may decrease posttransplant mortality in patients with a high MELD score. Transplant Proc. 2007;39:1164–1165. doi: 10.1016/j.transproceed.2007.02.048. [DOI] [PubMed] [Google Scholar]
- 83.Heaton N. Small-for-size liver syndrome after auxiliary and split liver transplantation: donor selection. Liver Transpl. 2003;9:S26–S28. doi: 10.1053/jlts.2003.50197. [DOI] [PubMed] [Google Scholar]
- 84.Lo CM, Fan ST, Liu CL, Chan JK, Lam BK, Lau GK, Wei WI, Wong J. Minimum graft size for successful living donor liver transplantation. Transplantation. 1999;68:1112–1116. doi: 10.1097/00007890-199910270-00009. [DOI] [PubMed] [Google Scholar]
- 85.Shimada M, Ijichi H, Yonemura Y, Harada N, Shiotani S, Ninomiya M, Yoshizumi T, Soejima Y, Suehiro T, Maehara Y. Is graft size a major risk factor in living-donor adult liver transplantation? Transpl Int. 2004;17:310–316. doi: 10.1007/s00147-004-0720-9. [DOI] [PubMed] [Google Scholar]
- 86.Selzner M, Kashfi A, Cattral MS, Selzner N, Greig PD, Lilly L, McGilvray ID, Therapondos G, Adcock LE, Ghanekar A, et al. A graft to body weight ratio less than 0.8 does not exclude adult-to-adult right-lobe living donor liver transplantation. Liver Transpl. 2009;15:1776–1782. doi: 10.1002/lt.21955. [DOI] [PubMed] [Google Scholar]
- 87.Li H, Li B. Using small-for-size grafts in living donor liver transplantation recipients with high MELD scores should not be considered a contraindication. Dig Dis Sci. 2013;58:3374–3375. doi: 10.1007/s10620-013-2797-4. [DOI] [PubMed] [Google Scholar]


