Abstract
Metabolism is the basic activity of live cells, and monitoring the metabolic state provides a dynamic picture of the cells or tissues, and how they respond to external changes, for in disease or treatment with drugs. NMR is an extremely versatile analytical tool that can be applied to a wide range of biochemical problems. Despite its modest sensitivity its versatility make it an ideal tool for analyzing biochemical dynamics both in vitro and in vivo, especially when coupled with its isotope editing capabilities, from which isotope distributions can be readily determined. These are critical for any analyses of flux in live organisms. This review focuses on the utility of NMR spectroscopy in metabolomics, with an emphasis on NMR applications in stable isotope-enriched tracer research for elucidating biochemical pathways and networks with examples from nucleotide biochemistry. The knowledge gained from this area of research provides a ready link to genomic, epigenomic, transcriptomic, and proteomic information to achieve systems biochemical understanding of living cells and organisms.
Keywords: Isotope editing, Stable Isotope Resolved Metabolomics (SIRM), isotopomer distribution analysis
1. Introduction
NMR spectroscopy is a very powerful and versatile tool for elucidating the structure and conformation of both small and macro molecules in the solution or solid state [3–11] (and this issue). In addition, NMR methods provide detailed information about molecular and internal dynamics over a wide range or timescales by determining rotational and translational diffusion coefficients and internal motions of molecules [13]. Furthermore, NMR can be applied to chemical dynamics, for studying mechanism as well as flux measurements both in vitro and in vivo [3, 14, 15].
A critical feature of NMR for molecular structural and quantitative analysis is its isotope-selective detection and sensitivity of nuclear spin properties to the intra- and inter-molecular environment, as well as to the robust and quantitative nature of NMR measurements. These advantages have made NMR an early choice for metabolite profiling efforts [16–19] and an excellent partner for mass spectrometry (MS)-based metabolite profiling [20]. For example, NMR analysis provides crucial structural parameters including functional groups, covalent linkages, and non-covalent interactions including stereochemistry, which are difficult to acquire by MS methods.
Metabolomics has become a vibrant field for elucidating the functional biochemistry of diverse organisms and model systems [12] and their responses to altered conditions and pathologies [21–30]. Although various mass spectrometry platforms are commonly used in metabolomics studies [31–37], the unique capabilities of NMR provide several advantages including isotope-selective editing of complex mixtures, detailed positional isotopomer analysis for enriched metabolites, de novo structure determination of unknown metabolites (both unenriched and enriched), accurate quantification without the need for standards, and in situ analysis of pathway dynamics from cells to whole organisms [2, 3, 6, 38, 39].
This review focuses on the utility of NMR spectroscopy in metabolomics, with an emphasis on NMR applications in stable isotope-enriched tracer research for elucidating biochemical pathways and networks such as those shown in Figures 1 and 2. The knowledge gained from this area of research provides a ready link to genomic, epigenomic, transcriptomic, and proteomic information to achieve systems biochemical understanding of living cells and organisms. Although many such basic pathways are often assumed, the techniques can in fact be used to determine the differences in tissue specific metabolism as well as discover unexpected metabolic transformations [40–43] that are not necessarily readily annotated from genomics. It is likely that in the fields of diverse speciation in the fungal and microbial worlds, many metabolic pathways remain to be discovered and characterized. Furthermore, with judicious use of stable isotope tracers (see below), it is possible in some instances to infer compartmentation even when the same metabolites are present in more than one [3, 44–46].
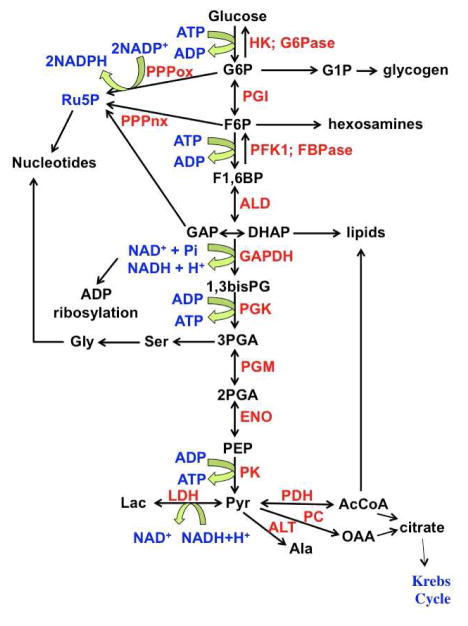
Figure 1. Biochemical network involving glucose metabolism.
Glucose is oxidized to pyruvate (glycolysis). Several intermediates are involved in parallel and intersecting pathways: glycogen metabolism, pentose phosphate pathway, serine pathways, lipid biosynthesis and the Krebs Cycle. G6P: glucose-6-phosphate; F6P fructose-6-phosphate; F1,6BP fructose-1,6-bisphosphate; GAP glyceraldehyde-3-phosphate; DHAP dihydroxyacetone phosphate; 1,3bisPG 1,3-bisphosphoglycerate; 2PGA 2-phosphogycerate; PEP phosphoenolpyruvate; Pyr pyruvate; OAA oxaloacetate; AcCoA acetyl CoA; Lac lactate; Ru5P ribose-5-phosphate; HK hexokinase; G6Pase glucose-6-phosphatase; PGI phosphoglucose isomerase; PFK1 phosphofructokinase 1; FBPase fructose 1,6 bisphosphatase; ALD aldolase; GAPDH glyceraldehyde-3- phosphate dehydrogenase; PGK phosphoglycerate kinase; PGM phosphoglycerate mutase; ENO enolase; PK pyruvate kinase; LDH lactate dehydrogenase; ALT alanine transaminase; PC pyruvate carboxylase; PDH pyruvate dehydrogenase; PPPox oxidative branch of the pentose phosphate pathway; PPPnx non-oxidative branch of the pentose phosphate pathway. Adapted from [12] “With permission of Springer”.
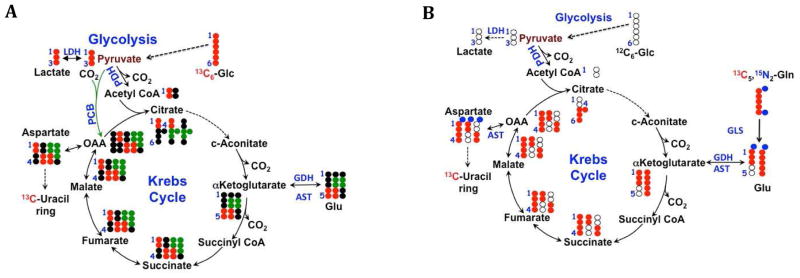
Figure 2. Glu isotopomers from Krebs cycling.
Isotopomers created via Krebs cycle activity by: (A) 13C6 glucose. Black dots are 12C. 13C6 glucose (red dots) produces 13C3 pyruvate which can enter the Krebs cycle either via PDH (2 13C) or PCB (3 13C, shown as green dots), which give rise to different isotopomers of Krebs cycle intermediates and anabolic products such as uracil and its precursor Asp, distinguishable by NMR as 13C1,2 + 13C3,4 via PDH in the forward direction, and 13C1,2,3 Asp via PCB (B) 13C5,15N2 Gln plus unlabeled glucose. Red dots are 13C atoms from Gln, blue dots are the two nitrogen atoms. Open circles are 12C. The anaplerotic input of fully labeled Gln produces the fully labeled Krebs cycle intermediates. Fully labeled OAA from Gln condenses with glucose-derived AcCoA to produce quadruple labeled citrate, which becomes via the Krebs cycle triply labeled αKG, and doubly labeled succinate. AST will transaminate OAA with Glu, transferring the amino nitrogen to Asp. The isotopomers produced evolve on further cycles, and differ with other inputs to the cycle. Isotopomer analysis is needed to sort out the resulting complex patterns.
2. Stable Isotope Resolved Metabolomics (SIRM)
Although NMR is intrinsically an accurate quantitative method such that steady state concentrations of metabolites can be reliably determined [3], changes in amounts of metabolites only tell part of the metabolic story. This is because not only are most intracellular metabolites maintained at fairly narrow concentration ranges (homeostasis) but also metabolic pathways are highly interconnected in a complex network of reactions. For example the central highways of carbohydrate metabolism, glycolysis and the pentose phosphate pathway and the Krebs cycle are not simple linear sequences of transformation, but many of the intermediates are also involved in numerous other pathways. Thus, glucose-6-phosphate, the first committed intermediate of glycolysis, has several possible fates including (i) transformation to fructose-6-phosphate by phosphoglucose isomerase (glycolysis), (ii) diversion into the oxidative branch of the pentose phosphate pathway and (iii) in glycogen synthesis after conversion to glucose-1-phosphate. This intermediate is also the entry point for galactose metabolism via the Leloir pathway [47, 48]. Similarly, in addition to being an intermediate in glycolysis, fructose-6-phosphate is the entry point to the hexosamine pathway, dihydroxyacetonephosphate (DHAP) is the entry point for production of glycerol-3-phosphate for glycerolipid synthesis, and 3-phosphoglycerate is the precursor of serine and glycine (Figure 1). The product of glycolysis, pyruvate, also has numerous fates, including reduction to lactate (lactic fermentation by lactate dehydrogenase, LDH), transamination with L-glutamate to alanine (via alanine amino transferase, ALT), oxidative decarboxylation to acetyl CoA (via pyruvate dehydrogenase, PDH) and ATP-dependent carboxylation to oxaloacetate (via pyruvate carboxylase, PC). The Krebs cycle receives carbon input not only from pyruvate, but also from amino acids and fatty acid oxidation. The degradation of the carbon skeleton of most amino acids is via the Krebs cycle (especially the liver) [12], and some cell types oxidize glutamine for energetic and anabolic purposes via the Krebs cycle [49–52]. The steady state concentration of the intermediates therefore depends on numerous inputs and outputs. To determine the sources and fates of the atoms that make up metabolites needs tracers. Commonly used stable isotope tracers are given in Table 1. These are commercially available compounds that probe different aspects of metabolism.
Table 1.
Commonly used stable isotope tracers
| Tracer | Pathways Probed | References |
|---|---|---|
| [U-13C]-glucose | Glycolysis, PPP, TCA cycle, hexosamine, nucleotide, and lipid biosynthesis | [3, 53] |
| [13C1,2]-glucose | Non-oxidative vs. oxidative PPP | [54, 55] |
| [13C3,4]-glucose | PC anaplerosis | [56, 57] |
| 13C-1 glucose | Glucose metabolism in vivo | |
| [U-13C,15N]-glutamine | Glutaminolysis, nucleotide biosynthesis, TCA cycle, fatty acid biosynthesis, amino acid metabolism | [53, 58, 59] |
| [U-13C]-fatty acids e.g. octanoate | β-oxidation, fatty acid biosynthesis | [26, 60] |
| [13C]-serine | Serine metabolism, one-carbon metabolism, lipid biosynthesis | [61] |
| [13C]-glycerol | Lipid biosynthesis | [62] |
| 13C1/13C2 pyr | LDH reaction/DNP | [63] |
| 13C6,2H7 | Glycolysis/PPP DNP | [64] |
Spin topology and isotopomers-spectral editing
A solution to sorting out the complexity is to use NMR-active stable isotope tracers, especially 13C and 15N, and in some cases also 2H. These tracers when incorporated into source compounds such as glucose, glutamine and others (cf. Table 1), generally have minimal influence on metabolic rates as primary isotope effects are small, and bond-breaking steps are not always wholly rate limiting in enzyme catalyzed reactions [65]. Isotope effects with perdeuterated substrates, however, have been observed [3, 66], indicating that some caution may be needed when comparing these substrates with the natural ones.
The metabolic transformations of stable isotope enriched compounds can be readily determined by mass spectrometry, which is routinely carried out in tracer studies [31, 67–70]. As MS measures mass, only the number of enriched atoms can be counted; without extensive fragmentation, the positional information is not readily available. In contrast, NMR has some unique capabilities including isotope editing (i.e. selection for molecules containing 13C, 15N or 31P for example), and direct determination of the positional enrichment either by direct integration or from the patterns generated by scalar coupling [2, 3, 39]. Furthermore, as the stable isotopes are fully compatible with live cells and organisms, it is possible to measure metabolic processes in vivo [71] [72, 73] [74] [63] [3, 64] and see below.
Spin topology of metabolites
A 5-carbon metabolite such as Glu has 32 carbon isotopomers, and if 15N is included, there are 64 isotopomers. As Glu is a hub metabolite that is involved in a large number of metabolic transformations, a significant number of these isotopomers may be present, and a quantitative analysis of the isotopomer distribution is needed to assess which pathways are active. For example, if uniformly 13C-enriched glucose ([U-13C]-glucose) is the 13C source in cells, then specific 13C isotopomers will be formed just from two turns of the Krebs cycle with pyruvate carboxylase input (Fig 2A). With [U-13C,15N]-Gln, different isotopomer distributions are produced, including the incorporation of 15N into Asp and Ala by transamination of Glu (Fig. 2B).
Spectral simplification by isotope and editing and chemoselection
Owing to the large number of metabolites and narrow frequency range,1D proton NMR spectra of cell and tissue extracts are highly overlapping in many regions, especially in the (phospho)sugars from 3.5 to 5 ppm, which is exacerbated by the presence of satellites from 13C.. There are two major routes to simplifying spectra while at the same time enabling extraction of specific information about metabolites and their function groups. The first is spectral editing using the metabolically incorporated stable isotope tracer. The editing then suppresses all protons that are not interacting with the isotope, so that the spectral display comprises resonances of atoms of metabolites that are part of the metabolic network accessed by the source. A simple approach is to use the HSQC experiment, which selects for protons directly attached to carbon or nitrogen, and in the case of nitrogen is useful for long range couplings (2–3 bonds) where 13C is absent such as in nucleotides [2, 75]. There are many variants of these experiments that can select for individual labeled atoms, adjacent atoms (e.g. HCCH experiments) and for doubly enriched experiments, where either triple resonance can be used such as HCN or HNC experiments [3], or the influence of the passive coupling of one isotope in a 2D experiments is detected.
Some of these isotopomers can be readily detected using TOCSY, which shows quite different cross-peak patterns for the protiated carbons of different isotopomers [65, 76]. It is possible to determine 13C-enrichment in carboxylate groups using HCCO experiments and discriminate molecules that are singly or multiply labeled and whether there are adjacent 13C atoms using HCCH via the 1JCC of 40–50 Hz [2, 3, 39, 77]. Similarly, where multiple labels such as 15N and 13C are used, it is possible to discern the position and enrichment of one label when editing for another. For example, phosphorylated compounds can be selected by HSQC using the 3JPH coupling, which is typically a few Hz. Such an HSQC spectrum of a mixture will select all of the (sufficiently abundant) phosphorylated compounds including the nucleotides [78] greatly simplifying the NMR spectrum. Taking the example of AMP or UMP from cells exposed to [U-13C]-glucose, then the ribose subunit will also be 13C-enriched, and the H5′/H‴ resonances will be split by the (passive) 1JCH ≈ 126 Hz. If the HSQC-TOCSY experiment is carried out, then other positions in the ribose ring will also be detected. The ratio of the satellite intensities to that of the central 12C peak provides an estimate of the fractional enrichment at that position. Similarly experiments can be carried out that utilize two stable isotopes simultaneously (multiplexing), for example 15N and 13C. We have used this approach to determine the positional 13C enrichment in γ-amino butyrate in rice coleoptiles grown in the presence both 15N nitrate and 13C acetate [65].
Chemoselection combines selection of metabolites in a mixture that are derivatized according to their functional group. If the derivatizing agent contains a stable isotope, then in those metabolites that have a particular derivatizable functional group will be observed in the NMR experiment. Gowda et al. [79] have developed the use of 15N-cholamine which reacts with free carboxylates in the presence of a catalyst forming a peptide bond. As 1JNH is around 91 Hz, the peptide unit is readily observed using 1H{15N}-HSQC, and a large number of resonances can be detected in complex cell extracts. Similarly, we have developed 15N aminooxy reagents that react specifically with aldehydes and ketone, but not carboxylates [38]. This reagent and 15N-cholamine are now available from the Metabolomics workbench (http://www.metabolomicsworkbench.org/standards/index.php). This includes the reducing sugars, which are otherwise difficult to assign by NMR because of the spectral overlap. Depending on the compound the spectra are edited using the 2JNH or 3JNH couplings, which are in the 2–3 Hz range. Although small, the long T2 in metabolites makes a long-range HSQC experiment sufficiently sensitive, and can be combined with TOCSY to obtain more information about the spin connectivity, and thus assignments, in the target metabolites. In both cases, only the target metabolite resonances are observed in the 15N-edited spectra. Other functional groups can be detected using appropriate reagents, including amino groups and sulfhydryl groups [80].
Considerations of analytical accuracy and precision
The absolute accuracy of NMR is very high if sufficient care is taken over the experimental conditions [3]. These include the sample quality, uniform excitation and relaxation periods, and the method of integration or resonances. As modern NMR spectrometers have a very high linear dynamic range (typically 105:1), only one internal standard is needed to determine the number of molecules present in the sample. In 1D 1H NMR it is common to use a peak deconvolution in most metabolomics applications, the relaxation delays are set long compared with most T1 values, so that correction for incomplete relaxation is small, and generally simple. With care, absolute concentrations can be determined with high accuracy (better than 5%, subject to certain caveats discussed in section 3 below) and precision (CoV of <1–2 %) which is far lower than any biological variance (reviewed in [3]).
Because of the extended time needed to record 2D NMR spectra, it is customary to reduce the recycle time close to the mean T1 value of the analytes to maximize sensitivity with 90° excitation pulses. This substantially distorts the intensities for spins having different T1 values. Also, in HSQC experiments recorded at high magnetic field strengths, uneven excitation occurs across the spectrum, in part owing to a shift-dependent variation of 1JCH values (from ca. >200 Hz for aromatic C-H pairs to ca. 125 Hz for methyl groups). For absolute quantification, this requires additional corrections, such as suggested by [81].
2D experiments, however, are more generally used to define the sites of enrichment (“isotopomer analysis”), as well as the degree of enrichment at each site. The main issue then is the differential relaxation rate constants for protons attached to 12C versus those bonded to 13C. Because the fractional enrichment is calculated as a ratio of peak intensities (e.g. volume (13C)/[volume(12C)+volume(13C)], such that even with relatively short recycle times, the quantitative accuracy and precision of the fractional 13C levels is high in TOCSY, as described [65, 76].
3. Metabolic pathway reconstruction
In order to proliferate, cells make the precursors of the components of biomass including nucleotides, lipids and some amino acids via endergonic anabolism. Furthermore, during proliferation, in order to maintain homeostasis, the metabolites must also be synthesized or obtained from the external medium. Complex metabolites such as the nucleotides (and nucleotide sugars) need multiple pathways to supply the carbon and nitrogen that comprise the product, and these processes are in fact complex metabolic networks of pathways [75].
Nucleotide synthesis
A large part of metabolism is mediated and enabled by nucleotide co-factors. Anabolic metabolism is endergonic, and for the most part is driven by the coupled hydrolysis of nucleoside triphosphates. ATP is the most widely used energy currency, but GTP, UTP and CTP are also used in specific contexts [75]. Furthermore in addition to driving thermodynamically unfavorable reactions, the nucleotides are also used to increase the chemical reactivity of otherwise unreactive metabolic subunits, by forming covalent linkages. This includes activation of acetate as AcCoA, a universal acetyl donor, and the methyl as S-Adenosyl Methionine (SAM), as well as in protein biosynthesis (cf. aminoadenylation), UDP-hexoses for glycosylation and complex carbohydrate synthesis, and CDP-choline for lipid synthesis among others. The nucleoside triphosphates are also the direct substrates for nucleic acid biosynthesis.
The redox dinucleotides, including NAD+/NADH and NADP+/NADPH are needed for catabolic oxidation (NAD+) and anabolic reduction (NADPH) as well as other functions such as regulation by ADP ribosylation (NAD+). The nucleotides are ubiquitous in metabolism, and unsurprisingly their concentrations are very tightly regulated [75].
(i) Free nucleotide and RNA synthesis
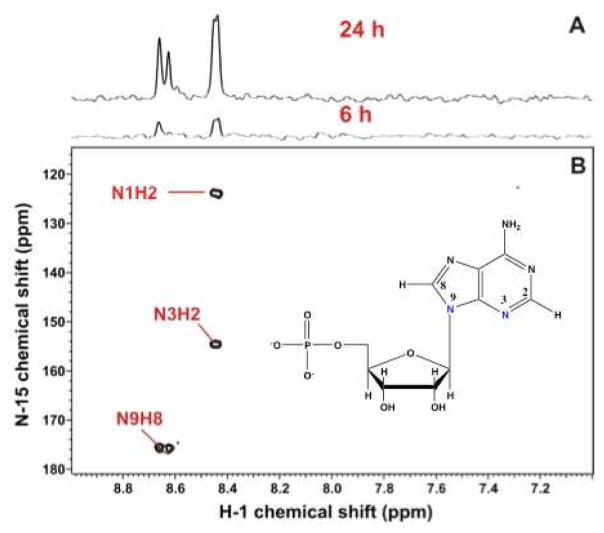
The concentration of ATP, ADP and AMP and their ratios regulate the function of live cells, as these nucleotides are used to maintain the non-equilibrium state of the cell in maintain membrane potentials via ion gradients coupled to ATP hydrolysis, protein turnover, and anabolic metabolism which is highly endergonic. Most of this is determined by phosphorylation at the substrate level and by mitochondrial oxidative phosphorylation. In addition, ATP along with GTP, UTP and CTP are needed as substrate for RNA and DNA biosynthesis, which remove the nucleotides from the free pools. This cells that are proliferating, or shift to a state of increased protein synthesis, must up regulate the de novo synthesis of the nucleotides to produce the macromolecules. The synthesis of the nucleotides is highly regulated [75]. Purine synthesis is cytoplasmic and the base is built directly on the ribose-phosphate subunit deriving from the pentose phosphate pathway, whereas the pyrimidine bases are built separately using both cytoplasmic and mitochondrial reactions, and then condensed onto the activate ribose phosphate (PRPP) as Uracil. SIRM studies can distinguish between the ribose subunit synthesis and the base synthesis. The ribose subunits in the free nucleotide pool can be detected by TOCSY and HSQC owing to the characteristic chemical shifts and spin topology [82], from which the 13C isotopomer distribution can be determined [20, 65, 76]. In most cases, this arises from glucose, unless there is significant gluconeogenesis, when 13C can be detected from glucogenic precursors such as lactate and some amino acids [83, 84]. Similarly, the H6 and H5 of the pyrimidine rings shows characteristic TOCSY and chemical shift patterns, and reveal the source of the carbons, which may be both glucose (via glycolysis and the Krebs cycle) or glutamine via glutaminolysis and the Krebs cycle (Figure 2)[82]. Similarly, the incorporation of 15N from Gln into the purine rings of nucleotides can be determined using the substantial long range 15N-1H scalar couplings to H8 and H2 (Figure 3) [2, 85]. This shows that as expected the Gln amido nitrogens were incorporated into the N3 and N9 positions, but also the N1 derived from the 15N Gln. This can be accounted for by the activity of AST which transaminates Glu (the product of glutaminase) with OAA to make Asp, the nitrogen donor for N1 [75] (cf. Fig. 2B).
Figure 3. 15N incorporation from Glutamine in purine nucleotides.
Spectral editing via 1H{15N}-HSQC detects 15N-isotopomers of nucleotides. A549 cells were grown for 24 h in the presence of 5 mM unlabeled glucose and 4 mM [U–13C,15N]-glutamine. The spectra of the polar extract were recorded at 14.1 T at 20 °C with acquisition times of 0.15 s in t2 and 0.021 s in t1 and a recycle time of 1.6 s. The value of the INEPT transfer delay was set to 10 ms.
A. 1D 1H{15N}-HSQC spectra at different time points. B. 2D HSQC shows editing of the complex proton spectrum. The long range 1H-15N couplings shows utilization of both the amido (blue) and amino N of Gln. Adapted from ref. [2] “With permission of Springer”
(ii) Redox dinucleotide NAD, NADH, NADP, NADPH quantification and synthesis/turnover
Generally the total concentration of NADH + NAD+ in mammalian cells is of the order 0.5–1 mM, with the free NAD+ concentration 700–1500 times higher than NADH [86], though the ratios may vary substantially in different compartments such as the cytosol and mitochondria. These ratios have been determined in vivo using the pyruvate/lactate redox couple and by NMR using DNP [86]. In contrast, the total NADPH + NADP+ is about 10 fold lower than NADH + NAD+, and the ratio of NADP+/NADPH has been variously estimated as 0.02 to 2 in mammalian cells [70, 87, 88] to >20 in yeast (free concentrations) or 1 (total concentrations) [89]. The source of the hydride varies according to cell type, and can also be followed using deutierated substrates [70, 87]
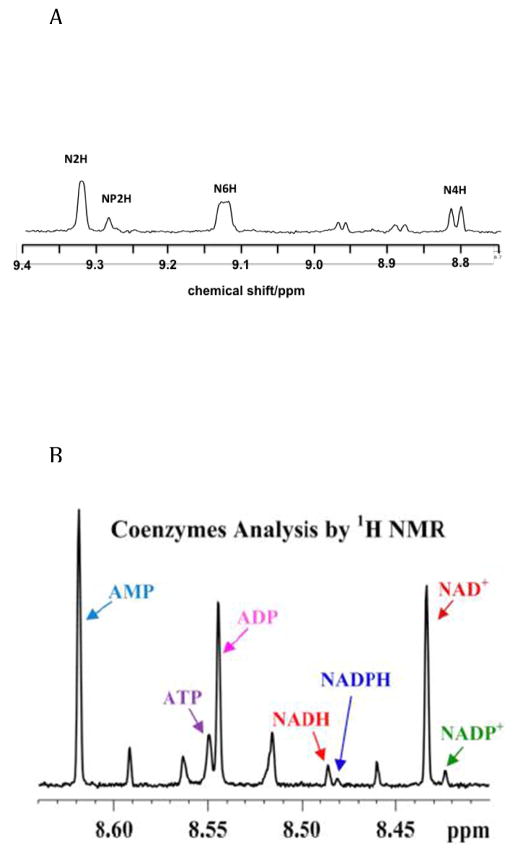
In cell extracts, the amounts of NAD+ and NADP+ can be readily determined by NMR, as the nicotinamide resonances are well resolved, and those of NAD and NADP can be easily integrated (Figure 4A). The nicotinamide resonances of the reduced forms however overlap with many other resonances [90]. Recently [1] et al. have shown that the adenine C8H resonance of ATP, ADP, AMP, NAD+, NADH, NADP+ and NADH are resolved in extracts (Fig 4B), permitting determination of the absolute amount of each nucleotide. In mouse cardiac muscle, for which the extraction process was optimized, after converting ng/mg tissue to mmol/kg tissue the ratios of ATP to ADP and AMP were ca. 7.5:1 and 10:1 respectively, while the NAD+/NADH = 2.8, NADPH/NADP+ ≈ 1.6. Furthermore, the NAD+/NADH ratio implies a lactate/pyruvate ratio of ca. 1300 at pH 7.4, which is much higher than is generally observed either in extracts or in vivo [59, 86, 91]. This method does however open up the possibility of using NMR to determine the rate of NAD(P)+ reduction using deuteriated substrates [70, 87].
Figure 4. Nucleotide analysis by 1H NMR.
1H NMR spectra of polar extracts of cells or tissues.
A. 1H NMR spectrum at 600 MHz of an extract of BEAS-2B cells (an immortalized bronchial epithelial cell line) showing the spectral region containing the resonances of the nicotinamide subunits of NAD+ (N) and NADP+ (NP). The spectrum was recorded at 288 K with an acquisition time of 2 s and a recycle time of 6 s (512 transients). The free induction decays were zero-filled once and apodized using an unshifted Gaussian function and a 05 Hz line broadening exponential.
B. 1H NMR spectrum of an extract of mouse heart showing the Adenine nucleotide region (C8-H) resonances) of different nucleotides including oxidized and reduced forms of NAD+ and NADP+. From [1]
The discrepancy between amounts and concentration ratios in vivo and those determined by extraction may be attributed to two main causes. First some of the nucleotides are unstable, and their integrity depends on the details of the extraction protocol, especially for the reduced nucleotides. The second is a different consequence of the extraction protocol. Many extraction techniques measure the total nucleotides as the various dehydrogenases are denatured during extraction, and release the bound nucleotides. Furthermore, the ratio of the free nucleotides versus the ratio of the bound nucleotides is in general not equal, because of differential affinity of the dehydrogenases for NAD+ and NADH, often an more than an order of magnitude [92]. In contrast, NMR of live cells gives estimates of amounts of common metabolites in the free state, that are significantly different from those obtained by denaturing extraction protocols, or by mass spectrometry, which explicitly requires a deproteinizing extraction prior to analysis [93]. Such differences attributable to binding of metabolites to proteins have also been observed for untreated plasma or serum [94, 95].
However, from the point of view of flux analysis and thermodynamics, it is more useful to determine the free concentrations. Consider the reaction catalyzed by lactate dehydrogenase (LDH):
| (1A) |
The equilibrium constant for this reaction, Keq, which can be measured in vitro, is:
| (1B) |
The concentrations refer to the free state, rather than the total (free plus bound).
The steady state reaction mechanism catalyzed by LDH can be approximated as a sequential ordered bi-bi enzyme mechanism with rapid quasi-equilibrium binding of substrates and coenzymes [96]. Ignoring the protons, the net flux, J, at steady state is given by:
| (2) |
where [S], [N], [P], [NH] are the free concentrations of substrate pyruvate, NAD+, product lactate and NADH, [E]t is the total enzyme concentration, KS, KN, KP, KNH are the dissociation constants of the substrate from the enzyme and Keq is the overall equilibrium constant. The functional form of the rate equation in terms of total concentrations of substrates and products requires knowing the concentrations of all of the bound forms of each, and leads to a far more complex rate equation. Furthermore, as there are several hundred dehydrogenases, this is unlikely to be determinable.
The term [1-[P][NH]/[S][N]Keq] is the thermodynamic term that describes how close the reaction is to equilibrium, and also the direction of net flux, and is independent of the details of the enzyme mechanism. Thus for nucleotides, the thermodynamic driving force and reaction kinetics may not be correctly analyzed using total concentrations determined from extracts; rather the free concentrations, as potentially determined from in vivo NMR, may be more reliable.
If the reaction in Eq. (1) is at equilibrium, then
| (3) |
The error in the ratio of [NAD+]/[NADH] is proportional to the errors in the ratio of [pyr]/[lac], to the equilibrium constant and to the relevant intracellular pH. Furthermore, this is correct strictly only at equilibrium, where there is no net flux, only an exchange flux [97]. The value of Keq has been estimated in vitro as 1–5 E-5 in the pH range from 6.8 to 7.0 [92, 98, 99]. The cytoplasmic pH is generally about 7.4, though lower values close to 7 [86] and higher ones in active cancer cells may occur [100]. These can in fact be estimated by 31P and 13C NMR in cells, with an estimated error of <0.1 pH unit in carefully calibrated systems [101, 102]. Thus the nucleotide ratio may be ill determined according to the range of the equilibrium constants estimated in vitro, the uncertainty of the relevant pH, as well as the analytical imprecision in the concentration ratio of lac and pyr. This might amount to an uncertainty in the [NAD+]/[NADH] ratio as much as 3–4 fold. However, the in vivo ratio is estimated in the range 700–1500, so even a factor of 3–4 fold might reduce this ratio to >200–500, which remains much higher than the in vitro estimates of ≈3 (see above).
4. Conclusions and Future Directions
The high quantitative accuracy of NMR, the lack of a need for standards, the extensive isotope editing capabilities, direct isotopomer distribution analysis, structural identification and applicability to metabolic studies in vivo make NMR an extremely valuable analytical tool for tracer-based metabolic studies.
Extraction is a problem for nucleotide analyses in terms of absolute concentrations. The errors in absolute concentration determination however do not affect isotopomer distributions as these are calculated as ratios. The isotopomer distributions carry much more information about pathways than steady state concentrations, and may thus be more robust. As nucleotides typically bind numerous proteins, the methods of extraction distort the relative amounts (free versus bound), which also complicates flux analysis. In vivo methods including NMR and for NAD(P)H fluorescence methods might be profitably combined with isotopomer analysis in vivo and in extracts to analyze quantitatively cellular fluxes [103].
On modern high field instruments equipped with cryoprobes, it is common to achieve SNR of 1500:1 on 2 mM sucrose in 90% H2O. In a 5 mm Shigemi tube, this is about 0.6 μmol solute, implying that a 1D proton spectrum with SNR>10 can be acquired on 0.4 nmol in around 10 minutes. At physiological salt concentration, this sensitivity decreases significantly (ca. 2 fold at 700 MHz), which can be partially offset using a 3 mm Shigemi tube.
Of particular interest for in vivo NMR studies there are methods that can enhance sensitivity thousands of fold including by Dynamic Nuclear Polarization or parahydrogen [104, 105][3, 14, 106–112] using a variety of commercially available polarizable substrates (cf Table 1) [3, 14]
Although much of metabolomics NMR is done using 1D acquisition [14, 113–115], the resolution in these experiments is relatively low, and isotopomer analysis may need higher dimensions, which can also make use of the isotope editing capabilities described above. However, using conventional sampling, even 2D methods with adequate digitization in the indirect dimension requires a large number of increments, making the experiment very lengthy which is not compatible with even medium throughput. For example, a conventionally sampled HSQC spectrum with resolution much better than the 1JCC coupling constants of 40–50 Hz, requires acquiring of the order 1000 fids (real + imaginary) at modest field strength (ca. 14.1 T), and with the need for signal averaging especially in weak samples, the time required for 16 transients per increment assuming a recycle time of 2 s is nearly 9 h. However, this can be reduced to <3 h with 33% sampling by nonuniform sampling (NUS), while ensuring that the resolution in F1 reaches the desired value. The time savings in 3D experiments can be even greater. For example, a conventionally sampled 3D spectrum with 64 increments in both indirect dimensions, and a recycle time of 1.5 sec, the experimental time with 16 scans/increment is about 27 h, with low resolutions in the indirect dimensions. The same experiment with 33% NUS in both indirect dimensions would need only 3 h with potentially higher resolution.
Furthermore, with dual receivers, it is possible to acquire parallel experiments with appropriate routing of coherences [116–119], which promises to speed up acquisition of multiple data sets significantly, making multidimensional NMR more routine, and expanding the capabilities of isotopomer analysis.
Highlights.
The metabolic state provides a dynamic functional readout of live cells and tissues
Stable Isotope Resolved Metabolomics (SIRM) permits tracing atoms through pathways
Isotope editing by NMR enables selection of labeled metabolites in complex mixtures
Flux measurements are possible both in vitro and in vivo using NMR-based SIRM
Acknowledgments
Funding: This work was supported by the National Institutes of Health [grant numbers 5P01CA163223, U24DK097215, 5R01ES022191].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gowda GAN, Abell L, Lee CF, Tian R, Raftery D. Simultaneous Analysis of Major Coenzymes of Cellular Redox Reactions and Energy Using ex Vivo 1H NMR Spectroscopy. Anal Chem. 2016;88:4817–4824. doi: 10.1021/acs.analchem.6b00442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fan TW-M, Lane AN. NMR-based Stable Isotope Resolved Metabolomics in Systems Biochemistry. J Biomolec NMR. 2011;49:267–280. doi: 10.1007/s10858-011-9484-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fan TW-M, Lane AN. Applications of NMR to Systems Biochemistry. Prog NMR Spectrosc. 2016;92:18–53. doi: 10.1016/j.pnmrs.2016.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schanda P, Meier BH, Ernst M. Quantitative Analysis of Protein Backbone Dynamics in Microcrystalline Ubiquitin by Solid-State NMR Spectroscopy. J Am Chem Soc. 2010;132:15957–15967. doi: 10.1021/ja100726a. [DOI] [PubMed] [Google Scholar]
- 5.Levitt MH. Spin Dynamics: Basics of Nuclear Magnetic Resonance. Chichester, United Kingdom: Wiley; [Google Scholar]
- 6.Fan TW-M, Lane AN. Assignment strategies for NMR resonances in metabolomics research. In: Lutz N, Sweedler JV, Weevers RA, editors. Methodologies for Metabolomics: Experimental Strategies and Techniques. Cambridge University Press; Cambridge: 2013. [Google Scholar]
- 7.Sanders JKM, Hunter BK. Modern NMR Spectroscopy. A guide for chemists. Oxford, U.K: Oxford University Press; 1987. [Google Scholar]
- 8.Wuthrich K. NMR of proteins and nucleic acids. New York: Wiley; 1986. [Google Scholar]
- 9.Kuszewski J, Schwieters CD, Garrett DS, Byrd RA, Tjandra N, Clore GM. Completely automated, highly error-tolerant macromolecular structure determination from multidimensional nuclear overhauser enhancement spectra and chemical shift assignments. Journal of the American Chemical Society. 2004;126(20):6258–6273. doi: 10.1021/ja049786h. [DOI] [PubMed] [Google Scholar]
- 10.Lopez-Mendez B, Guntert P. Automated protein structure determination from NMR spectra. Journal of the American Chemical Society. 2006;128(40):13112–13122. doi: 10.1021/ja061136l. [DOI] [PubMed] [Google Scholar]
- 11.Rance M, Cavanagh J, Fairbrother WJ, Palmer AG, Skelton NJ. Protein NMR spectroscopy: principles and practice. 2. Boston: Academic Press; 2007. [Google Scholar]
- 12.Lane AN, Higashi RM, Fan TW-M. Preclinical models for interrogating drug action in human cancers using Stable Isotope Resolved Metabolomics (SIRM) Metabolomics. 2016;12:1–15. doi: 10.1007/s11306-016-1065-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palmer AG., III Enzyme Dynamics from NMR Spectroscopy. Acc Chem Res. 2015;48(2):457–465. doi: 10.1021/ar500340a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brindle KM. Imaging Metabolism with Hyperpolarized C-13-Labeled Cell Substrates. Journal of the American Chemical Society. 2015;137(20):6418–6427. doi: 10.1021/jacs.5b03300. [DOI] [PubMed] [Google Scholar]
- 15.Carvalho RA, Rodrigues TB, Zhao PY, Jeffrey FMH, Malloy CR, Sherry AD. A C-13 isotopomer kinetic analysis of cardiac metabolism: influence of altered cytosolic redox and [Ca2+](o) American Journal of Physiology-Heart and Circulatory Physiology. 2004;287(2):H889–H895. doi: 10.1152/ajpheart.00976.2003. [DOI] [PubMed] [Google Scholar]
- 16.Fan TWM, Higashi RM, Lane AN, Jardetzky O. Combined use of proton NMR and gas chromatography-mass spectra for metabolite monitoring and in vivo proton NMR assignments. Biochimica et Biophysica Acta. 1986;882(2):154–167. doi: 10.1016/0304-4165(86)90150-9. [DOI] [PubMed] [Google Scholar]
- 17.Evanochko WT, Ng TC, Glickson JD. Application of in vivo NMR spectroscopy to cancer. Magnetic resonance in medicine. 1984;1:508–534. doi: 10.1002/mrm.1910010410. [DOI] [PubMed] [Google Scholar]
- 18.Bales JR, Higham DP, Howe I, Nicholson JK, Sadler PJ. Use of high-resolution proton nuclear magnetic resonance spectroscopy for rapid multi-component analysis of urine. Clin Chem. 1984;30(3):426–432. [PubMed] [Google Scholar]
- 19.Nicholson JK, Buckingham MJ, Sadler PJ. High-Resolution H-1-Nmr Studies of Vertebrate Blood and Plasma. Biochemical Journal. 1983;211(3):605–615. doi: 10.1042/bj2110605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lane AN, Fan TW, Higashi RM. Isotopomer-based metabolomic analysis by NMR and mass spectrometry. Biophysical Tools for Biologists. 2008;84:541–588. doi: 10.1016/S0091-679X(07)84018-0. [DOI] [PubMed] [Google Scholar]
- 21.Serkova NJ, Spratlin JL, Eckhardt SG. NMR-based metabolomics: Translational application and treatment of cancer. Current Opinion in Molecular Therapeutics. 2007;9:572–585. [PubMed] [Google Scholar]
- 22.Shaykhutdinov RA, MacInnis GD, Dowlatabadi R, Weljie AM, Vogel HJ. Quantitative analysis of metabolite concentrations in human urine samples using C-13{H-1} NMR spectroscopy. Metabolomics. 2009;5(3):307–317. [Google Scholar]
- 23.Simon G, Kervarec N, Cerantola S. HRMAS NMR Analysis of Algae and Identification of Molecules of Interest via Conventional 1D and 2D NMR: Sample Preparation and Optimization of Experimental Conditions. Methods in molecular biology (Clifton, NJ) 2015;1308:191–205. doi: 10.1007/978-1-4939-2684-8_12. [DOI] [PubMed] [Google Scholar]
- 24.Whitehead TL, Ekman DR, Durhan EJ, Jensen KM, Kahl MD, Makynen EA, Villeneuve DL, Ankley GT. H-1-NMR metabolomics analysis of zebrafish (Danio rerio) exposed to the environmentally-relevant EDC 17a-ethinylestradiol (EE2) Abstracts of Papers of the American Chemical Society. 2006:231. [Google Scholar]
- 25.Whitfield PD, German AJ, Noble PJM. Metabolomics: an emerging post-genomic tool for nutrition. British Journal of Nutrition. 2004;92(4):549–555. doi: 10.1079/bjn20041243. [DOI] [PubMed] [Google Scholar]
- 26.Winnike JH, Pediaditakis P, Wolak JE, McClelland RW, Watkins PB, Macdonald JM. Stable isotope resolved metabolomics of primary human hepatocytes reveals a stressed phenotype. Metabolomics. 2012;8:34–49. [Google Scholar]
- 27.Wishart DS. Metabolomics: The principles and potential applications to transplantation. American Journal of Transplantation. 2005;5(12):2814–2820. doi: 10.1111/j.1600-6143.2005.01119.x. [DOI] [PubMed] [Google Scholar]
- 28.Beger RD, Dunn W, Schmidt MA, Gross SS, Kirwan JA, Cascante M, Brennan L, Wishart DS, Oresic M, Hankemeier T, Broadhurst DI, Hankemeier, Lane AN, Suhre K, Zanetti K, Kastenmüller G, Kaddurah-Daouk R. Metabolomics Enables Precision Medicine - “A White Paper, Community Perspective”. Metabolomics. 2016;12:149. doi: 10.1007/s11306-016-1094-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fan TW-M, Lorkiewicz P, Sellers K, Moseley HNB, Higashi RM, Lane AN. Stable isotope-resolved metabolomics and applications to drug development. Pharmacology and Therapeutics. 2012;133:366–391. doi: 10.1016/j.pharmthera.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beger RD. A Review of Applications of Metabolomics in Cancer. Metabolites. 2013;3:552–574. doi: 10.3390/metabo3030552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Higashi RM, Fan TW-M, Lorkiewicz PK, Moseley HNB, Lane AN. Stable Isotope Labeled Tracers for Metabolic Pathway Elucidation by GC-MS and FT-MS. In: Raftery D, editor. Mass Spectrometry Methods in Metabolomics. Humana Press; USA: 2014. pp. 147–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kind T, Tolstikov V, Fiehn O, Weiss RH. A comprehensive urinary metabolomic approach for identifying kidney cancer. Analytical Biochemistry. 2007;363(2):185–195. doi: 10.1016/j.ab.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 33.Wang J, Christison TT, Misuno K, Lopez L, Huhmer AF, Huang Y, Hu S. Metabolomic Profiling of Anionic Metabolites in Head and Neck Cancer Cells by Capillary Ion Chromatography with Orbitrap Mass Spectrometry. Analytical Chemistry. 2014;86(10):5116–5124. doi: 10.1021/ac500951v. [DOI] [PubMed] [Google Scholar]
- 34.Tang DQ, Zou L, Yin XX, Ong CN. HILIC-MS for metabolomics: An attractive and complementary approach to RPLC-MS. Mass Spectrometry Reviews. 2016;35(5):574–600. doi: 10.1002/mas.21445. [DOI] [PubMed] [Google Scholar]
- 35.Dwivedi P, Schultz AJ, Hill HH. Metabolic profiling of human blood by high-resolution ion mobility mass spectrometry (IM-MS) International Journal of Mass Spectrometry. 2010;298(1–3):78–90. doi: 10.1016/j.ijms.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scalbert A, Brennan L, Fiehn O, Hankemeier T, Kristal BS, van Ommen B, Pujos-Guillot E, Verheij E, Wishart D, Wopereis S. Mass-spectrometry-based metabolomics: limitations and recommendations for future progress with particular focus on nutrition research. Metabolomics. 2009;5(4):435–458. doi: 10.1007/s11306-009-0168-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lorkiewicz PK, Higashi RM, Lane AN, Fan TW-M. High information throughput analysis of nucleotides and their isotopically enriched isotopologues by direct-infusion FTICR-MS. Metabolomics. 2012;8:930–939. doi: 10.1007/s11306-011-0388-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lane AN, Arumugam S, Lorkiewicz PK, Higashi RM, Laulhe S, Nantz MH, Moseley HNB, Fan TW-M. Chemoselective detection of carbonyl compounds in metabolite mixtures by NMR. Mag Res Chem. 2015;53:337–343. doi: 10.1002/mrc.4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lane AN. NMR applications in metabolomics. In: Fan TW-M, Lane AN, Higashi RM, editors. Handbook of Metabolomics. Humana; 2012. [Google Scholar]
- 40.Dang L, White DW, Gross S, Bennett BD, Bittinger MA, Driggers EM, Fantin VR, Jang HG, Jin S, Keenan MC, Marks KM, Prins RM, Ward PS, Yen KE, Liau LM, Rabinowitz JD, Cantley LC, Thompson CB, Heiden MGV, Su SM. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature. 2009;462(7274):739–U52. doi: 10.1038/nature08617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yoo H, Antoniewicz MR, Stephanopoulos G, Kelleher JK. Quantifying reductive carboxylation flux of glutamine to lipid in a brown adipocyte cell line. Journal of Biological Chemistry. 2008;283(30):20621–20627. doi: 10.1074/jbc.M706494200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Michelucci A, Cordes T, Ghelfi J, Pailot A, Reiling N, Goldmann O, Binz T, Wegner A, Tallam A, Rausell A, Buttini M, Linster CL, Medina E, Balling R, HK Immune-responsive gene 1 protein links metabolism to immunity by catalyzing itaconic acid production. Proc Natl Acad Sci USA. 2013;110:7820–7825. doi: 10.1073/pnas.1218599110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clasquin MF, Melamud E, Singer A, Gooding JR, Xu X, Dong A, Cui H, Campagna SR, Savchenko A, Yakunin AF, Rabinowitz JD, Caudy AM. Riboneogenesis in Yeast. Cell. 2011;145:969–980. doi: 10.1016/j.cell.2011.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Le A, Lane AN, Hamaker M, Bose S, Barbi J, Tsukamoto T, Rojas CJ, Slusher BS, Zhang H, Zimmerman LJ, Liebler DC, Slebos RJC, Lorkiewicz PK, Higashi RM, Fan TW-M, Dang CV. Myc induction of hypoxic glutamine metabolism and a glucose-independent TCA cycle in human B lymphocytes. Cell Metabolism. 2012;15:110–121. doi: 10.1016/j.cmet.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Serres S, Bezancon E, Franconi JM, Merle M. Brain pyruvate recycling and peripheral metabolism: an NMR analysis ex vivo of acetate and glucose metabolism in the rat. Journal of Neurochemistry. 2007;101(5):1428–1440. doi: 10.1111/j.1471-4159.2006.04442.x. [DOI] [PubMed] [Google Scholar]
- 46.Patel AB, de Graaf RA, Mason GF, Rothman DL, Shulman RG, Behar KL. The contribution of GABA to glutamate/glutamine cycling and energy metabolism in the rat cortex in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(15):5588–5593. doi: 10.1073/pnas.0501703102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Holden HM, Rayment I, Thoden JB. Structure and Function of Enzymes of the Leloir Pathway for Galactose Metabolism. BJ Biol Chem. 2003;278:43885–43888. doi: 10.1074/jbc.R300025200. [DOI] [PubMed] [Google Scholar]
- 48.Frey PA. The Leloir pathway: a mechanistic imperative for three enzymes to change the stereochemical configuration of a single carbon in galactose. FASEB J. 1996;10:461–470. [PubMed] [Google Scholar]
- 49.Newsholme EA, Crabtree B, Ardawij MSM. Glutamine metabolism in lymphocytes:Its biochemical, physiological and clinical importance. Quarterly Journal of Experimental Physiology. 1985;70:473–489. doi: 10.1113/expphysiol.1985.sp002935. [DOI] [PubMed] [Google Scholar]
- 50.Brennan L, Corless M, Hewage C, Malthouse JPG, McClenaghan NH, Flatt PR, Newsholme P. C-13 NMR analysis reveals a link between L-glutamine metabolism, D-glucose metabolism and gamma-glutamyl cycle activity in a clonal pancreatic beta-cell line. Diabetologia. 2003;46(11):1512–1521. doi: 10.1007/s00125-003-1184-7. [DOI] [PubMed] [Google Scholar]
- 51.Yuneva M, Zamboni N, Oefner P, Sachidanandam R, Lazebnik Y. Deficiency in glutamine but not glucose induces MYC-dependent apoptosis in human cells. Journal of Cell Biology. 2007;178(1):93–105. doi: 10.1083/jcb.200703099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.DeBerardinis RJ, Cheng T. Q’s next: the diverse functions of glutamine in metabolism, cell biology and cancer. Oncogene. 2010;29(3):313–324. doi: 10.1038/onc.2009.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sellers K, Fox MP, Bousamra M, Slone S, Higashi RM, Miller DM, Wang Y, Yan J, Yuneva MO, Deshpande R, Lane AN, Fan TW-M. Pyruvate carboxylase is critical for non-small-cell lung cancer proliferation. J Clin Invest. 2015;125(2):687–698. doi: 10.1172/JCI72873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boros LG, Lerner MR, Morgan DL, Taylor SL, Smith BJ, Postier RG, Brackett DJ. [1,2-13C2]-D-glucose profiles of the serum, liver, pancreas, and DMBA-induced pancreatic tumors of rats. Pancreas. 2005;31(4):337–43. doi: 10.1097/01.mpa.0000186524.53253.fb. [DOI] [PubMed] [Google Scholar]
- 55.Lee W-NP, Boros LG, Puigjaner J, Bassilian S, Lim S, Cascante M. Mass isotopomer study of the nonoxidative pathways of the pentose cycle with [1,2-13C2]glucose. Am J Physiol Endocrinol Metab. 1998;274(5):E843–851. doi: 10.1152/ajpendo.1998.274.5.E843. [DOI] [PubMed] [Google Scholar]
- 56.Cheng T, Sudderth J, Yang C, Mullen AR, Jin ES, Mates JM, DeBerardinis RJ. Pyruvate carboxylase is required for glutamine-independent growth of tumor cells. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(21):8674–8679. doi: 10.1073/pnas.1016627108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Crown SB, Ahn WS, Antoniewicz MR. Rational design of (1)(3)C-labeling experiments for metabolic flux analysis in mammalian cells. BMC Syst Biol. 2012;6:43. doi: 10.1186/1752-0509-6-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mullen AR, Wheaton WW, Jin ES, Chen PH, Sullivan LB, Cheng T, Yang Y, Linehan WM, Chandel NS, DeBerardinis RJ. Reductive carboxylation supports growth in tumour cells with defective mitochondria. Nature. 2012;481(7381):385–8. doi: 10.1038/nature10642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xie H, Hanai J, Ren J-G, Kats L, Burgess K, Bhargava P, Signoretti S, Billiard J, Duffy KJ, Grant A, Wang X, Lorkiewicz PK, Schatzman S, Bousamra M, II, Lane AN, Higashi RM, Fan TW-M, Pandolfi PPP, Sukhatme VP, Seth P. Targeting lactate dehydrogenase-A (LDH-A) inhibits tumorigenesis and tumor progression in mouse models of lung cancer and impacts tumor initiating cells. Cell Metabolism. 2014;19:795–809. doi: 10.1016/j.cmet.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kasumov T, Adams JE, Bian F, David F, Thomas KR, Jobbins KA, Minkler PE, Hoppel CL, Brunengraber H. Probing peroxisomal beta-oxidation and the labelling of acetyl-CoA proxies with [1-(13C)]octanoate and [3-(13C)]octanoate in the perfused rat liver. Biochem J. 2005;389(Pt 2):397–401. doi: 10.1042/BJ20050144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cowin GJ, Willgoss DA, Bartley J, Endre ZH. Serine isotopmer analysis by 13C-NMR defines glycine-serine interconversion in situ in the renal proximal tubule. Biochim Biophys Acta. 1996;1310(1):32–40. doi: 10.1016/0167-4889(95)00142-5. [DOI] [PubMed] [Google Scholar]
- 62.Qi J, Lang W, Geisler JG, Wang P, Petrounia I, Mai S, Smith C, Askari H, Struble GT, Williams R, Bhanot S, Monia BP, Bayoumy S, Grant E, Caldwell GW, Todd MJ, Liang Y, Gaul MD, Demarest KT, Connelly MA. The use of stable isotope-labeled glycerol and oleic acid to differentiate the hepatic functions of DGAT1 and -2. J Lipid Res. 2012;53(6):1106–16. doi: 10.1194/jlr.M020156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hu S, Yoshihara HAI, Bok R, Zhou J, Zhu MH, Kurhanewicz J, Vigneron DB. Use of hyperpolarized 1-C-13 pyruvate and 2-C-13 pyruvate to probe the effects of the anticancer agent dichloroacetate on mitochondrial metabolism in vivo in the normal rat. Magnetic Resonance Imaging. 2012;30(10):1367–1372. doi: 10.1016/j.mri.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rodrigues TB, Serrao EM, Kennedy BWC, Hu DE, Kettunen MI, Brindle KM. Magnetic resonance imaging of tumor glycolysis using hyperpolarized C-13-labeled glucose. Nature Medicine. 2014;20(1):93-+. doi: 10.1038/nm.3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fan TW, Lane AN. Structure-based profiling of Metabolites and Isotopomers by NMR. Progress in NMR Spectroscopy. 2008;52:69–117. [Google Scholar]
- 66.Rose IA. The Use of Kinetic Isotope Effects in the Study of Metabolic Control. J Biol Chem. 1961;236:603–609. [PubMed] [Google Scholar]
- 67.Higashi RM. Structural Mass Spectrometry for Metabolomics. In: Fan TW, Higashi RM, Lane AN, editors. Handbook of Metabolomics Methods. Humana Press; New York: 2011. [Google Scholar]
- 68.Boros LG. Metabolic targeted therapy of cancer: current tracer technologies and future drug design strategies in the old metabolic network. Metabolomics. 2005;1(1):11–15. [Google Scholar]
- 69.Metallo CM, Gameiro PA, Bell EL, Mattaini KR, Yang J, Hiller K, Jewell CM, Johnson ZR, Irvine DJ, Guarente L, Kelleher JK, Vander Heiden MGV, Iliopoulos O, Stephanopoulos G. Reductive glutamine metabolism by IDH1 mediates lipogenesis under hypoxia. Nature. 2011;481:380–384. doi: 10.1038/nature10602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lewis CA, Parker SJ, Fiske BP, McCloskey D, Gui DY, Green CR, Vokes NI, Feist AM, Vander Heiden MG, Metallo CM. Tracing Compartmentalized NADPH Metabolism in the Cytosol and Mitochondria of Mammalian Cells. Molecular Cell. 2014;55(2):253–263. doi: 10.1016/j.molcel.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Singer S, Okunieff P, Gostin C, Thilly WG, Chen LB, Neuringer LJ. 13C- and 31P-NMR studies of human colon cancer in-vitro and in-vivo. Surgical Oncology. 1993;2(1):7–18. doi: 10.1016/0960-7404(93)90039-2. [DOI] [PubMed] [Google Scholar]
- 72.Gadian DG. NMR and its applications to living systems. 2. Oxford, U.K: Oxford University Press; 1995. p. 283. [Google Scholar]
- 73.Fan TWM, Lane AN, Higashi RM. An electrophoretic profiling method for thiol-rich phytochelatins and metallothioneins. Phytochemical Analysis. 2004;15(3):175–183. doi: 10.1002/pca.765. [DOI] [PubMed] [Google Scholar]
- 74.Burgess SC, Babcock EE, Jeffrey FM, Sherry AD, Malloy CR. NMR indirect detection of glutamate to measure citric acid cycle flux in the isolated perfused mouse heart. FEBS Lett. 2001;505(1):163–7. doi: 10.1016/s0014-5793(01)02799-5. [DOI] [PubMed] [Google Scholar]
- 75.Lane AN, Fan TW-M. Regulation of mammalian nucleotide metabolism and biosynthesis. Nucleic Acids Res. 2015;43:2466–2485. doi: 10.1093/nar/gkv047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lane AN, Fan TW. Quantification and identification of isotopomer distributions of metabolites in crude cell extracts using 1H TOCSY. Metabolomics. 2007;3:79–86. [Google Scholar]
- 77.Fan TW-M, Kucia M, Jankowski K, Higashi RM, Rataczjak MZ, Rataczjak J, Lane AN. Proliferating Rhabdomyosarcoma cells shows an energy producing anabolic metabolic phenotype compared with Primary Myocytes. Molecular Cancer. 2008;7:79. doi: 10.1186/1476-4598-7-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gradwell MJ, Fan TWM, Lane AN. Analysis of phosphorylated metabolites in crayfish extracts by two-dimensional H-1-P-31 NMR heteronuclear total correlation spectroscopy (heteroTOCSY) Analytical Biochemistry. 1998;263(2):139–149. doi: 10.1006/abio.1998.2789. [DOI] [PubMed] [Google Scholar]
- 79.Tayyari F, Gowda G, Gu H, Raftery D. 15N-cholamine--a smart isotope tag for combining NMR- and MS-based metabolite profiling. Anal Chem. 2013;85:8715–8721. doi: 10.1021/ac401712a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gori SS, Lorkiewicz PK, Ehringer DS, Belshoff AC, Higashi RM, Fan TW-M, Nantz MH. Profiling Thiol Metabolites and Quantification of Cellular Glutathione using FT-ICR-MS Spectrometry. Analytical & Bioanalytical Chemistry. 2014;406:4371–4379. doi: 10.1007/s00216-014-7810-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lewis IA, Shortreed MR, Hegeman AD, Markley JL. Novel NMR and MS Approaches to Metabolomics. In: Fan TW-M, Lane AN, Higashi RM, editors. The Handbook of Metabolomics. Humana; New York: 2012. pp. 199–230. [Google Scholar]
- 82.Fan TW-M, Tan JL, McKinney MM, Lane AN. Stable Isotope Resolved Metabolomics Analysis of Ribonucleotide and RNA Metabolism in Human Lung Cancer Cells. Metabolomics. 2012;8:517–527. doi: 10.1007/s11306-011-0337-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yang L, Kombu RS, Kasumov T, Zhu S-H, Cendrowski AV, David F, Anderson VE, Kelleher JK, Brunengraber H. Metabolomic and mass isotopomer analysis of liver gluconeogenesis and citric acid cycle - I. Interrelation between gluconeogenesis and cataplerosis; Formation of methoxamates from aminooxyacetate and ketoacids. Journal of Biological Chemistry. 2008;283(32):21978–21987. doi: 10.1074/jbc.M803454200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yuneva MO, Fan TW-M, Allen TD, Higashi RM, Ferraris DV, Tsukamoto T, Matés JM, Alonso FJ, Wang C, Seo Y, Chen X, Bishop JM. The Metabolic Profile of Tumors Depends on Both the Responsible Genetic Lesion and Tissue Type. Cell Metabolism. 2012;15:157–170. doi: 10.1016/j.cmet.2011.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ippel J, Wijmenga S, de Jong B, Heus H, Hilbers C, Vroom E, van de Marel C, van Boom J. Heteronuclear scalar couplings in the bases and sugar rings of nucleic acids: their determination and application in assignment and conformational analysis. Magnetic Resonance in Chemistry. 1996;34:S156–S176. [Google Scholar]
- 86.Christensen CE, Karlsson M, Winther JR, Jensen PR, Lerche MH. Non-invasive In-cell Determination of Free Cytosolic NAD(+)/NADH Ratios Using Hyperpolarized Glucose Show Large Variations in Metabolic Phenotypes. Journal of Biological Chemistry. 2014;289(4):2344–2352. doi: 10.1074/jbc.M113.498626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fan J, Ye JB, Kamphorst JJ, Shlomi T, Thompson CB, Rabinowitz JD. Quantitative flux analysis reveals folate-dependent NADPH production. Nature. 2014;510(7504):298-+. doi: 10.1038/nature13236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jeon S-M, Chandel NS, Hay N. AMPK regulates NADPH homeostasis to promote tumour cell survival during energy stress. Nature. 2012;485(7400):661-+. doi: 10.1038/nature11066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang J, ten Pierick A, van Rossum HM, Seifar RM, Ras C, Daran J-M, Heijnen JJ, Wahl SA. Determination of the Cytosolic NADPH/NADP Ratio in Saccharomyces cerevisiae using Shikimate Dehydrogenase as Sensor Reaction. Scientific Reports. 2015;5:12846. doi: 10.1038/srep12846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fan TW-M, Lane AN. Assignment strategies for NMR resonances in metabolomics research. In: Lutz N, Sweedler JV, Weevers RA, editors. Methodologies for Metabolomics: Experimental Strategies and Techniques. Cambridge University Press; Cambridge: 2012. [Google Scholar]
- 91.Witney TH, Kettunen MI, Brindle KM. Kinetic Modeling of Hyperpolarized C-13 Label Exchange between Pyruvate and Lactate in Tumor Cells. Journal of Biological Chemistry. 2011;286(28):24572–24580. doi: 10.1074/jbc.M111.237727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zewe V, Fromm HJ. Kinetic Studies of Rabbit Muscle Lactate Dehydrogenase. H Biol Chem. 1962;237:1668–1675. [PubMed] [Google Scholar]
- 93.Gowda GAN, Raftery D. Quantitating metabolites in protein precipitated serum using NMR spectroscopy. Anal Chem. 2014;86:5433–5440. doi: 10.1021/ac5005103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jupin M, Michiels PJ, Girard FC, Spraul M, Wijmenga SS. NMR metabolomics profiling of blood plasma mimics shows that medium- and long-chain fatty acids differently release metabolites from human serum albumin. J Magn Reson. 2014;239:34–43. doi: 10.1016/j.jmr.2013.11.019. [DOI] [PubMed] [Google Scholar]
- 95.Jupin M, Michiels PJ, Girard FC, Spraul M, Wijmenga SS. NMR identification of endogenous metabolites interacting with fatted and nonfatted human serum albumin in blood plasma: fatty acids influence the HSAmetabolite interaction. J Magn Reson. 2014;228:81–94. doi: 10.1016/j.jmr.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 96.Zewe V, Fromm HJ. Kinetic Studies of Rabbit Muscle Lactate Dehydrogenase. J Biol Chem. 1962;237:1668–1675. [PubMed] [Google Scholar]
- 97.Sun F, Dai C, Xie J, Hu X. Biochemical Issues in Estimation of Cytosolic Free NAD/NADH Ratio. PLoS ONE. 2012;7:e34525. doi: 10.1371/journal.pone.0034525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Williamson DH, Lund P, Krebs HA. The Redox State of Free Nicotinamide-Adenine Dinucleotide in the Cytoplasm and Mitochondria of Rat Liver. Biochem J. 1967;103:514–526. doi: 10.1042/bj1030514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Schwert GW. The Estimation of Kinetic Constants for the Lactate Dehydrogenase System by the Use of Integrated Rate Equations. J Biol Chem. 1969;244:1285–1290. [PubMed] [Google Scholar]
- 100.Gillies RJ, Gatenby RA. Adaptive landscapes and emergent phenotypes: why do cancers have high glycolysis? Journal of Bioenergetics and Biomembranes. 2007;39(3):251–257. doi: 10.1007/s10863-007-9085-y. [DOI] [PubMed] [Google Scholar]
- 101.Roberts JKM, Wadejardetzky N, Jardetzky O. Intracellular Ph Measurements by P-31 Nuclear Magnetic-Resonance Influence of Factors Other Than Ph on P-31 Chemical-Shifts. Biochemistry. 1981;20(19):5389–5394. doi: 10.1021/bi00522a006. [DOI] [PubMed] [Google Scholar]
- 102.Gallagher FA, Kettunen MI, Brindle KM. Imaging pH with hyperpolarized C-13. Nmr in Biomedicine. 2011;24(8):1006–1015. doi: 10.1002/nbm.1742. [DOI] [PubMed] [Google Scholar]
- 103.Ishikawa-Ankerhold HC, Ankerhold R, Drummen GPC. Advanced Fluorescence Microscopy Techniques—FRAP, FLIP, FLAP, FRET and FLIM. Molecules. 2012;17:4047–4132. doi: 10.3390/molecules17044047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nikolaou P, Goodson BM, Chekmenev EY. NMR Hyperpolarization Techniques for Biomedicine. Chemistry-a European Journal. 2015;21(8):3156–3166. doi: 10.1002/chem.201405253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Clatworthy MR, Kettunen MI, Hu DE, Mathews RJ, Witney TH, Kennedy BWC, Bohndiek SE, Gallagher FA, Jarvis LB, Smith KGC, Brindle KM. Magnetic resonance imaging with hyperpolarized 1,4-C-13(2) fumarate allows detection of early renal acute tubular necrosis. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(33):13374–13379. doi: 10.1073/pnas.1205539109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nelson SJ, Kurhanewicz J, Vigneron DB, Larson PEZ, Harzstark AL, Ferrone M, van Criekinge M, Chang JW, Bok R, Park I, Reed G, Carvajal L, Small EJ, Munster P, Weinberg VK, Ardenkjaer-Larsen JH, Chen AP, Hurd RE, Odegardstuen L-I, Robb FJ, Tropp J, Murray JA. Metabolic Imaging of Patients with Prostate Cancer Using Hyperpolarized 1-C-13 Pyruvate. Science Translational Medicine. 2013;5(198) doi: 10.1126/scitranslmed.3006070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lerche MH, Jensen PR, Karlsson M, Meier S. NMR Insights into the Inner Workings of Living Cells. Analytical Chemistry. 2015;87(1):119–132. doi: 10.1021/ac501467x. [DOI] [PubMed] [Google Scholar]
- 108.Shchepin RV, Coffey AM, Waddell KW, Chekmenev EY. PASADENA Hyperpolarized C-13 Phospholactate. Journal of the American Chemical Society. 2012;134(9):3957–3960. doi: 10.1021/ja210639c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gallagher FA, Kettunen MI, Hu DE, Jensen PR, in’t Zandt R, Karlsson M, Gisselsson A, Nelson SK, Witney TH, Bohndiek SE, Hansson G, Peitersen T, Lerche MH, Brindle KM. Production of hyperpolarized 1,4-C-13(2) malate from 1,4-C-13(2) fumarate is a marker of cell necrosis and treatment response in tumors. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(47):19801–19806. doi: 10.1073/pnas.0911447106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Reile I, Eshuis N, Hermkens NKJ, van Weerdenburg BJA, Feiters MC, Rutjes F, Tessari M. NMR detection in biofluid extracts at sub-mu M concentrations via para-H-2 induced hyperpolarization. Analyst. 2016;141(13):4001–4005. doi: 10.1039/c6an00804f. [DOI] [PubMed] [Google Scholar]
- 111.Eshuis N, Hermkens N, van Weerdenburg BJA, Feiters MC, Rutjes F, Wijmenga SS, Tessari M. Toward Nanomolar Detection by NMR Through SABRE Hyperpolarization. Journal of the American Chemical Society. 2014;136(7):2695–2698. doi: 10.1021/ja412994k. [DOI] [PubMed] [Google Scholar]
- 112.Green RA, Adams RW, Duckett SB, Mewis RE, Williamson DC, Green GGR. The theory and practice of hyperpolarization in magnetic resonance using parahydrogen. Progress in Nuclear Magnetic Resonance Spectroscopy. 2012;67:1–48. doi: 10.1016/j.pnmrs.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 113.Bollard ME, Stanley EG, Lindon JC, Nicholson JK, Holmes E. NMR-based metabonomic approaches for evaluating physiological influences on biofluid composition. Nmr in Biomedicine. 2005;18(3):143–162. doi: 10.1002/nbm.935. [DOI] [PubMed] [Google Scholar]
- 114.Dona AC, Jimenez B, Schaefer H, Humpfer E, Spraul M, Lewis MR, Pearce JTM, Holmes E, Lindon JC, Nicholson JK. Precision High-Throughput Proton NMR Spectroscopy of Human Urine, Serum, and Plasma for Large-Scale Metabolic Phenotyping. Analytical Chemistry. 2014;86(19):9887–9894. doi: 10.1021/ac5025039. [DOI] [PubMed] [Google Scholar]
- 115.Solinas A, Chessa M, Culeddu N, Porcu MC, Virgilio G, Arcadu F, Deplano A, Cossu S, Scanu D, Migaleddu V. High resolution-magic angle spinning (HR-MAS) NMR-based metabolomic fingerprinting of early and recurrent hepatocellular carcinoma. Metabolomics. 2014;10(4):616–626. [Google Scholar]
- 116.Pudakalakatti SM, Dubey A, Jaipuria G, Shubhashree U, Adiga SK, Moskau D, Atreya HS. A fast NMR method for resonance assignments: application to metabolomics. Journal of Biomolecular Nmr. 2014;58(3):165–173. doi: 10.1007/s10858-014-9814-6. [DOI] [PubMed] [Google Scholar]
- 117.Kupce E. NMR with multiple receivers. Top Curr Chem. 2013;335:71–96. doi: 10.1007/128_2011_226. [DOI] [PubMed] [Google Scholar]
- 118.Le Guennec A, Giraudeau P, Caldarelli S. Evaluation of Fast 2D NMR for Metabolomics. Analytical Chemistry. 2014;86(12):5946–5954. doi: 10.1021/ac500966e. [DOI] [PubMed] [Google Scholar]
- 119.Akoka S, Giraudeau P. Fast hybrid multi-dimensional NMR methods based on ultrafast 2D NMR. Magn Reson Chem. 2015;53:986–994. doi: 10.1002/mrc.4237. [DOI] [PubMed] [Google Scholar]