Abstract
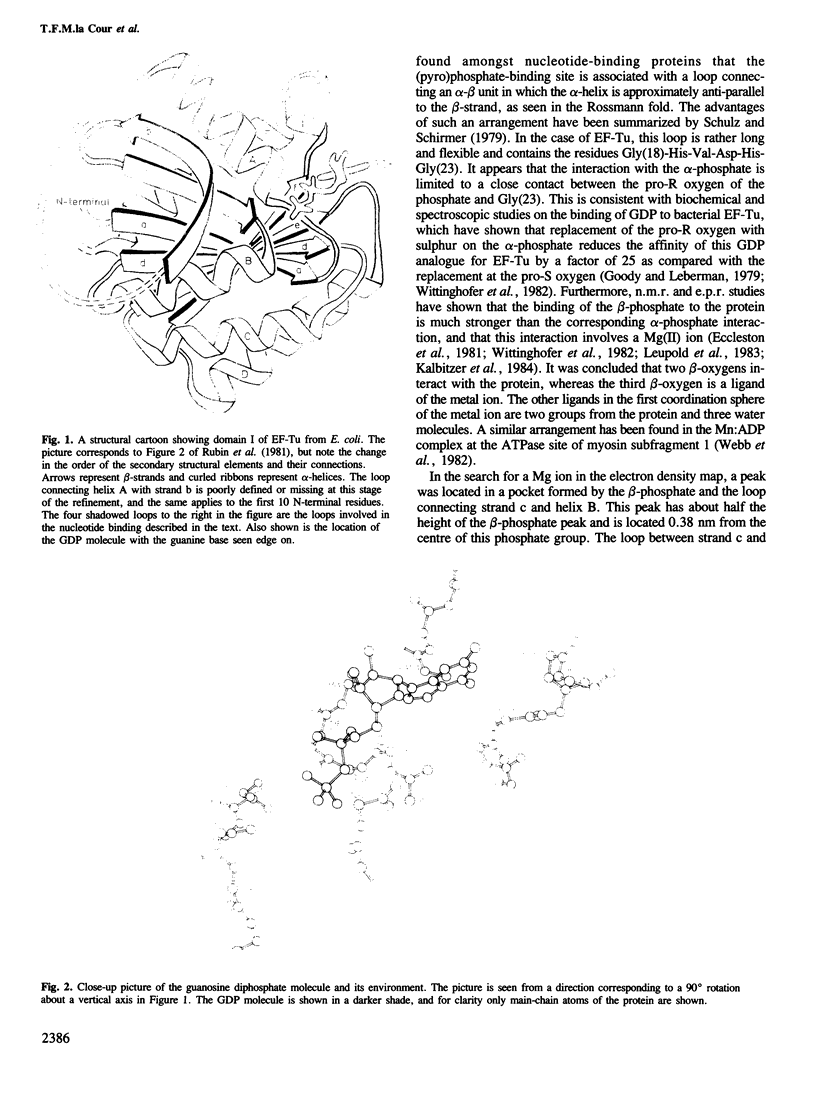
Structural details of the guanosine diphosphate binding to a modified form of elongation factor Tu from Escherichia coli, resulting from X-ray crystallographic studies, are reported. The protein elements that take part in the nucleotide binding are located in four loops connecting beta-strands with alpha-helices. These loops correspond to regions in primary sequences which show a high degree of homology when compared with other prokaryotic and eukaryotic elongation factors and initiation factor 2.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antonsson B., Leberman R. Modification of amino groups in EF-Tu.GTP and the ternary complex EF-Tu.GTP.valyl-tRNAVal. Eur J Biochem. 1984 Jun 15;141(3):483–487. doi: 10.1111/j.1432-1033.1984.tb08218.x. [DOI] [PubMed] [Google Scholar]
- Arai K., Kawakita M., Nakamura S., Ishikawa K., Kaziro Y. Studies on the polypeptide elongation factors form E. coli. VI. Characterization of sulfhydryl groups in EF-Tu and EF-Ts. J Biochem. 1974 Sep;76(3):523–534. doi: 10.1093/oxfordjournals.jbchem.a130596. [DOI] [PubMed] [Google Scholar]
- Eccleston J. F. Spectroscopic studies of the nucleotide binding site of elongation factor Tu from Escherichia coli. An approach to characterizing the elementary steps of the elongation cycle of protein biosynthesis. Biochemistry. 1981 Oct 13;20(21):6265–6272. doi: 10.1021/bi00524a055. [DOI] [PubMed] [Google Scholar]
- Eccleston J. F., Webb M. R., Ash D. E., Reed G. H. EPR studies of the Mn(II) complex with elongation factor Tu and GDP Identification of oxygen ligands to Mn(II) by observation of 17O superhyperfine coupling. J Biol Chem. 1981 Nov 10;256(21):10774–10777. [PubMed] [Google Scholar]
- Gay N. J., Walker J. E. Homology between human bladder carcinoma oncogene product and mitochondrial ATP-synthase. Nature. 1983 Jan 20;301(5897):262–264. doi: 10.1038/301262a0. [DOI] [PubMed] [Google Scholar]
- Goody R. S., Leberman R. The preparation of thiophosphate analogs of GDP and their interaction with EF-Tu. FEBS Lett. 1979 Jun 15;102(2):269–272. doi: 10.1016/0014-5793(79)80016-2. [DOI] [PubMed] [Google Scholar]
- Halliday K. R. Regional homology in GTP-binding proto-oncogene products and elongation factors. J Cyclic Nucleotide Protein Phosphor Res. 1983;9(6):435–448. [PubMed] [Google Scholar]
- Hol W. G., van Duijnen P. T., Berendsen H. J. The alpha-helix dipole and the properties of proteins. Nature. 1978 Jun 8;273(5662):443–446. doi: 10.1038/273443a0. [DOI] [PubMed] [Google Scholar]
- Jones M. D., Petersen T. E., Nielsen K. M., Magnusson S., Sottrup-Jensen L., Gausing K., Clark B. F. The complete amino-acid sequence of elongation factor Tu from Escherichia coli. Eur J Biochem. 1980 Jul;108(2):507–526. doi: 10.1111/j.1432-1033.1980.tb04748.x. [DOI] [PubMed] [Google Scholar]
- Jonák J., Smrt J., Holý A., Rychlík I. Interaction of Escherichia coli EF-Tu.GTP and EF-Tu.GDP with analogues of the 3' terminus of aminoacyl-tRNA. Eur J Biochem. 1980 Apr;105(2):315–320. doi: 10.1111/j.1432-1033.1980.tb04503.x. [DOI] [PubMed] [Google Scholar]
- Kalbitzer H. R., Goody R. S., Wittinghofer A. Electron-paramagnetic-resonance studies of manganese(II) complexes with elongation factor Tu from Bacillus stearothermophilus. Observation of a GTP hydrolysis intermediate state complex. Eur J Biochem. 1984 Jun 15;141(3):591–597. doi: 10.1111/j.1432-1033.1984.tb08234.x. [DOI] [PubMed] [Google Scholar]
- Leberman R., Egner U. Homologies in the primary structure of GTP-binding proteins: the nucleotide-binding site of EF-Tu and p21. EMBO J. 1984 Feb;3(2):339–341. doi: 10.1002/j.1460-2075.1984.tb01808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leupold C. M., Goody R. S., Wittinghofer A. Stereochemistry of the elongation factor Tu X GTP complex. Eur J Biochem. 1983 Sep 15;135(2):237–241. doi: 10.1111/j.1432-1033.1983.tb07643.x. [DOI] [PubMed] [Google Scholar]
- Levitt M., Chothia C. Structural patterns in globular proteins. Nature. 1976 Jun 17;261(5561):552–558. doi: 10.1038/261552a0. [DOI] [PubMed] [Google Scholar]
- Miller D. L., Hachmann J., Weissbach H. The reactions of the sulfhydryl groups on the elongation factors Tu and Ts. Arch Biochem Biophys. 1971 May;144(1):115–121. doi: 10.1016/0003-9861(71)90460-7. [DOI] [PubMed] [Google Scholar]
- Montandon P. E., Stutz E. Nucleotide sequence of a Euglena gracilis chloroplast genome region coding for the elongation factor Tu; evidence for a spliced mRNA. Nucleic Acids Res. 1983 Sep 10;11(17):5877–5892. doi: 10.1093/nar/11.17.5877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morikawa K., la Cour T. F., Nyborg J., Rasmussen K. M., Miller D. L., Clark B. F. High resolution x-ray crystallographic analysis of a modified form of the elongation factor Tu: guanosine diphosphate complex. J Mol Biol. 1978 Nov 5;125(3):325–338. doi: 10.1016/0022-2836(78)90406-0. [DOI] [PubMed] [Google Scholar]
- Möller W., Amons R. Phosphate-binding sequences in nucleotide-binding proteins. FEBS Lett. 1985 Jul 1;186(1):1–7. doi: 10.1016/0014-5793(85)81326-0. [DOI] [PubMed] [Google Scholar]
- Nagata S., Nagashima K., Tsunetsugu-Yokota Y., Fujimura K., Miyazaki M., Kaziro Y. Polypeptide chain elongation factor 1 alpha (EF-1 alpha) from yeast: nucleotide sequence of one of the two genes for EF-1 alpha from Saccharomyces cerevisiae. EMBO J. 1984 Aug;3(8):1825–1830. doi: 10.1002/j.1460-2075.1984.tb02053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata S., Tsunetsugu-Yokota Y., Naito A., Kaziro Y. Molecular cloning and sequence determination of the nuclear gene coding for mitochondrial elongation factor Tu of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6192–6196. doi: 10.1073/pnas.80.20.6192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovchinnikov YuA, Alakhov YuB, Bundulis YuP, Bundule M. A., Dovgas N. V., Kozlov V. P., Motuz L. P., Vinokurov L. M. The primary structure of elongation factor G from Escherichia coli. A complete amino acid sequence. FEBS Lett. 1982 Mar 8;139(1):130–135. doi: 10.1016/0014-5793(82)80503-6. [DOI] [PubMed] [Google Scholar]
- Rubin J. R., Morikawa K., Nyborg J., la Cour T. F., Clark B. F., Miller D. L. Structural features of the GDP binding site of elongation factor Tu from Escherichia coli as determined by x-ray diffraction. FEBS Lett. 1981 Jun 29;129(1):177–179. doi: 10.1016/0014-5793(81)80784-3. [DOI] [PubMed] [Google Scholar]
- Sacerdot C., Dessen P., Hershey J. W., Plumbridge J. A., Grunberg-Manago M. Sequence of the initiation factor IF2 gene: unusual protein features and homologies with elongation factors. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7787–7791. doi: 10.1073/pnas.81.24.7787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih T. Y., Papageorge A. G., Stokes P. E., Weeks M. O., Scolnick E. M. Guanine nucleotide-binding and autophosphorylating activities associated with the p21src protein of Harvey murine sarcoma virus. Nature. 1980 Oct 23;287(5784):686–691. doi: 10.1038/287686a0. [DOI] [PubMed] [Google Scholar]
- Suck D., Kabsch W. X-ray determination of the GDP-binding site of Escherichia coli elongation factor Tu by substitution with ppGpp. FEBS Lett. 1981 Apr 6;126(1):120–122. doi: 10.1016/0014-5793(81)81048-4. [DOI] [PubMed] [Google Scholar]
- Webb M. R., Ash D. E., Leyh T. S., Trentham D. R., Reed G. H. Electron paramagnetic resonance studies of MN(II) complexes with myosin subfragment 1 and oxygen 17-labeled ligands. J Biol Chem. 1982 Mar 25;257(6):3068–3072. [PubMed] [Google Scholar]
- Wierenga R. K., Hol W. G. Predicted nucleotide-binding properties of p21 protein and its cancer-associated variant. Nature. 1983 Apr 28;302(5911):842–844. doi: 10.1038/302842a0. [DOI] [PubMed] [Google Scholar]
- Wittinghofer A., Goody R. S., Roesch P., Kalbitzer H. R. The structure of the EF-Tu . GDP . Me2+ complex. Eur J Biochem. 1982 May;124(1):109–115. doi: 10.1111/j.1432-1033.1982.tb05912.x. [DOI] [PubMed] [Google Scholar]
- Wittinghofer A., Leberman R. Elongation factor T from Bacillus stearothermophilus and Escherichia coli. Purification and some properties of EF-Tu and EF-Ts from Bacillus stearothermophilus. Eur J Biochem. 1976 Feb 16;62(2):373–382. doi: 10.1111/j.1432-1033.1976.tb10169.x. [DOI] [PubMed] [Google Scholar]
- Wittinghofer A., Warren W. F., Leberman R. Structural requirements of the GDP binding site of elongation factor Tu. FEBS Lett. 1977 Mar 15;75(1):241–243. doi: 10.1016/0014-5793(77)80095-1. [DOI] [PubMed] [Google Scholar]
- van Hemert F. J., Amons R., Pluijms W. J., van Ormondt H., Möller W. The primary structure of elongation factor EF-1 alpha from the brine shrimp Artemia. EMBO J. 1984 May;3(5):1109–1113. doi: 10.1002/j.1460-2075.1984.tb01937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]