Abstract
AIM
To sythesize the available literature on hand dysfunction after transradial catheterization.
METHODS
We searched MEDLINE and EMBASE. The search results were reviewed by two independent judicators for studies that met the inclusion criteria and relevant reviews. We included studies that evaluated any transradial procedure and evaluated hand function outcomes post transradial procedure. There were no restrictions based on sample size. There was no restriction on method of assessing hand function which included disability, nerve damage, motor or sensory loss. There was no restriction based on language of study. Data was extracted, these results were narratively synthesized.
RESULTS
Out of 555 total studies 13 studies were finally included in review. A total of 3815 participants with mean age of 62.5 years were included in this review. A variety of methods were used to assess sensory and motor dysfunction of hand. Out of 13 studies included, only 3 studies reported nerve damage with a combined incidence of 0.16%, 5 studies reported sensory loss, tingling and numbness with a pooled incidence of 1.52%. Pain after transradial access was the most common form of hand dysfunction (6.67%) reported in 3 studies. The incidence of hand dysfunction defined as disability, grip strength change, power loss or any other hand complication was incredibly low at 0.26%. Although radial artery occlusion was not our primary end point for this review, it was observed in 2.41% of the participants in total of five studies included.
CONCLUSION
Hand dysfunction may occur post transradial catheterisation and majority of symptoms resolve without any clinical sequel.
Keywords: Transradial access, Transfemoral access, Hand dysfunction
Core tip: Transradial access (TRA) is default access site in many countries to perform coronary procedures. Hand function may occur post TRA, however our review shows that its incidence is exceedingly low and most symptoms resolve without any clinical sequel.
INTRODUCTION
Coronary angiography is the current gold standard in providing anatomical information regarding the extent and severity of coronary artery disease[1,2]. Access site practice has changed in a number of European and Asian countries from mainly being transfemoral (TFA) to transradial (TRA)[3,4] in view of less access site related bleeding complications, mortality and shorter hospital stay associated with TRA[5-11]. For instance, in the United Kingdom use of radial access has increased from 14% to 80% between 2005 and 2014 in patients undergoing percutaneous coronary intervention (PCI) and it is estimated that this practice change has saved an estimated 450 lives nationally[12]. In the most recent European Society of Cardiology guidelines for management of non-ST elevation myocardial infarction (NSTEMI), TRA received class 1A indication for invasive management of NSTEMI with PCI[2]. Furthermore national bodies have formulated recommendations to prevent and minimize procedure related complications of TRA such as reducing the risk of radial artery occlusion (RAO), minimizing patient and operator radiation exposure and transitioning to TRA for primary PCI[13,14].
Nevertheless, despite of its clear advantages over TFA, TRA is not without limitations and is associated with longer operator learning curve[15,16], increased radiation exposure in individual operators at the start of their learning curves[17,18] and higher case radial proportion to translate the better results of randomized trials into clinical practice[11,19,20]. Moreover, vascular complications such as RAO[21] and radial artery spasm[22] are not uncommon and very recently concerns have been raised that patients undergoing TRA PCI may encounter hand dysfunction[23].
Whether access site related complications can lead to hand dysfunction is unclear and studies have reported inconsistence results. A study by van Leeuwen et al[24] investigated the impact of TRA on limb function at long term follow up, reported 9% and 11% of the patients develop temporary or permanent hand dysfunction respectively. Whereas Zwaan et al[25] reported a pooled incidence of 0.32% in 14 studies evaluating hand dysfunction post TRA.
Considering that the TRA is the predominant access site for cardiac catheterization procedures in many countries, there is little data around hand dysfunction post procedure. In view of the limited published data we conducted a systematic review to evaluate the hand dysfunction post TRA.
MATERIALS AND METHODS
We searched MEDLINE and EMBASE on 23 August 2016 using the search terms: [(radial or transradial or radial artery) AND (catheterisation or catheterization or angiography or angiogram or angioplasty or percutaneous coronary intervention or PCI)] AND (hand function or grip strength or disability or dysfunction or sensation or paraesthesia or paralysis). The search results were reviewed by two independent adjudicators (MAU, CWW) for studies that met the inclusion criteria and relevant reviews. The bibliographies of included studies and relevant reviewers were screened for additional studies.
We included studies with patients undergoing transradial procedure and evaluated hand function outcomes post procedure. No control group was required so studies could be single arm. There were no restrictions based on language, sample size or method of assessing hand function which included disability, nerve damage, motor or sensory loss. These results were then narratively synthesized.
RESULTS
Our search yielded 555 related studies out of which after screening and reviewing the full manuscripts, 13[24,26-38] studies were included in the final review. Detail process of inclusion and exclusion is illustrated in Figure 1.
Figure 1.
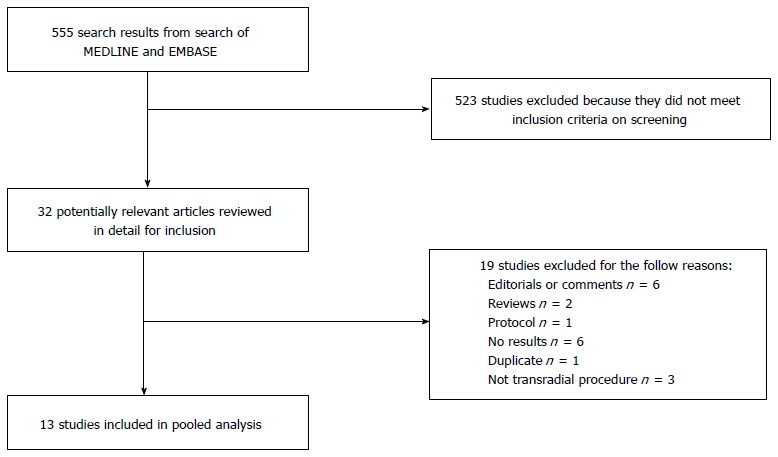
Flow diagram of study inclusion/ exclusion.
Table 1 provides the description of studies, year of study, percentage of males and number of participants. A total of 3815 participants with mean age of 62.5 years were included in the studies. Table 2 describes the various methods of assessment employed to assess hand dysfunction, follow up time and results. We observed significant heterogeneity in the methods of assessment hand function and follow up time. For instance, the follow up of assessment varied from anytime between the day procedure was undertaken up to a year post TRA. Similarly, an array of methods were employed to assess the sensory and motor component of hand function such as questionnaire based surveys in the form of Disabilities of Arm, Shoulder and Hand (Quick DASH) or Cold Intolerance and Symptom Severity (CISS) or postal surveys, electromyography (EMG), dynamometer and forefinger pinch grip tests.
Table 1.
Study design and participant characteristics
| Ref. | Study design/country/year | No. of participants | Mean age | % male | Participant inclusion criteria and procedural details |
| Benit et al[26] | Randomized trial; Belgium; 1994-1995 | 50 | 57.7 | 100% | Participants had transradial coronary angioplasty with 6-Fr catheters and Palmaz-Schatz stent |
| Campeau et al[27] | Cohort study; Canada; Unclear | 100 | 58 (median) | 90% | Participants had transradial coronary angiogram with 5-Fr, 6-Fr and 7-Fr sheath |
| Chatelain et al[28] | Cohort study; Switzerland; 1995-1997 | 159 | 60 | 82% | Participants had transradial diagnostic and interventional cardiac procedures with 4-Fr, 5-Fr or 6-Fr introducer sheath and guide catheters with RadiStop radial compression system |
| De Belder et al[29] | Cohort study; United Kingdom; Unclear | 75 | Unclear | 69% | Participants had transradial coronary angiography and intervention and severe peripheral vascular disease with 5-Fr or 6-Fr sheath and 6-Fr guide catheter |
| Kiemeneij et al[30] | Cohort study; The Netherlands; 1992-1993 | 100 | 62 | 77% | Participants had transradial coronary angiography with 6-Fr introducer and 6-Fr-guide catheters |
| Lotan et al[31] | Cohort study; Israel; 1994 | 100 | 61 | 79% | Participants had transradial coronary angiography and angioplasty with 6-Fr introducer and 6-Fr guide catheters |
| Prull et al[32] | Cohort study; Germany; Unclear | 93 | 62.5 | 80.6% | Participants had transradial diagnostic cardiac catheterization with 5-Fr or 6-Fr sheath or transradial coronary intervention with 7-Fr sheath |
| Sciahbasi et al[33] | Prospective cohort study; Italy; Unclear | 99 | 65 | 72% | Participants had transradial coronary angiography and angioplasty with 6-Fr introducer sheath |
| Tharmaratnam et al[35] | Retrospective case control study; United Kingdom; 2005-2006 | 1283 | 65.5 | 79% | Participants had transradial coronary angiography and angioplasty |
| Valgimigli et al[39] | Prospective cohort study; The Netherlands, Italy; 2014 | 942 | 70 | 73% | Participants had transradial coronary angiography and angioplasty |
| Van Leeuwen et al[24] | Prospective cohort study; The Netherlands; 2015 | 286 | 64 | 72% | Participants had transradial coronary angiography and angioplasty with 6-Fr introducer sheath |
| Wu et al[37] | Cohort study; United States; 1996-1998 | 40 | 65 | 88% | Participants underwent 6-Fr and 8-Fr transradial procedure |
| Zankl et al[34] | Prospective cohort study; Germany; 2010 | 488 | Unclear | Unclear | Participants had transradial coronary angiography and angioplasty with 5- and 6-Fr introducer, 4-, 5- and 6-Fr catheters |
Table 2.
Results of studies
| Ref. | Measure of hand function and vascular complications | Follow up post procedure | Results |
| Benit et al[26] | Local complications assessed in clinic by history and EMG | 1 mo | Nerve damage documented by EMG: 0/50 Local pain: 0/50 |
| Campeau et al[27] | Patients were re-examined or questioned over telephone about local complications | 1 to 3 mo | No nerve injury: 0/100 |
| Chatelain et al[28] | Physicians assessed for any clinical events | Assessment prior to discharge | Paraesthesia of right thumb during exercise: 1/159 |
| De Belder et al[29] | Clinical evaluation | 4-6 wk | Haematoma and paraethesia post procedure: 1/75 Hand sensation and function at 4-6 wk: 0/75 |
| Kiemeneij et al[30] | Examination and ultrasound study performed if radial artery pulsations or flow were absent | 1 to 3 mo | Functional disability of the hand: 0/100 |
| Lotan et al[31] | Assessment methods unclear | 1 mo follow up | Small hematoma in wrist: 3/100 Small pseudoaneurysm: 2/100 Numbness of the thumb and index finger: 1/100 No flow on Doppler: 2/100 |
| Prull et al[32] | Clinical evaluation with ultrasound | Post-procedure assessment | Vascular complication: 9/93 Motor skills, coordination or force reduction of hand after procedure: 0/93 No pseudoaneurysm: 0/93 |
| Sciahbasi et al[33] | Radial artery occlusion by ultrasound test. Handgrip strength by Jamar Plus dynamometer. Thumb and forefinger pinch test by Jamar Plus electronic pinch gauge | Day of procedure and at least 30 d follow up | Radial artery occlusion: 9/99 Hand grip strength change at follow up: 0/99 Thumb and forefinger pinch test change at follow up: 0/99 |
| Tharmaratnam et al[35] | Questionnaire posted to address and clinical notes for significant clinical events | Unclear | Problem with radial access site: 166/1283 (12.9%) Pain at puncture site: 95/1283 (7.4%) Swelling: 46/1283 (3.6%) Bruising: 30/1283 (2.3%) Non-specific sensory abnormalities either pain or paraesthesia in hand: 22/1283 (1.71%) |
| Valgimigli et al[39] | Radial artery occlusion by duplex echocardiographic examination. Hand grip strength test with dynanometer | Just after procedure, 1 d, 30 d and 1 yr | Radial artery occlusions at day 1: 5/942 Radial artery occlusions at 1 year: 3/942 Change in handgrip strength test: 0/942 Ischemic vascular or bleeding complications: 0/942 |
| Van Leeuwen et al[24] | Quick DASH questionnaire and CISS questionnaire. Patients were asked to describe any procedure-related extremity complaints or loss of function at 1 mo | Pre, 30 d and 1 yr post procedure | Temporary upper limb complaint (< 30 d): 26/286 (9%) Persisting upper limb complaint (> 30 d): 31/286 (11%) Pain: 13/286 Numbness: 2/286 Tingling: 3/286 Stiffness: 2/286 Less power: 2/286 Upper limb function by QuickDASH at 30 d: No change over time, baseline 4.55 (IQR 0-13.64), follow up 2.27 (IQR 0-9.32) Upper limb function by CISS at 30 d: No change over time Upper limb function by QuickDASH at 1 yr: no change over time, baseline 2.39 (IQR 0-13.64), follow up 0 (0-11.02) Cold intolerance was not associated with access route at 1 yr |
| Wu et al[37] | Ultrasound assessment for radial artery occlusion, aneurysm or dissection. Grip strength based on dynamometer results. Palmar pinch, key pinch and tip pinch strength tests were assessed by dynamic endurance test | Late follow up 315 d | Hand complication in hospital: 0/40 Radial occlusion: 1/40 Late radial occlusions: 5/34 Radial artery aneurysm: 0/40 Radial artery dissection 0/40 Grip strength: Baseline 68 ± 34, post-catheterization 69 ± 35 Palmar pinch: Baseline 18 ± 10, post-catheterization 17 ± 6 Key pinch: Baseline 19 ± 7, post-catheterization 19 ± 6 Tip pinch: Baseline 14 ± 6, post-catheterization 14 ± 4 Endurance: Median for 6 Fr and 8 Fr is 78 (IQR 53, 108) and 58 (IQR 32, 68) respectively, post-catheterization 58 (IQR 47, 84) and 56 (IQR 38, 80), respectively |
| Zankl et al[34] | Assessment with ultrasound | 4 wk follow up | Radial artery occlusion at 1 d: 51/488 Persistent radial artery occlusion at 4 wk: 21/488 Radial nerve paralysis: 1/488 |
CISS: Cold intolerance symptom severity; EMG: Electromyography.
Table 3 presents pooled results of various form of limb dysfunction described by the studies. Out of 13 studies included, only 3 studies reported nerve damage[26,27,34] with a combined incidence of 0.16%, 5 studies reported sensory loss, tingling and numbness[24,28,29,31,35] with a pooled incidence of 1.52%. Pain after TRA was the most common form of hand dysfunction (6.67%) reported in 3 studies[24,26,35]. The incidence of hand dysfunction defined as disability, grip strength change, power loss or any other hand complication was incredibly low at 0.26%[24,30,32,33,37,39]. Although RAO was not our primary end point for this review, it was observed in 2.41% of the participants in total of five studies included[31,33,34,37,39].
Table 3.
Summary of pooled results for hand dysfunction or vascular complications post transradial procedure
| Hand dysfunction or vascular complication | No. of studies | No of events | No of participants | Percentage of events |
| Nerve damage | 3[26,27,34] | 1 | 638 | 0.16% |
| Sensory loss, tingling and numbness | 5[24,28,29,31,35] | 29 | 1903 | 1.52% |
| Pain | 3[24,26,35] | 108 | 1619 | 6.67% |
| Hand function, disability, grip strength change, stiffness, power loss and hand complications | 6[24,30,32,33,37,39] | 4 | 1560 | 0.26% |
| Vascular complications including occlusions, hematoma, pseudoaneurysm and dissection | 6[29,31,32,35,37,39] | 54 | 1762 | 3.06% |
| Radial artery occlusion | 5[31,33,34,37,39] | 40 | 1663 | 2.41% |
In one the very early studies from pre-stent era, Campeau et al[27] assessed the neurological damage to hand following TRA using 5 Fr, 6 Fr or 7 Fr sheath. Patients were assessed at 1 and 3 mo either clinically or via telephone reported no nerve injury. It is not clear how the nerve damage was assessed in patients reviewed by telephone. Another study employing a more subjective assessment of nerve function using EMG in 150 patients receiving TRA using a 6 Fr sheath reported no damage to the median nerve at 1 mo follow up. In a large retrospective analysis of 1283 patients undergoing TRA using hydrophilic sheaths, 13.2% patients reported non-specific sensory symptoms post procedure[35]. However, the results were dependent on a questionnaire based postal survey and no objective method was used to assess for the sensory loss. Similarly two other studies[28,29] assessing the neurological dysfunction post TRA, reported only 1 case of paraesthesia of right thumb and 1 case of forearm haematoma resulting in some sensory disturbance of hand but no loss of function. More importantly, both cases made full recovery without any clinical sequel.
In a prospective study of 203 patients after TRA, Valgimigli et al[39] assessed the motor component of hand function by performing handgrip strength tests using a dynaomometer at 30 d and 1 year, maximal isometric strength on handgrip test did not change over time. Van Leeuwen et al[24] conducted a randomised study of 338 patients to evaluate motor component of upper limb function using self-reported shortened version of Disabilities of Arm, Shoulder and Hand (Quick DASH, Table 4) and sensory component using Cold Intolerance and Symptom Severity (CISS, Table 5) questionnaires at baseline and 30 d. There was no statistically significant change in Quick DASH score at baseline to follow up in patients undergoing TRA (baseline 4.55; IQR: 0.00 to 13.64; follow-up 2.27 IQR: 0.00 to 9.32, P = 0.06). Similarly there was no change in the CISS score over time. An important feature of the study was they included patients undergoing TFA to make a comparison between the two access sites. More recently, HANGAR (HANd Grip test After tRansradial percutaneous coronary procedures) study investigated 108 patients with stable angina undergoing PCI using 6Fr sheath with a primary endpoint of variation in hand grip strength measured with the Jamar Plus dynamometer after the procedure[33]. The secondary endpoints of interest were thumb and forefinger pinch measured using key pinch and electronic pinch gauge respectively. Out of 99 patients, 9 patients developed radial artery occlusion after the procedure, the patients were then divided in two groups according to the radial patency (group 1) or occlusion (group 2) The hand grip test values were significantly reduced compared with baseline values (40 ± 11 kg in group 1, P < 0.0001 and 37 ± 17 kg in group 2, P = 0.007) after the procedure but returned back to baseline at follow up. Interestingly thumb and finger pinch function was unaffected at baseline, after the procedure and follow up. Finally ARCUS (Effects of transradial percutaneous coronary intervention on upper extremity function) is an ongoing trial assessing the effects of TRA on hand function by taking various measurement such as Echo Doppler for radial artery occlusion, Questionnaires testing including Quick DASH (Table 4), Boston Carpal Tunnel Questionnaire (BCTQ, Table 6) and Visual Analogue Scale (VAS), volumetry of hand and forearm, sensibility of fingertips, key and palmar grips and isometric strength of wrist and elbow[40]. The interim results were published recently suggesting that 143 of 191 (74.9%) patients had some form of upper limb dysfunction defined as a complied binary score of various measurements taken[38]. Furthermore, RAO was 9.8% in upper limb dysfunction group as compared to 0% RAO in non-upper limb dysfunction group.
Table 4.
Disabilities of Arm, Shoulder and Hand (QuickDASH) Questionnaire
| No difficulty | Mild difficulty | Moderate difficulty | Severe difficulty | Unable | |
| 1 Open a tight or new jar | 1 | 2 | 3 | 4 | 5 |
| 2 Do heavy house hold chores eg. Wash walls, floors | 1 | 2 | 3 | 4 | 5 |
| 3 Carry a shopping bag or briefcase | 1 | 2 | 3 | 4 | 5 |
| 4 Wash your back | 1 | 2 | 3 | 4 | 5 |
| 5 Use a knife to cut food | 1 | 2 | 3 | 4 | 5 |
| 6 Recreational activities in which you take some force or impact through your arm shoulder or hand | 1 | 2 | 3 | 4 | 5 |
| 7 During the past week to what extent has your arm, shoulder or hand problem interfered with your normal social activities with family, friends, neighbors or groups? | 1 | 2 | 3 | 4 | 5 |
| 8 During the past week, were you limited in your work or other daily activities as a result of your arm, shoulder or hand problem? | 1 | 2 | 3 | 4 | 5 |
| 9 Arm, shoulder or hand pain | 1 | 2 | 3 | 4 | 5 |
| 10 Tingling | 1 | 2 | 3 | 4 | 5 |
| 11 Sleep | 1 | 2 | 3 | 4 | 5 |
Table 5.
Cold Intolerance symptoms severity Questionnaire
| Questions | Score |
| Which of the following symptoms of cold intolerance do you experience in your injured limb on exposure to cold? | |
| Pain, numbness, stiffness, weakness, aching, skin colour change (white/bluish white/blue) | |
| How often do you experience these symptoms? (Please tick) | |
| Continuously/all the time | |
| Several times a day | |
| Once a day | |
| Once a week | |
| Once a month or less | |
| Never | |
| When you develop cold induced symptoms, on your return to a warm environment are the symptoms relieved? (Please tick) | |
| Not applicably | |
| Within a few minutes | |
| Within 30 min | |
| After more than 30 min | |
| What do you do to ease or prevent your symptoms occurring? (Please tick) | |
| Take no special action | |
| Keep hand in pocket | |
| Wear gloves in cold weather | |
| Wear gloves all the time | |
| Avoid cold weather/stay indoors | |
| Other (please specify) | |
| How much does cold bother your injured hand in the following situations? (Please score 0-10) | |
| Holding a glass of ice water | |
| Holding a frozen package from the freezer | |
| Washing in cold water | |
| When you get out of a hot bath/shower with air room temperature | |
| During cold wintry weather | |
| Please state how each of the following activities have been affected as a consequence of cold induced symptoms in your injured hand and score each (please score 0-4) | |
| Domestic chores | |
| Hobbies and interests | |
| Dressing and undressing | |
| Tying your |
Table 6.
Boston Carpal Tunnel Syndrome Questionnaire
| 1 | 2 | 3 | 4 | 5 | |
| A: Symptom severity scale (11 items) | |||||
| 1 How severe is the hand or wrist pain that you have at night? | Normal | Slight | Medium | Serious | Very serious |
| 2 How often did hand or wrist pain wake you up during a typical night in the past two weeks? | Normal | Once | 2-3 | 4-5 | > 5 |
| 3 Do you typically have pain in your hand or wrist during the daytime? | No Pain | Slight | Medium | Serious | Very Serious |
| 4 How often do you have hand or wrist pain during daytime? | Normal | 1-2 times/d | 1 times/d | > 5 times/d | Continued |
| 5 How long on average does an episode of pain last during the daytime? | Normal | < 10 min | 10-60 continued | > 60 min | Continued |
| 6 Do you have numbness (loss of sensation) in your hand? | Normal | Slight | Medium | Severe | Very Serious |
| 7 Do you have weakness in your hand or wrist? | Normal | Slight | Medium | Severe | Very Serious |
| 8 Do you have tingling sensations in your hand? | Normal | Slight | Medium | Severe | Very Serious |
| 9 How severe is numbness (loss of sensation) or tingling at night? | Normal | Slight | Medium | Severe | Very Serious |
| 10 How often did hand numbness or tingling wake you up during a typical night during the past two weeks? | Normal | Once | 2-3 times | 4-5 times | > 5 |
| 11 Do you have difficulty with the grasping and use of small objects such as keys or pens? | Without difficulty | Little difficulty | Moderate difficulty | Very difficulty | Very difficult |
| B: Functional status scale (8 items) | |||||
| Writing | |||||
| Buttoning of cloths | |||||
| Holding a book while reading | |||||
| Gripping of a telephone handle | |||||
| Opening of jars | |||||
| House hold chores | |||||
| Carrying of grocery basket | |||||
| Bathing and dressing |
DISCUSSION
In the current review, we synthesize the evidence on the incidence and clinical impact of hand dysfunction after TRA. We observe a very low incidence of hand dysfunction in limited literature and importantly, we observe significant heterogeneity in the definition and method of assessment of hand dysfunction amongst the studies, with no internationally accepted measure of hand dysfunction that can be used as the gold standard for such studies. Many of these studies are poorly conducted and subjective reports of sensory/hand dysfunction with only few studies quantifying any changes in a robust manner. Finally, we find no evidence of widespread clinically significant hand dysfunction post TRA and the potential benefits of TRA in reducing major bleeding, access site related complications and mortality outweigh such rare events.
The majority of studies that reported cases of neurological deficits following TRA were underpowered[26,29,37]. In most circumstances, studies relied on subjective reporting of symptoms by patients, rather than quantifying the neurologic deficit with proper neurophysiological or other robust objective testing[24,27-31,34,35]. Benit et al[26] assessed nerve damage clinically and quantified this using EMG. Valgimigli et al[39] and Sciahbasi et al[33] used dynamometer to assess hand grip function whereas only Sciahbasi et al[33] used electronic pinch gauge to check for thumb and finger pinch tests. Van Leeuwen et al[24] used QuickDASH questionnaire and Cold Intolerance Symptom Severity (CISS) questionnaire based assessment of hand function post TRA.
The clinical significance of neurological and motor injuries leading to hand dysfunction must be considered. Many neurological injuries are known to be transient and resolve over time. For instance, van Leeuwen et al[24] reported that almost 20% patients developed subjective neurological complications in the form of numbness, tingling, stiffness and less power, more importantly nearly 50% resolved by 30 d at follow up. Similarly, pain is commonly reported by patients regardless of the access site practice but long term sequel of such symptoms is unclear. In addition, there is no consensus on the optimal method of assessing hand function and studies so far have used various methods such VAS, BCTQ, Disabilities of Arm, Shoulder and Hand (QuickDASH) and CISS (Tables 4-6).
Visual analogue scale is measure of pain intensity on a continuous scale anchored by pain descriptor ranging from “no pain (0 score)” to worst pain (score 10)[41]. BCTQ questionnaire comprises of a symptom severity scale and a functional status scale (Table 6). The symptom severity scale has 11 questions scored from 1 point (mildest) to 5 points (most severe). Likewise, functional status scale has eight questions scored from 1 point (no difficulty with activity) to 5 points (cannot perform the activity at all)[42]. Similarly, CISS score is usually employed to detect cold intolerance. It consists of 6 questions and based on response, patient with a score of 30 or higher is said to have pathological CISS score[43,44]. There is a need of internationally agreed, sensitive method of assessing hand function amongst the radial community to evaluate and monitor for such complications.
The mechanisms that may underlie hand dysfunction after TRA remains unclear though there are several possible explanations. For instance, Flexor Carpi Radialis, Flexor Pollices Longus tendons and Median nerve lies next to radial artery at wrist from lateral to medial respectively. Neurological deficits may occur from direct damage to these structures during cannulation of the radial artery. There also may be indirect extrinsic compression of these structures due to haematomas which may result in motor or sensory deficit of the hand. Endothelial dysfunction, intimal hyperplasia and medial dissections resulting in radial artery stenosis and occlusion are well known complications associated with TRA[45,46]. Haematoma or pseudoaneurysm is another relatively rare complications encountered after TRA. There is a possibility that such vascular complications may lead to transient or permanent ischemia of the nervous supply of hand leading to sensory deficit or directly cause motor dysfunction of small muscles of hand. Additionally, there are anatomic variations of neurovascular bundles of hand[47] which might be injured during the puncture leading to hand dysfunction such as sensory or motor symptoms. There are isolated case reports that describe this mechanism of nerve damage[48-50]. RAO may occur post TRA[21], however it is usually asymptomatic and rarely causes ischemia due to the excellent collateral supply of hand from ulnar and intermediate artery[45,51]. Notably, recent results of ACRUS trial suggested that hand dysfunction was very common in patients developing RAO compared to the ones with a patent radial artery post procedure[38]. However, in the study conducted by Valgimigli et al[39] across whole spectrum of Allen test, there were no differences in serial lactate measurement after the procedure suggesting that it is unlikely such mechanism can lead to clinically significant hand dysfunction.
It is unclear what factors are associated with hand dysfunction after TRA. It could very well be that certain patient factors, such as baseline hand muscle strength, history of musculoskeletal disorders, gender, atypical anatomy may be a risk factor but no studies have evaluated such predictors. Another important point how minor changes in hand function may impact on a patient’s life. For example individuals that require very fine manual dexterity for their profession such as watchmakers, pianists, and surgeons may notice very minor changes in hand function whilst in other patient groups this may be less relevant. Finally, the way in which complications are managed may also affect hand function such as how quickly a haematoma is identified and compressed. Future studies should be focused in assessing both patient and procedure related factors which may lead to development of hand dysfunction with clinically relevant end points. Finally, current literature does not provide an insight around the prevalence and significance of lower limb function in patients undergoing transfemoral access. Adequately powered randomized trial with a control group is required to better understand the incidence and mechanisms involved in the development of hand dysfunction post TRA.
In conclusion, hand dysfunction is an exceedingly rare complication post TRA. There is significant heterogeneity in the methodology and reporting of the studies investigating hand function after TRA. Patients may develop non-specific sensory symptoms or muscle weakness but majority of these symptoms resolve over time. Future studies should be focused around assessing such complications using robust methodology and more importantly reporting on the clinical relevance of hand function. Given the reductions in mortality, MACE and major bleeding complications associated with use of TRA in high risk groups undergoing PCI, TRA should remain the default access site for PCI in such high risk groups of patients at risk of bleeding complications, in line with international guidelines and consensus statements.
COMMENTS
Background
The uptake of transradial access (TRA) for cardiac procedures is growing with both observational and randomized controlled trial data showing decreases in mortality and access site related bleeding complications across the whole spectrum of acute coronary syndromes compared to procedures undertaken through the femoral approach.
Research frontiers
Recently, concerns have been raised around hand dysfunction following transradial procedures.
Innovations and breakthroughs
The review of the literature suggests that hand dysfunction after TRA has been reported in several studies and case reports. The quality of the evidence describing these complications is poor as many studies are underpowered and do not report any events. These complications appear to be rare and of uncertain clinical impact in most cases. Isolated case reports have reported rare complications such as compartment syndrome requiring emergency surgery or complex regional pain syndrome which can be disabling due to chronic pain.
Applications
The current literature is limited as there is no standardized method of assessment of hand function with very few studies that provide mechanistic insight. Higher quality studies with clinically relevant endpoints are needed to better understand the incidence and clinical significance of the hand dysfunction following TRA.
Terminology
TRA: Transradial access; TFA: Transfemoral access; PCI: Percutaneous coronary intervention; UED: Upper Extremity dysfunction.
Peer-review
It is an excellent review.
Footnotes
Conflict-of-interest statement: None.
Data sharing statement: None.
Manuscript source: Invited manuscript
Specialty type: Cardiac and cardiovascular systems
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Peer-review started: February 1, 2017
First decision: May 11, 2017
Article in press: June 13, 2017
P- Reviewer: Falconi M, Gong KZ, Said SAM S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
References
- 1.Valgimigli M, Patrono C, Collet JP, Mueller C, Roffi M. Questions and answers on coronary revascularization: a companion document of the 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2015 doi: 10.1093/eurheartj/ehv408. [DOI] [PubMed] [Google Scholar]
- 2.Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F, Bax JJ, Borger MA, Brotons C, Chew DP, et al. [2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC)] G Ital Cardiol (Rome) 2016;17:831–872. doi: 10.1714/2464.25804. [DOI] [PubMed] [Google Scholar]
- 3.Feldman DN, Swaminathan RV, Kaltenbach LA, Baklanov DV, Kim LK, Wong SC, Minutello RM, Messenger JC, Moussa I, Garratt KN, et al. Adoption of radial access and comparison of outcomes to femoral access in percutaneous coronary intervention: an updated report from the national cardiovascular data registry (2007-2012) Circulation. 2013;127:2295–2306. doi: 10.1161/CIRCULATIONAHA.112.000536. [DOI] [PubMed] [Google Scholar]
- 4.Ludwig J, Achenbach S, Daniel WG, Arnold M. The transradial approach. An increasingly used standard for coronary diagnosis and interventions. Herz. 2011;36:386–395. doi: 10.1007/s00059-011-3483-y. [DOI] [PubMed] [Google Scholar]
- 5.Cruden NL, Teh CH, Starkey IR, Newby DE. Reduced vascular complications and length of stay with transradial rescue angioplasty for acute myocardial infarction. Catheter Cardiovasc Interv. 2007;70:670–675. doi: 10.1002/ccd.21182. [DOI] [PubMed] [Google Scholar]
- 6.Jolly SS, Yusuf S, Cairns J, Niemelä K, Xavier D, Widimsky P, Budaj A, Niemelä M, Valentin V, Lewis BS, et al. Radial versus femoral access for coronary angiography and intervention in patients with acute coronary syndromes (RIVAL): a randomised, parallel group, multicentre trial. Lancet. 2011;377:1409–1420. doi: 10.1016/S0140-6736(11)60404-2. [DOI] [PubMed] [Google Scholar]
- 7.Kwok CS, Khan MA, Rao SV, Kinnaird T, Sperrin M, Buchan I, de Belder MA, Ludman PF, Nolan J, Loke YK, et al. Access and non-access site bleeding after percutaneous coronary intervention and risk of subsequent mortality and major adverse cardiovascular events: systematic review and meta-analysis. Circ Cardiovasc Interv. 2015;8:pii: e001645. doi: 10.1161/CIRCINTERVENTIONS.114.001645. [DOI] [PubMed] [Google Scholar]
- 8.Mamas MA, Ratib K, Routledge H, Neyses L, Fraser DG, de Belder M, Ludman PF, Nolan J. Influence of arterial access site selection on outcomes in primary percutaneous coronary intervention: are the results of randomized trials achievable in clinical practice? JACC Cardiovasc Interv. 2013;6:698–706. doi: 10.1016/j.jcin.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 9.Mamas MA, Anderson SG, Ratib K, Routledge H, Neyses L, Fraser DG, Buchan I, de Belder MA, Ludman P, Nolan J. Arterial access site utilization in cardiogenic shock in the United Kingdom: is radial access feasible? Am Heart J. 2014;167:900–908.e1. doi: 10.1016/j.ahj.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 10.Ratib K, Mamas MA, Anderson SG, Bhatia G, Routledge H, De Belder M, Ludman PF, Fraser D, Nolan J. Access site practice and procedural outcomes in relation to clinical presentation in 439,947 patients undergoing percutaneous coronary intervention in the United kingdom. JACC Cardiovasc Interv. 2015;8:20–29. doi: 10.1016/j.jcin.2014.06.026. [DOI] [PubMed] [Google Scholar]
- 11.Valgimigli M, Gagnor A, Calabró P, Frigoli E, Leonardi S, Zaro T, Rubartelli P, Briguori C, Andò G, Repetto A, et al. Radial versus femoral access in patients with acute coronary syndromes undergoing invasive management: a randomised multicentre trial. Lancet. 2015;385:2465–2476. doi: 10.1016/S0140-6736(15)60292-6. [DOI] [PubMed] [Google Scholar]
- 12.Mamas MA, Nolan J, de Belder MA, Zaman A, Kinnaird T, Curzen N, Kwok CS, Buchan I, Ludman P, Kontopantelis E. Changes in Arterial Access Site and Association With Mortality in the United Kingdom: Observations From a National Percutaneous Coronary Intervention Database. Circulation. 2016;133:1655–1667. doi: 10.1161/CIRCULATIONAHA.115.018083. [DOI] [PubMed] [Google Scholar]
- 13.Hamon M, Pristipino C, Di Mario C, Nolan J, Ludwig J, Tubaro M, Sabate M, Mauri-Ferré J, Huber K, Niemelä K, et al. Consensus document on the radial approach in percutaneous cardiovascular interventions: position paper by the European Association of Percutaneous Cardiovascular Interventions and Working Groups on Acute Cardiac Care** and Thrombosis of the European Society of Cardiology. EuroIntervention. 2013;8:1242–1251. doi: 10.4244/EIJV8I11A192. [DOI] [PubMed] [Google Scholar]
- 14.Rao SV, Tremmel JA, Gilchrist IC, Shah PB, Gulati R, Shroff AR, Crisco V, Woody W, Zoghbi G, Duffy PL, et al. Best practices for transradial angiography and intervention: a consensus statement from the society for cardiovascular angiography and intervention’s transradial working group. Catheter Cardiovasc Interv. 2014;83:228–236. doi: 10.1002/ccd.25209. [DOI] [PubMed] [Google Scholar]
- 15.Ball WT, Sharieff W, Jolly SS, Hong T, Kutryk MJ, Graham JJ, Fam NP, Chisholm RJ, Cheema AN. Characterization of operator learning curve for transradial coronary interventions. Circ Cardiovasc Interv. 2011;4:336–341. doi: 10.1161/CIRCINTERVENTIONS.110.960864. [DOI] [PubMed] [Google Scholar]
- 16.Barbash IM, Minha S, Gallino R, Lager R, Badr S, Loh JP, Kitabata H, Pendyala LK, Torguson R, Satler LF, et al. Operator learning curve for transradial percutaneous coronary interventions: implications for the initiation of a transradial access program in contemporary US practice. Cardiovasc Revasc Med. 2014;15:195–199. doi: 10.1016/j.carrev.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 17.Dobies DR, Barber KR, Cohoon AL. Analysis of safety outcomes for radial versus femoral access for percutaneous coronary intervention from a large clinical registry. Open Heart. 2016;3:e000397. doi: 10.1136/openhrt-2015-000397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simard T, Hibbert B, Natarajan MK, Mercuri M, Hetherington SL, Wright R, Delewi R, Piek JJ, Lehmann R, Ruzsa Z, et al. Impact of Center Experience on Patient Radiation Exposure During Transradial Coronary Angiography and Percutaneous Intervention: A Patient-Level, International, Collaborative, Multi-Center Analysis. J Am Heart Assoc. 2016;5 doi: 10.1161/JAHA.116.003333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hulme W, Sperrin M, Rushton H, Ludman PF, De Belder M, Curzen N, Kinnaird T, Kwok CS, Buchan I, Nolan J, et al. Is There a Relationship of Operator and Center Volume With Access Site-Related Outcomes? An Analysis From the British Cardiovascular Intervention Society. Circ Cardiovasc Interv. 2016;9:e003333. doi: 10.1161/CIRCINTERVENTIONS.115.003333. [DOI] [PubMed] [Google Scholar]
- 20.Rashid M, Sperrin M, Ludman PF, O’Neill D, Nicholas O, de Belder MA, Mamas MA. Impact of operator volume for percutaneous coronary intervention on clinical outcomes: what do the numbers say? Eur Heart Jqual Care Clin Outcomes. 2016;2:16–22. doi: 10.1093/ehjqcco/qcv030. [DOI] [PubMed] [Google Scholar]
- 21.Rashid M, Kwok CS, Pancholy S, Chugh S, Kedev SA, Bernat I, Ratib K, Large A, Fraser D, Nolan J, et al. Radial Artery Occlusion After Transradial Interventions: A Systematic Review and Meta-Analysis. J Am Heart Assoc. 2016;5:pii: e002686. doi: 10.1161/JAHA.115.002686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kwok CS, Rashid M, Fraser D, Nolan J, Mamas M. Intra-arterial vasodilators to prevent radial artery spasm: a systematic review and pooled analysis of clinical studies. Cardiovasc Revasc Med. 2015;16:484–490. doi: 10.1016/j.carrev.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 23.Hassell ME, Piek JJ. Upper-extremity dysfunction following transradial percutaneous procedures: an overlooked and disregarded complication? Neth Heart J. 2015;23:510–513. doi: 10.1007/s12471-015-0749-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Leeuwen MA, van Mieghem NM, Lenzen MJ, Selles RW, Hoefkens MF, Zijlstra F, van Royen N. The effect of transradial coronary catheterization on upper limb function. JACC Cardiovasc Interv. 2015;8:515–523. doi: 10.1016/j.jcin.2014.10.025. [DOI] [PubMed] [Google Scholar]
- 25.Zwaan EM, Koopman AG, Holtzer CA, Zijlstra F, Ritt MJ, Amoroso G, Moerman E, Kofflard MJ, IJsselmuiden AA. Revealing the impact of local access-site complications and upper extremity dysfunction post transradial percutaneous coronary procedures. Neth Heart J. 2015;23:514–524. doi: 10.1007/s12471-015-0747-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benit E, Missault L, Eeman T, Carlier M, Muyldermans L, Materne P, Lafontaine P, De Keyser J, Decoster O, Pourbaix S, et al. Brachial, radial, or femoral approach for elective Palmaz-Schatz stent implantation: a randomized comparison. Cathet Cardiovasc Diagn. 1997;41:124–130. doi: 10.1002/(sici)1097-0304(199706)41:2<124::aid-ccd3>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 27.Campeau L. Percutaneous radial artery approach for coronary angiography. Cathet Cardiovasc Diagn. 1989;16:3–7. doi: 10.1002/ccd.1810160103. [DOI] [PubMed] [Google Scholar]
- 28.Chatelain P, Arceo A, Rombaut E, Verin V, Urban P. New device for compression of the radial artery after diagnostic and interventional cardiac procedures. Cathet Cardiovasc Diagn. 1997;40:297–300. doi: 10.1002/(sici)1097-0304(199703)40:3<297::aid-ccd18>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 29.de Belder AJ, Smith RE, Wainwright RJ, Thomas MR. Transradial artery coronary angiography and intervention in patients with severe peripheral vascular disease. Clin Radiol. 1997;52:115–118. doi: 10.1016/s0009-9260(97)80103-1. [DOI] [PubMed] [Google Scholar]
- 30.Kiemeneij F, Laarman GJ. Transradial artery Palmaz-Schatz coronary stent implantation: results of a single-center feasibility study. Am Heart J. 1995;130:14–21. doi: 10.1016/0002-8703(95)90229-5. [DOI] [PubMed] [Google Scholar]
- 31.Lotan C, Hasin Y, Mosseri M, Rozenman Y, Admon D, Nassar H, Gotsman MS. Transradial approach for coronary angiography and angioplasty. Am J Cardiol. 1995;76:164–167. doi: 10.1016/s0002-9149(99)80050-2. [DOI] [PubMed] [Google Scholar]
- 32.Prull MW, Brandts B, Rust H, Trappe H. Vaskuläre Komplikationen der perkutanen transradialen Koronarangiographie und Koronarintervention. Med Klin. 2005;100:377–382. doi: 10.1007/s00063-005-1049-6. [DOI] [PubMed] [Google Scholar]
- 33.Sciahbasi A, Rigattieri S, Sarandrea A, Cera M, Di Russo C, Fedele S, Romano S, Penco M, Rocco Pugliese F. Radial artery occlusion and hand strength after percutaneous coronary procedures: Results of the HANGAR study. Catheter Cardiovasc Interv. 2016;87:868–874. doi: 10.1002/ccd.26142. [DOI] [PubMed] [Google Scholar]
- 34.Zankl AR, Andrassy M, Volz C, Ivandic B, Krumsdorf U, Katus HA, Blessing E. Radial artery thrombosis following transradial coronary angiography: incidence and rationale for treatment of symptomatic patients with low-molecular-weight heparins. Clin Res Cardiol. 2010;99:841–847. doi: 10.1007/s00392-010-0197-8. [DOI] [PubMed] [Google Scholar]
- 35.Tharmaratnam D, Webber S, Owens P. Adverse local reactions to the use of hydrophilic sheaths for radial artery canulation. Int J Cardiol. 2010;142:296–298. doi: 10.1016/j.ijcard.2008.11.117. [DOI] [PubMed] [Google Scholar]
- 36.Spaulding C, Lefèvre T, Funck F, Thébault B, Chauveau M, Ben Hamda K, Chalet Y, Monségu H, Tsocanakis O, Py A, et al. Left radial approach for coronary angiography: results of a prospective study. Cathet Cardiovasc Diagn. 1996;39:365–370. doi: 10.1002/(SICI)1097-0304(199612)39:4<365::AID-CCD8>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 37.Wu SS, Galani RJ, Bahro A, Moore JA, Burket MW, Cooper CJ. 8 french transradial coronary interventions: clinical outcome and late effects on the radial artery and hand function. J Invasive Cardiol. 2000;12:605–609. [PubMed] [Google Scholar]
- 38.Zwaan E, Ijsselmuiden A, Kofflard M, Van Woerkens O, Holtzer C. Upper extremity function after transradial PCI: preliminary results (EuroIntervention 2016) Available from: https://www.pcronline.com/eurointervention/AbstractsEuroPCR2016_issue/abstracts-europcr-2016/Euro16A-POS1035/upper-extremity-function-after-transradial-pci-preliminary-results.html.
- 39.Valgimigli M, Campo G, Penzo C, Tebaldi M, Biscaglia S, Ferrari R. Transradial coronary catheterization and intervention across the whole spectrum of Allen test results. J Am Coll Cardiol. 2014;63:1833–1841. doi: 10.1016/j.jacc.2013.12.043. [DOI] [PubMed] [Google Scholar]
- 40.Zwaan EM, IJsselmuiden AJ, van Rosmalen J, van Geuns RM, Amoroso G, Moerman E, Ritt MJ, Schreuders TA, Kofflard MJ, Holtzer CA. Rationale and design of the ARCUS: Effects of trAnsRadial perCUtaneouS coronary intervention on upper extremity function. Catheter Cardiovasc Interv. 2016;88:1036–1043. doi: 10.1002/ccd.26525. [DOI] [PubMed] [Google Scholar]
- 41.Hawker GA, Mian S, Kendzerska T, French M. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP) Arthritis Care Res (Hoboken) 2011;63 Suppl 11:S240–S252. doi: 10.1002/acr.20543. [DOI] [PubMed] [Google Scholar]
- 42.Leite JC, Jerosch-Herold C, Song F. A systematic review of the psychometric properties of the Boston Carpal Tunnel Questionnaire. BMC Musculoskelet Disord. 2006;7:78. doi: 10.1186/1471-2474-7-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ruijs AC, Jaquet JB, Daanen HA, Hovius SE. Cold intolerance of the hand measured by the CISS questionnaire in a normative study population. J Hand Surg Br. 2006;31:533–536. doi: 10.1016/j.jhsb.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 44.Klocker J, Peter T, Pellegrini L, Mattesich M, Loescher W, Sieb M, Klein-Weigel P, Fraedrich G. Incidence and predisposing factors of cold intolerance after arterial repair in upper extremity injuries. J Vasc Surg. 2012;56:410–414. doi: 10.1016/j.jvs.2012.01.060. [DOI] [PubMed] [Google Scholar]
- 45.Mamas MA, Fraser DG, Ratib K, Fath-Ordoubadi F, El-Omar M, Nolan J, Neyses L. Minimising radial injury: prevention is better than cure. EuroIntervention. 2014;10:824–832. doi: 10.4244/EIJV10I7A142. [DOI] [PubMed] [Google Scholar]
- 46.Yonetsu T, Kakuta T, Lee T, Takayama K, Kakita K, Iwamoto T, Kawaguchi N, Takahashi K, Yamamoto G, Iesaka Y, et al. Assessment of acute injuries and chronic intimal thickening of the radial artery after transradial coronary intervention by optical coherence tomography. Eur Heart J. 2010;31:1608–1615. doi: 10.1093/eurheartj/ehq102. [DOI] [PubMed] [Google Scholar]
- 47.Mitchell R, Chesney A, Seal S, McKnight L, Thoma A. Anatomical variations of the carpal tunnel structures. Can J Plast Surg. 2009;17:e3–e7. [PMC free article] [PubMed] [Google Scholar]
- 48.Araki T, Itaya H, Yamamoto M. Acute compartment syndrome of the forearm that occurred after transradial intervention and was not caused by bleeding or hematoma formation. Catheter Cardiovasc Interv. 2010;75:362–365. doi: 10.1002/ccd.22282. [DOI] [PubMed] [Google Scholar]
- 49.Sugimoto A, Iwamoto J, Tsumuraya N, Nagaoka M, Ikari Y. Acute compartment syndrome occurring in forearm with relatively small amount of hematoma following transradial coronary intervention. Cardiovasc Interv Ther. 2016;31:147–150. doi: 10.1007/s12928-015-0328-2. [DOI] [PubMed] [Google Scholar]
- 50.Mouawad NJ, Capers Q, Allen C, James I, Haurani MJ. Complete “in situ” avulsion of the radial artery complicating transradial coronary rotational atherectomy. Ann Vasc Surg. 2015;29:123.e7–123.11. doi: 10.1016/j.avsg.2014.07.024. [DOI] [PubMed] [Google Scholar]
- 51.Agostoni P, Zuffi A, Biondi-Zoccai G. Pushing wrist access to the limit: homolateral right ulnar artery approach for primary percutaneous coronary intervention after right radial failure due to radial loop. Catheter Cardiovasc Interv. 2011;78:894–897. doi: 10.1002/ccd.23192. [DOI] [PubMed] [Google Scholar]


