Abstract
Introduction
Advances in the development of long acting antiretroviral therapy (ART) can revolutionize current treatments for HIV/AIDS. We have coined the term long active slow effective release ART (LASER ART) based on properties of slow drug dissolution, poor water-solubility, excellent bioavailability, limited off target systemic toxicities, and excellent patient treatment adherence. Drug carrier technologies characterized by high payload of antiretroviral drugs (ARVs) in a single carrier are being developed to improve the pharmacokinetics and pharmacodynamics of the nanoformulated ART (nanoART). Additionally, surface modification of slow release antiretroviral carriers with targeting ligands has facilitated receptor-mediated transport across physiological barriers serves to improve therapeutic outcomes.
Areas covered
This review highlights current developments of reservoir targeted LASER ART delivery platforms that have the potential to improve HIV/AIDS therapeutic outcomes. Such nanoART delivery platforms include decorated multifunctional nano- and micro- particles, prodrugs and polymer conjugates. Therapeutic strategies such as anti-inflammatory and neuroprotective agents and CRISPR/Cas 9 based gene-editing technologies that affect drug depots, boost ART effectiveness and facilitate viral clearance are discussed.
Expert opinion
The persistence of HIV-1 in its lymphoid, gut and nervous system reservoirs poses a major challenge to viral eradication. Emerging innovative strategies for effective medicines and slow release products to target intracellular pathogens, immune based interventions, genome-editing technologies, compounds that sustain drug depots and combinations of nanoART and image contrast agents have the potential to meet the unmet clinical needs of HIV patients. Such efforts will bring the medicines to sites of active viral replication and accelerate viral clearance.
Keywords: Antiretroviral therapy, viral reservoirs, long acting slow effective release, nanoformulated ART, targeted drug delivery, HIV-1 proviral excision, anti-inflammatory activities, neuroprotection, theranostics, prodrugs
1. Introduction
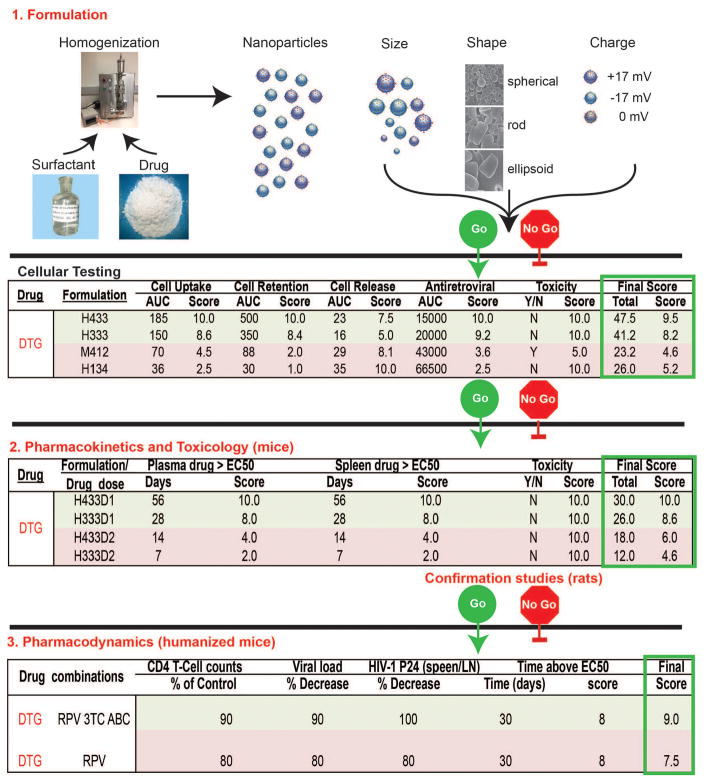
Human immunodeficiency virus-1 (HIV-1) infects then depletes CD4+ T lymphocytes resulting in immunodeficiency and host susceptibility to a range of opportunistic infections and malignancies [1, 2, 3]. While antiretroviral therapy (ART) has reduced disease morbidity and mortality it has thus far failed to clear virus from anatomical reservoir sites. Such sites include the central nervous system, gut-associated amongst other lymphoid tissues (gut-associated lymphoid tissue (GALT), lymph nodes and spleen). Thus achieving virologic cure through antiretroviral drugs (ARVs) currently remains a formidable and perhaps an improbable obstacle to overcome [4, 5, 6]. This is further complicated by limited access to ART seen commonly in resource-limited countries, adverse drug side effects, viral mutation rates that have led to emergence of resistant viral strains and poor patient compliance to lifelong therapy due to psychological, physical and drug addictions [7, 8, 9, 10]. Thus, the need for the development of reservoir targeted long acting slow effective release (LASER) ART delivery platforms cannot be overstated. Parallel efforts in academic laboratories and the pharmaceutical industry are directed towards developing personalized drug delivery systems that can selectively target body areas where virus hides in a latent, restricted or productive state. The inevitable outcome is to maximize the therapeutic index, improve ARV compliance and maximize viral control [11, 12, 13]. The question, though, is how best to achieve such a goal. This has led, in measure, to a reassessment of drug development. This can occur by modification of existing compounds to affect improved drug encapsulation into delivery systems or to re-evaluate drugs that were overlooked during prior evaluations. Indeed the latter compounds could be brought forward from new drug discovery efforts that have not moved ahead in development due to their poor physicochemical properties such as limited oral absorption, low dissolution at physiological pH and rapid clearance [14, 15, 16]. To overcome these delivery barriers, drug delivery designs and dosage forms, delivery routes, nano and micro carrier systems such as liposomes, lipid nano-constructs, polymeric nanoparticles, dendrimers, and nanocapsules may be used to facilitate carriage of drugs and affect their biodistribution and half-life. Indeed, for example, nanomedicine-based drug delivery systems are being developed with promising profiles that enable improved targeting and imaging capacities and specifically target HIV reservoirs. Our own laboratories have developed go / no go criteria in an ambitious plan to develop a library of known approved ARVs as long acting medicines. A description of product synthesis, quality control, laboratory and animal testing towards clinical implementation is outlined in the accompanying Figure 1. The development of ARV nanoparticles begins with assigning designations. Here all of the formulations are designated with specific letters and numbers that outline each step of formulation production. This includes whether the particles are homogenized or milled; the particle size (1 < 200 nm; 2 200–300 nm; 3 300–400 nm; and 4 >400 nm); their shape (1 cuboid; 2 box; 3 rod) and the excipients employed for each of the crystal hydrophobic ARV encasements. The nanoformulations produced are evaluated, in kind, within human macrophage cultures before being tested in rodent pharmacokinetic (PK) tests. Nanoformulations with “best” scores based on the criteria of drug particle macrophage entry, cell retention and release, antiretroviral activities and cell vitality are subsequently selected for animal investigations. PK measurements are rank scored by criteria that include plasma and tissue drug concentrations. The acceptability scores include drug plasma levels of the half maximal inhibitory drug concentration (IC50) scored as a measure of effectiveness in inhibiting viral growth as. measured over weeks of testing. These tests included toxicological evaluation done, in parallel, by hematologic, renal, hepatic, metabolic and histological tests. Pharmacodynamic studies conducted using drug combinations in humanized mice are then used to determine plasma viral load and intracellular drug reservoirs for the chosen formulations as uncovered by the PK tests. These are our go no-go criteria. Taken together, the current review is more extensive that what is ongoing in our own laboratory and highlights “all drug delivery platforms” that have already shown or have the potential to improve therapeutic outcomes for HIV/AIDS not and in future years.
Figure 1.
Go no-go criteria for the development of dolutegravir (DTG) LASER ART. While DTG is provided as an example it represents one of many ARVs being developed into long acting formulations. Using high-pressure homogenization (H) or wet milling (M) ARVs are packaged into particles with surfactant and ligand coatings designed to target circulating mononuclear phagocytes (MP; dendritic cells, monocytes and macrophages). The size, shape, charge and surfactant coating of the particles facilitate optimal MP uptake into subcellular autophagosomes (numbers under formulation are the poloxamer, particle shape and size). MPs serve as drug depots and scavenge particles serving as delivery vehicles. Laboratory cell-based tests are used to measure MP particle uptake, retention, and release of nanoART and determine antiretroviral activity (defined by area under the curve, AUC, for each activity over time) and cytotoxicity. A ranked scoring system for the made formulations determines those best suited to be moved forward for PK testing in mice and rats at two doses (D1 and 2). Select formulations are used for pharmacodynamic tests to demonstrate extended antiretroviral efficacy in humanized rodent models of HIV disease. Final therapeutic scores and consideration for human use are dependent upon toxicity measures, dosing, and end organ toxicities that include no adverse hematologic, renal, hepatic, immune or other systemic events. Pharmacodynamics screens are used to assess clearance of virus from its tissue reservoirs by ultrasensitive viral RNA and DNA detection systems. A range of drug tissue distribution, viral, pharmacologic and immune tests with end organ histology evaluations are performed to determine drug efficacy.
2. Nanoparticle mediated ART delivery
Delivery of therapeutic agents using solid lipid nanoparticles has received considerable interest in recent years. This is owing to the abilities to introduce targeting ligands, improve biocompatibility, limit drug degradation and reduce systemic toxicity. Solid lipid nanoparticles comprise biocompatible lipids stabilized by emulsifiers. The use of lipid nanoparticles to deliver multiple therapeutic agents has drawn increased interest in recent years. Packaging multiple ARVs into one particle is important for targeting different stages of viral replication and reducing the risk of viral resistance. In addition to facilitating residual viral clearance, co-delivery of multiple antiretroviral drugs could potentially enhance patient adherence. Most recently, multi-drug lipid nanoparticles encapsulating the ARVs lopinavir (LPV), ritonavir (RTV) and tenofovir (PMPA) were developed [17]. The lipid ARV nanoparticles were administered subcutaneously to rhesus macaques at drug doses of 25, 14.3 and 17.1 mg/kg, respectively, and compared to native drug treatments. Primates dosed with lipid nanoparticles exhibited extended and higher drug concentrations in plasma and tissues. The three-drug lipid nanoparticle combinations increased intracellular drug concentrations of LPV and RTV in the lymph nodes by 50 times compared to the native drug treatment group. Additionally, both plasma and intracellular drug levels in the nanoparticle treatment group were sustained for 7 days, as opposed to administration of native drugs, which were undetectable at day 2. These findings demonstrated that multifunctional long acting ARV nanoparticles holds promise to overcome inherent compliance limitations of the current drug regimens.
3. Theranostics and nanoART
Despite significant improvements in ART regimens a major obstacle for bench to bedside translation of nanoparticle delivery systems rests in an assessment of drug depots in relationship to viral reservoirs. In recent years, magnetic resonance imaging (MRI) has been extended to the development of contrast agents to enable in vivo tracking of drug carrying nanoparticles. Our laboratory first developed small magnetite antiretroviral therapy (SMART) [18]. SMART is defined by the use of alendronate polyethylene glycol superparamagnetic iron oxide nanoparticles (ALN-PEG SPIO) and used for screening of targeted nanoformulations. The particles were used to assess tissue biodistribution of ARVs using conventional MRI tests. The surface of the magnetite particles was coated with ALN-PEG to generate stable and tunable particles capable of carrying more than one targeting ligand. Folic acid (FA) was conjugated onto the ALN particles to test whether the particles could reflect FA-targeted nanoART biodistribution. The particles were administered intramuscularly into mice, where FA coating was shown to enhance nanoparticle uptake and retention. While these SMART particles do reflect drug tissue distribution they have thus far not enabled precise drug distributions to viral reservoirs. Such reservoirs include the lymph nodes, GALT and brain. One additional limitation of this system is related to the low sensitivity of the SMART particle system. To overcome these limitations, we recently explored combined fluorescence and magnetic properties of europium to generate MRI sensitive cobalt ferrite europium core shell silica magnetic nanoparticles [19]. The resultant monodispersed CFEus formed crystals with a unique inverse-spinel structure and particle sizes equivalent to conventional nanoART. Importantly, the transverse relaxivity of the Si-CFEu particles were increased up to a log when compared to SMART particles. Ligand decorated Si-CFEu particles hold considerable promise for rapid assessment of nanoparticle drug biodistribution and efficiency for improving drug distribution strategies to HIV reservoirs
4. Sustained-release ARV formulations
A major obstacle to the goal of HIV eradication lies in targeting latently infected cells from a spectrum of tissue sites. While several therapeutic interventions are being developed with the goal of targeting reservoirs that harbor latent virus none have delivered enough cargo at long enough intervals with optimal efficacy to exert viral clearance [20, 21]. The directives include a broad number of direct and indirect measures to affect the viral replication cycle. The ultimate end point is designed to eliminate infected resting CD4+ T lymphocytes [22, 23]. In this manner, a number of drugs were tested that would affect viral clearance and potential elimination. For example, CD4+ T cell-activating agents were investigated but proven unsuccessful in eliminating virus and preventing its rebound as measured at two or three weeks affect ARV cessation. Ongoing research investigations are focused towards generating slow release products that combine latency-breaking agents and antiretroviral drugs in the same carrier. In a recent study, tenofovir was co-encapsulated with the HIV-1 latency reversing agent vorinostat into magnetically guided layer-by-layer nanoparticles to allow for simultaneous activation and clearance of the virus [24]. The ultrasmall magnetic nanoparticles were synthesized and characterized for physicochemical properties, cytotoxicity, intracellular uptake, release and antiviral responses using astrocytes and brain microvascular endothelial cells. In addition, the effects of an external magnetic field on the transmigration ability and blood brain barrier (BBB) integrity were investigated. Tenofovir-vorinostat nanoparticles demonstrated antiretroviral efficacy over a period of five days after infection, an indication that the external field facilitated intracellular accumulation of nanoparticles.
Delivery of ARV to the central nervous system (CNS) is critical for effective treatment of HIV/AIDS patients but always challenging due to the limited permeability of the BBB [25]. Recent years have seen advancements in the development of promising nanotechnologies for delivery of antiretroviral drugs to the CNS [26]. Notably, amphiphilic polymer coated iron oxide nanoparticles synthesized by solvothermal methods and functionalized with the antiretroviral peptide enfuvirtide were shown to facilitate drug carriage across the BBB [27]. The mechanism of nanoparticle transport across the endothelial barrier was investigated using both in vitro and in vivo models demonstrating intracellular distribution of enfuvirtide-modified iron oxide nanoparticles. Comparisons were made against free peptide or non-modified iron oxide particle controls using confocal microscopy methods. The results showed that coating the iron oxide nanoparticles with enfuvirtide modified the amphilic polymer and markedly improved endothelial cell permeation of the drug. The data showed up to 170% increases in permeation. Similarly, increased fluorescence intensity was observed in brains of mice treated with nanoformulated enfuvirtide iron oxide compared to free peptide or non-modified nanoparticle treatment groups. The data, all together, showed peptide penetration of the modified nanoparticles across endothelial cells with strong interactions between the polymer coating and cell membrane. This was followed by internalization and dissociation of the nanocomplex in the brain parenchyma. These encouraging findings suggest that amphiphilic polymer iron oxide nanoconstructs could potentially enhance CNS drug delivery.
Nanosuspensions are aqueous suspensions containing poorly water-soluble drug crystals and stabilizers [15, 20, 28]. One or several excipients and appropriate buffers are used to prevent crystal growth or particle aggregation. Particle size reduction can be achieved through high-pressure homogenization, wet milling or precipitation to generate nanoparticles in the range of 50 to 1000 nm. Nanoparticles offer numerous advantages that include high drug loading capacity, controllable size, charge and tunable surfaces for targeting ligand conjugation and can be lyophilized for long term storage [28, 29, 30, 31]. Antiretroviral drugs could be nanoformulated to extend the drug half-life and for various routes of administration. Cabotegravir (CAB) is an integrase strand-transfer inhibitor with high potency (IC50 of 0.22 nM), low aqueous solubility and long half-life [32]. CAB-LAP is a long-acting injectable nanosuspension with a half-life ranging from 21 to 50 days after intramuscular (IM) administration and is currently under clinical evaluation [33]. In a study by Andrews et al., pharmacokinetics and efficacy of CAB-LAP for pre-exposure prophylaxis against repeat high-dose intravaginal SHIV challenge was evaluated in female rhesus macaques [34]. The study was conducted with eight female rhesus macaques that were exposed to CAB-LAP at week 0 and four positive controls. The animals were then chronically infected with SHIV162P3 at week 1 and monitored for viral loads. CAB-LAP was found to protect the animals from infection, whereas viremia was detected at 2 weeks after SHIV challenge in all control animals. The animals in the experimental treatment arm were given a boost of CAB-LAP at week 4 and further challenged with the virus at weeks 5 and 7. Interestingly, six out of eight animals in the CAB-LAP arm were protected against three high-dose SHIV challenges, whereas all control animals showed infection after a single challenge. These encouraging results highlight the potential of long acting injectable nanosuspensions in the management of HIV.
The use of antiretroviral drugs for pre-exposure prophylaxis (PrEP) in high-risk populations has been shown to be highly effective at preventing HIV transmission [35, 36, 37, 38]. Current studies evaluated long acting injectable medicines for PrEP that included raltegravir nanosuspensions prepared by milling and subsequent reconstitution in polyethylene glycol, polysorbate 80 and mannitol. Pharmacokinetic and biodistribution profiles of the drug were evaluated by replicate formulations administered to BALB/c, NSG (NOD–scid–gamma) and humanized bone marrow–liver–thymus (BLT) mice and also in rhesus macaques [39]. Furthermore, in vivo assays were used to assess the ability of the nanoformulated drug to protect humanized mice against vaginal virus transmission and during acute infection. Plasma drug concentrations at two weeks after a single injection of nanoformulated raltegravir at doses of 7.5 mg and 160 mg to BLT mice and rhesus macaques, respectively, were equivalent to a twice-daily oral dose of 400 mg in humans. Effective suppression of viral plasma RNA was achieved with a single dose of nanoformulated raltegravir. A single subcutaneous dose of nanoformulated raltegravir protected BLT mice from HIV infection following two high-dose vaginal challenges with virus at one week and four weeks post administration of long acting nanoformulated raltegravir. The outcomes of this study highlight the translational potential of long acting injectable antiretroviral drug delivery systems for HIV prevention and treatment. Rilpivirine is an FDA approved non-nucleoside reverse transcriptase inhibitor (NNRTI) for use in HIV-1 infected naive patients in combination with other antiretroviral agents. A nanosuspensions of rilpivirine, TMC278 LA, has been developed and is under clinical investigation as a long acting injectable formulation [40]. Preclinical studies have demonstrated sustained therapeutic concentration of rilpivirine in plasma for up to three months in dogs, two months in rats or three weeks in mice after a single dose of TMC278 LA [40, 41].
Others assessed the pharmacokinetics, safety and tolerability profiles of TMC278 following single IM doses of 300 to 1200 mg in HIV seronegative volunteers [42]. Rilpivirine concentrations in plasma, cervicovaginal fluid, rectal fluid and tissues from female genital tract and male rectum were measured over 84 days after drug treatment. Results from this study demonstrated that higher doses of nanoformulated rilpivirine provide sustained release and enhanced fluid and tissue biodistribution of the drug for up to 84 days. Higher rilpivirine concentrations corresponded to enhanced ex vivo inhibition of the virus replication in infected cervicogenital tissues from participants. These encouraging clinical findings suggest that long acting ARVs could improve efficacy and adherence to PrEP treatments. In another study, the pharmacokinetics, safety, and tolerability profiles of CAB and TMC278 were assessed after repeated dosing of long-acting injectable formulations in healthy subjects [43]. According to the study design, the subjects received oral doses of 30 mg/day of CAB during the initial two weeks, followed by a seven-day wash out period prior to administration of nanoformulated drugs. The subjects were then divided into four treatment groups: the first group received 800 mg of CAB-LAP via IM followed by quarterly booster doses of 200 mg via SC, the second group was dosed with 800 mg CAB-LAP via IM followed by quarterly booster doses of 200 mg via IM, the third group was administered with 800 mg of CAB-LAP via IM followed by quarterly booster doses of 400 mg via IM, while the fourth group received 800 mg CAB-LAP IM followed by a booster dose of 800 mg 12 weeks later. Subjects in the second and third groups also received IM doses of TMC278 LA at the third (1200 mg) and fourth (900 or 600 mg) months. Spreen et al. noted that co-administration of CAB-LAP and TMC278 LA was well tolerated and therapeutically sustained plasma concentrations of the two drugs were achieved. Clinical trial studies that are still in progress or have been completed using CAB LAP and RPV LA are summarized in the Table.[44, 45, 46]
Dextran electrospan microconfetti fibers loaded with the protease inhibitor saquinavir were synthesized from acetylated dextran by grinding techniques to affect drug release kinetics [47]. The drug loading capacities and properties of the fibers were compared against other polymers such as poly lactic-co-glycolic acid and polycaprolactone. The results showed that the release profile of saquinavir from the microconfetti formulations was dependent on the stability of the polymer and drug concentration. A single subcutaneous administration of the dextran microconfetti formulations at a dose of 80 mg/kg of saquinavir into mice sustained serum and tissue drug concentrations for seven days. These encouraging data sets suggest injectable microconfetti carriers could be used for delivery of other hydrophobic small molecule drugs.
5. Targeted delivery
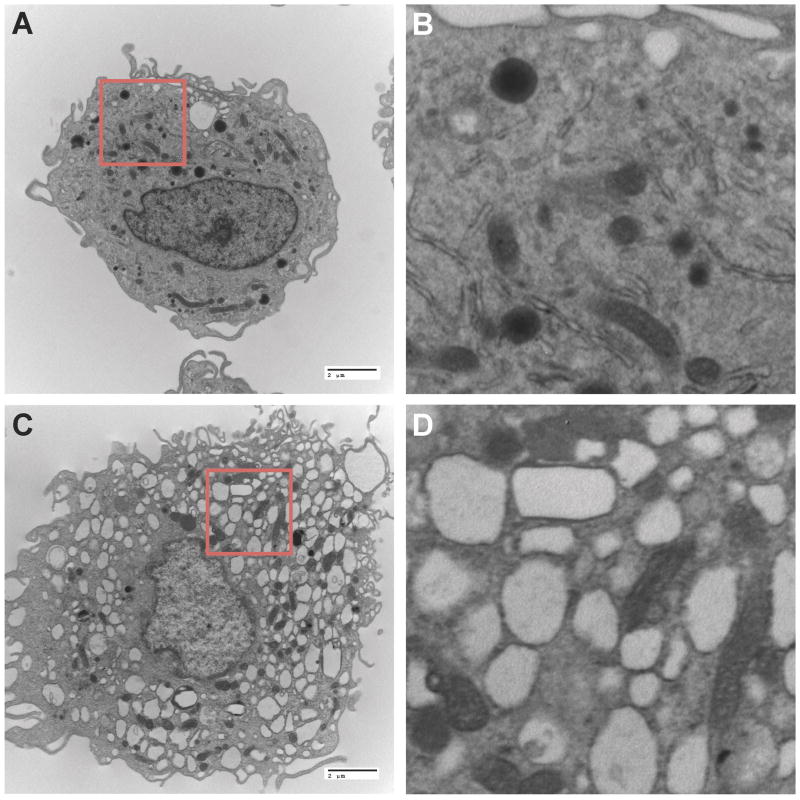
Active targeting is accomplished by attachment of specific molecules, peptides or proteins on the surface of the delivery system, thereby maximizing binding and interactions with receptors expressed on target cells or tissues [31, 48, 49]. The choice of the appropriate ligand is based on its specificity, stability, availability and selectivity on the target cells or tissues. Targeted co-delivery of ARVs to infected CD4+ T cells and macrophages has great potential in the management of HIV-1 infection. Ramana et al. developed anti-CD4 modified liposomes loaded with nevirapine and saquinavir and evaluated them for cellular uptake and antiretroviral responses in Jurkat T cell lines [50]. The liposomes were prepared by thin film hydration and covalently linked to anti-CD4 antibody via thiol-maleimide chemistry. Entrapment and association of each drug within the lipid layers of the liposome was influenced by physicochemical properties of each compound, with the aqueous core associated hydrophilic saquinavir exhibiting a slower release rate compared to the hydrophobic nevirapine localized in the surrounding lipid bilayers. The dual drug loaded anti-CD4 modified liposomes exhibited enhanced drug uptake and improved antiretroviral efficacy compared to equimolar concentrations of native drugs. These in vitro experiments demonstrated that active targeting of CD4+ T cells could facilitate intracellular localization of medicines and improve the efficacy of antiretroviral drugs. Harnessing macrophage transport properties for drug delivery can improve clinical drug responses. Indeed, cell-based nanocarriers have been developed for not only cancer chemotherapy but also a wide range of microbial infections [49, 51, 52]. Cell targeted nanomedicines may offer several advantages over conventional drug delivery methods including enhanced efficacy, reduced side effects, increased drug stability and effective subcellular targeting [27, 53, 54]. To facilitate macrophage targeting, our laboratory developed injectable ART nanoformulations and evaluated drug delivery to macrophage subcellular compartments and tissues, as well as the pharmacokinetic profile [28, 29, 30, 53, 54, 55, 56, 57]. We observed rapid uptake and accumulation of hydrophobic drug nanocrystals throughout the macrophage cytoplasm without notable changes in cell morphology (Figure 2). It is noteworthy that despite the significant accumulation of particles in the cytoplasm the locale was identified in endosomal vesicles. The co-localization studies were amenable to confocal microscopic evaluation of the particle subcellular locale. There were no significant changes in cell viability in cells which contained nanocrystals. These were readily seen during any of the prolonged (measured in weeks) drug depot formation. Folic acid (FA) was covalently conjugated onto poloxamer 407 and used to manufacture nanosuspensions of the protease inhibitor atazanavir, (ATV; FA-nanoATV) by high-pressure homogenization [53, 58, 59]. Our results showed that in comparison with non-targeted formulation, FA-nanoATV increased macrophage ATV uptake and retention 2-fold, and provided robust anti-HIV efficacy in the Rab 5, 7 and 11 endosomal compartments. Pharmacokinetic evaluation in mice showed that a single IM injection of FA-nanoATV enhanced ATV concentration in plasma nearly 10-fold at 14 days post-injection. Furthermore, ATV concentration in lymph nodes increased nearly 4-fold and in liver and kidneys by up to 5-fold over non-targeted nanoformulations at day 14 [53]. Antiretroviral efficacy of ritonavir boosted ATV nanoformuations administered to non-obese diabetic severe combined immunodeficient mice reconstituted with human peripheral blood lymphocytes then infected with HIV-1ADA showed viral suppression by measures of viral load, number of HIV-1p24+ cells in lymphoid tissue and polymerase chain reaction for viral RNA [58]. These results demonstrated the role played by FA targeting of ARV nanoparticles in improving the pharmacokinetics and pharmacodynamics of long acting drug nanoformulations.
Figure 2.
Macrophage uptake and storage of nanoformulated DTG prodrug crystals. A) Transmission electron microscopy (TEM) images of a human monocyte-derived macrophage (MDM) displaying eccentric nuclei, abundant cytoplasm, well-develop endoplasmic reticulum, lysosomes, and Golgi with intracytoplasmic vacuoles and a villus plasma membrane (magnified 6,500x). B) Higher magnification (30,333x) of highlighted area from panel A. C) Replicate human MDM treated for two hours with 100 μM nanoformulated DTG prodrug displays abundant intracellular vesicles within the cytoplasm rich with drug nanocrystals (magnified 6,500x). D) Higher magnification (30,333x) of highlighted region from panel C.
6. Prodrugs and drug polymer conjugates
Yet another approach that has been utilized to improve physiochemical properties of drug molecules rests in prodrug design. Prodrugs themselves do not possess intrinsic biological activity but are capable of generating biologically active drugs during their metabolism [60, 61]. Typically, prodrug strategies are aimed at conferring improved properties to the parent drug that would enhance delivery across physiological barriers or allow for encapsulation into delivery systems [62]. The success of this approach relies upon cleavage of the chemical linkage between the active parent drug and the derivatizing moiety in order to elicit a pharmacological effect [61]. The nature of the chemical linkage is therefore an important consideration. Also, prodrugs should not elicit toxicity. Various ester, carbamate and amide prodrugs can be hydrolyzed by carboxyesterases [61]. Even though prodrug strategies have successfully been utilized in many medicines, only a few prodrug products for HIV therapy have been marketed to date. Only fosamprenavir and tenofovir disoproxil fumarate anti-HIV prodrugs have been approved by the FDA [63, 64]. The majority of pharmaceutical research into the utility of long acting prodrug injectable formulations has been in the area of antipsychotics. Several long acting antipsychotic parenteral agents are clinically available [65]. These formulations include prolixin decanoate sesame oil formulation, marketed by Bristol Myers Squibb as a biweekly injectable, and paliperidone palmitate nanosuspension, marketed by Janssen as a monthly injectable for acute treatment of schizophrenia [66, 67, 68]. Prodrug based drug delivery systems are an attractive strategy for the development of long acting formulations that might significantly simplify the frequency of dosing of ART [64]. Nucleoside reverse transcriptase inhibitors (NRTIs) are the backbone of combination antiretroviral therapy in the treatment of HIV infection [69]. However, these drugs have short half-lives and often require daily or twice daily dosing to maintain therapeutic drug levels. The hydrophilic nature of NRTIs pose further challenges to the development of long acting nanoformulations. A recent article described the synthesis of a library of lamivudine prodrug polymer conjugates and evaluated their drug release kinetics through measurement of glutathione-mediated release of lamivudine (3TC) and in vitro antiviral efficacy against HIV entry and polymerase activity [70]. The copolymers were assembled by reversible addition-fragmentation chain transfer (RAFT) polymerization reactions. The release of 3TC from the polymer occurred over 5 and 10 hours for non-sulfonated and sulfonated polymers, respectively. The polymer conjugates studied by others exhibit potent kinase independent reverse transcriptase inhibition as well as activity against DNA–DNA polymerase. Prior works had shown sulfonic acid polymers to exhibit antiretroviral responses through interaction with the viral glycoprotein gp120 preventing cell membrane fusion [71, 72, 73]. These in vitro data demonstrate that surfactants that exhibit anti-HIV activity could be used in the design of multimodal carriers that might protect cells from invasion by the virus. To overcome limitations of short acting NRTIs, our laboratory recently developed slow release products of 3TC (NMTC) and abacavir (ABC; NMABC) extending their half-lives from hours to weeks [29, 57]. Myristoylated prodrugs of 3TC (M3TC) and ABC (MABC) were produced then encapsulated into poloxamer 407 excipients. Both non-targeted and FA-modified poloxamer nanoformulations of MABC and MTC were manufactured by high-pressure homogenization to generate particles that were characterized by stable physical properties. The prodrug nanosuspensions were then tested to assess uptake and retention of MABC and MTC particles in macrophages. An up to 2.5-fold increase was observed in ABC uptake of FA decorated nanoformulated MABC (FA-NMABC) when compared to replicate undecorated formulation. Blocking the FA receptor decreased FA-NMABC uptake. Increased MABC retention in macrophages over 15 days was seen for FA-NMABC. Similarly, uptake of FA-NM3TC increased over 24 hours. This mirrored improved antiretroviral efficacy of the nanoformulated drugs. FA-NMABC and NM3TC suppressed RT activity for up to 15 days. To determine whether improved hydrophobicity and encapsulation of MABC and M3TC into nanoformulations would translate into sustained plasma drug levels in vivo, mice were treated IM with native drugs or nanoformulated prodrugs (equivalent to 50 mg/kg active drug). Mice were maintained on folate deficient diet prior to drug administration to reduce circulating folate levels. Blood levels of ABC were detectable over 14 days following treatment with nanoformulated MABC. Similarly, 3TC levels were detectable over 10 days following treatment with nanoformulated MTC. At day 14, the plasma 3TC level for FA-NM3TC was 22.7± 12.5 ng/mL and that of NM3TC was at the limit of quantitation. Similarly, 3TC levels in the liver, spleen and lymph nodes were greater than 2-fold higher for the FA-NM3TC treated group compared to the NMTC group. These exciting results demonstrate that short acting drugs can be converted into slow release products and packaged into macrophages to improve bioavailability and pharmacokinetics of the parent drugs. Such delivery systems bring the drugs to sites of active viral replication and therefore hold great potential for clinical application.
7. Anti-inflammatory and neuroprotective agents affecting combination ART depots
Efforts have been made to improve ART delivery across the BBB. However, recent studies have also demonstrated that improved CNS penetrance could itself contribute to neurocognitive dysfunction [74, 75, 76]. In an effort to overcome such limitations, neuroprotective agents could serve to protect vulnerable neurons against viral and cellular neurotoxins [77]. To date, none of the neuroprotective clinical trials have moved forward to clinical practice [78]. However, one compound in particular has been shown to affect nanoART efficacy while at the same time providing anti-inflammatory and neuroprotective activities. This compound is the mixed-lineage kinase 3 (MLK3) inhibitor, URMC-099, that has been shown to play a key role in attenuating pro-inflammatory responses. Inhibition of MLK3 activation in HIV infection has also been shown to neuroprotective properties [79]. In a phase I clinical study the MLK3 inhibitor CEP-1347 given together with ritonavir boosted atazanavir (ATV/r) enhanced plasma drug levels and extended the antiretroviral drug half-life in infected patients [80]. A next generation MLK3 inhibitor, URMC-099 is brain-penetrant and has been shown to facilitate nanoART depots in HIV-1 tissue reservoirs. While URMC-099 alone had no antiretroviral effect its co-administration with nanoATV demonstrated enhanced suppression of HIV-1 [81]. Combination of URMC-099 and nanoATV potentiated antiretroviral responses compared to the ARV treatment alone. The antiretroviral responses paralleled increased drug levels in early, late and recycling endosomes. Interestingly, such endosomal compartments are known to be major subcellular HIV reservoirs [82, 83, 84]. We recently demonstrated that the mechanisms underlying URMC-099 enhancement of antiretroviral responses was based on stimulation of autophagy and later sequestration of drug particles into autophagosomes [85]. Since autophagosomes are used for HIV-1 assembly and maturation, URMC-099 induced autophagy could be harnessed to facilitate viral clearance, improve cell health and facilitate accumulation and retention of ARV nanoparticles at viral action sites.
8. Excision and elimination of HIV proviral DNA
Previously developed “shock and kill” strategies have failed to eradicate latent viral reservoirs [86]. Such past strategies are not efficient nor are they specifically acting leading to eradication failures and cell and tissue toxicities. We have begun to combine efforts to improve drug delivery to viral reservoirs by novel decorated nanomedicines by enabling molecular discoveries that remove proviral DNA from infected T cells and in coordinate efforts prevent ongoing viral infection by mutating CCR5. To such ends, short palindromic repeat (CRISPR)-associated protein-9 nuclease (Cas9) systems were developed for HIV-1 proviral excision in order to eliminate latent provirus [87, 88]. The means to deliver this cargo to lymphoid tissues such as GALT and lymph nodes as well as the CNS and specifically to CD4+ T cells and macrophages is being sought [49, 50, 53, 59]. Certainly the needs to develop novel polymer and medicinal chemistry approaches for optimal manufacture of receptor-targeted nanoformulated Cas9 is of utmost importance and inevitably to use such approaches to efficiency use such formulations in reducing provirus DNA in infected cell reservoirs of virus.
9. Conclusions
Increased interest in ARV and viral eradication strategies birthed the development of long acting ART nanocarriers. The emergence of LASER ART as part of any clinical regimen provides real potential to impact improved therapeutic outcomes. Such drug delivery systems for treatment and prevention of HIV-1 and other infections are of immediate need. A number of nanomedicines were investigated to optimize drug pharmacokinetics and biodistribution, mitigate off target toxicity, and improve penetrance into viral reservoirs. While progress was made in extending the half-life of ART, the main limitations of the existing drug carriers include requirements for high injectable doses and suspension volumes. LASER products that enable ART delivery to anatomical reservoirs while maintaining therapeutic drug concentrations over time periods measured in months are of immediate need. This will require carriers with appropriate surface chemistry for cell and tissue recognitions. Current approaches to HIV elimination are focused on designing products that are co-administered with adjunctive medicines that boost autophagosomal depots to ensure sustained antiviral activities in HIV sanctuaries. These advancements provide strong support of nanomedicine-based platforms for delivery of anti-retroviral drugs and can be taken with medicines that facilitate elimination of infected cells or excise integrated proviral DNA towards establishing a viral cure.
10. Expert Opinion
The persistence of HIV-1 in its anatomical tissue sanctuaries represents a major challenge to final viral eradication. New agents have been developed and designed to eliminate infected cells, induce effective immune antiretroviral responses, excise latent integrated proviral DNA, offer decoys to the viral receptor or better bring drugs to reservoir sites by prodrug modification and encapsulation in targeted particles. These can be seen through currently licensed products or others that have been overlooked by compositions thought not relevant for prior use. Combined therapies that target various stages of the virus life cycle and bring the drug to action sites with particular properties are being sought. Such discovery efforts, we envision, will result in new targets to combat latent or restricted viral infections. To date, there are few commercial slow release products of antiretroviral therapies, in part due to inherent poor physicochemical properties of the drug compounds. With the advent of promising anti-HIV compounds, molecules that induce autophagy, latency reversing agents and gene therapy strategies, it is time to invest in the development of slow release products that would bring us closer to a functional cure. Slow release products have the potential to enable delivery of ART with inherent poor physiochemical properties. Carriers with high drug loading capacities, extended circulation times and active targeting capabilities hold promise to improve the delivery of currently licensed short acting ART, enhance drug dissolution rates and improve bioavailability, protect drugs from rapid clearance through metabolism, and localize therapeutic concentrations of ART at intracellular and tissue sites of infection. The next generation of ART slow release products will also be characterized by infrequent parenteral administration. This will enable reductions in toxicities, viral loads and resistance patterns while improving drug regimen adherence. Such advances hold great potential to accelerate viral clearance in infectious reservoirs that include GALT, lymph nodes, the genitourinary tract and the CNS. Innovative approaches to bridge bench to bedside development of ART slow release products and other strategies to eradicate HIV-1 reservoirs will require collaboration across academia, industry and healthcare providers.
Table 1.
Clinical Trial Data Sets for LA ARVs
| Pre-Exposure Prophylaxis (PrEP) | |||
|---|---|---|---|
| Drug | Study ID | Study description | Comments/Status |
| CAB | NCT02178800 | Phase IIa study designed to evaluate the safety, tolerability and pharmacokinetics of oral CAB and IM CAB LA as PrEP for HIV-1 negative adults. | Estimated study completion date is July 2017 |
| ÉCLAIR | Phase IIa study designed to evaluate safety, tolerability and acceptability of oral CAB and IM CAB LA for PrEP in HIV-1 negative adult males. | Both CAB and CAB LA were well tolerated Site of injection reactions were pain, swelling and itching The absorption and clearance rate of CAB LA was found to be faster than predicted by the PK models. |
|
| CAPRISA 014 | Phase IIa study designed to evaluate safety and acceptability of oral CAB and IM CAB LA for PrEP in HIV-1 negative women at high risk of being infected with HIV-1. | Estimated study completion date is September 2018 | |
| NCT02720094 | Phase IIb/III study designed to compare the safety and efficacy of IM CAB LA to daily oral tenofovir disoproxil fumarate/emtricitabine for PrEP in HIV-1 negative cisgender men and transgender women at high risk of being infected with HIV-1. | Estimated study completion date is June 2020 | |
| RPV LA | SSAT 040 | Phase I study designed to evaluate the safety, pharmacokinetics and metabolism of various doses of IM RPV LA in low risk HIV-1 negative females. | All doses administered were well tolerated RPV was detectable in all samples through day 84. A single 300 mg dose of RPV LA was found not to be effective at providing protection against HIV-1 infection |
| MWR1-01 | Phase I study designed to evaluate the safety, acceptability, pharmacokinetics and ex-vivo pharmacodynamics of IM RPV LA in adults | Rectal tissue concentrations of RPV (five times above the therapeutic concentration) were found to be higher than concentrations in vaginal tissue (2.5 times below the therapeutic concentration). Significant suppression of viral replication by RPV LA was only seen in the rectal tissue | |
| HPTN 076 | Phase II study designed to evaluate the safety and tolerability of IM RPV LA for PrEP in HIV-1 negative adult women | Estimated study completion date is 2017 | |
| HIV/AIDS Treatment | |||
|---|---|---|---|
| Drug | Study ID | Study description | Comments/Status |
| RPV LA & CAB LA | LATTE-2 | Phase IIb study designed to compare the safety and efficacy of intramuscular two drug regimen of CAB LA + RPV LA with daily triple oral CAB+ABC/3TC in naïve adult patients. | At 32 weeks, antiviral activity of the two long acting injectable nano-suspensions was comparable to daily oral three-drug dose. Injection site reactions were noted |
Article highlights box.
Long acting slow effective release of antiretroviral therapy for patient regimen adherence
Macrophage carriage of antiretroviral therapy for drug delivery
Particle decoration with targeting ligands that promote cell and tissue entry
Polymer encased hydrophobic prodrugs and conjugates
Magnetite nanoparticles as theranostic tools for assessment of ART biodistribution
Autophagy stimulation facilitates intracellular accumulation of ARV nanoparticles
Acknowledgments
This work was supported by National Institutes of Health grants P01 DA028555, R01 NS36126, P01 NS31492, 2R01 NS034239, P01 MH64570, P01 NS43985, P30 MH062261 and R01 AG043540.
Footnotes
Declaration of interest
The authors state no conflict of interest.
Bibliography
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Schneider T, Ullrich R, Jahn HU, Bergs C, Schmidt W, Dormann A, Zeitz M. Loss of activated CD4-positive T cells and increase in activated cytotoxic CD8-positive T cells in the duodenum of patients infected with human immunodeficiency virus. Berlin Diarrhea/Wasting Syndrome Study Group. Adv Exp Med Biol. 1995;371B:1019–21. [PubMed] [Google Scholar]
- 2.Pawlowski A, Jansson M, Skold M, Rottenberg ME, Kallenius G. Tuberculosis and HIV co-infection. PLoS Pathog. 2012;8:e1002464. doi: 10.1371/journal.ppat.1002464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ong EL. Common AIDS-associated opportunistic infections. Clin Med (Lond) 2008;8:539–43. doi: 10.7861/clinmedicine.8-5-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lorenzo-Redondo R, Fryer HR, Bedford T, Kim EY, Archer J, Kosakovsky Pond SL, Chung YS, Penugonda S, Chipman JG, Fletcher CV, Schacker TW, Malim MH, Rambaut A, Haase AT, McLean AR, Wolinsky SM. Persistent HIV-1 replication maintains the tissue reservoir during therapy. Nature. 2016;530:51–6. doi: 10.1038/nature16933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deeks SG, Lewin SR, Ross AL, Ananworanich J, Benkirane M, Cannon P, Chomont N, Douek D, Lifson JD, Lo YR, Kuritzkes D, Margolis D, Mellors J, Persaud D, Tucker JD, Barre-Sinoussi F, Alter G, Auerbach J, Autran B, Barouch DH, Behrens G, Cavazzana M, Chen Z, Cohen EA, Corbelli GM, Eholie S, Eyal N, Fidler S, Garcia L, Grossman C, Henderson G, Henrich TJ, Jefferys R, Kiem HP, McCune J, Moodley K, Newman PA, Nijhuis M, Nsubuga MS, Ott M, Palmer S, Richman D, Saez-Cirion A, Sharp M, Siliciano J, Silvestri G, Singh J, Spire B, Taylor J, Tolstrup M, Valente S, van Lunzen J, Walensky R, Wilson I, Zack J International ASTaCWG. International AIDS Society global scientific strategy: towards an HIV cure 2016. Nat Med. 2016;22:839–50. doi: 10.1038/nm.4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hill AL, Rosenbloom DI, Goldstein E, Hanhauser E, Kuritzkes DR, Siliciano RF, Henrich TJ. Real-Time Predictions of Reservoir Size and Rebound Time during Antiretroviral Therapy Interruption Trials for HIV. PLoS Pathog. 2016;12:e1005535. doi: 10.1371/journal.ppat.1005535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chibanda D, Benjamin L, Weiss HA, Abas M. Mental, neurological, and substance use disorders in people living with HIV/AIDS in low- and middle-income countries. J Acquir Immune Defic Syndr. 2014;67(Suppl 1):S54–67. doi: 10.1097/QAI.0000000000000258. [DOI] [PubMed] [Google Scholar]
- 8.Nyberg CR, Patterson BY, Williams MM. When patients cannot take pills: antiretroviral drug formulations for managing adult HIV infection. Top Antivir Med. 2011;19:126–31. [PMC free article] [PubMed] [Google Scholar]
- 9.Azzam R, Lal L, Goh SL, Kedzierska K, Jaworowski A, Naim E, Cherry CL, Wesselingh SL, Mills J, Crowe SM. Adverse effects of antiretroviral drugs on HIV-1-infected and -uninfected human monocyte-derived macrophages. J Acquir Immune Defic Syndr. 2006;42:19–28. doi: 10.1097/01.qai.0000214809.83218.88. [DOI] [PubMed] [Google Scholar]
- 10.Schneider MT, Birger M, Haakenstad A, Singh L, Hamavid H, Chapin A, Murray CJ, Dieleman JL. Tracking development assistance for HIV/AIDS: the international response to a global epidemic. AIDS. 2016;30:1475–9. doi: 10.1097/QAD.0000000000001081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barnhart M, Shelton JD. ARVs: the next generation. Going boldly together to new frontiers of HIV treatment. Glob Health Sci Pract. 2015;3:1–11. doi: 10.9745/GHSP-D-14-00243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vitoria M, Ford N, Doherty M, Flexner C. Simplification of antiretroviral therapy: a necessary step in the public health response to HIV/AIDS in resource-limited settings. Antivir Ther. 2014;19(Suppl 3):31–7. doi: 10.3851/IMP2898. [DOI] [PubMed] [Google Scholar]
- 13.Calmy A, Klement E, Teck R, Berman D, Pecoul B, Ferradini L. Simplifying and adapting antiretroviral treatment in resource-poor settings: a necessary step to scaling-up. AIDS. 2004;18:2353–60. [PubMed] [Google Scholar]
- 14.Persson EM, Gustafsson AS, Carlsson AS, Nilsson RG, Knutson L, Forsell P, Hanisch G, Lennernas H, Abrahamsson B. The effects of food on the dissolution of poorly soluble drugs in human and in model small intestinal fluids. Pharm Res. 2005;22:2141–51. doi: 10.1007/s11095-005-8192-x. [DOI] [PubMed] [Google Scholar]
- 15.Rabinow BE. Nanosuspensions in drug delivery. Nat Rev Drug Discov. 2004;3:785–96. doi: 10.1038/nrd1494. [DOI] [PubMed] [Google Scholar]
- 16.Singh SS. Preclinical pharmacokinetics: an approach towards safer and efficacious drugs. Curr Drug Metab. 2006;7:165–82. doi: 10.2174/138920006775541552. [DOI] [PubMed] [Google Scholar]
- 17•.Freeling JP, Koehn J, Shu C, Sun J, Ho RJ. Anti-HIV drug-combination nanoparticles enhance plasma drug exposure duration as well as triple-drug combination levels in cells within lymph nodes and blood in primates. AIDS Res Hum Retroviruses. 2015;31:107–14. doi: 10.1089/aid.2014.0210. Describes development and co-delivery of ARV lipid nanoparticles to rhesus macaques. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li T, Gendelman HE, Zhang G, Puligujja P, McMillan JM, Bronich TK, Edagwa B, Liu XM, Boska MD. Magnetic resonance imaging of folic acid-coated magnetite nanoparticles reflects tissue biodistribution of long-acting antiretroviral therapy. Int J Nanomedicine. 2015;10:3779–90. doi: 10.2147/IJN.S83279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19•.Kevadiya BD, Bade AN, Woldstad C, Edagwa BJ, McMillan JM, Sajja BR, Boska MD, Gendelman HE. Development of europium doped core-shell silica cobalt ferrite functionalized nanoparticles for magnetic resonance imaging. Acta Biomater. 2016 doi: 10.1016/j.actbio.2016.11.071. The listed articles describe image contrast agents that would enable in vivo tracking of drug carrying nanoparticles for theranostics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spreen WR, Margolis DA, Pottage JC., Jr Long-acting injectable antiretrovirals for HIV treatment and prevention. Curr Opin HIV AIDS. 2013;8:565–71. doi: 10.1097/COH.0000000000000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21••.Landovitz RJ, Kofron R, McCauley M. The promise and pitfalls of long-acting injectable agents for HIV prevention. Curr Opin HIV AIDS. 2016;11:122–8. doi: 10.1097/COH.0000000000000219. Key reviews on long acting injectable ART agents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chomont N, El-Far M, Ancuta P, Trautmann L, Procopio FA, Yassine-Diab B, Boucher G, Boulassel MR, Ghattas G, Brenchley JM, Schacker TW, Hill BJ, Douek DC, Routy JP, Haddad EK, Sekaly RP. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat Med. 2009;15:893–900. doi: 10.1038/nm.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23••.Richman DD, Margolis DM, Delaney M, Greene WC, Hazuda D, Pomerantz RJ. The challenge of finding a cure for HIV infection. Science. 2009;323:1304–7. doi: 10.1126/science.1165706. Highlight the challenges of eliminating the virus in restricted anatomical reservoirs. [DOI] [PubMed] [Google Scholar]
- 24•.Jayant RD, Atluri VS, Agudelo M, Sagar V, Kaushik A, Nair M. Sustained-release nanoART formulation for the treatment of neuroAIDS. Int J Nanomedicine. 2015;10:1077–93. doi: 10.2147/IJN.S76517. Describes carrier systems for latency-breaking agents and antiretroviral drugs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cory TJ, Schacker TW, Stevenson M, Fletcher CV. Overcoming pharmacologic sanctuaries. Curr Opin HIV AIDS. 2013;8:190–5. doi: 10.1097/COH.0b013e32835fc68a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nair M, Jayant RD, Kaushik A, Sagar V. Getting into the brain: Potential of nanotechnology in the management of NeuroAIDS. Adv Drug Deliv Rev. 2016;103:202–17. doi: 10.1016/j.addr.2016.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27•.Fiandra L, Colombo M, Mazzucchelli S, Truffi M, Santini B, Allevi R, Nebuloni M, Capetti A, Rizzardini G, Prosperi D, Corsi F. Nanoformulation of antiretroviral drugs enhances their penetration across the blood brain barrier in mice. Nanomedicine. 2015;11:1387–97. doi: 10.1016/j.nano.2015.03.009. This reference describes drug carriage across the blood brain barrier. [DOI] [PubMed] [Google Scholar]
- 28.Balkundi S, Nowacek AS, Veerubhotla RS, Chen H, Martinez-Skinner A, Roy U, Mosley RL, Kanmogne G, Liu X, Kabanov AV, Bronich T, McMillan J, Gendelman HE. Comparative manufacture and cell-based delivery of antiretroviral nanoformulations. Int J Nanomedicine. 2011;6:3393–404. doi: 10.2147/IJN.S27830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29•.Singh D, McMillan J, Hilaire J, Gautam N, Palandri D, Alnouti Y, Gendelman HE, Edagwa B. Development and characterization of a long-acting nanoformulated abacavir prodrug. Nanomedicine (Lond) 2016;11:1913–27. doi: 10.2217/nnm-2016-0164. The characterization and development of slow release carriers for the hydrophilic drug abacavir is described. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nowacek AS, Miller RL, McMillan J, Kanmogne G, Kanmogne M, Mosley RL, Ma Z, Graham S, Chaubal M, Werling J, Rabinow B, Dou H, Gendelman HE. NanoART synthesis, characterization, uptake, release and toxicology for human monocyte-macrophage drug delivery. Nanomedicine (Lond) 2009;4:903–17. doi: 10.2217/nnm.09.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31••.Moghimi SM, Hunter AC, Murray JC. Long-circulating and target-specific nanoparticles: theory to practice. Pharmacol Rev. 2001;53:283–318. A key reference that reviews rational approaches to long circulating nanoparticles. [PubMed] [Google Scholar]
- 32.Kobayashi M, Yoshinaga T, Seki T, Wakasa-Morimoto C, Brown KW, Ferris R, Foster SA, Hazen RJ, Miki S, Suyama-Kagitani A, Kawauchi-Miki S, Taishi T, Kawasuji T, Johns BA, Underwood MR, Garvey EP, Sato A, Fujiwara T. In Vitro antiretroviral properties of S/GSK1349572, a next-generation HIV integrase inhibitor. Antimicrob Agents Chemother. 2011;55:813–21. doi: 10.1128/AAC.01209-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andrews CD, Heneine W. Cabotegravir long-acting for HIV-1 prevention. Curr Opin HIV AIDS. 2015;10:258–63. doi: 10.1097/COH.0000000000000161. [DOI] [PubMed] [Google Scholar]
- 34•.Andrews CD, Yueh YL, Spreen WR, St Bernard L, Boente-Carrera M, Rodriguez K, Gettie A, Russell-Lodrigue K, Blanchard J, Ford S, Mohri H, Cheng-Mayer C, Hong Z, Ho DD, Markowitz M. A long-acting integrase inhibitor protects female macaques from repeated high-dose intravaginal SHIV challenge. Sci Transl Med. 2015;7:270ra4. doi: 10.1126/scitranslmed.3010298. Highlights the potential of long acting injectable nanosuspensions in the management of HIV. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J, Tappero JW, Bukusi EA, Cohen CR, Katabira E, Ronald A, Tumwesigye E, Were E, Fife KH, Kiarie J, Farquhar C, John-Stewart G, Kakia A, Odoyo J, Mucunguzi A, Nakku-Joloba E, Twesigye R, Ngure K, Apaka C, Tamooh H, Gabona F, Mujugira A, Panteleeff D, Thomas KK, Kidoguchi L, Krows M, Revall J, Morrison S, Haugen H, Emmanuel-Ogier M, Ondrejcek L, Coombs RW, Frenkel L, Hendrix C, Bumpus NN, Bangsberg D, Haberer JE, Stevens WS, Lingappa JR, Celum C Partners Pr EPST. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367:399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, Goicochea P, Casapia M, Guanira-Carranza JV, Ramirez-Cardich ME, Montoya-Herrera O, Fernandez T, Veloso VG, Buchbinder SP, Chariyalertsak S, Schechter M, Bekker LG, Mayer KH, Kallas EG, Amico KR, Mulligan K, Bushman LR, Hance RJ, Ganoza C, Defechereux P, Postle B, Wang F, McConnell JJ, Zheng JH, Lee J, Rooney JF, Jaffe HS, Martinez AI, Burns DN, Glidden DV iPrEx Study T. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587–99. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abdool Karim Q, Abdool Karim SS, Frohlich JA, Grobler AC, Baxter C, Mansoor LE, Kharsany AB, Sibeko S, Mlisana KP, Omar Z, Gengiah TN, Maarschalk S, Arulappan N, Mlotshwa M, Morris L, Taylor D, Group CT. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329:1168–74. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38•.Thigpen MC, Kebaabetswe PM, Paxton LA, Smith DK, Rose CE, Segolodi TM, Henderson FL, Pathak SR, Soud FA, Chillag KL, Mutanhaurwa R, Chirwa LI, Kasonde M, Abebe D, Buliva E, Gvetadze RJ, Johnson S, Sukalac T, Thomas VT, Hart C, Johnson JA, Malotte CK, Hendrix CW, Brooks JT, Group TDFS. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012;367:423–34. doi: 10.1056/NEJMoa1110711. Highlight the translational potential of long acting injectable antiretroviral delivery systems for pre-exposure prophylaxis. [DOI] [PubMed] [Google Scholar]
- 39.Kovarova M, Council OD, Date AA, Long JM, Nochi T, Belshan M, Shibata A, Vincent H, Baker CE, Thayer WO, Kraus G, Lachaud-Durand S, Williams P, Destache CJ, Garcia JV. Nanoformulations of Rilpivirine for Topical Pericoital and Systemic Coitus-Independent Administration Efficiently Prevent HIV Transmission. PLoS Pathog. 2015;11:e1005075. doi: 10.1371/journal.ppat.1005075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baert L, van’t Klooster G, Dries W, Francois M, Wouters A, Basstanie E, Iterbeke K, Stappers F, Stevens P, Schueller L, Van Remoortere P, Kraus G, Wigerinck P, Rosier J. Development of a long-acting injectable formulation with nanoparticles of rilpivirine (TMC278) for HIV treatment. Eur J Pharm Biopharm. 2009;72:502–8. doi: 10.1016/j.ejpb.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 41.van’t Klooster G, Hoeben E, Borghys H, Looszova A, Bouche MP, van Velsen F, Baert L. Pharmacokinetics and disposition of rilpivirine (TMC278) nanosuspension as a long-acting injectable antiretroviral formulation. Antimicrob Agents Chemother. 2010;54:2042–50. doi: 10.1128/AAC.01529-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jackson AG, Else LJ, Mesquita PM, Egan D, Back DJ, Karolia Z, Ringner-Nackter L, Higgs CJ, Herold BC, Gazzard BG, Boffito M. A compartmental pharmacokinetic evaluation of long-acting rilpivirine in HIV-negative volunteers for pre-exposure prophylaxis. Clin Pharmacol Ther. 2014;96:314–23. doi: 10.1038/clpt.2014.118. [DOI] [PubMed] [Google Scholar]
- 43.Spreen W, Williams P, Margolis D, Ford SL, Crauwels H, Lou Y, Gould E, Stevens M, Piscitelli S. Pharmacokinetics, safety, and tolerability with repeat doses of GSK1265744 and rilpivirine (TMC278) long-acting nanosuspensions in healthy adults. J Acquir Immune Defic Syndr. 2014;67:487–92. doi: 10.1097/QAI.0000000000000365. [DOI] [PubMed] [Google Scholar]
- 44.Jackson A, McGowan I. Long-acting rilpivirine for HIV prevention. Curr Opin HIV AIDS. 2015;10:253–7. doi: 10.1097/COH.0000000000000160. [DOI] [PubMed] [Google Scholar]
- 45•.McGowan I, Dezzutti CS, Siegel A, Engstrom J, Nikiforov A, Duffill K, Shetler C, Richardson-Harman N, Abebe K, Back D, Else L, Egan D, Khoo S, Egan JE, Stall R, Williams PE, Rehman KK, Adler A, Brand RM, Chen B, Achilles S, Cranston RD. Long-acting rilpivirine as potential pre-exposure prophylaxis for HIV-1 prevention (the MWRI-01 study): an open-label, phase 1, compartmental, pharmacokinetic and pharmacodynamic assessment. Lancet HIV. 2016;3:e569–e78. doi: 10.1016/S2352-3018(16)30113-8. Highlight the potential of long acting ARVs to improve efficacy and adherence to PrEP treatments. [DOI] [PubMed] [Google Scholar]
- 46.Cabotegravir. Clinical Trials, Side Effects, AIDSinfo Drug Database. 2016 Available from: https://aidsinfo.nih.gov/drugs/513/cabotegravir/0/patient.
- 47.Collier MA, Gallovic MD, Bachelder EM, Sykes CD, Kashuba A, Ainslie KM. Saquinavir Loaded Acetalated Dextran Microconfetti - a Long Acting Protease Inhibitor Injectable. Pharm Res. 2016;33:1998–2009. doi: 10.1007/s11095-016-1936-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Edagwa BJ, Zhou T, McMillan JM, Liu XM, Gendelman HE. Development of HIV reservoir targeted long acting nanoformulated antiretroviral therapies. Curr Med Chem. 2014;21:4186–98. doi: 10.2174/0929867321666140826114135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49••.Sapsford KE, Algar WR, Berti L, Gemmill KB, Casey BJ, Oh E, Stewart MH, Medintz IL. Functionalizing nanoparticles with biological molecules: developing chemistries that facilitate nanotechnology. Chem Rev. 2013;113:1904–2074. doi: 10.1021/cr300143v. Two key reviews that describe active targeting strategies. [DOI] [PubMed] [Google Scholar]
- 50.Ramana LN, Sharma S, Sethuraman S, Ranga U, Krishnan UM. Stealth anti-CD4 conjugated immunoliposomes with dual antiretroviral drugs--modern Trojan horses to combat HIV. Eur J Pharm Biopharm. 2015;89:300–11. doi: 10.1016/j.ejpb.2014.11.021. [DOI] [PubMed] [Google Scholar]
- 51.Edagwa BJ, Guo D, Puligujja P, Chen H, McMillan J, Liu X, Gendelman HE, Narayanasamy P. Long-acting antituberculous therapeutic nanoparticles target macrophage endosomes. FASEB J. 2014;28:5071–82. doi: 10.1096/fj.14-255786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Luo Y, Zhou H, Krueger J, Kaplan C, Lee SH, Dolman C, Markowitz D, Wu W, Liu C, Reisfeld RA, Xiang R. Targeting tumor-associated macrophages as a novel strategy against breast cancer. J Clin Invest. 2006;116:2132–41. doi: 10.1172/JCI27648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Puligujja P, McMillan J, Kendrick L, Li T, Balkundi S, Smith N, Veerubhotla RS, Edagwa BJ, Kabanov AV, Bronich T, Gendelman HE, Liu XM. Macrophage folate receptor-targeted antiretroviral therapy facilitates drug entry, retention, antiretroviral activities and biodistribution for reduction of human immunodeficiency virus infections. Nanomedicine. 2013;9:1263–73. doi: 10.1016/j.nano.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54••.Dou H, Destache CJ, Morehead JR, Mosley RL, Boska MD, Kingsley J, Gorantla S, Poluektova L, Nelson JA, Chaubal M, Werling J, Kipp J, Rabinow BE, Gendelman HE. Development of a macrophage-based nanoparticle platform for antiretroviral drug delivery. Blood. 2006;108:2827–35. doi: 10.1182/blood-2006-03-012534. First description of macrophage targeted nanomedicines for CNS drug delivery. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dou H, Grotepas CB, McMillan JM, Destache CJ, Chaubal M, Werling J, Kipp J, Rabinow B, Gendelman HE. Macrophage delivery of nanoformulated antiretroviral drug to the brain in a murine model of neuroAIDS. J Immunol. 2009;183:661–9. doi: 10.4049/jimmunol.0900274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gautam N, Roy U, Balkundi S, Puligujja P, Guo D, Smith N, Liu XM, Lamberty B, Morsey B, Fox HS, McMillan J, Gendelman HE, Alnouti Y. Preclinical pharmacokinetics and tissue distribution of long-acting nanoformulated antiretroviral therapy. Antimicrob Agents Chemother. 2013;57:3110–20. doi: 10.1128/AAC.00267-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57•.Guo D, Zhou T, Arainga M, Palandri D, Gautam N, Bronich T, Alnouti Y, McMillan J, Edagwa B, Gendelman HE. Creation of a Long-Acting Nanoformulated 2′,3′-Dideoxy-3′-Thiacytidine. J Acquir Immune Defic Syndr. 2016 doi: 10.1097/QAI.0000000000001170. The article describes the development of slow release carriers for hydrophilic drugs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Puligujja P, Balkundi SS, Kendrick LM, Baldridge HM, Hilaire JR, Bade AN, Dash PK, Zhang G, Poluektova LY, Gorantla S, Liu XM, Ying T, Feng Y, Wang Y, Dimitrov DS, McMillan JM, Gendelman HE. Pharmacodynamics of long-acting folic acid-receptor targeted ritonavir-boosted atazanavir nanoformulations. Biomaterials. 2015;41:141–50. doi: 10.1016/j.biomaterials.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Puligujja P, Arainga M, Dash P, Palandri D, Mosley RL, Gorantla S, Poluektova L, McMillan J, Gendelman HE. Pharmacodynamics of folic acid receptor targeted antiretroviral nanotherapy in HIV-1-infected humanized mice. Antiviral Res. 2015;120:85–8. doi: 10.1016/j.antiviral.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huttunen KM, Raunio H, Rautio J. Prodrugs--from serendipity to rational design. Pharmacol Rev. 2011;63:750–71. doi: 10.1124/pr.110.003459. [DOI] [PubMed] [Google Scholar]
- 61.Anastasi C, Quelever G, Burlet S, Garino C, Souard F, Kraus JL. New antiviral nucleoside prodrugs await application. Curr Med Chem. 2003;10:1825–43. doi: 10.2174/0929867033457034. [DOI] [PubMed] [Google Scholar]
- 62.Rautio J, Kumpulainen H, Heimbach T, Oliyai R, Oh D, Jarvinen T, Savolainen J. Prodrugs: design and clinical applications. Nat Rev Drug Discov. 2008;7:255–70. doi: 10.1038/nrd2468. [DOI] [PubMed] [Google Scholar]
- 63.Ray AS, Fordyce MW, Hitchcock MJ. Tenofovir alafenamide: A novel prodrug of tenofovir for the treatment of Human Immunodeficiency Virus. Antiviral Res. 2016;125:63–70. doi: 10.1016/j.antiviral.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 64.Palombo MS, Singh Y, Sinko PJ. Prodrug and conjugate drug delivery strategies for improving HIV/AIDS therapy. J Drug Deliv Sci Technol. 2009;19:3–14. doi: 10.1016/s1773-2247(09)50001-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Park EJ, Amatya S, Kim MS, Park JH, Seol E, Lee H, Shin YH, Na DH. Long-acting injectable formulations of antipsychotic drugs for the treatment of schizophrenia. Arch Pharm Res. 2013;36:651–9. doi: 10.1007/s12272-013-0105-7. [DOI] [PubMed] [Google Scholar]
- 66.Lamb YN, Keating GM. Paliperidone Palmitate Intramuscular 3-Monthly Formulation: A Review in Schizophrenia. Drugs. 2016;76:1559–66. doi: 10.1007/s40265-016-0645-5. [DOI] [PubMed] [Google Scholar]
- 67.Spanarello S, La Ferla T. The pharmacokinetics of long-acting antipsychotic medications. Curr Clin Pharmacol. 2014;9:310–7. doi: 10.2174/15748847113089990051. [DOI] [PubMed] [Google Scholar]
- 68.Baweja R, Sedky K, Lippmann S. Long-acting antipsychotic medications. Curr Drug Targets. 2012;13:555–60. doi: 10.2174/138945012799499785. [DOI] [PubMed] [Google Scholar]
- 69.Ibbotson T, Perry CM. Lamivudine/zidovudine/abacavir: triple combination tablet. Drugs. 2003;63:1089–98. doi: 10.2165/00003495-200363110-00010. discussion 99–100. [DOI] [PubMed] [Google Scholar]
- 70•.Danial M, Andersen AH, Zuwala K, Cosson S, Riber CF, Smith AA, Tolstrup M, Moad G, Zelikin AN, Postma A. Triple Activity of Lamivudine Releasing Sulfonated Polymers against HIV-1. Mol Pharm. 2016;13:2397–410. doi: 10.1021/acs.molpharmaceut.6b00156. Demonstrates the use of surfactants that exhibit anti-HIV activity to design multimodal carriers. [DOI] [PubMed] [Google Scholar]
- 71.Baba M, De Clercq E, Schols D, Pauwels R, Snoeck R, Van Boeckel C, Van Dedem G, Kraaijeveld N, Hobbelen P, Ottenheijm H, et al. Novel sulfated polysaccharides: dissociation of anti-human immunodeficiency virus activity from antithrombin activity. J Infect Dis. 1990;161:208–13. doi: 10.1093/infdis/161.2.208. [DOI] [PubMed] [Google Scholar]
- 72.Mahalingam A, Geonnotti AR, Balzarini J, Kiser PF. Activity and safety of synthetic lectins based on benzoboroxole-functionalized polymers for inhibition of HIV entry. Mol Pharm. 2011;8:2465–75. doi: 10.1021/mp2002957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mohan P, Schols D, Baba M, De Clercq E. Sulfonic acid polymers as a new class of human immunodeficiency virus inhibitors. Antiviral Res. 1992;18:139–50. doi: 10.1016/0166-3542(92)90034-3. [DOI] [PubMed] [Google Scholar]
- 74.Winston A, Duncombe C, Li PC, Gill JM, Kerr SJ, Puls R, Petoumenos K, Taylor-Robinson SD, Emery S, Cooper DA Altair Study G. Does choice of combination antiretroviral therapy (cART) alter changes in cerebral function testing after 48 weeks in treatment-naive, HIV-1-infected individuals commencing cART? A randomized, controlled study. Clin Infect Dis. 2010;50:920–9. doi: 10.1086/650743. [DOI] [PubMed] [Google Scholar]
- 75.Marra CM, Zhao Y, Clifford DB, Letendre S, Evans S, Henry K, Ellis RJ, Rodriguez B, Coombs RW, Schifitto G, McArthur JC, Robertson K Team ACTGS. Impact of combination antiretroviral therapy on cerebrospinal fluid HIV RNA and neurocognitive performance. AIDS. 2009;23:1359–66. doi: 10.1097/QAD.0b013e32832c4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schweinsburg BC, Taylor MJ, Alhassoon OM, Gonzalez R, Brown GG, Ellis RJ, Letendre S, Videen JS, McCutchan JA, Patterson TL, Grant I, Group H. Brain mitochondrial injury in human immunodeficiency virus-seropositive (HIV+) individuals taking nucleoside reverse transcriptase inhibitors. J Neurovirol. 2005;11:356–64. doi: 10.1080/13550280591002342. [DOI] [PubMed] [Google Scholar]
- 77.Rumbaugh JA, Steiner J, Sacktor N, Nath A. Developing neuroprotective strategies for treatment of HIV-associated neurocognitive dysfunction. Futur HIV Ther. 2008;2:271–80. doi: 10.2217/17469600.2.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stein DG. Embracing failure: What the Phase III progesterone studies can teach about TBI clinical trials. Brain Inj. 2015;29:1259–72. doi: 10.3109/02699052.2015.1065344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Marker DF, Tremblay ME, Puccini JM, Barbieri J, Gantz Marker MA, Loweth CJ, Muly EC, Lu SM, Goodfellow VS, Dewhurst S, Gelbard HA. The new small-molecule mixed-lineage kinase 3 inhibitor URMC-099 is neuroprotective and anti-inflammatory in models of human immunodeficiency virus-associated neurocognitive disorders. J Neurosci. 2013;33:9998–10010. doi: 10.1523/JNEUROSCI.0598-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ma Q, Gelbard HA, Maggirwar SB, Dewhurst S, Gendelman HE, Peterson DR, DiFrancesco R, Hochreiter JS, Morse GD, Schifitto G. Pharmacokinetic interactions of CEP-1347 and atazanavir in HIV-infected patients. J Neurovirol. 2013;19:254–60. doi: 10.1007/s13365-013-0172-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81•.Zhang G, Guo D, Dash PK, Arainga M, Wiederin JL, Haverland NA, Knibbe-Hollinger J, Martinez-Skinner A, Ciborowski P, Goodfellow VS, Wysocki TA, Wysocki BJ, Poluektova LY, Liu XM, McMillan JM, Gorantla S, Gelbard HA, Gendelman HE. The mixed lineage kinase-3 inhibitor URMC-099 improves therapeutic outcomes for long-acting antiretroviral therapy. Nanomedicine. 2016;12:109–22. doi: 10.1016/j.nano.2015.09.009. This report highlights co-administration of slow release carriers and autophagy agents to facilitate accumulation and retention of nanoparticles at viral action sites. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Arainga M, Guo D, Wiederin J, Ciborowski P, McMillan J, Gendelman HE. Opposing regulation of endolysosomal pathways by long-acting nanoformulated antiretroviral therapy and HIV-1 in human macrophages. Retrovirology. 2015;12:5. doi: 10.1186/s12977-014-0133-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kadiu I, Gendelman HE. Human immunodeficiency virus type 1 endocytic trafficking through macrophage bridging conduits facilitates spread of infection. J Neuroimmune Pharmacol. 2011;6:658–75. doi: 10.1007/s11481-011-9298-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84•.Kadiu I, Nowacek A, McMillan J, Gendelman HE. Macrophage endocytic trafficking of antiretroviral nanoparticles. Nanomedicine (Lond) 2011;6:975–94. doi: 10.2217/nnm.11.27. Describe intracellular traficking mechanisms of antiretroviral nanoparticles. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85••.Gnanadhas DP, DPK, Sillman B, Bade AN, Lin Z, Palandri DL, Gautam N, Alnouti Y, Gelbard HA, McMillan J, Mosley RL, Edagwa B, Gendelman HE, Gorantla S. Autophagy facilitates macrophage depots of sustained release nanoformulated antiretroviral drugs. J Clin Invest. 2017 doi: 10.1172/JCI90025. in press. Describes how autophagy stimulation facilitates autophagosome drug depots. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Archin NM, Margolis DM. Emerging strategies to deplete the HIV reservoir. Curr Opin Infect Dis. 2014;27:29–35. doi: 10.1097/QCO.0000000000000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.White MK, Hu W, Khalili K. The CRISPR/Cas9 genome editing methodology as a weapon against human viruses. Discov Med. 2015;19:255–62. [PMC free article] [PubMed] [Google Scholar]
- 88•.Kaminski R, Chen Y, Fischer T, Tedaldi E, Napoli A, Zhang Y, Karn J, Hu W, Khalili K. Elimination of HIV-1 Genomes from Human T-lymphoid Cells by CRISPR/Cas9 Gene Editing. Sci Rep. 2016;6:22555. doi: 10.1038/srep22555. Describe excision of integrated proviral DNA towards establishing a viral cure. [DOI] [PMC free article] [PubMed] [Google Scholar]