Abstract
E-cigarette derived inhaled nicotine may contribute to the pathogenesis of periodontal and pulmonary diseases in particular via lung inflammation, injurious and dysregulated repair responses. Nicotine is shown to have anti-proliferative properties and affects fibroblasts in vitro, which may interfere in tissue myofibroblast differentiation in e-cig users. This will affect the ability to heal wounds by decreasing wound contraction. In periodontics, direct exposure to e-vapor has been shown to produce harmful effects in periodontal ligament and gingival fibroblasts in culture. This is due to the generation of reactive oxygen species/aldehydes/carbonyls from e-cig aerosol, leading to protein carbonylation of extracellular matrix and DNA adducts/damage. A limited number of studies regarding the effects of e-cig in oral and lung health are available. However, no reports are available to directly link the deleterious effects on e-cigs, inhaled nicotine, and flavorings aerosol on oral periodontal and pulmonary health in particular to identify the risk of oral diseases by e-cigarettes and nicotine aerosols. This mini-review summarizes the recent perspectives on e-cigarettes including inhaled nicotine effects on several pathophysiological events, such as oxidative stress, DNA damage, innate host response, inflammation, cellular senescence, pro-fibrogenic and dysregulated repair, leading to lung remodeling, oral submucous fibrosis and periodontal diseases.
Keywords: e-cigarettes, fibrosis, inflammation, lung, oxidative stress, periodontium
Introduction
Electronic cigarettes (E-cigs) are battery-operated devices, which consist of a metal heating element in a stainless steel shell, a cartridge, an atomizer and a battery. The heating element vaporizes a solution containing a mixture of chemicals including nicotine and other additives/humectants, such as base/carrying agents, propylene glycol, glycerin/glycerol, and hundreds of flavoring agents including fruit and candy flavors (Cheng, 2014, Barrington-Trimis et al., 2014). Apart of high concentration of nicotine (up to 24 mg), numerous chemicals including aldehydes (as carbonyls), heavy metals (nickel, chromium, copper-coated with silver), metal particle/ultrafine/nano-particles, and tobacco specific nitrosamines as well as diacetyl, 2,3-pentanedione, and acetoin (buttery) are found in e-cig aerosols (Kosmider et al., 2014, Cheng, 2014). Other flavoring chemicals include ortho-vanilin (vanilla), maltol (malt), cinnamaldehyde and coumarin (Gerloff et al., 2017). Variable levels of carbonyls (e.g., up to 380 µg formaldehyde/10 puffs) have been detected in e-cig aerosols during vaporizations (Jensen et al., 2015, Kosmider et al., 2014). Moreover, a general lack of oversight in manufacturing and marketing of e-liquid/e-juices has been reported (Lisko et al., 2015). Therefore, significant concerns exist regarding the purity and variety (e.g., flavor additives) of ingredients employed.
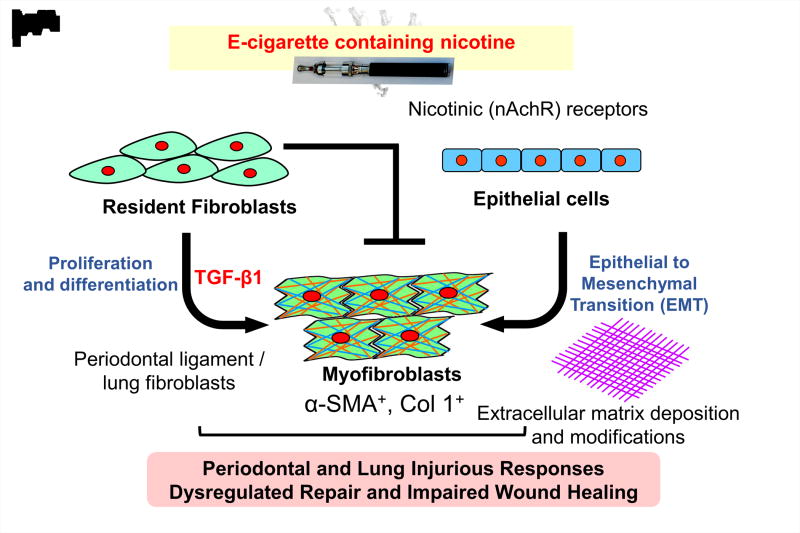
The use of e-cigs has increased in the United States (U.S.) and worldwide particularly among young adults (Regan et al., 2013, Krishnan-Sarin et al., 2014). In the U.S., approximately 11% to 21% of adult smokers have reported to have ever used E-cigs (Varlet et al., 2015). Despite rising e-cig use, only a limited number of studies have addressed the potential toxicological effect of e-cig smoking on oral health (Sundar et al., 2016, Harrison & Hicklin, 2016, Rouabhia et al., 2016). Exposure to e-cig aerosol mixtures with flavorings may increase oxidative/carbonyl stress and inflammatory cytokine release in human periodontal ligament fibroblasts, human gingival epithelium progenitors, pooled cells (HGEPp), and 3D EpiGingival tissues (Sundar et al., 2016). Various aldehydes including acrolein and formaldehyde are found in the aerosols from e-cigs (Cheng, 2014, Kosmider et al., 2014). E-cig-derived aldehydes cause carbonyl/oxidative stress and DNA adducts/damage, which may lead to dysregulated repair and impaired wound healing, in particular in smokers (Figure 1) (Pradeep et al., 2013, Baltacioglu et al., 2008, Lei et al, 2017).
Fig. 1. Possible mechanism of e-cigarette induced inflammatory and dysregulated repair responses to inhaled nicotine.
Inhaled nicotine impact effects on lung and systemic inflammatory mediators in oral fluids and causes dysregulated repair responses via its receptor α7 nicotinic acetylcholine receptor (α7nAChR). Nicotine also causes impaired wound healing due to inhibition of myofibroblasts differentiation and/or epithelial-mesenchymal transition. Oxidative stress and vascular remodeling by inhaled nicotine may trigger inflammatory responses in periodontal tissues. Oxidative stress can lead to carbonylation of extracellular matrix and further deposition of modified matrix. All these responses are associated with initiation of oral submucosal fibrosis.
While the contribution of smoking tobacco to the progression of periodontal diseases and other adverse oral health outcomes is well described (Reibel, 2003, Javed et al., 2014, Brown et al., 1996, Albandar et al., 1999); there is currently no information available regarding the impact of e-cig aerosols vaping on oral and systemic health. The aim of the present review is to briefly review and summarize the available evidence about the effects of e-cig aerosols on periodontal and pulmonary health.
PubMed (National Library of Medicine), Google-Scholar, Scopus, EMBASE, MEDLINE (OVID) and Web of Knowledge databases were searched to identify articles that assessed the effects of e-cig on periodontal and pulmonary health. All levels of available evidence (including in vitro studies, studies in animal models, case reports and case series) were included. Commentaries and letters to the editor were however not sought.
Electronic nicotine delivery system and inhaled nicotine
Nicotine is a main bioactive component of tobacco-derived products, including conventional cigarettes, cigars, cigarillos, e-cigarettes, and waterpipes (ranges from 0 mg–100 mg/ml). Nicotine is well known for its addictive properties. Nicotine delivery systems (electronic nicotine-delivery systems [ENDS]) have recently emerged. These ENDS are proposed to reduce craving for conventional cigarettes, but are not regulated like tobacco (Regan et al., 2013, Giovino et al., 2012). Recently, a rapid growth has taken place in both marketing and consumption of e-cigs (Regan et al., 2013). With each “puff,” the heating element vaporizes a small amount of liquid. In this format, the ENDS user is not inhaling smoke, but an aerosol/vapor of nicotine (up to 24–100 mg) as mist/vapor (Jorenby et al., 2016, Ruhe et al., 2017). Hence, ENDS will deliver a significant amount of nicotine compared to tobacco cessation devices available commercially.
E-cig aerosols and respiratory system
ENDS are unique in their ability to deliver a nicotine laden aerosol to the lung by inhalation (Cheng, 2014, Barrington-Trimis et al., 2014). Concentration of nicotine varies in commercial e-fluids/e-juices (Pisinger & Dossing, 2014). With the recent emergence and increasing popularity among youth/adults of multiple devices for the recreational inhalation of non-combustible nicotine, e.g., e-cigarettes, the importance of understanding effects of inhaled nicotine is needed. There are increasing numbers of reports regarding the direct effect of ENDS aerosol on health in recent years (Lerner et al., 2015b, Schweitzer et al., 2015). Although carcinogens appear to be reduced or eliminated in e-cigs, health concerns surrounding nicotine have been raised (Cahn & Siegel, 2011, Cobb & Abrams, 2011). Currently, listed as a reproductive or developmental toxicant, some studies suggest that nicotine may increase cardiovascular stress (Benowitz & Gourlay, 1997, Girdler et al., 1997), but the toxicological effects of inhaled nicotine delivered in to the lung are not well known. Nicotine binds to a family of nicotinic acetylcholine receptors (nAChRs), similar to acetylcholine (ACh) (Jensen et al., 2012, Carlisle et al., 2004). nAChRs are abundantly expressed in fibroblasts and epithelial cells of the lung (Wilk et al., 2012, Sekhon et al., 2002). Moreover, these receptors trigger protease expression (Li & Dai, 2012), mucin production (Fu et al., 2011) and smooth muscle contraction (Hahn et al., 1992), which mediate airway obstruction in chronic obstructive pulmonary disease (COPD). Short-term use of ENDS causes an increase in impedance, peripheral airway flow resistance, and oxidative stress among healthy smokers (Vardavas et al., 2012, Flouris et al., 2013). ENDS inhalation increases allergen-induced airway hyper-responsiveness, alters innate immunity/host response, and increases virulence of colonizing bacteria and virus infection (Wu et al., 2014), hence affecting local microbiota/microbiome. Nicotine acts via α7nAChR to induce MUC5AC-expression and increases mucus production (Gundavarapu et al., 2012, Chen et al., 2014). This supports the notion that nicotine imparts its effect via the nicotinic receptors in downstream signaling pathways.
Inhaled nicotine effects on airway remodeling, pro-fibrogenic response and dysregulated repair
Tobacco smoking is associated with chronic airways disease and lung remodeling (Ji et al., 2016); however, the role of nicotine (particularly inhaled nicotine) in this regard remains unclear. Nicotine can also promote airway remodeling via its receptor in airway smooth muscle (Hahn et al., 1992). Small airway remodeling is a key feature in the development of COPD (Hogg et al., 2004, McDonough et al., 2011). These studies suggest that ENDS aerosol causes adverse health effects in users, but there are no long-term studies of ENDS use are available especially in airway obstruction/emphysematous and pro-fibrogenic remodeling/dysregulated repair responses. Thus, it is possible that inhaled nicotine can have repercussions on cellular homeostasis and the pulmonary system. Further, inhaled nicotine can lead to airway remodeling, pro-fibrogenic and dysregulated repair by e-cig aerosols (Lei et al., 2017). Inhaled nicotine may affect the ability of mesenchymal stromal/stem cells (MSCs) for their ability to heal the wounds or in general repair processes.
Inhaled nicotine, oxidative stress and DNA damage
Recent studies have indicated lung cellular toxicity by e-cig products (Shimosato et al., 2012, Cahn & Siegel, 2011, Wu et al., 2014, Cervellati et al., 2014). Lerner et al. reported increased mitochondrial ROS, DNA nuclear fragmentation and impaired stability of electron transport chain complex IV subunit on human lung fibroblasts exposed to e-cig and end-products (copper nanoparticles) (Lerner et al., 2016). Likewise, Schweitzer et al. reported that components of e-cig (acrolein, propylene glycol, glycerol, and nicotine) produced a dose-dependent loss of lung endothelial barrier function and inflammation associated with increased intracellular ceramides and myosin light chain phosphorylation (Schweitzer et al., 2015). Gerloff and co-workers have recently shown that e-cig and various flavoring agents can trigger inflammatory response and barrier dysfunction in human lung epithelial cells (Gerloff et al., 2017). Therefore, it is feasible that the exposure to e-cig and its products might lead to augmented oxidative stress and inflammatory responses in lung cells and tissues in chronic exposure conditions.
E-cig aerosols and oral health effects: impact on cellular senescence
Carbonyl/oxidative stress lead to stress-induced cellular senescence (a state of irreversible growth arrest which re-enforces chronic inflammation) and impaired myofibroblast differentiation and epithelial mesenchymal transition (Figure 1). E-cig aerosols upregulate the receptors for advanced glycation end-products (RAGE) in human oral fibroblasts and gingival epithelial cells, which is regulated by histone deacetylase 2 (HDAC2) (Sundar et al., 2016). Both RAGE and HDAC2 are implicated in regulation of inflammation and cellular senescence. However, no information is available regarding the role of RAGE and HDAC2 in regulating cellular senescence and inflammatory responses by e-cig aerosol in oral tissues (Table 1). E-cig aerosols may affect cellular signaling in periodontal ligament fibroblasts and MSCs.
Table 1.
Markers and targets for periodontal and lung diseases by inhaled e-cig aerosol containing nicotine
| Markers | Targets |
|---|---|
| Oxidative stress | Lipid peroxidation products 4-hydroxy-2-nonenal, malondialdehyde, F2-isoprostanes |
| Inflammatory responses (cytokines and prostaglandins) |
NF-kappa B, Toll like receptors, NLRP3 inflammasome |
| Antioxidants | Glutathione, superoxide dismutases, antioxidant enzymes, lipid peroxidation inhibitors |
| Innate host defense | RAGE receptors (S100A8 and S100A9) Advanced glycation end products Histone deacetylases (HDACs) |
| Lipid mediators | Resolvins, polyunsaturated fatty acids (omega 3 fatty acids) |
| Proteases | Matrix metalloproteases (MMP-9, MMP-12) |
| Growth factors | VEGF, FGF, fibroblast growth factor (FGF), PDGF, TGF-β |
| Myofibroblast differentiation/wound healing | TGF-β, PGE2, GM-CSF, prostacyclins |
E-cigarette aerosol, inhaled nicotine, and periodontal complications
Periodontal disease is characterized by chronic inflammation of the supporting tissues of the teeth (Albandar et al., 1999, Brown et al., 1996, Hajishengallis, 2015). Periodontal ligament cells and gingival fibroblasts as well as epithelial cells are the most abundant structural cells in periodontal tissues playing a fundamental role in periodontal regeneration. Upon stimulation or stress, these cells are able to incite and maintain inflammatory responses (Ara et al., 2009). There is an association between smoking and tooth loss, periodontal attachment level, deeper periodontal pockets, and more extensive alveolar bone loss along with the destruction of connective tissue and matrix (Giorgetti et al., 2012, Correa et al., 2010, Cesar-Neto et al., 2006), leading to increased risk of periodontitis (Reibel, 2003, Javed et al., 2014). Oxidants/reactive oxygen species reactivity from e-cig aerosols is comparable to conventional cigarette smoke (Lerner et al., 2015a). Moreover, direct exposure to e-liquids has also been shown to produce harmful effects in periodontal ligament cells and gingival fibroblasts in culture (Willershausen et al., 2014, Sancilio et al., 2015). Reactive aldehydes/carbonyls derived from e-cig aerosol can cause protein carbonylation and DNA adducts/damage, and carbonyls are cleaved by aldehyde dehydrogenase (ALDH). Protein carbonylation leads to autoantibody production, which may lead to destruction of matrix and bone loss during periodontitis (Pradeep et al., 2013, Baltacioglu et al., 2008). Hence, it is possible that carbonyls/aldehydes play an important role in e-cig aerosol-induced oral toxicity. Nicotine is shown to have anti-proliferative properties and affects fibroblasts in vitro (Rothem et al., 2009, Frazer-Abel et al., 2004). This implicates that E-cig containing nicotine affects oral myofibroblast differentiation in e-cig users; and hence may affect their ability to heal wounds by decreasing wound contraction by myofibroblasts (Lei et al., 2017). This may be due to the release of prostaglandins (PGE2) and matrix metalloproteases (MMP-9, MMP-12) as well as their effects on MSCs. Likewise, Holliday et al. reported that e-cigarette-exposed cells presented reduced viability and clonogenic survival, along with increased rates of apoptosis and necrosis in vitro (Holliday et al., 2016). Further, the nicotine exposed cells presented significantly increased comet tail length and accumulation of γ-H2AX foci, demonstrating increased DNA strand breaks (Sundar et al., 2016).
Resolvins, pro-resolving lipid mediators including resolvin D1 (derivatives of omega-3 polyunsaturated fatty acids ω-3-PUFAs), are shown to resolve inflammation in periodontitis in vivo and in vitro models including animal model of periodontitis (Odusanwo et al., 2012, Mustafa et al., 2013, Hasturk et al., 2007). However, the effects of e-cig aerosols on carbonyl stress, inflammation, antioxidants, pro-resolving mediators, pro-fibrogenic response, and cellular senescence have not been mechanistically studied (Table 1).
E-cigarette devices or ENDS deliver nicotine at varying concentrations. Nicotine has been associated with impaired leukocyte activity and healing by inhibiting neovascularization and osteoblastic differentiation (Levin & Schwartz-Arad, 2005). Similarly, tobacco smoking including nicotine is associated with an increased risk of implant failure, impaired healing, poor papilla regeneration, and increased bone loss (Twito & Sade, 2014, Raes et al., 2015, Al Amri et al., 2016). Therefore, it is likely that nicotine derived from e-cig may impair healing potential at the bone/implant interface. This may also be due to impair functions of MSCs or resident stem cells by nicotine. Berley et al. reported decreased bone to implant contact after 4 weeks of implant placement in rats’femur receiving subcutaneous nicotine (Berley et al., 2010). Likewise, Yamano et al. reported a down-regulation in the expression of bone matrix-related genes around implants in rats receiving nicotine for 8 weeks (Yamano et al., 2010). However, the effects of nicotine delivery by e-cig on peri-implant soft and hard tissues as well as other periodontal complications have not been studied.
E-cig aerosols and oral submucous fibrosis
Oral submucous fibrosis (OSF) is a chronic potentially malignant disorder, characterized by progressive submucosal fibrosis of the oral tissues and the oropharynx. Approximately 7% to 13% of patients with OSF develop in oral squamous cell carcinoma (Liu et al., 2015). Tobacco smoking has been associated with higher risks of OSF. Furthermore, the risk increases among smokers consuming chewable tobacco (Liu et al., 2015). It has been suggested that nicotine and arecoline might induce the over-expression of human telomerase reverse transcriptase (hTERT) mRNA in oral keratinocytes (affecting cellular senescence due to telomerase and telomere length), which may lead to the malignancy of OSF (Gao et al., 2007). Arecoline has also shown to induce fibroblast proliferation by the up-regulation of growth factors expression and endothelial necrosis (Ullah et al., 2015). It is hypothesized that e-cig and end-products might play a role in the manifestation, progression and malignancy of OSF via cellular senescence. However, no information is available regarding the e-cig effects on OSF.
Conclusion
E-cigs and/or inhaled nicotine along with various flavoring chemicals may contribute to the pathogenesis of periodontal and pulmonary diseases in particular via lung inflammation, injurious, and dysregulated repair responses via its effect on oral myofibroblast differentiation. This may have an affect their ability to heal wounds by decreasing wound contraction by release of various pro-inflammatory mediators. E-cig and its flavoring agents along with their chemical interactions with nicotine may produce harmful effects in periodontal ligament, stem cells, and gingival fibroblasts in cultures due generation of aldehydes/carbonyls from e-cig aerosol, leading to protein carbonylation of extracellular matrix and DNA adducts/damage, and cellular senescence. However, the association between E-cig and impaired would healing, oral fibrosis and bronchiolitis obliterans (popcorn lung) remains unknown. The research findings discussed in this review will not only provide information for further research on e-cigs and inhaled nicotine, but also for other tobacco products including conventional tobacco and waterpipe/hookah smoking alone or in combinations i.e. poly-use of these products. Further research is required to establish the risk of using e-cig on oral, systemic and pulmonary responses, and could help the public health community to identify and deliver appropriate messages about e-cigarettes’ (inhaled nicotine) safety and promote future product regulation.
Acknowledgments
This study was supported by the NIH 1R01HL135613, NIH 2R01HL085613 and 3R01HL085613-07S1.
Footnotes
Author contributions:
FJ, SVK, ISK, IR: Conceived the idea and wrote the manuscript; FJ, GER and IR: Edited revised critically the manuscript.
Conflicting interests: The authors have declared that no conflict of interest exists and none to declare.
References
- Al Amri MD, Kellesarian SV, Abduljabbar TS, Al-Rifaiy MQ, Al Baker AM, Al-Keraif AA. Comparison of Peri-Implant Soft Tissue Parameters and Crestal Bone Loss Around Immediately-Loaded and Delayed Loaded Implants Among Smokers and Nonsmokers: 5-Year Follow-Up Results. J Periodontol. 2016:1–12. doi: 10.1902/jop.2016.160427. [DOI] [PubMed] [Google Scholar]
- Albandar JM, Brunelle JA, Kingman A. Destructive periodontal disease in adults 30 years of age and older in the United States, 1988–1994. Journal of periodontology. 1999;70:13–29. doi: 10.1902/jop.1999.70.1.13. [DOI] [PubMed] [Google Scholar]
- Ara T, Kurata K, Hirai K, Uchihashi T, Uematsu T, Imamura Y, Furusawa K, Kurihara S, Wang PL. Human gingival fibroblasts are critical in sustaining inflammation in periodontal disease. Journal of periodontal research. 2009;44:21–27. doi: 10.1111/j.1600-0765.2007.01041.x. [DOI] [PubMed] [Google Scholar]
- Baltacioglu E, Akalin FA, Alver A, Deger O, Karabulut E. Protein carbonyl levels in serum and gingival crevicular fluid in patients with chronic periodontitis. Archives of oral biology. 2008;53:716–722. doi: 10.1016/j.archoralbio.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Barrington-Trimis JL, Samet JM, McConnell R. Flavorings in electronic cigarettes: an unrecognized respiratory health hazard? JAMA. 2014;312:2493–2494. doi: 10.1001/jama.2014.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL, Gourlay SG. Cardiovascular toxicity of nicotine: implications for nicotine replacement therapy. Journal of the American College of Cardiology. 1997;29:1422–1431. doi: 10.1016/s0735-1097(97)00079-x. [DOI] [PubMed] [Google Scholar]
- Berley J, Yamano S, Sukotjo C. The effect of systemic nicotine on osseointegration of titanium implants in the rat femur. The Journal of oral implantology. 2010;36:185–193. doi: 10.1563/AAID-JOI-D-09-00050. [DOI] [PubMed] [Google Scholar]
- Brown LJ, Brunelle JA, Kingman A. Periodontal status in the United States, 1988–1991: prevalence, extent, and demographic variation. Journal of dental research. 1996;75:672–683. doi: 10.1177/002203459607502S07. Spec No. [DOI] [PubMed] [Google Scholar]
- Cahn Z, Siegel M. Electronic cigarettes as a harm reduction strategy for tobacco control: a step forward or a repeat of past mistakes? Journal of public health policy. 2011;32:16–31. doi: 10.1057/jphp.2010.41. [DOI] [PubMed] [Google Scholar]
- Carlisle DL, Hopkins TM, Gaither-Davis A, Silhanek MJ, Luketich JD, Christie NA, Siegfried JM. Nicotine signals through muscle-type and neuronal nicotinic acetylcholine receptors in both human bronchial epithelial cells and airway fibroblasts. Respiratory research. 2004;5:27. doi: 10.1186/1465-9921-5-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervellati F, Muresan XM, Sticozzi C, Gambari R, Montagner G, Forman HJ, Torricelli C, Maioli E, Valacchi G. Comparative effects between electronic and cigarette smoke in human keratinocytes and epithelial lung cells. Toxicology in vitro : an international journal published in association with BIBRA. 2014;28:999–1005. doi: 10.1016/j.tiv.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesar-Neto JB, Benatti BB, Sallum EA, Casati MZ, Nociti FH., Jr The influence of cigarette smoke inhalation and its cessation on the tooth-supporting alveolar bone: a histometric study in rats. Journal of periodontal research. 2006;41:118–123. doi: 10.1111/j.1600-0765.2005.00844.x. [DOI] [PubMed] [Google Scholar]
- Chen EY, Sun A, Chen CS, Mintz AJ, Chin WC. Nicotine alters mucin rheological properties. American journal of physiology. Lung cellular and molecular physiology. 2014;307:L149–L157. doi: 10.1152/ajplung.00396.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng T. Chemical evaluation of electronic cigarettes. Tob Control. 2014;23(Suppl 2):ii11–ii17. doi: 10.1136/tobaccocontrol-2013-051482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb NK, Abrams DB. E-cigarette or drug-delivery device? Regulating novel nicotine products. The New England journal of medicine. 2011;365:193–195. doi: 10.1056/NEJMp1105249. [DOI] [PubMed] [Google Scholar]
- Correa MG, Campos ML, Benatti BB, Marques MR, Casati MZ, Nociti FH, Jr, Sallum EA. The impact of cigarette smoke inhalation on the outcome of enamel matrix derivative treatment in rats: histometric analysis. Journal of periodontology. 2010;81:1820–1828. doi: 10.1902/jop.2010.100200. [DOI] [PubMed] [Google Scholar]
- Flouris AD, Chorti MS, Poulianiti KP, Jamurtas AZ, Kostikas K, Tzatzarakis MN, Wallace Hayes A, Tsatsaki AM, Koutedakis Y. Acute impact of active and passive electronic cigarette smoking on serum cotinine and lung function. Inhalation toxicology. 2013;25:91–101. doi: 10.3109/08958378.2012.758197. [DOI] [PubMed] [Google Scholar]
- Frazer-Abel AA, Baksh S, Fosmire SP, Willis D, Pierce AM, Meylemans H, Linthicum DS, Burakoff SJ, Coons T, Bellgrau D, Modiano JF. Nicotine activates nuclear factor of activated T cells c2 (NFATc2) and prevents cell cycle entry in T cells. The Journal of pharmacology and experimental therapeutics. 2004;311:758–769. doi: 10.1124/jpet.104.070060. [DOI] [PubMed] [Google Scholar]
- Fu XW, Wood K, Spindel ER. Prenatal nicotine exposure increases GABA signaling and mucin expression in airway epithelium. American journal of respiratory cell and molecular biology. 2011;44:222–229. doi: 10.1165/rcmb.2010-0109OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao YJ, Ling TY, Yin XM, Li X, Huang Y. Effects of arecoline and nicotine on the expression of hTERT in oral keratinocytes. Zhonghua kou qiang yi xue za zhi = Zhonghua kouqiang yixue zazhi = Chinese journal of stomatology. 2007;42:26–30. [PubMed] [Google Scholar]
- Gerloff J, Sundar IK, Freter R, Sekera ER, Friedman AE, Robinson RJ, Pagano T, Rahman I. Inflammatory response and barrier dysfunction by different e-cigarette flavoring chemicals identified by GC-MS in e-liquids and e-vapors on human lung epithelial cells and fibroblasts. Applied In Vitro Toxicology. 2017 doi: 10.1089/aivt.2016.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgetti AP, Cesar Neto JB, Casati MZ, Sallum EA, Nociti Junior FH. Cigarette smoke inhalation influences bone healing of post-extraction tooth socket: a histometric study in rats. Brazilian dental journal. 2012;23:228–234. doi: 10.1590/s0103-64402012000300008. [DOI] [PubMed] [Google Scholar]
- Giovino GA, Mirza SA, Samet JM, Gupta PC, Jarvis MJ, Bhala N, Peto R, Zatonski W, Hsia J, Morton J, Palipudi KM, Asma S, Group GC. Tobacco use in 3 billion individuals from 16 countries: an analysis of nationally representative cross-sectional household surveys. Lancet. 2012;380:668–679. doi: 10.1016/S0140-6736(12)61085-X. [DOI] [PubMed] [Google Scholar]
- Girdler SS, Jamner LD, Jarvik M, Soles JR, Shapiro D. Smoking status and nicotine administration differentially modify hemodynamic stress reactivity in men and women. Psychosomatic medicine. 1997;59:294–306. doi: 10.1097/00006842-199705000-00012. [DOI] [PubMed] [Google Scholar]
- Gundavarapu S, Wilder JA, Mishra NC, Rir-Sima-Ah J, Langley RJ, Singh SP, Saeed AI, Jaramillo RJ, Gott KM, Pena-Philippides JC, Harrod KS, McIntosh JM, Buch S, Sopori ML. Role of nicotinic receptors and acetylcholine in mucous cell metaplasia, hyperplasia, and airway mucus formation in vitro and in vivo. The Journal of allergy and clinical immunology. 2012;130:770–780. e11. doi: 10.1016/j.jaci.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn HL, Lang M, Bleicher S, Zwerenz S, Rausch C. Nicotine-induced airway smooth muscle contraction: neural mechanisms involving the airway epithelium. Functional and histologic studies in vitro. The Clinical investigator. 1992;70:252–262. doi: 10.1007/BF00184659. [DOI] [PubMed] [Google Scholar]
- Hajishengallis G. Periodontitis: from microbial immune subversion to systemic inflammation. Nature reviews. Immunology. 2015;15:30–44. doi: 10.1038/nri3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison R, Hicklin D., Jr Electronic cigarette explosions involving the oral cavity. Journal of the American Dental Association (1939) 2016;147:891–896. doi: 10.1016/j.adaj.2016.03.018. [DOI] [PubMed] [Google Scholar]
- Hasturk H, Kantarci A, Goguet-Surmenian E, Blackwood A, Andry C, Serhan CN, Van Dyke TE. Resolvin E1 regulates inflammation at the cellular and tissue level and restores tissue homeostasis in vivo. Journal of immunology. 2007;179:7021–7029. doi: 10.4049/jimmunol.179.10.7021. [DOI] [PubMed] [Google Scholar]
- Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, Cherniack RM, Rogers RM, Sciurba FC, Coxson HO, Pare PD. The nature of small-airway obstruction in chronic obstructive pulmonary disease. The New England journal of medicine. 2004;350:2645–2653. doi: 10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- Holliday R, Kist R, Bauld L. E-cigarette vapour is not inert and exposure can lead to cell damage. Evid Based Dent. 2016;17:2–3. doi: 10.1038/sj.ebd.6401143. [DOI] [PubMed] [Google Scholar]
- Javed F, Bashir Ahmed H, Romanos GE. Association between environmental tobacco smoke and periodontal disease: a systematic review. Environ Res. 2014;133:117–122. doi: 10.1016/j.envres.2014.05.008. [DOI] [PubMed] [Google Scholar]
- Jensen K, Nizamutdinov D, Guerrier M, Afroze S, Dostal D, Glaser S. General mechanisms of nicotine-induced fibrogenesis. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2012;26:4778–4787. doi: 10.1096/fj.12-206458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen RP, Luo W, Pankow JF, Strongin RM, Peyton DH. Hidden Formaldehyde in E-Cigarette Aerosols. The New England journal of medicine. 2015;372:392–394. doi: 10.1056/NEJMc1413069. [DOI] [PubMed] [Google Scholar]
- Ji W, Lim MN, Bak SH, Hong SH, Han SS, Lee SJ, Kim WJ, Hong Y. Differences in chronic obstructive pulmonary disease phenotypes between non-smokers and smokers. The clinical respiratory journal. 2016 doi: 10.1111/crj.12577. [DOI] [PubMed] [Google Scholar]
- Jorenby DE, Smith SS, Fiore MC, Baker TB. Nicotine levels, withdrawal symptoms, and smoking reduction success in real world use: A comparison of cigarette smokers and dual users of both cigarettes and E-cigarettes. Drug and alcohol dependence. 2016;170:93–101. doi: 10.1016/j.drugalcdep.2016.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosmider L, Sobczak A, Fik M, Knysak J, Zaciera M, Kurek J, Goniewicz ML. Carbonyl compounds in electronic cigarette vapors: effects of nicotine solvent and battery output voltage. Nicotine Tob Res. 2014;16:1319–1326. doi: 10.1093/ntr/ntu078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan-Sarin S, Morean ME, Camenga DR, Cavallo DA, Kong G. E-cigarette Use Among High School and Middle School Adolescents in Connecticut. Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco. 2014 doi: 10.1093/ntr/ntu243. pii: ntu243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei W, Lerner C, Sundar IK, Rahman I. Myofibroblast differentiation and its functional properties are inhibited by nicotine and e-cigarette via mitochondrial OXPHOS complex III. Scientific Reports. 2017 doi: 10.1038/srep43213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner CA, Rutagarama P, Ahmad T, Sundar IK, Elder A, Rahman I. Electronic cigarette aerosols and copper nanoparticles induce mitochondrial stress and promote DNA fragmentation in lung fibroblasts. Biochem Biophys Res Commun. 2016;477:620–625. doi: 10.1016/j.bbrc.2016.06.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner CA, Sundar IK, Watson RM, Elder A, Jones R, Done D, Kurtzman R, Ossip DJ, Robinson R, McIntosh S, Rahman I. Environmental health hazards of e-cigarettes and their components: Oxidants and copper in e-cigarette aerosols. Environ Pollut. 2015a;198:100–107. doi: 10.1016/j.envpol.2014.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner CA, Sundar IK, Yao H, Gerloff J, Ossip DJ, McIntosh S, Robinson R, Rahman I. Vapors produced by electronic cigarettes and e-juices with flavorings induce toxicity, oxidative stress, and inflammatory response in lung epithelial cells and in mouse lung. PloS one. 2015b;10:e0116732. doi: 10.1371/journal.pone.0116732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin L, Schwartz-Arad D. The effect of cigarette smoking on dental implants and related surgery. Implant Dent. 2005;14:357–361. doi: 10.1097/01.id.0000187956.59276.f8. [DOI] [PubMed] [Google Scholar]
- Li ZZ, Dai QY. Pathogenesis of abdominal aortic aneurysms: role of nicotine and nicotinic acetylcholine receptors. Mediators of inflammation. 2012;2012:103120. doi: 10.1155/2012/103120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisko JG, Tran H, Stanfill SB, Blount BC, Watson CH. Chemical Composition and Evaluation of Nicotine, Tobacco Alkaloids, pH, and Selected Flavors in E-Cigarette Cartridges and Refill Solutions. Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco. 2015 doi: 10.1093/ntr/ntu279. pii: ntu279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Shen M, Xiong J, Yuan Y, Wu X, Gao X, Xu J, Guo F, Jian X. Synergistic effects of betel quid chewing, tobacco use (in the form of cigarette smoking), and alcohol consumption on the risk of malignant transformation of oral submucous fibrosis (OSF): a case-control study in Hunan Province, China. Oral surgery, oral medicine, oral pathology and oral radiology. 2015;120:337–345. doi: 10.1016/j.oooo.2015.04.013. [DOI] [PubMed] [Google Scholar]
- McDonough JE, Yuan R, Suzuki M, Seyednejad N, Elliott WM, Sanchez PG, Wright AC, Gefter WB, Litzky L, Coxson HO, Pare PD, Sin DD, Pierce RA, Woods JC, McWilliams AM, Mayo JR, Lam SC, Cooper JD, Hogg JC. Small-airway obstruction and emphysema in chronic obstructive pulmonary disease. The New England journal of medicine. 2011;365:1567–1575. doi: 10.1056/NEJMoa1106955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafa M, Zarrough A, Bolstad AI, Lygre H, Mustafa K, Hasturk H, Serhan C, Kantarci A, Van Dyke TE. Resolvin D1 protects periodontal ligament. American journal of physiology. Cell physiology. 2013;305:C673–C679. doi: 10.1152/ajpcell.00242.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odusanwo O, Chinthamani S, McCall A, Duffey ME, Baker OJ. Resolvin D1 prevents TNF-alpha-mediated disruption of salivary epithelial formation. American journal of physiology. Cell physiology. 2012;302:C1331–C1345. doi: 10.1152/ajpcell.00207.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisinger C, Dossing M. A systematic review of health effects of electronic cigarettes. Preventive medicine. 2014;69C:248–260. doi: 10.1016/j.ypmed.2014.10.009. [DOI] [PubMed] [Google Scholar]
- Pradeep AR, Ramchandraprasad MV, Bajaj P, Rao NS, Agarwal E. Protein carbonyl: An oxidative stress marker in gingival crevicular fluid in healthy, gingivitis, and chronic periodontitis subjects. Contemporary clinical dentistry. 2013;4:27–31. doi: 10.4103/0976-237X.111589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raes S, Rocci A, Raes F, Cooper L, De Bruyn H, Cosyn J. A prospective cohort study on the impact of smoking on soft tissue alterations around single implants. Clin Oral Implants Res. 2015;26:1086–1090. doi: 10.1111/clr.12405. [DOI] [PubMed] [Google Scholar]
- Regan AK, Promoff G, Dube SR, Arrazola R. Electronic nicotine delivery systems: adult use and awareness of the 'e-cigarette' in the USA. Tobacco control. 2013;22:19–23. doi: 10.1136/tobaccocontrol-2011-050044. [DOI] [PubMed] [Google Scholar]
- Reibel J. Tobacco and oral diseases. Update on the evidence, with recommendations. Medical principles and practice : international journal of the Kuwait University, Health Science Centre. 2003;12(Suppl 1):22–32. doi: 10.1159/000069845. [DOI] [PubMed] [Google Scholar]
- Rothem DE, Rothem L, Soudry M, Dahan A, Eliakim R. Nicotine modulates bone metabolism-associated gene expression in osteoblast cells. Journal of bone and mineral metabolism. 2009;27:555–561. doi: 10.1007/s00774-009-0075-5. [DOI] [PubMed] [Google Scholar]
- Rouabhia M, Park HJ, Semlali A, Zakrzewski A, Chmielewski W, Chakir J. E-Cigarette Vapor Induces an Apoptotic Response in Human Gingival Epithelial Cells Through the Caspase-3 Pathway. Journal of cellular physiology. 2016 doi: 10.1002/jcp.25677. [DOI] [PubMed] [Google Scholar]
- Ruhe M, Haller-Stevenson E, Roulston K, Jourdan M. Strengthening the Capacity of Local Health Departments to Reduce Exposure to Electronic Nicotine Delivery Systems. Journal of public health management and practice : JPHMP. 2017;23:93–94. doi: 10.1097/PHH.0000000000000521. [DOI] [PubMed] [Google Scholar]
- Sancilio S, Gallorini M, Cataldi A, di Giacomo V. Cytotoxicity and apoptosis induction by e-cigarette fluids in human gingival fibroblasts. Clinical oral investigations. 2015 doi: 10.1007/s00784-015-1537-x. [DOI] [PubMed] [Google Scholar]
- Schweitzer KS, Chen SX, Law S, Van Demark M, Poirier C, Justice MJ, Hubbard WC, Kim ES, Lai X, Wang M, Kranz WD, Carroll CJ, Ray BD, Bittman R, Goodpaster J, Petrache I. Endothelial disruptive proinflammatory effects of nicotine and e-cigarette vapor exposures. American journal of physiology. Lung cellular and molecular physiology. 2015;309:L175–L187. doi: 10.1152/ajplung.00411.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekhon HS, Keller JA, Proskocil BJ, Martin EL, Spindel ER. Maternal nicotine exposure upregulates collagen gene expression in fetal monkey lung. Association with alpha7 nicotinic acetylcholine receptors. American journal of respiratory cell and molecular biology. 2002;26:31–41. doi: 10.1165/ajrcmb.26.1.4170. [DOI] [PubMed] [Google Scholar]
- Shimosato T, Geddawy A, Tawa M, Imamura T, Okamura T. Chronic administration of nicotine-free cigarette smoke extract impaired endothelium-dependent vascular relaxation in rats via increased vascular oxidative stress. Journal of pharmacological sciences. 2012;118:206–214. doi: 10.1254/jphs.11187fp. [DOI] [PubMed] [Google Scholar]
- Sundar IK, Javed F, Romanos GE, Rahman I. E-cigarettes and flavorings induce inflammatory and pro-senescence responses in oral epithelial cells and periodontal fibroblasts. Oncotarget. 2016 doi: 10.18632/oncotarget.12857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twito D, Sade P. The effect of cigarette smoking habits on the outcome of dental implant treatment. PeerJ. 2014;2:e546. doi: 10.7717/peerj.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullah M, Cox S, Kelly E, Moore MA, Zoellner H. Arecoline increases basic fibroblast growth factor but reduces expression of IL-1, IL-6, G-CSF and GM-CSF in human umbilical vein endothelium. J Oral Pathol Med. 2015;44:591–601. doi: 10.1111/jop.12276. [DOI] [PubMed] [Google Scholar]
- Vardavas CI, Anagnostopoulos N, Kougias M, Evangelopoulou V, Connolly GN, Behrakis PK. Short-term pulmonary effects of using an electronic cigarette: impact on respiratory flow resistance, impedance, and exhaled nitric oxide. Chest. 2012;141:1400–1406. doi: 10.1378/chest.11-2443. [DOI] [PubMed] [Google Scholar]
- Varlet V, Farsalinos K, Augsburger M, Thomas A, Etter JF. Toxicity assessment of refill liquids for electronic cigarettes. International journal of environmental research and public health. 2015;12:4796–4815. doi: 10.3390/ijerph120504796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilk JB, Shrine NR, Loehr LR, Zhao JH, Manichaikul A, Lopez LM, Smith AV, Heckbert SR, Smolonska J, Tang W, Loth DW, Curjuric I, Hui J, Cho MH, Latourelle JC, Henry AP, Aldrich M, Bakke P, Beaty TH, Bentley AR, Borecki IB, Brusselle GG, Burkart KM, Chen TH, Couper D, Crapo JD, Davies G, Dupuis J, Franceschini N, Gulsvik A, Hancock DB, Harris TB, Hofman A, Imboden M, James AL, Khaw KT, Lahousse L, Launer LJ, Litonjua A, Liu Y, Lohman KK, Lomas DA, Lumley T, Marciante KD, McArdle WL, Meibohm B, Morrison AC, Musk AW, Myers RH, North KE, Postma DS, Psaty BM, Rich SS, Rivadeneira F, Rochat T, Rotter JI, Soler Artigas M, Starr JM, Uitterlinden AG, Wareham NJ, Wijmenga C, Zanen P, Province MA, Silverman EK, Deary IJ, Palmer LJ, Cassano PA, Gudnason V, Barr RG, Loos RJ, Strachan DP, London SJ, Boezen HM, Probst-Hensch N, Gharib SA, Hall IP, O'Connor GT, Tobin MD, Stricker BH. Genome-wide association studies identify CHRNA5/3 and HTR4 in the development of airflow obstruction. American journal of respiratory and critical care medicine. 2012;186:622–632. doi: 10.1164/rccm.201202-0366OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willershausen I, Wolf T, Weyer V, Sader R, Ghanaati S, Willershausen B. Influence of E-smoking liquids on human periodontal ligament fibroblasts. Head & face medicine. 2014;10:39. doi: 10.1186/1746-160X-10-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Jiang D, Minor M, Chu HW. Electronic cigarette liquid increases inflammation and virus infection in primary human airway epithelial cells. PLoS One. 2014;9:e108342. doi: 10.1371/journal.pone.0108342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamano S, Berley JA, Kuo WP, Gallucci GO, Weber HP, Sukotjo C. Effects of nicotine on gene expression and osseointegration in rats. Clinical oral implants research. 2010;21:1353–1359. doi: 10.1111/j.1600-0501.2010.01955.x. [DOI] [PubMed] [Google Scholar]