Abstract
Study Design
Temporal immunohistochemistry analysis of spinal cord tissue from a rat model of cervical radiculopathy.
Objective
The goal was to measure spinal endothelial and astrocytic vimentin expression following a painful nerve root compression to define spinal cellular expression of vimentin in the context of pain.
Summary of Background Data
The intermediate filament, vimentin, is expressed in a variety of cell types in the spinal cord and is modulated in response to neural pathologies. Early after nerve root compression spinal astrocytes become activated and blood-spinal cord barrier (BSCB) breakdown occurs in parallel with development of pain-related behaviors; these spinal responses remain activated as does the presence of pain. In addition to vimentin, glial fibrillary acidic protein (GFAP) expression is a hallmark of astrocyte activation. In contrast, vascular endothelial cells down-regulate vimentin expression in parallel with vascular breakdown. It is not known whether spinal astrocytes and endothelial cells modulate their expression of vimentin in response to a painful neural injury.
Methods
Mechanical hyperalgesia was measured and spinal cord tissue was harvested at days 1 and 7 following a unilateral nerve root compression in rats. Vimentin was co-immunolabeled with GFAP to label astrocytes and von Willebrand factor (VWF) for endothelial cells in the spinal cord on the side of injury.
Results
Spinal astrocytic vimentin increases by day 7 after nerve root compression, corresponding to when mechanical hyperalgesia is maintained. Spinal endothelial vimentin increases as early as day 1 after a painful compression and is even more robust at day 7.
Conclusions
The delayed elevation in spinal astrocytic vimentin corresponding to sustained mechanical hyperalgesia supports its having a relationship with pain maintenance. Further, since BSCB integrity has been shown to be reestablished by day 7 after a painful compression, endothelial expressed vimentin may help to fortify spinal vasculature contributing to BSCB stability.
Keywords: vimentin, spinal, astrocyte, endothelial cell, pain, nerve root, radiculopathy, neuropathic pain, pain
Introduction
Intermediate filaments, such as vimentin, perform many roles; they maintain cell shape, resist tensile loads, transmit mechanotransductive signals, act as a substrate for phosphorylation reactions in the cytoplasm and stabilize connectivity between cells within a tissue [1–3]. Intermediate filaments have a structure rich in α-helices, which facilitates a range of molecular unfolding events with applied stretch [4]. This structural feature enables intermediate filaments to resist much higher tensile loads than other cytoskeletal filaments [1, 3, 4]. One intermediate filament in particular, vimentin, greatly influences the mechanical integrity of the cell cytoplasm. Highlighting this, fibroblasts derived from vimentin deficient (vim−/−) mice exhibit half the cytoplasmic stiffness of wild type fibroblasts [1]. Vimentin is expressed by a variety of cells within the CNS including astrocytes and endothelial cells, and its expression is modulated by pathological insults [5–7]. Since neuronal function and dysfunction at least partially depends on the stiffness of the local environment [8–10], changes in the stiffness of astrocytes, which comprise much of the mechanical substrate for neurons in the CNS, is hypothesized to facilitate aberrant signaling by neurons and other cells in the spinal cord that contribute to nociception.
Although the expression of intermediate filaments, including vimentin and glial fibrillary acidic protein (GFAP), correlates with astrocyte stiffness in vitro [6], increased astrocytic vimentin expression is more often associated with glial activation [7, 11]. Both vimentin and GFAP are upregulated in the spinal cord by day 7 after chronic constriction of the sciatic nerve and remain elevated for up to 28 days, which parallels persistent pain [12]. However, GFAP and vimentin expression in astrocytes does not always follow the same temporal profile. Ischemic injury induced in the middle cerebral artery increases the number of GFAP-positive and vimentin-positive cells in nearby cortical regions within 48 hours [13]. However, only GFAP-positive cell populations remain elevated for 30 days, whereas the number of vimentin positive cells returns to normal by that time [13]. After a nerve root compression, spinal GFAP is upregulated by day 1 when pain-like behaviors develop after that injury; spinal GFAP remains elevated for up to 7 days in parallel with pain-like behaviors [14, 17]. Although both GFAP and vimentin intermediate filaments are established markers of astrocyte activation, it is not known if spinal vimentin expression within astrocytes is associated with pain from neuropathic injury.
Vimentin is also an important cytoskeletal filament in endothelial cells and regulates the integrity of individual endothelial cells, as well as cell monolayers. Vimentin filament networks maintain cellular integrity when expressed as long stable filaments dispersed throughout the cytoplasm [1, 4]. Phosphorylation of vimentin filaments disassembles their intracellular organization and causes nuclear aggregation [18]. That disruption in vimentin stability also corresponds to increased endothelial barrier permeability [5, 19]. A role for endothelial vimentin in vascular stability is further supported by vim−/− mice exhibiting increased paracellular transmigration of blood mononuclear cells into the lymph nodes and spleen as an indicator of elevated vascular permeability [20]. The permeability of the blood-spinal cord barrier (BSCB) transiently increases early after a painful nerve root compression and stabilizes by day 7 [21], suggesting that microvascular endothelial function is compromised in response to peripheral neural compression. Yet, the structural changes that endothelial cells undergo in order to facilitate that breakdown and subsequent recovery are not fully understood. Modifications in endothelial vimentin expression over time might contribute to the changes in BSCB integrity that are induced by a painful nerve root compression injury.
A peripheral neuropathic injury, applied via transient compression of the nerve root, induces BSCB permeability, spinal astrocyte activation and pain along different time courses [15, 16, 21]. Compressing the nerve root for just 15 minutes induces a substantial breakdown in the BSCB, which occurs at the same time as the development of mechanical hyperalgesia by day 1 [21]. Due to the apparent roles of vimentin in both astrocytic activation and vascular permeability [6, 13, 19, 22–24], this study investigates whether modifications in spinal astrocytic and endothelial vimentin expression are associated with the development and/or maintenance of pain after a nerve root compression injury.
Materials & Methods
Nerve Root Compression & Tissue Harvest Procedures
Surgical procedures were performed as previously described [15, 16]. Briefly, adult male Holtzman rats were anesthetized using inhalation isoflurane anesthesia and the right C6 and C7 vertebrae were cleared of the paraspinal muscle. The right C7 dorsal nerve root was then exposed via a C6/C7 hemilaminectomy and partial facetectomy. The nerve root was compressed for 15 minutes (injury, n=10) with a 10gf microvascular clip [15, 16]. Separate rats underwent the same surgical procedures, but did not receive a nerve root compression (sham, n=10) to serve as a surgical control. Spinal cord tissue from the C7 spinal level was harvested from rats on day 1 (injury, n=6; sham n=4) or day 7 (injury, n=4; sham n=6) after surgery, fixed with 4% paraformaldehyde and cryosectioned for immunohistochemical processing and analysis.
Measuring Mechanical Hyperalgesia
Mechanical hyperalgesia is an increased response to a stimulus, which is normally painful [25–28]. Because the C7 nerve root contains afferent sensory neurons that innervate the plantar surface of the forepaw, sensitivity to a mechanical stimulus was measured in the forepaw ipsilateral to the nerve root compression. Mechanical hyperalgesia was measured to determine the mechanical threshold at which a rat responds to an applied mechanical stimulus; a decrease in threshold corresponds to an increase in pain-related behaviors. Briefly, rats were acclimated to the testing apparatus, which consisted of an elevated mesh-floored cage. Mechanical stimuli were applied to the forepaw ipsilateral to injury using a graded series of von Frey filaments of increasing strength (1.4, 2, 4, 6, 8, 10, 15, and 26 g) (Stoelting; Wood Dale, IL). The filament strength eliciting a response, such as shaking or licking of the paw, was recorded as the threshold [29–31]. Each filament was applied five times and if a rat responded to two consecutive filaments, the lower strength filament was recorded as the paw withdrawal threshold. If no filament elicited a response, then the highest magnitude filament (26g) was recorded as the threshold. On each testing day, the mechanical threshold was measured three times for each forepaw tested and averaged for each rat across the rounds.
Mechanical hyperalgesia was measured on day 0 (baseline) prior to surgery and on days 1 and 7 following the root compression or sham surgery. Rats from which tissue was harvested on day 1 only underwent behavioral testing on days 0 and 1. The difference in paw withdrawal threshold was determined between the injury and sham groups at day 1 and day 7 using a two-way ANOVA (group × day) with Tukey’s test.
Spinal Cord Immunohistochemistry
Astrocytic and endothelial vimentin were measured by co-immunolabeling for vimentin with either GFAP, as a marker for astrocytes, or von Willebrand factor (VWF), to label endothelial cells, in the spinal cord at days 1 and 7 after a painful nerve root compression. Fixed spinal cord tissue was cryosectioned axially at 14μm and mounted on slides. Spinal cord sections were incubated in a blocking solution consisting of 5% normal goat serum (Vector Laboratories; Burlingame, CA) with 0.3% Triton-X100 (Bio-Rad Laboratories; Hercules, CA) in PBS for 1 hour at room temperature. Slides were then incubated with goat-anti-vimentin raised in rabbit (1μg/ml; Abcam; Cambridge, MA) and goat-anti-GFAP raised in mouse (1:750; Millipore; Billerica, MA) overnight at 4°C. Tissue sections were incubated in goat-anti-rabbit Alexa Flour 488 and goat-anti-mouse Alexa Fluor 568 (1:1000; Life Technologies; Carlsbad, CA) for 2 hours at room temperature. Slides were washed with PBS and cover slipped with TRIS buffer (Electron Microscopy Sciences; Hatfield, PA) for imaging.
The ipsilateral dorsal horn was imaged at 20× for 2–6 spinal sections for each rat (day 1: injury, n=6; sham n=4, day 7: injury, n=4; sham n=6). Total spinal vimentin was quantified using densitometry methods [16, 32] to measure the percent of the tissue pixels positively labeled for vimentin. The percent of pixels labeling vimentin in tissue sections was then normalized to the percent labeling in spinal cord tissue from normal naïve rats (n=2). Spinal astrocytic vimentin was measured in the same tissue sections by quantifying the percent of co-localized pixels positively labeled for both vimentin and GFAP [33]. The percent of co-localized vimentin and GFAP pixels was then compared to the percent of co-localized pixels in spinal tissue from normal rats.
To quantify endothelial vimentin, spinal cord sections were co-immunolabeled for vimentin with VWF. Slides were blocked in a solution consisting of 5% normal goat serum (Vector Laboratories; Burlingame, CA) with 0.3% Triton-X100 (Bio-Rad Laboratories; Hercules, CA) in PBS for 1 hour at room temperature and then incubated with goat-anti-vimentin raised in rabbit (1μg/ml; Abcam; Cambridge, MA) and goat-anti-VWF raised in mouse (1:75; Abcam; Cambridge, MA) overnight at 4°C. Tissue sections were then incubated in goat-anti-rabbit Alexa Flour 488 (1:1000; Life Technologies; Carlsbad, CA) and goat-anti-mouse Alexa Fluor 568 (1:500; Life Technologies; Carlsbad, CA) for 2 hours at room temperature. The ipsilateral dorsal horn was imaged at 20× in 2–6 spinal sections from each rat. Spinal endothelial vimentin expression was quantified by normalizing the percent of co-localized vimentin and VWF pixels to the percent of co-localized pixels in spinal tissue from naïve rats [33].
Statistical differences in the quantified percent vimentin, percent pixels for vimentin co-localized with GFAP, and percent pixels for vimentin co-localized with VWF in the spinal cord were compared between injury and sham at day 1 and day 7 using two-way ANOVAs (group × day) with Tukey’s honestly significant difference test.
Results
The withdrawal threshold in the forepaw ipsilateral to injury significantly decreases (p<0.004), corresponding to increased mechanical hyperalgesia, on days 1 and 7 following a nerve root compression injury compared to baseline (Figure 1). Nerve root injury also induces a significant decrease in the withdrawal threshold compared to sham overall (p<0.001), as well as on each of days 1 and 7 (p<0.010) (Figure 1).
Figure 1. Transient root injury increases mechanical hyperalgesia.
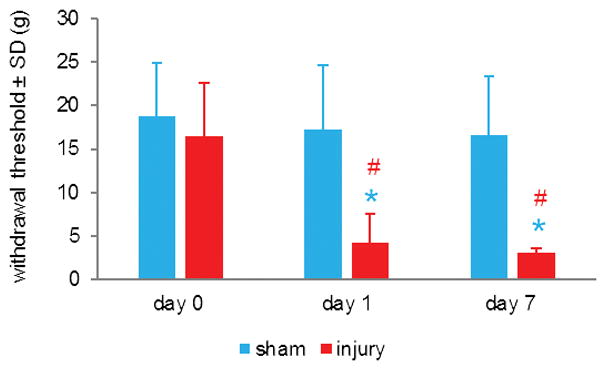
C7 nerve root compression significantly decreases the withdrawal threshold of the forepaw ipsilateral to injury by day 1 (#p=0.004), for at least 7 days (#p<0.001) compared to baseline (day 0) thresholds. Compression also induces a significant drop (*p<0.010) in withdrawal threshold compared to sham on days 1 and 7. Data are represented as mean ± standard deviation (SD).
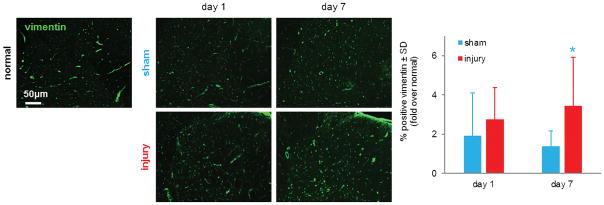
Vimentin is expressed in the spinal cord of normal rats, as well those undergoing a nerve root injury or sham operation (Figure 2). A nerve root compression injury induces a robust increase in total vimentin expression in the spinal cord ipsilateral to injury by day 7 (Figure 2). That injury-induced increase in spinal vimentin is significantly elevated (p<0.001) over sham overall and on day 7 (Figure 2). At day 1, both a nerve root injury and sham operation increase spinal vimentin to approximately 2-fold over normal expression levels.
Figure 2. Root injury increases spinal vimentin.
Vimentin labeling (green) is elevated in the ipsilateral spinal dorsal horn by day 1 relative to normal levels and is more robustly increased by day 7 compared to normal and sham levels at either time. The percent of spinal vimentin expression, normalized to normal expression, significantly increases after root injury compared to sham overall (p<0.001) and on day 7 (*p<0.001). Data are represented as mean ± standard deviation (SD).
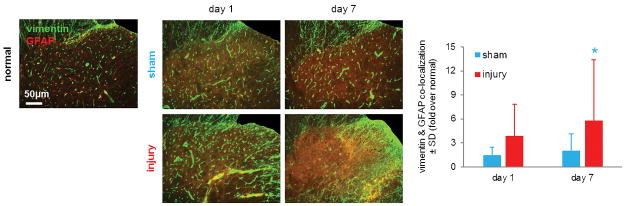
Spinal astrocytic expression of vimentin is visually increased over normal levels for both groups at both days 1 and 7 (Figure 3). Vimentin expression in spinal astrocytes also increases by day 7 after a root compression over sham at that time point and either procedure at day 1 (Figure 3). Indeed, spinal astrocytic vimentin is significantly elevated after a compression injury compared to sham overall (p=0.001) and on day 7 (p=0.036) (Figure 3).
Figure 3. Spinal astrocytic vimentin expression is elevated after root compression.
Spinal vimentin (green) labeling co-localizes (yellow) with GFAP (red) for all groups at all time points. The quantified co-localization of vimentin with GFAP labeling normalized to expression in tissue from normal rats is significantly increased compared to sham overall (p=0.001) and on day 7 (*p=0.036). Data are represented as mean ± standard deviation (SD).
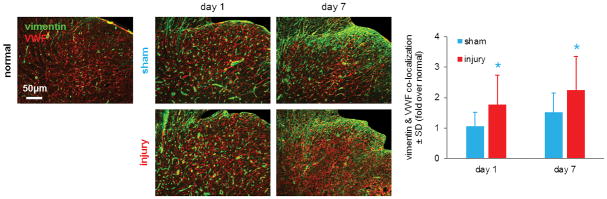
Spinal vimentin expression also localizes to vascular endothelial cells (Figure 4). Endothelial vimentin expression in the ipsilateral spinal cord is greater at day1 and day 7 after injury compared to its expression in sham tissue at the same times and normal tissue. When quantified, spinal endothelial vimentin is significantly greater after injury than sham overall (p<0.001) (Figure 4). Spinal endothelial vimentin is significantly elevated over sham levels by day 1 (p=0.030) and that injury-induced increase is maintained until day 7 (p=0.036) (Figure 4).
Figure 4. Spinal endothelial vimentin increases early and remains elevated after nerve root compression.
Spinal vimentin (green) co-localizes with von Willebrand factor (VWF; green) and is shown (yellow) in the representative images. When quantified, normalized spinal endothelial vimentin expression is significantly elevated after root injury compared to a sham procedure overall (p<0.001) and on days 1 and 7 (*p<0.036). Data are represented as mean ± standard deviation (SD).
Discussion
Spinal endothelial vimentin increases after mechanical trauma to the nerve root (Figure 4), paralleling the development and maintenance of mechanical hyperalgesia evident after a compressive nerve root injury (Figure 1) [14]. A transient nerve root compression induces mechanical hyperalgesia and mechanical allodynia by day 1, both of which are maintained for at least 7 days (Figure 1) [16, 21, 29]. A painful root compression also increases spinal astrocytic vimentin; however, that increase is not observed until day 7 (Figure 3), when pain persists (Figure 1).
Elevated spinal endothelial vimentin paralleling the development of pain-like behaviors after a nerve root compression (Figures 1 & 4) suggests that endothelial vimentin may contribute to pain. The same painful injury used in this study induces BSCB breakdown early after injury, which is restored to normal permeability by day 7 after injury [21]. Because increased endothelial vimentin expression has been associated with a strengthened endothelial barrier integrity in vitro [5, 19], the compression-induced increase in endothelial vimentin that is even more robust at day 7 than day 1 might contribute to restoring BSCB stability, which is evident at day 7 after painful compression [21]. Although spinal endothelial vimentin is also upregulated after a compression at day 1 when the BSCB is disrupted [21], it may not control the increased vascular permeability at that early time point. Instead, endothelial tight junction disruption may contribute to the decreased integrity of the spinal vasculature at day 1 after a compression [34–36].
The fact that vimentin expression increases in both spinal endothelial cells and astrocytes after painful root compression suggests that spinal cells may respond by changing their mechanical properties after a neural injury, even when that trauma is remote from the spinal cord itself. Increases in astrocytic and endothelial vimentin independently correlate with increases in cytoplasmic stiffness [1, 6]. Astrocytes comprise a majority of the cellular makeup of the spinal cord and significantly contribute to the tensile stiffness of the spinal cord [37, 38]. The elevated astrocytic vimentin observed at day 7 (Figure 3) may contribute to an increase in the stiffness of the spinal cord after a painful nerve root compression. In contrast, compressive stiffness of the spinal cord decreases for up to 8 weeks after a hemisection injury [39]. A hemisection injury compromises the local cellular and extracellular integrity of the spinal cord itself and the lesion is repopulated by activated astrocytes, oligodendrocytes, immune cells, and connective tissue elements that collectively form a glial scar [40, 41]. Yet, the macroscale changes in mechanical integrity of the spinal parenchyma after spinal cord injury are robust and likely dominate any contributions from changes in the mechanical properties of the resident cells. Since a nerve root compression is remote from the spinal cord and does not produce bulk structural changes to the spinal cord, any mechanical changes in the spinal parenchyma must be due to changes of the mechanical responses of the cells, or of the ECM proteins, in the spinal cord.
There are several limitations in the current study. Measuring vimentin expression alone does not provide information about the functionality of this intermediate filament. For example, the subcellular organization of vimentin, as well as the state of its phosphorylation, both contribute to its control over cytoplasmic stiffness [4, 19, 42]. However, since the mechanical properties of the spinal parenchyma were not measured directly, the hypothesis that changes in spinal vimentin expression modulated spinal parenchymal stiffness was not tested. Microscopic techniques to measure compressive stiffness, such as microindentation or atomic force microscopy (AFM), allow measurement of regional mechanical properties of biological tissues [43, 44]. Measuring the micromechanics of the spinal cord in regions that exhibit increased vimentin at day 7 after painful nerve root compression (Figure 2), would determine whether the spinal parenchyma undergoes stiffness changes that correspond to changes in cell mechanics. It is important to note that the studies presented here only begin to investigate the complex responses of spinal vimentin in the context of a neuropathic injury. Cause and effect relationships, as well as the specific upstream and downstream cellular pathways that are involved in vimentin modifications are necessary to define before the full influence of vimentin on pain is elucidated. Moreover, although the findings of this study point to an increase in spinal vimentin after painful neural trauma, they may be associated with the repair of the BSCB rather than modulating pain. Additional studies looking at these outcomes and at later time points will particularly add utility in the context of translating these findings to human patients.
Nevertheless, this is the first study to investigate the response of the intermediate filament, vimentin, within spinal astrocytes and endothelial cells after a peripheral neural injury. Astrocytic vimentin undergoes a delayed elevation at day 7 after a painful nerve root compression (Figure 3), which occurs along the same temporal profile as spinal GFAP elevation [16, 17] and the production of mechanical hyperalgesia (Figure 1). Endothelial vimentin also increases in the spinal cord at day 7 after a nerve root compression (Figure 4). The increase in endothelial vimentin at day 7 after painful injury corresponds with the delayed recovery of the BSCB stability [21], suggesting that an increase in endothelial vimentin might even be protective after neuropathic injury.
Acknowledgments
The Catherine Sharpe Foundation, the Department of Defense (W81XWH-10-1-1002), and by fellowships from the National Institutes of Health (T32-AR007132) and the Ashton Foundation funds were received in support of this work.
Footnotes
The manuscript submitted does not contain information about medical device(s)/drug(s).
No relevant financial activities outside the submitted work.
Level of Evidence: N/A
References
- 1.Guo M, Ehrlicher AJ, Mahammad S, et al. The role of vimentin intermediate filaments in cortical and cytoplasmic mechanics. Biophys J. 2013;105(7):1562–8. doi: 10.1016/j.bpj.2013.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herrmann H, Bar H, Kreplak L, et al. Intermediate filaments: from cell architecture to nanomechanics. Nat Rev Mol Cell Biol. 2007;8(7):562–73. doi: 10.1038/nrm2197. [DOI] [PubMed] [Google Scholar]
- 3.Janmey PA, Euteneuer U, Traub P, et al. Viscoelastic properties of vimentin compared with other filamentous biopolymer networks. J Cell Biol. 1991;113(1):155–60. doi: 10.1083/jcb.113.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buehler MJ. Mechanical players-The role of intermediate filaments in cell mechanics and organization. Biophys J. 2013;105(8):1733–4. doi: 10.1016/j.bpj.2013.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu T, Guevara OE, Warburton RR, et al. Regulation of vimentin intermediate filaments in endothelial cells by hypoxia. Am J Physiol Cell Physiol. 2010;299(2):C363–73. doi: 10.1152/ajpcell.00057.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu YB, Iandiev I, Hollborn M, et al. Reactive glial cells: increased stiffness correlates with increased intermediate filament expression. FASEB J. 2011;25(2):624–31. doi: 10.1096/fj.10-163790. [DOI] [PubMed] [Google Scholar]
- 7.Pekny M, Pekna M. Astrocyte intermediate filaments in CNS pathologies and regeneration. J Pathol. 2004;204(4):428–37. doi: 10.1002/path.1645. [DOI] [PubMed] [Google Scholar]
- 8.Ahmed WW, Li TC, Rubakhin SS, et al. Mechanical tension modulates local and global vesicle dynamics in neurons. Cell Mol Bioeng. 2012;5(2):155–64. doi: 10.1007/s12195-012-0223-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flanagan LA, Ju Y-E, Marg B, et al. Neurite branching on deformable substrates. Neuroreport. 2002;13(18):2411–5. doi: 10.1097/01.wnr.0000048003.96487.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koch D, Rosoff William J, Jiang J, et al. Strength in the Periphery: Growth Cone Biomechanics and Substrate Rigidity Response in Peripheral and Central Nervous System Neurons. Biophysical J. 2012;102(3):452–60. doi: 10.1016/j.bpj.2011.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calvo JL, Carbonell AL, Boya J. Co-expression of glial fabrillary acidic protein and vimentin in reactive astrocytes following brain injury in rats. Brain Res. 1991;566(1):333–6. doi: 10.1016/0006-8993(91)91720-l. [DOI] [PubMed] [Google Scholar]
- 12.Cao J, Wang JS, Ren XH, et al. Spinal sample showing p-JNK and P38 associated with the pain signaling transduction of glial cell in neuropathic pain. Spinal Cord. 2015;53(2):92–7. doi: 10.1038/sc.2014.188. [DOI] [PubMed] [Google Scholar]
- 13.Barreto GE, White RE, Xu L, et al. Effects of heat shock protein 72 (Hsp72) on evolution of astrocyte activation following stroke in the mouse. Exp Neurol. 2012;238(2):284–96. doi: 10.1016/j.expneurol.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hubbard RD, Winkelstein BA. Transient cervical nerve root compression in the rat induces bilateral forepaw allodynia and spinal glial activation: mechanical factors in painful neck injuries. Spine. 2005;30(17):1924–32. doi: 10.1097/01.brs.0000176239.72928.00. [DOI] [PubMed] [Google Scholar]
- 15.Nicholson KJ, Quindlen JC, Winkelstein BA. Development of a duration threshold for modulating evoked neuronal responses after nerve root compression injury. Stapp Car Crash J. 2011;55:1–24. doi: 10.4271/2011-22-0001. [DOI] [PubMed] [Google Scholar]
- 16.Rothman SM, Nicholson KJ, Winkelstein BA. Time-dependent mechanics and measures of glial activation and behavioral sensitivity in a rodent model of radiculopathy. J Neurotrauma. 2010;27(5):803–14. doi: 10.1089/neu.2009.1045. [DOI] [PubMed] [Google Scholar]
- 17.Rothman SM, Winkelstein BA. Chemical and mechanical nerve root insults induce differential behavioral sensitivity and glial activation that are enhanced in combination. Brain Res. 2007;1181:30–43. doi: 10.1016/j.brainres.2007.08.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grin B, Mahammad S, Wedig T, et al. Withaferin a alters intermediate filament organization, cell shape and behavior. PLoS One. 2012;7(6):e39065. doi: 10.1371/journal.pone.0039065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu T, Ghamloush MM, Aldawood A, et al. Modulating endothelial barrier function by targeting vimentin phosphorylation. J Cell Physiol. 2014;229(10):1484–93. doi: 10.1002/jcp.24590. [DOI] [PubMed] [Google Scholar]
- 20.Nieminen M, Henttinen T, Merinen M, et al. Vimentin function in lymphocyte adhesion and transcellular migration. Nat Cell Biol. 2006;8(2):156–62. doi: 10.1038/ncb1355. [DOI] [PubMed] [Google Scholar]
- 21.Smith JR, Galie PA, Slochower DR, et al. Salmon-derived thrombin inhibits development of chronic pain through an endothelial barrier protective mechanism dependent on ACP. Biomaterials. 2016;80:96–105. doi: 10.1016/j.biomaterials.2015.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gonzales M, Weksler B, Tsuruta D, et al. Structure and function of a vimentin-associated matrix adhesion in endothelial cells. Mol Biol Cell. 2001;12(1):85–100. doi: 10.1091/mbc.12.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haseloff RF, Krause E, Bigl M, et al. Differential protein expression in brain capillary endothelial cells induced by hypoxia and posthypoxic reoxygenation. Proteomics. 2006;6(6):1803–9. doi: 10.1002/pmic.200500182. [DOI] [PubMed] [Google Scholar]
- 24.Liu T, Guevara OE, Warburton RR, et al. Modulation of HSP27 alters hypoxia-induced endothelial permeability and related signaling pathways. J Cell Physiol. 2009;220(3):600–10. doi: 10.1002/jcp.21773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baron R, Binder A, Wasner G. Neuropathic pain: diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol. 2010;9(8):807–19. doi: 10.1016/S1474-4422(10)70143-5. [DOI] [PubMed] [Google Scholar]
- 26.Merksey H, Bogduk N. Classification of chronic pain. Seattle: IASP Press; 1994. [Google Scholar]
- 27.Winkelstein BA. How can animal models inform on the transition to chronic symptoms in whiplash? Spine. 2010;36(25 Suppl):S218–25. doi: 10.1097/BRS.0b013e3182387f96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Woolf CJ, Mannion RJ. Neuropathic pain: aetiology, symptoms, mechanisms, and management. Lancet. 1994;353(9168):1959–64. doi: 10.1016/S0140-6736(99)01307-0. [DOI] [PubMed] [Google Scholar]
- 29.Chang YW, Winkelstein BA. Schwann cell proliferation and macrophage infiltration are evident at day 14 after painful cervical nerve root compression in the rat. J Neurotrauma. 2011;28(12):2429–38. doi: 10.1089/neu.2011.1918. [DOI] [PubMed] [Google Scholar]
- 30.Chaplan SR, Bach FW, Pogrel JW, et al. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53(1):55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 31.Lee KE, Winkelstein BA. Joint distraction magnitude is associated with different behavioral outcomes and substance P levels for cervical facet joint loading in the rat. J Pain. 2009;10(4):436–45. doi: 10.1016/j.jpain.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 32.Nicholson KJ, Guarino BB, Winkelstein BA. Transient nerve root compression load and duration differentially mediate behavioral sensitivity and associated spinal astrocyte activation and mGLuR5 expression. Neuroscience. 2012;209:187–95. doi: 10.1016/j.neuroscience.2012.02.027. [DOI] [PubMed] [Google Scholar]
- 33.Dong L, Smith JR, Winkelstein BA. Ketorolac reduces spinal astrocytic activation and PAR1 expression associated with attenuation of pain after facet joint injury. J Neurotrauma. 2013;30(10):818–25. doi: 10.1089/neu.2012.2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis. 2004;16(1):1–13. doi: 10.1016/j.nbd.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 35.Echeverry S, Shi XQ, Rivest S, et al. Peripheral nerve injury alters blood-spinal cord barrier functional and molecular integrity through a selective inflammatory pathway. J Neurosci. 2011;31(30):10819–28. doi: 10.1523/JNEUROSCI.1642-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57(2):173–85. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- 37.Shreiber DI, Hao H, Elias RA. Probing the influence of myelin and glia on the tensile properties of the spinal cord. Biomech Model Mechanobiol. 2009;8(4):311–21. doi: 10.1007/s10237-008-0137-y. [DOI] [PubMed] [Google Scholar]
- 38.Vallejo R, Tilley DM, Vogel L, et al. The role of glia and the immune system in the development and maintenance of neuropathic pain. Pain Pract. 2010;10(3):167–84. doi: 10.1111/j.1533-2500.2010.00367.x. [DOI] [PubMed] [Google Scholar]
- 39.Saxena T, Gilbert J, Stelzner D, et al. Mechanical characterization of the injured spinal cord after lateral spinal hemisection injury in the rat. J Neurotrauma. 2012;29(9):1747–57. doi: 10.1089/neu.2011.1818. [DOI] [PubMed] [Google Scholar]
- 40.Fitch MT, Silver J. CNS injury, glial scars, and inflammation: Inhibitory extracellular matrices and regeneration failure. Exp Neurol. 2008;209(2):294–301. doi: 10.1016/j.expneurol.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hausmann ON. Post-traumatic inflammation following spinal cord injury. Spinal Cord. 2003;41(7):369–78. doi: 10.1038/sj.sc.3101483. [DOI] [PubMed] [Google Scholar]
- 42.Li QF, Spinelli AM, Wang R, et al. Critical role of vimentin phosphorylation at Ser-56 by p21-activated kinase in vimentin cytoskeleton signaling. J Biol Chem. 2006;281(45):34716–24. doi: 10.1074/jbc.M607715200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Levental I, Levental KR, Klein EA, et al. A simple indentation device for measuring micrometer-scale tissue stiffness. J Phys Condens Matter. 2010;22(19):194120. doi: 10.1088/0953-8984/22/19/194120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin DC, Horkay F. Nanomechanics of polymer gels and biological tissues: a critical review of analytical approaches in the Hertzian regime and beyond. Soft Matter. 2008;4(4):669–82. doi: 10.1039/b714637j. [DOI] [PubMed] [Google Scholar]