Abstract
Cardiac and pericardial masses may be neoplastic, benign and malignant, non-neoplastic such as thrombus or simple pericardial cysts, or normal variants cardiac structure can also be a diagnostic challenge. Currently, there are several imaging modalities for diagnosis of cardiac masses; each technique has its inherent advantages and disadvantages. Echocardiography, is typically the initial test utilizes in such cases, Echocardiography is considered the test of choice for evaluation and detection of cardiac mass, it is widely available, portable, with no ionizing radiation and provides comprehensive evaluation of cardiac function and valves, however, echocardiography is not very helpful in many cases such as evaluation of extracardiac extension of mass, poor tissue characterization, and it is non diagnostic in some cases. Cross sectional imaging with cardiac computed tomography provides a three dimensional data set with excellent spatial resolution but utilizes ionizing radiation, intravenous iodinated contrast and relatively limited functional evaluation of the heart. Cardiac magnetic resonance imaging (CMR) has excellent contrast resolution that allows superior soft tissue characterization. CMR offers comprehensive evaluation of morphology, function, tissue characterization. The great benefits of CMR make CMR a highly useful tool in the assessment of cardiac masses. (Fluorine 18) fluorodeoxygluocse (FDG) positron emission tomography (PET) has become a corner stone in several oncological application such as tumor staging, restaging, treatment efficiency, FDG is a very useful imaging modality in evaluation of cardiac masses. A recent advance in the imaging technology has been the development of integrated PET-MRI system that utilizes the advantages of PET and MRI in a single examination. FDG PET-MRI provides complementary information on evaluation of cardiac masses. The purpose of this review is to provide several clinical scenarios on the incremental value of PET and MRI in the evaluation of cardiac masses.
Keywords: Cardiac, Pericardial tumors, Echocardiography
Core tip: With the commercial availability of positron emission tomography- magnetic resonance imaging (PET-MRI) true simultaneous PET and MRI in a single study is real. Several studies have demonstrated the feasibility and incremental value of combined PET and MRI in many clinical applications. A combination of PET and MRI can provide incremental information in many cardiovascular scenarios. Evaluation of cardiac tumors may be most straightforward application for PET-MRI because it offers a unique opportunity to evaluate the tumor morphology, characterization, infiltration to adjacent structures, local and M staging and comprehensive cardiac evaluation in a single study. The purpose of this review is to provide several clinical scenarios on the incremental value of PET and MRI in the evaluation of cardiac masses.
INTRODUCTION
Primary cardiac tumors, Benign and Malignant, are rare with an estimated prevalence of 0.002%-0.3% at autopsy[1]. The primary benign cardiac tumors are common and include myxomas, fibromas, rhabdomyomas, lipoma, fibroelastomas, hemangioma, and paragngliomas. The primary malignant cardiac neoplasm is generally sarcomas, mesotheliomas, or lymphoma. Cardiac metastases involving the heart and pericardium (secondary cardiac tumors) from direct invasion or hematological spread are 20%-40% more common than primary cardiac tumors and usually associated with poor prognosis primary related to advanced stage of primary malignancy[2]. However, the most common type of cardiac mass is in fact psedutumors or tumors like structures such as intracardiac thrombus, pericardial cyst, valvular vegetation, perivalvular abscess, or normal cardiac variant such as crista terminalis.
Patients with primary cardiac tumors may present with a range of symptoms that can simulate cardiac disease. The clinical personation is determined mostly by location, size, and texture, the rate of growth and invasiveness of the tumor. For example, patients with mechanical obstruction related to cardiac mass in outflow tract may present with heart failure or valvular disease, systolic dysfunction or diastolic dysfunction related to impaired contractility. Pericardial disease may present as pericardial effusion, pericardial tamponade, or pericardial thickening and nodularties.
Several imaging modalities are used in the evaluation of cardiac and Pericardiac tumors with their inherent advantages and disadvantages. Transthoracic echocardiography is widely available and is considered the procedure of choice for diagnosis of intracardiac tumors. It provides information on the size, mobility, shape and location of the cardiac mass but limited information on mass tissue characterization. In certain patients, such as obese patients or patients with chronic obstructive lung disease trans esophageal echocardiography offers a diagnostic alternative[3]. Cardiac computed tomography (CT) is a noninvasive technique which offers a high spatial resolution and sufficient temporal resolution. Both cardiac and intracardiac mass can be very clearly depicted as well as their degree of myocardial and pericardial involvement; however, radiation and iodinated contrast injection are some of CT imaging[4]. Cardiac magnetic resonance (CMR) has several advantages in the diagnosis of patients with cardiac tumors. The greatest advantages of CMR over various imaging modalities, is that CMR have a unique ability to characterize tissue composition based on the inherent T1 and T2 relaxation of different tissue; in addition CMR provides excellent temporal and spatial resolution, multiplanar 3D imaging capabilities, large field of view, evaluating adjacent vascular structures, lymph nodes involvement and mediastinum[5,6]. Positron emissions tomography (PET) offers an accurate evaluation of the metabolic activity of the tumors via utilizing (fluorine 18) fluorodeoxygluocse (FDG). FDG PET is very helpful for staging malignancies, optimizing biopsy location, radiation therapy planning and detection of tumors recurrence and response to therapy. In cases of cardiac metastases FDG pet can detect both primary lesions such as lung cancer and cardiac metastases. The extent of FDG uptake by tumors is useful for differentiation between benign and malignant tumors[7].
Positron emission tomography-magnetic resonance imaging (PET-MRI) hybrid scanners are a newly developed type of clinical imaging system. PET-MRI benefits from the advantages of both PET and MRI. MRI provides tumors tissue characterization with T1 and T2, and different pulse sequence with and without gadolinium injection, extent of the tumors invasion and local metastasis, in addition, CMR offers a comprehensive examination of the heart and any complication related to the tumors such as pericardial effusion or valvular dysfunction. PET asses the metabolic evaluation of the tumors, and evaluation of the primary extracardiac tumors or other distant metastases. Integrated PET-MRI systems provide improved spatial and temporal alignment, CMR image based motion correction is also improved, artefacts such as motion and partial volume effect in PET scanning. Integrated PET-MRI system significantly reduces time of imaging acquisition compared to performing two separate examinations, improve throughput and also reduce patient’s discomfort[8,9]. In this review, we will discuss several clinical scenarios in which CMR and PET provides additive information, these illustrated cases performed as sequential examinations, not on integrated PET-MRI scan, these includes: (1) characterization and Localization of abnormal FDG uptake in the heart; (2) characterization and Localization of abnormal FDG uptake adjacent to the heart; (3) differentiation between tumoral thrombus from bland thrombus; (4) distant Metastasis (M staging); (5) evaluation of aggressiveness of the lesion and assessment of cardiac involvement; and (6) other potential indications.
CHARACTERIZATION AND LOCALIZATION OF ABNORMAL FDG UPTAKE IN THE HEART
The patterns and distribution of FDG in the normal myocardium may be classified into three types; no to faint uptake, regional uptake, and diffuse uptake. There is no specific pattern myocardial FDG uptake[10]. Furthermore, myocardial FDG uptake in the same individual is neither stable nor reproducible unless under the same fasting condition. The PM has a characteristic location on axial and coronal images. PM uptake can occasionally be seen without myocardial uptake, this appearance can mimic an intraventricular thrombus or tumor[11]. Focal intense FDG uptake is frequently observed in the basal septal region in patients with active cardiac sarcoidosis[12]. Several reports describing FDG patterns in cardiac tumors have been published[13,14]. However, the usefulness of FDG in the differentiation between benign (including thrombus) and malignant tumors.
Different imaging modalities are often necessary for approaching final diagnosis of primary or metastatic cardiac tumors. The differential diagnosis can be narrowed down by clinical history, signs and symptoms of the clinical presentation, history of primary neoplasm. Normal cardiac variants and variable myocardial uptake of FDG may raise the suspension of cardiac metastasis in some PET-CT studies in patients with cancer. In such clinical circumstances, CMR is very useful (Figures 1 and 2).
Figure 1.
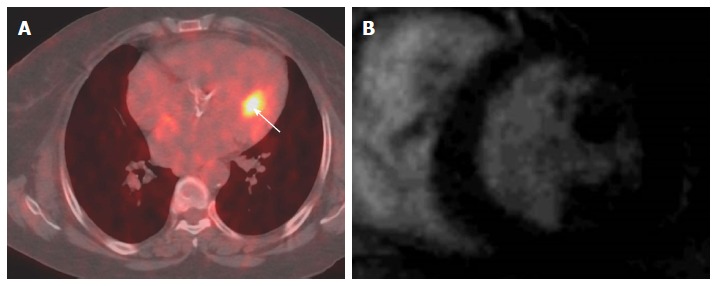
Localization of abnormal fluorodeoxygluocse activity in the heart. A: Axial fused PET-CT image shows focal intense FDG uptake in the heart (arrow) in patient with melanoma that thought represents cardiac metastases; B: Selected delayed enhancement short axis image of the same patient with other images (not shown) shows no abnormal enhancement or tissue infiltration, the FDG uptake was corresponding to hypertrophic papillary muscle, follow PET-CT was normal. PET: Positron emission tomography; CT: Computed tomography; FDG: Fluorodeoxygluocse.
Figure 2.
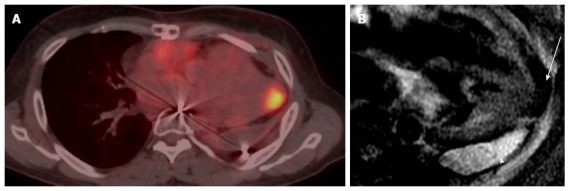
Localization and characterization of abnormal fluorodeoxygluocse activity in the heart. A: Axial PET-CT images in patient with history of invasive thymoma underwent surgical resection shows intense FDG focal uptake in the cardiac apex highly suspicious for cardiac metastases, review CT component of PET-CT shows area of and soft tissue; B: Four-chamber DE image with triple inversion recovery shows complete fat suppression of the apical activity in keeping with brown fat (arrow), with high signal fluid intensity consistent with post-operative lobulated fluid collection (arrowhead). PET: Positron emission tomography; CT: Computed tomography; FDG: Fluorodeoxygluocse.
CHARACTERIZATION AND LOCALIZATION OF ABNORMAL FDG UPTAKE ADJACENT TO THE HEART
One of the most and frequent artefact in PET-CT is due to respiratory motion during scanning. It typically creates mismatch between a specific stage of breath cycle during the CT and average of many breath cycles of the PET images. The diaphragm that is visualized in a single position during fast CT acquisition is different from mean position of PET images or in case of respiratory motion. misregistration of CT and PET images disrupts images fusion of normal organ and may cause erroneous localization of FDG avid lesion in the liver, upper abdomen, base of the lung, or adjacent to the heart. The best way to correct for respiratory motion between CT and PET images would be acquired gated images to discriminate different interval for a breath cycle[15]. There are some techniques in integrated PET-MRI that correct for motion artifact, motion can be derived from MR data without the need for navigators, an approach called self-navigation, and these techniques require that K-space be sampled in a motion sensitive scanner[16]. The motion control and gating approaches will continue to be used in integrated PET-MRI scanner, the filed will likely move towards data-derived approaches[17]. Oncologic PET-CT scans may reveal abnormal focus of hypermetabolism in the chest, either in or adjacent to the heart. It is often not possible to accurately localize this lesion using PET or the corresponding low-dose CT scan. In such situations, MRI can provide additional information in localizing and characterizing these masses (Figures 3 and 4).
Figure 3.
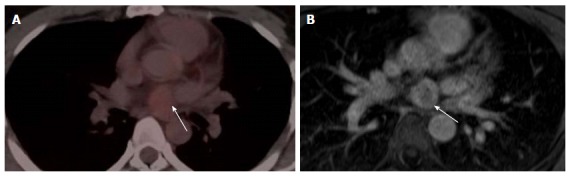
Localization and characterization of abnormal fluorodeoxygluocse activity adjacent to the heart. A: Localization and characterization of abnormal FDG activity adjacent to the heart. Axial PET-CT image of the heart shows a non-FDG avid nodule near the base of the heart (arrow), PET-CT was performed for incidentally discovered nodule mass on Echocardiography. The study was otherwise unremarkable; B: Axial first pass perfusion image shows highly vascular intrapericardial tumors with no evidence of tissue invasion (arrow); this lesion was surgically removed and pathologically proven schwannoma. PET: Positron emission tomography; CT: Computed tomography; FDG: Fluorodeoxygluocse.
Figure 4.
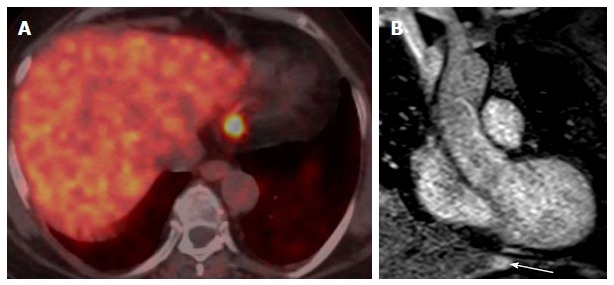
Localization and characterization of abnormal fluorodeoxygluocse activity adjacent to the heart. A: Axial Ga-68 DOTATATE PET/CT shows a focal intense radiotracer uptake adjacent to the heart with difficulty to accurately localize; patient is known to have history of ileocecal area carcinoid and underwent surgery; B: Coronal first pass perfusion images show highly vascular lesion in the tip of the left lobe of the liver corresponding to abnormal radiotracer uptake, this location is very common for PET-CT misregistration and there was no focal lesion identified in CT component of PET-CT. PET: Positron emission tomography; CT: Computed tomography; FDG: Fluorodeoxygluocse.
DIFFERENTIATION BETWEEN TUMORAL THROMBUS FROM BLAND THROMBUS
Tumor thrombosis is often clinically asymptomatic, the diagnosis of tumoral thrombus is usually made incidentally, discrimination between benign, and tumor thrombus can have significant implications in patient’s management. In general, tumor thrombus is a rare complication of solid cancers, with occult inferior vena cava tumor thrombosis having a reported incidence of 0.11%. Few case reports have described the diagnosis of tumor thrombus by PET-CT in various cancer including pancreatic, colon, renal cell cancer and adrenocortical cancer. In contrast, venous thromboembolism (VTE) is relatively common than tumor thrombosis in patients with cancer. Cancer has been associated with 18% of all cases of incidental VTE. Across all patients with cancer, the risk for VTE has elevated seven folds, in certain malignances the risk for VTE may increase up to 28-folds[18].
Tumor thrombosis is composed mainly of viable tumor cells and usually has high metabolic neoplastic activity[19] with subsequent high FDG uptake. FDG uptake in malignant cells is mediated through glucose transporters receptors. In contrast, benign thrombus is composed of activated of platelets, macrophages and fibrin. Therefore, benign thrombus is none metabolically active, simple, non-infected thrombus called bland thrombus. On PET-CT images, bland thrombus appear as intravascular filling defect without FDG uptake and can be found in veins of any size and location.
Distinguishing between benign thrombus and tumor thrombus on basis of presence or absence of FDG uptake may be difficult, it is quite reasonable for inflammatory or infectious thrombus to demonstrate some degree of FDG uptake. It is reported that inflammatory cells cause significant increase in FDG uptake in presence of platelet-aggregation factors and cytokines including growth factors[20]. In addition, expression of the glucose transporter is also increased in activated granulocytes and macrophages[21]. The differential diagnosis can be narrowed down by correlating clinical presentation, distinctive clinical features, demographics, and relevant laboratory findings in association with imaging characterization of the thrombus.
Different imaging modalities are available for distinguishing between benign and malignant thrombus. PET-CT offers comprehensive anatomical and metabolic evaluation of the thrombus, particularly, if CT component performed with IV iodinated contrast. On MRI, the signal intensity of the thrombus may vary depending on the age of the thrombus. Gadolinium contrast is useful for differentiating thrombus from tumor, Thrombus typically does not enhance but tumors usually enhance on delayed imaging[22]. One study reported, diffusion weighted (DW) imaging enables differentiation between portal vein tumoral thrombus from bland thrombus in patients with hepatocellular carcinoma (HCC), when apparent diffusion coefficient (ADC) of the thrombus to ADC of HCC is less than 2 and when the thrombus showed signal intensity similar to HCC, DW imaging may be very helpful in certain patients, such as patients with contraindication for contrast material injection and/or history of previous reaction to contrast media[23] (Figures 5 and 6).
Figure 5.
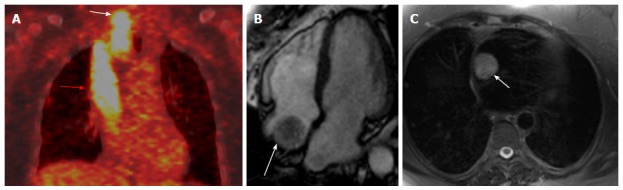
Differnation between tumoral thrombus from bland thrombus. A: Differnation between tumoral thrombus from bland thrombus in patient with history of thyroid cancer treated with total thyroidectomy presented with neck mass. Coronal fused FDG-PET image shows intense linear FDG uptake from in the thyroid bed (white arrow) and spreading through SCV to the right atrium (red arrow); B: Four chamber cine image shows irregularly defined mass in the right atrium (arrow), the rest of cardiac examination was unremarkable; C: Axial HASTE at the junction of superior vena cava and right atrium shows a high signal intensity mass (arrow). PET: Positron emission tomography; CT: Computed tomography; FDG: Fluorodeoxygluocse.
Figure 6.
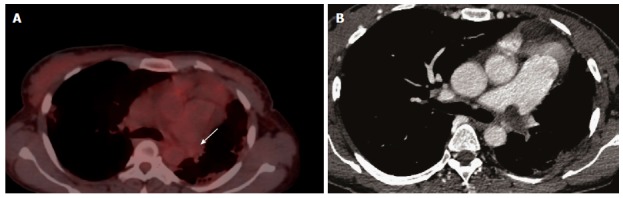
Differnation between tumoral thrombus from bland thrombus. A: Differnation between tumoral thrombus from bland thrombus in female patient with history ovarian cancer, patient was known to have chronic pulmonary embolism, there was a bland thrombus almost occulting the left pulmonary artery was no FDG uptake (arrow); B: Axial CT image shows a left pulmonary artery thrombus. CT: Computed tomography; FDG: Fluorodeoxygluocse.
M STAGING
CMR is a very helpful imaging technique for diagnosis of cardiac tumors which enables evaluation of anatomy, function, tissue type, and vascularity and relationship to adjacent structures. It also allows searching for primary tumors in case of cardiac metastases and detection for metastases in the thorax and liver/abdomen. However, CMR is not adequate for M staging, PET-CT is useful technique for M staging of wide varieties of cancers, several studies reported that PET-CT is more accurate than other conventional imaging staging in several cancer such as several types of lymphoma, solid cancer such as breast, lung, ovary, and head and neck cancers. The newer MRI technique, whole body diffusion weighted imaging is used for tumors detection, characterization, therapy monitoring. In certain with low FDG, uptakes such as neuroendocrine tumors, thyroid cancer and several low malignancy lymphomas, whole body DW is additive for PET-CT for tumors detection, staging, and assessment for response for therapy[24,25] (Figure 7).
Figure 7.

M staging. A: M staging: Coronal PET-CT image of patient with lymphoma with unexpected cardiac involvement; B: Axial T1-weighted image at the level of interatrial septum shows well defined mass attached to the atrial septum and nearly fills the right atrium and was proved to be lymphoma. PET: Positron emission tomography; CT: Computed tomography.
EVALUATION OF AGGRESSIVENESS OF THE LESION AND ASSESSMENT OF CARDIAC INVOLVEMENT
FDG PET uptake reflects the metabolic glycolysis in tumors and supplies additional information to morphological imaging. Generally, there is correlation between glucose accumulation in tumors tissue and degree of malignancy, with some exceptions[26,27]. However, PET has not yet systematically evaluated for characterization of cardiac tumors, furthermore, PET imaging can assess the local extent of the tumors and tumor infiltration to adjacent tissue. In addition, it is not often possible to accurately localize the lesion using low dose CT component of PET-CT. Contrast-enhanced CT visualizes several morphological features of cardiac tumors and helps to discriminate between benign and malignant tumors. CT high sensitive markers for malignancy includes location of the tumors outside the heart, tissue inhomogeneity and contrast enhancement. The presence of pericardial effusion is a high specific feature for malignancy, but tumors size is neither specific nor sensitive for malignancy[28]. A well-defined tumor without infiltration to adjacent structures is highly suggestive for benignity. CMR may have benefit over CT in the assessment of local tumor extension because MRI provides high soft tissue contrast[29,30]. Therefore PET-MRI can have role in the detection of T-stage and assessment of local invasion and infiltration (Figures 8 and 9).
Figure 8.
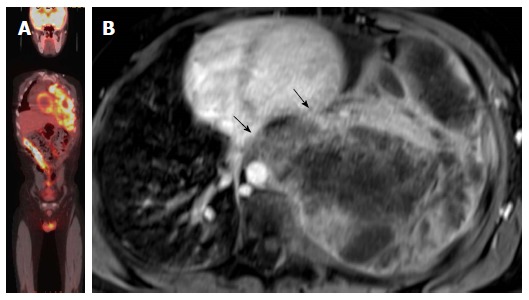
Evaluation of the aggressiveness of the lesion and assessment of cardiac involvement. A: Evaluation of the aggressiveness of the lesion and assessment of cardiac involvement; whole body PET-CT image of patient with extensive Ewing sarcoma of the left hemithorax, PET-CT images are not sufficient to evaluate local extension of the tumor to the heart; B: Axial delayed enhancement image shows large necrotic mass occupying the left hemithorax with direct left ventricle (upper arrow) the arrow without circle and left atrial invasion (lower arrow). PET: Positron emission tomography; CT: Computed tomography.
Figure 9.
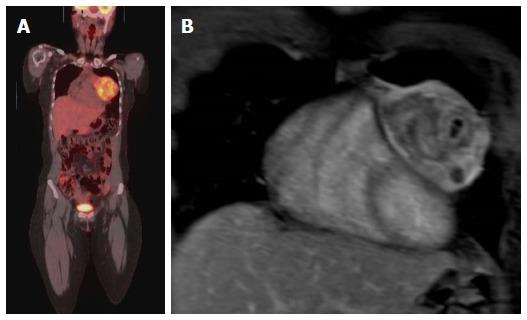
Evaluation of the aggressiveness of the lesion and assessment of cardiac involvement. A: Evaluation of the aggressiveness of the lesion and assessment of cardiac involvement; Coronal PET-CT image in a patent with Ewing sarcoma of the left chest wall with direct compression of the left side of the heart; B: Coronal Post contrast T1-weighted image of the heart shows no evidence of cardiac invasion with clear separation of the mass from the heart, the mass was surgically removed and there was no evidence of cardiac invasion. PET: Positron emission tomography; CT: Computed tomography.
OTHER POTENTIAL INTEGRATED PET-MRI APPLICATIONS RELATED TO CARDIAC TUMORS
PET-MRI with optimal coregistration is essential to differentiate between residual scar tissue and tumors relapse, integrated PET-MRI imaging obviously combines the advantage of both methods in a single examination. FDG PET- and MRI-imaging yielded 100% senstivity and 92% specificity in detecting tumors malignancy, but combined PET-MR yielded 100% sensitivity and specificity in one small study, one of the limitations of this study was small ample size[31]. In addition, in integrated PET-MRI, MRI component can assess the cardiac function, volume, morphology, and metabolism, and accurately assess tumors infiltration to various cardiac struts such as valves, papillary muscle, or coronary artery (Figure 10).
Figure 10.
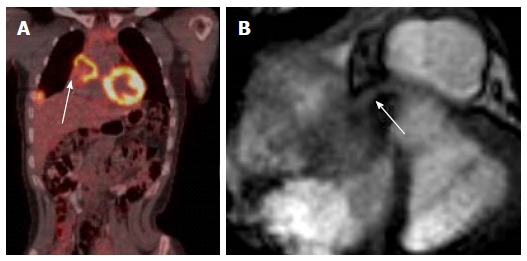
Evaluation of coronary artery involvement by the tumor. A: Coronal PET-CT image in a female patient with history of breast cancer with mediastinal and lung metastasis and recurrent chest pain; B: Axial cine image at the base of the heart shows metastatic lesion invading the heart causing mechanical obstruction of the right coronary artery (arrow). PET: Positron emission tomography; CT: Computed tomography.
CONCLUSION
With the commercial availability of PET-MRI true simultaneous PET and MRI in a single study is real. Several studies have demonstrated the feasibility and incremental value of combined PET and MRI in many clinical applications. A combination of PET and MRI can provide incremental information in many cardiovascular scenarios. Evaluation of cardiac tumors may be most straightforward application for PET-MRI because it offers a unique opportunity to evaluate the tumor morphology, characterization, infiltration to adjacent structures, local and M staging and comprehensive cardiac evaluation in a single study.
Footnotes
Conflict-of-interest statement: We declare we do not have any conflict of interest.
Manuscript source: Unsolicited manuscript
Specialty type: Cardiac and cardiovascular systems
Country of origin: Saudi Arabia
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Peer-review started: December 31, 2016
First decision: March 28, 2017
Article in press: May 15, 2017
P- Reviewer: den Uil CA, Said SAM, Suzuki G S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
References
- 1.Amano J, Nakayama J, Yoshimura Y, Ikeda U. Clinical classification of cardiovascular tumors and tumor-like lesions, and its incidences. Gen Thorac Cardiovasc Surg. 2013;61:435–447. doi: 10.1007/s11748-013-0214-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Butany J, Leong SW, Carmichael K, Komeda M. A 30-year analysis of cardiac neoplasms at autopsy. Can J Cardiol. 2005;21:675–680. [PubMed] [Google Scholar]
- 3.Douglas PS, Garcia MJ, Haines DE, Lai WW, Manning WJ, Patel AR, Picard MH, Polk DM, Ragosta M, Parker Ward R, et al. ACCF/ASE/AHA/ASNC/HFSA/HRS/SCAI/SCCM/SCCT/SCMR 2011 Appropriate Use Criteria for Echocardiography. A Report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, American Society of Echocardiography, American Heart Association, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Critical Care Medicine, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance American College of Chest Physicians. J Am Soc Echocardiogr. 2011;24:229–267. doi: 10.1016/j.echo.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 4.den Harder AM, Willemink MJ, de Jong PA, Schilham AM, Rajiah P, Takx RA, Leiner T. New horizons in cardiac CT. Clin Radiol. 2016;71:758–767. doi: 10.1016/j.crad.2016.01.022. [DOI] [PubMed] [Google Scholar]
- 5.O’Donnell DH, Abbara S, Chaithiraphan V, Yared K, Killeen RP, Cury RC, Dodd JD. Cardiac tumors: optimal cardiac MR sequences and spectrum of imaging appearances. AJR Am J Roentgenol. 2009;193:377–387. doi: 10.2214/AJR.08.1895. [DOI] [PubMed] [Google Scholar]
- 6.Hundley WG, Bluemke DA, Finn JP, Flamm SD, Fogel MA, Friedrich MG, Ho VB, Jerosch-Herold M, Kramer CM, Manning WJ, et al. ACCF/ACR/AHA/NASCI/SCMR 2010 expert consensus document on cardiovascular magnetic resonance: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. J Am Coll Cardiol. 2010;55:2614–2662. doi: 10.1016/j.jacc.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rahbar K, Seifarth H, Schäfers M, Stegger L, Hoffmeier A, Spieker T, Tiemann K, Maintz D, Scheld HH, Schober O, et al. Differentiation of malignant and benign cardiac tumors using 18F-FDG PET/CT. J Nucl Med. 2012;53:856–863. doi: 10.2967/jnumed.111.095364. [DOI] [PubMed] [Google Scholar]
- 8.Nekolla SG, Martinez-Moeller A, Saraste A. PET and MRI in cardiac imaging: from validation studies to integrated applications. Eur J Nucl Med Mol Imaging. 2009;36 Suppl 1:S121–S130. doi: 10.1007/s00259-008-0980-1. [DOI] [PubMed] [Google Scholar]
- 9.Chalian H, O’Donnell JK, Bolen M, Rajiah P. Incremental value of PET and MRI in the evaluation of cardiovascular abnormalities. Insights Imaging. 2016;7:485–503. doi: 10.1007/s13244-016-0494-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fukuchi K, Ohta H, Matsumura K, Ishida Y. Benign variations and incidental abnormalities of myocardial FDG uptake in the fasting state as encountered during routine oncology positron emission tomography studies. Br J Radiol. 2007;80:3–11. doi: 10.1259/bjr/92105597. [DOI] [PubMed] [Google Scholar]
- 11.Lin EC. Isolated papillary muscle uptake on FDG PET/CT. Clin Nucl Med. 2007;32:76–78. doi: 10.1097/01.rlu.0000249516.53979.ed. [DOI] [PubMed] [Google Scholar]
- 12.Yamagishi H, Shirai N, Takagi M, Yoshiyama M, Akioka K, Takeuchi K, Yoshikawa J. Identification of cardiac sarcoidosis with (13)N-NH(3)/(18)F-FDG PET. J Nucl Med. 2003;44:1030–1036. [PubMed] [Google Scholar]
- 13.Nguyen JD, Carrasquillo JA, Little RF, Ryan QC, Wilson W, Chen CC. Fluorodeoxyglucose positron emission tomography in the presence of cardiac metastases. Clin Nucl Med. 2003;28:979–980. doi: 10.1097/01.rlu.0000099808.30653.06. [DOI] [PubMed] [Google Scholar]
- 14.Shimotsu Y, Ishida Y, Fukuchi K, Hayashida K, Toba M, Hamada S, Takamiya M, Satoh T, Nakanishi N, Nishimura T. Fluorine-18-fluorodeoxyglucose PET identification of cardiac metastasis arising from uterine cervical carcinoma. J Nucl Med. 1998;39:2084–2087. [PubMed] [Google Scholar]
- 15.Pettinato C, Nanni C, Farsad M, Castellucci P, Sarnelli A, Civollani S, Franchi R, Fanti S, Marengo M, Bergamini C. Artefacts of PET/CT images. Biomed Imaging Interv J. 2006;2:e60. doi: 10.2349/biij.2.4.e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uribe S, Muthurangu V, Boubertakh R, Schaeffter T, Razavi R, Hill DL, Hansen MS. Whole-heart cine MRI using real-time respiratory self-gating. Magn Reson Med. 2007;57:606–613. doi: 10.1002/mrm.21156. [DOI] [PubMed] [Google Scholar]
- 17.Kesner AL, Schleyer PJ, Büther F, Walter MA, Schäfers KP, Koo PJ. On transcending the impasse of respiratory motion correction applications in routine clinical imaging - a consideration of a fully automated data driven motion control framework. EJNMMI Phys. 2014;1:8. doi: 10.1186/2197-7364-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blom JW, Doggen CJ, Osanto S, Rosendaal FR. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA. 2005;293:715–722. doi: 10.1001/jama.293.6.715. [DOI] [PubMed] [Google Scholar]
- 19.Ozülker T, Ozpaçaci T, Ozülker F, Ozekici U, Bilgiç R, Mert M. Incidental detection of Sertoli-Leydig cell tumor by FDG PET/CT imaging in a patient with androgen insensitivity syndrome. Ann Nucl Med. 2010;24:35–39. doi: 10.1007/s12149-009-0321-x. [DOI] [PubMed] [Google Scholar]
- 20.Bhargava P, Zhuang H, Hickeson M, Alavi A. Pelvic kidney mimicking recurrent colon cancer on FDG positron emission tomographic imaging. Clin Nucl Med. 2002;27:602–603. doi: 10.1097/00003072-200208000-00015. [DOI] [PubMed] [Google Scholar]
- 21.Mochizuki T, Tsukamoto E, Kuge Y, Kanegae K, Zhao S, Hikosaka K, Hosokawa M, Kohanawa M, Tamaki N. FDG uptake and glucose transporter subtype expressions in experimental tumor and inflammation models. J Nucl Med. 2001;42:1551–1555. [PubMed] [Google Scholar]
- 22.Paydarfar D, Krieger D, Dib N, Blair RH, Pastore JO, Stetz JJ, Symes JF. In vivo magnetic resonance imaging and surgical histopathology of intracardiac masses: distinct features of subacute thrombi. Cardiology. 2001;95:40–47. doi: 10.1159/000047342. [DOI] [PubMed] [Google Scholar]
- 23.Kwee TC, Takahara T, Ochiai R, Koh DM, Ohno Y, Nakanishi K, Niwa T, Chenevert TL, Luijten PR, Alavi A. Complementary roles of whole-body diffusion-weighted MRI and 18F-FDG PET: the state of the art and potential applications. J Nucl Med. 2010;51:1549–1558. doi: 10.2967/jnumed.109.073908. [DOI] [PubMed] [Google Scholar]
- 24.Wilhelm T, Stieltjes B, Schlemmer HP. Whole-body-MR-diffusion weighted imaging in oncology. Rofo. 2013;185:950–958. [PubMed] [Google Scholar]
- 25.Li B, Li Q, Nie W, Liu S. Diagnostic value of whole-body diffusion-weighted magnetic resonance imaging for detection of primary and metastatic malignancies: a meta-analysis. Eur J Radiol. 2014;83:338–344. doi: 10.1016/j.ejrad.2013.11.017. [DOI] [PubMed] [Google Scholar]
- 26.Weber WA. Positron emission tomography as an imaging biomarker. J Clin Oncol. 2006;24:3282–3292. doi: 10.1200/JCO.2006.06.6068. [DOI] [PubMed] [Google Scholar]
- 27.Fischman AJ. Positron emission tomography in the clinical evaluation of metastatic cancer. J Clin Oncol. 1996;14:691–696. doi: 10.1200/JCO.1996.14.3.691. [DOI] [PubMed] [Google Scholar]
- 28.Araoz PA, Mulvagh SL, Tazelaar HD, Julsrud PR, Breen JF. CT and MR imaging of benign primary cardiac neoplasms with echocardiographic correlation. Radiographics. 2000;20:1303–1319. doi: 10.1148/radiographics.20.5.g00se121303. [DOI] [PubMed] [Google Scholar]
- 29.Sparrow PJ, Kurian JB, Jones TR, Sivananthan MU. MR imaging of cardiac tumors. Radiographics. 2005;25:1255–1276. doi: 10.1148/rg.255045721. [DOI] [PubMed] [Google Scholar]
- 30.Zhu D, Yin S, Cheng W, Luo Y, Yang D, Lin K, An Q, Sun J, Chen Y. Cardiac MRI-based multi-modality imaging in clinical decision-making: Preliminary assessment of a management algorithm for patients with suspected cardiac mass. Int J Cardiol. 2016;203:474–481. doi: 10.1016/j.ijcard.2015.09.021. [DOI] [PubMed] [Google Scholar]
- 31.Nensa F, Tezgah E, Poeppel TD, Jensen CJ, Schelhorn J, Köhler J, Heusch P, Bruder O, Schlosser T, Nassenstein K. Integrated 18F-FDG PET/MR imaging in the assessment of cardiac masses: a pilot study. J Nucl Med. 2015;56:255–260. doi: 10.2967/jnumed.114.147744. [DOI] [PubMed] [Google Scholar]


