Abstract
Activating mutations in the GLUD1 gene, which encodes glutamate dehydrogenase (GDH), result in the hyperinsulinism-hyperammonemia syndrome. GDH is an allosterically regulated enzyme responsible for amino acid-mediated insulin secretion via the oxidative deamination of glutamate to 2-oxoglutarate, leading to ATP production and insulin release. This study characterizes a novel combination of mutations in GLUD1 found in a neonate who presented on the first day of life with severe hypoglycemia, hyperammonemia, and seizures. Mutation analysis revealed a novel frameshift mutation (c.37delC) inherited from the asymptomatic mother that results in a truncated protein and a de novo activating mutation (p.S445L) close to the GTP binding site that has previously been reported. GTP inhibition of GDH enzyme activity in 293T cells expressing the p.S445L or wild type GDH showed that the half-maximal inhibitory concentration (IC50) for GTP was approximately 800 times higher for p.S445L compared to wild type. GTP inhibition of GDH activity in lymphoblasts from the patient, from a heterozygote for the p.S445L mutation, and in wild type lymphoblasts showed that the IC50 for GTP of the patient was approximately 200 times that of wild type and 7 times that of heterozygote. However, while the patient had a loss of GTP inhibition of GDH that was more severe than that of heterozygotes, the patient’s clinical phenotype is similar to typical heterozygous mutations of GDH. This is the first time we have observed a functionally homozygous activating mutation of GDH in a human.
Introduction
Activating mutations in GLUD1, which encodes glutamate dehydrogenase (GDH), cause the hyperinsulinemia-hyperammonemia syndrome (HI/HA) and constitute the second most common cause of congenital hyperinsulinism (1–3). GDH, a protein expressed in all organisms, catalyzes the reversible oxidative deamination of L-glutamate to 2-oxoglutarate and ammonia using NAD(P)+ as a coenzyme (4). In humans, GDH resides in the mitochondrial matrix, is expressed predominantly in the pancreatic islets, liver, kidney, and brain, and is extensively allosterically regulated (5). Primary allosteric activators of GDH are ADP and leucine, and inhibitors are GTP, palmitoyl CoA, and ATP. Epigallocatechin gallate (EGCG), a polyphenol found in green tea, has also been found to be a potent inhibitor of GDH via competitive binding to the ADP site (6).
As demonstrated by the phenotype of individuals with genetic mutations to allosteric GDH sites, tight regulation of GDH activity is critical to plasma glucose and ammonia homeostasis in humans. Activating mutations result in HI/HA, with almost all reported cases resulting from dominant heterozygous missense mutations to GLUD1 that impair the ability of GTP to inhibit GDH activity (7). Children with HI/HA present at an average age of 4 months with symptomatic protein-sensitive and fasting hypoglycemia and plasma ammonia concentrations that are persistently elevated (7, 8). The KATP channel agonist diazoxide inhibits insulin secretion and prevents hypoglycemia in individuals with HI/HA.
Here we describe an infant with a novel combination of mutations in GLUD1 who presented on the first day of life with hypoglycemia, hyperammonemia, and seizures. She was found to have a de novo activating mutation on the paternal allele in addition to a novel inactivating frameshift mutation, inherited from the asymptomatic mother, which is predicted to cause a stop codon 77 amino acids downstream from the mutation. We hypothesized that this very rare novel combination of mutations generate homohexamers of activated proteins, as would be found in a homozygote, and explains the patient’s severe phenotype.
Subject and Methods
Clinical Data
Clinical information was obtained by chart review. Written informed consent was obtained from parents of the proband included in this study. The study was reviewed and approved by the Children's Hospital of Philadelphia (CHOP) Institutional Review Board.
DNA Mutation Analysis
DNA was extracted from peripheral blood and mutation analysis was performed in a commercial laboratory. Mutations were numbered according to the published sequence NM_005271 (http://www.ncbi.nlm.nih.gov/), with nucleotide numbers beginning at the first ATG start site in exon 1 and amino acid numbers beginning at the start of the mature protein. SeqBuilder (DNASTAR, INC, Madison, Wisconsin) was used to predict the result of the frameshift mutation. RNA was extracted from peripheral blood lymphoblasts using the RNeasy Mini Kit (Qiagen, Germantown, Maryland) and converted to cDNA using the Superscript First Strand Synthesis System for RT-PCR (Invitrogen, Carlsbad, California). The cDNA was amplified and directly sequenced on an ABI 3730 DNA analyzer (Applied Biosystems, Carlsbad, California).
Expression studies in 293T cells
Site-directed mutagenesis was used to reproduce the p.S445L and p.H454Y mutations, which are well characterized HI/HA-causing mutations with impaired GTP inhibition (1, 9). The construct was then packed into a lentiviral vector (pUL-CMV-MCS) with mCherry as the reporter protein. Wild type or mutant GDH were then expressed in 293T cells by lentivirus transfection with about 80% transfection efficiency. GDH enzyme kinetics was determined spectrophotometrically in the cell homogenates (10).
Enzyme kinetic studies in lymphoblast cells
Lymphocytes isolated from the proband, a heterozygous individual with the p.S445L mutation, and a healthy control were transformed with Epstein-Barr virus to establish lymphoblast cultures (7, 9). GDH enzyme kinetics in the homogenized lymphoblasts was measured.
Statistical Analyses
Student t tests were done when two groups were compared. Differences were considered significant when p<0.05.
Results
Clinical Data
The proband was a girl born at 39 weeks of gestation to a 27-year-old mother after an uncomplicated pregnancy weighing 3700 g (85th percentile), slightly above the average birth weight of 3500 g for GDH HI newborns (8). The infant presented with hypoglycemia, hyperammonemia, and seizures at 12 hours of life. The infant’s plasma glucose on the second day of life was 20 mg/dL with a plasma insulin concentration of 2.5 μU/mL. She required a high glucose infusion rate of 23–26 mg/kg/min in addition to continuous intravenous infusion of glucagon to maintain a normal plasma glucose concentration. The plasma ammonia concentration was elevated between 75 and 150 μmol/L (normal <40 μmol/L).
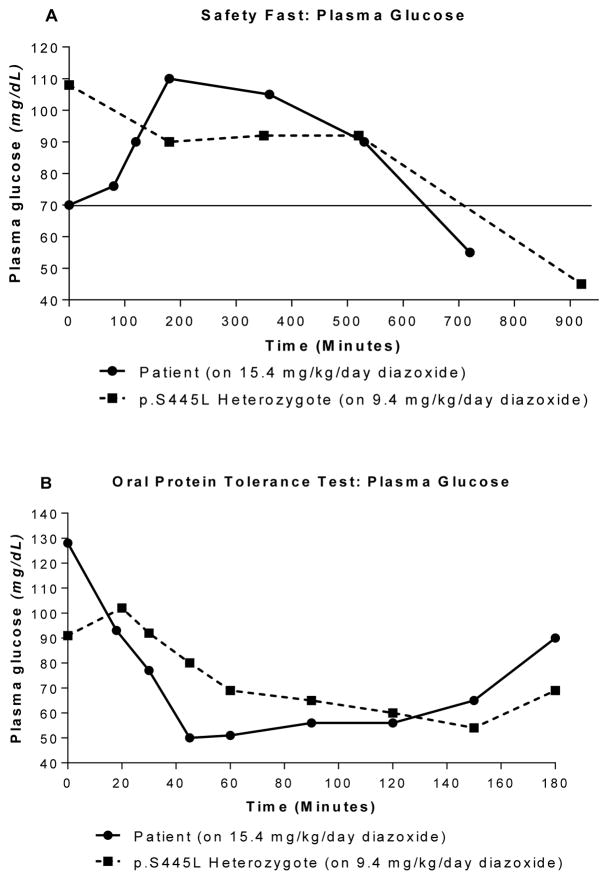
At two weeks of age, a fasting test showed the patient’s plasma glucose falling to 38 mg/dL within four hours with a suppressed plasma β-hydroxybutyrate concentration (<0.3 mmol/L). Diazoxide was initiated on day of life 16 and had to be raised to 15.4 mg/kg/day to maintain euglycemia. Figure 1 compares the patient’s glycemic control while on this high dose of diazoxide to a patient who was heterozygous for the GLUD1: p.S445L mutation, which is one the same mutations identified in the patient presented here, and was being treated with a more typical dose of diazoxide (9.4 mg/kg/day). As shown, despite being on a 50% higher dose of diazoxide, the patient became hypoglycemic more quickly in response to fasting and, particularly, in response to oral protein loading than the comparison child with hyperinsulinism-hyperammonemia syndrome. The proband’s ammonia during the oral protein tolerance test remained constant at around 105 μmol/L for the first 40 minutes, rose to 120 μmol/L over the next hour, then dropped sharply to 56 μmol/L by the end of the test at 130 minutes; this degree of hyperammonemia is similar to that of typical patients with the hyperammonemia syndrome (11).
Figure 1.
Glucose levels in fasting and oral protein tolerance test. Panel A: Patient was able to fast for less time on 15.4 mg/kg/day diazoxide treatment before blood glucose fell below 70 mg/dL compared to the p.S445L heterozygote on 9.4 mg/kg/day. Panel B: Patient demonstrates a greater decrease in blood glucose on 15.4 mg/kg/day diazoxide than p.S445L heterozygote on 9.4 mg/kg/day in an oral protein tolerance test.
Mutation Analysis
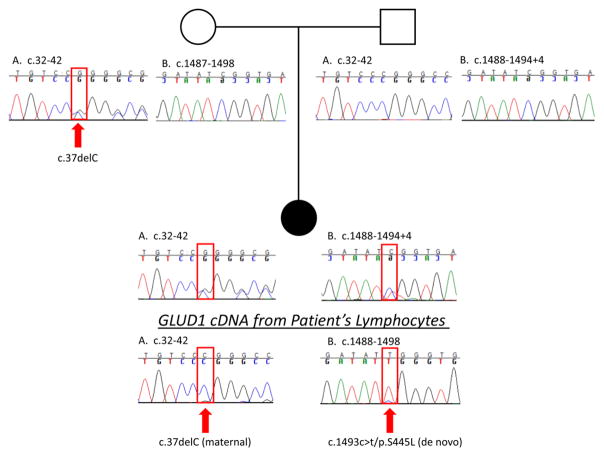
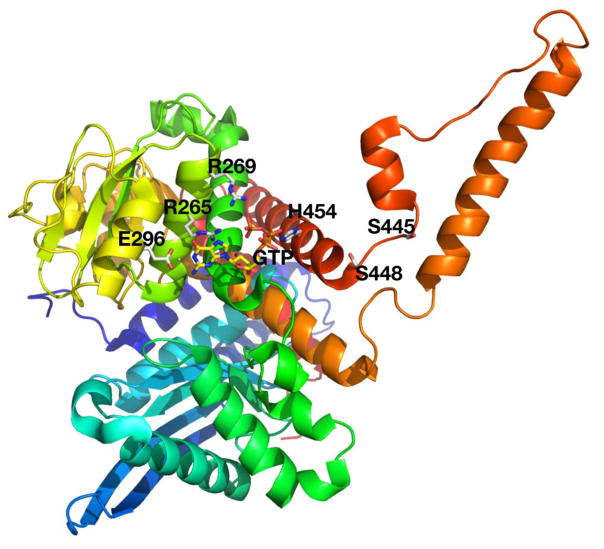
Mutation analysis of the proband’s genomic DNA revealed two mutations in GDH (Figure 2): a novel frameshift mutation, c.37delC, inherited from the asymptomatic mother and a de novo mutation, c.1493C>T/p.S445L, that has been previously described (5). The frameshift mutation is located in the mitochondrial targeting leader peptide sequence and results in the introduction of a stop codon 77 amino acids downstream (53 amino acids into the mature peptide). The point mutation is located at the base of the antenna region of GDH close to the GTP binding site (Figure 3). Direct sequencing of cDNA from the patient’s lymphoblasts showed a higher proportion of normal sequence in the region of the maternal mutation (c.37delC) and a higher proportion of the mutant sequence in the region of the de novo c.1493c>t/p.S445L mutation (Figure 2). These data suggest that the c.1493c>t/p.S445L de novo mutation arose on the paternal allele and is observed at a higher proportion in the cDNA due to instability of mRNA transcripts from the maternal allele resulting from the c.37delC mutation. Additionally, the c.37delC mutation, on the maternal allele, is predicted to introduce an early stop codon resulting in a truncated protein. Functionally, therefore, the proband is hemizygous for the p.S445L activating mutation in GDH.
Figure 2. Family Pedigree.
Shown are GLUD1 genomic DNA sequences of the maternal mutation site (c.37) and the de novo mutation site (c.1493) for the patient (black circle), patient’s mother (open circle) and patient’s father (open square). Note that the patient’s genomic DNA shows the maternally transmitted c.37delC heterozygous mutation plus a de novo c.1493c>t/p.S445L mutation.
Figure 3.
Crystal structure of GDH showing the location of the patient’s missense mutation close to the GTP binding site. The other GTP binding site mutations are also shown in the structure, including positions of E296, R265, R269, S448, H454.
GDH Enzyme Activity
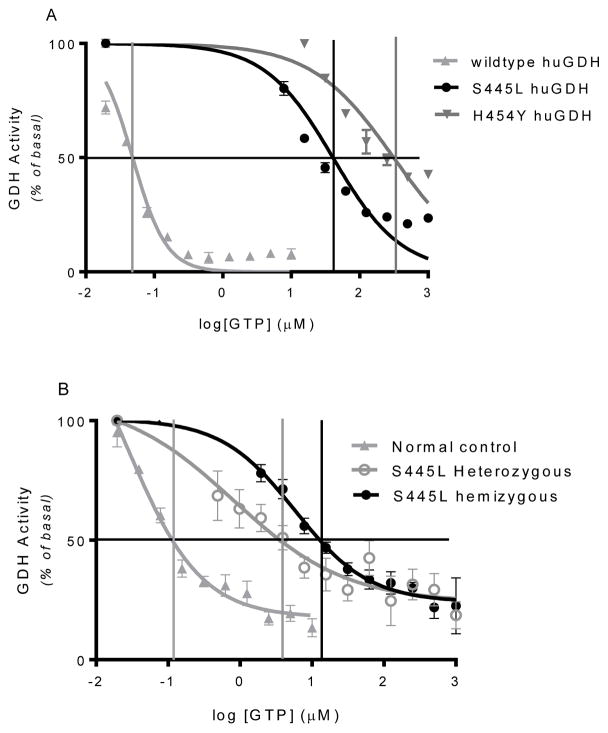
Table 1 shows enzyme kinetics for wild type and for p.S445L and p.H454Y mutant forms of GDH expressed in 293T cells and for lymphoblast GDH from the proband, a patient heterozygous for the p.S445L mutation, and a normal control. The Km for the enzyme substrate, alpha-ketoglutarate, was similar for all three expressed forms of GDH and for all three lymphoblasts samples. Inhibition by EGCG, which interacts with the ADP binding site on GDH, was similar for wild type, p.S445L, and p.H454Y expressed forms of GDH. However, sensitivity to GTP inhibition was markedly reduced in the expressed forms of mutant GDH in 293T cells: as shown in Table 1 and in Figure 4A, half maximal inhibition of GDH activity was 850 times greater than wild type for the p.S445L GDH and 6,500 times higher for p.H454Y GDH. These values for GTP EC50 of wild type and p.H454Y GDH expressed in 293T cells are similar to those previously obtained with the purified forms of GDH expressed in E. coli. Table 1 and Figure 4B show that the IC50 for GTP inhibition of GDH activity from the proband’s lymphoblasts was approximately 200 times that of wild type and seven times that of lymphoblasts from a S445L heterozygote (5,800 nM versus wild type 27 nM and heterozygote 810 nM) (Figure 4B).
Table 1.
Comparison of mutant and wild type GDH enzyme kinetics in 293T cells and lymphoblasts.
| α-ketoglutarate (Km, mM) | IC50 | ||
|---|---|---|---|
| EGCG (uM) | GTP (nM) | ||
| 293T cells | |||
| Wild type | 0.30 | 1.05 | 48 |
| p.S445L | 0.19 | 1.07 | 40,700 |
| p.H454Y | 0.32 | 1.02 | 314,000 |
| Lymphoblasts | |||
| Wild type | 0.12 | 27 | |
| p.S445L heterozygote | 0.39 | 810 | |
| Proband | 0.23 | 5,800 | |
Figure 4.
GTP inhibition is impaired in both 293T cells expressing GDH mutations and in patient’s lymphoblasts. Panel A shows GTP inhibition curve in 293T cells expressing wild type, p.S445L and p.H454Y GDH mutations. Panel B shows GTP inhibition in lymphoblasts from healthy control, and patient with either heterozygous or hemizygous p.S445L GDH mutation. Vertical lines indicate the log of the IC50 for GTP.
Discussion
This child with hemizygosity for an activating mutation of GDH provides a unique opportunity to understand the potential range of clinical phenotypes of the HI/HA syndrome. Children with the typical form of the disease due to heterozygous activating GDH mutations have a consistent phenotype of fasting and protein-induced hypoglycemia that can be controlled with moderate doses of diazoxide and persistently elevated plasma ammonia concentrations in the range of 80–120 μmol/L. While it might have been anticipated that homozygosity for an activating mutation in GDH would be associated with devastatingly severe hypoglycemia and hyperammonemia, the observations in our patient with a hemizygous activating mutation show only a rather small increase in severity of manifestations. Compared to typical cases of GDH hyperinsulinism, our patient had earlier onset of recognized hypoglycemic symptoms and needed higher doses of diazoxide to control hypoglycemia, but did not have a greater degree of hyperammonemia than other GDH-HI cases.
Activating mutations of GDH in patients with HI/HA interfere with the GTP inhibitory allosteric site either directly or indirectly via the adjacent antenna loop (Figure 3). These mutations increase GDH activity by impairing sensitivity to GTP inhibition with IC50 values ranging from ~100 to 500 nmol/L in heterozygous lymphoblasts from patients compared to 30–60 nmol/L in normal cells. In the purified expressed mutant forms of GDH, the IC50 values are much higher than in heterozygous mutant/wild type cells and the range of EC50 values is much greater: e.g., from as low as 3,100 nmol/L for p.S448P to as high as 210,000 for p.H454Y (9). The narrow range of IC50 in patient cells reflects the fact that GDH is a homohexamer in which overall enzyme activity reflects cooperative interactions among mutant and wild type subunits. This explains why the GTP IC50 for GTP among different mutations does not precisely reflect the properties of the pure mutant enzyme and probably accounts for the fact that various HI/HA mutations result in quite similar impairments in GTP inhibition. Cooperative interactions between subunits within the GDH hexamer also explains why the IC50 for GTP inhibition in our patient cells was nearly two orders of magnitude greater than that of a heterozygote with the same mutation. The fact that her phenotype was not dramatically worse than usual may indicate that impairment of GTP sensitivity is already essentially complete in the typical heterozygous GDH mutant subject.
Of note, plasma insulin concentrations did not correlate with our patient’s greater susceptibility to hypoglycemia. Although insulin was elevated during hypoglycemia on the second day of life, insulin was appropriately suppressed to undetectable levels during a diagnostic fast performed before starting diazoxide treatment. Insulin levels were also low during fasting and protein-induced hypoglycemia. Hypoglycemia in the context of appropriately suppressed insulin levels has been seen previously in HI/HA patients and in other forms of congenital HI (7, 12, 13). In addition, recent studies in a transgenic mouse model with expression of p.H454Y GDH mutation in beta-cells suggest that inhibition of glucagon release may play a role in the mechanism of hypoglycemia associated with GDH activating mutation (14).
It is interesting that, despite evidence of more severe hypoglycemia, our patient’s plasma ammonia concentrations were not higher than in patients with p.S445L heterozygous mutants or other GDH activating mutants in general. Recent work suggests that the elevated ammonia in HI/HA is caused by increased production of ammonia from GDH overactivity in the kidney, rather than impaired hepatic ureagenesis (15). Thus, it appears that that homohexamers of mutant GDH enzyme subunits are not significantly more active compared to heterohexamers of wild type and mutant GDH in kidney cells.
In summary, this patient has HI/HA syndrome caused by the combination of a missense and a frame-shift mutation that make her functionally homozygous for an activating GLUD1 mutation. Despite having markedly more severe impairment in GTP inhibitory allosteric control of GDH, the patient’s phenotype is remarkably similar to that of patients with more typical heterozygous mutations of GDH with the exception of earlier presentation and slightly more severe hypoglycemia requiring a higher-than-usual dose of diazoxide.
Acknowledgments
This work was supported by National Institutes of Health Grants 1RO1DK098517-01A1 and R01 DK098517-03S1 (to C. L. & D. D. L.), R37-DK056268 (C.A.S). National Institute of Diabetes and Digestive and Kidney Diseases medical student research program NIH DK007314-35 (M. BA.)
References
- 1.Stanley CA, Lieu YK, Hsu BY, Burlina AB, Greenberg CR, Hopwood NJ, Perlman K, Rich BH, Zammarchi E, Poncz M. Hyperinsulinism and hyperammonemia in infants with regulatory mutations of the glutamate dehydrogenase gene. N Engl J Med. 1998;338(19):1352–7. doi: 10.1056/NEJM199805073381904. [DOI] [PubMed] [Google Scholar]
- 2.De Leon DD, Stanley CA. Congenital Hypoglycemia Disorders: New Aspects of Etiology, Diagnosis, Treatment and Outcomes. Pediatr Diabetes; Highlights of the Proceedings of the Congenital Hypoglycemia Disorders Symposium; Philadelphia. April 2016; 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gong C, Huang S, Su C, Qi Z, Liu F, Wu D, Cao B, Gu Y, Li W, Liang X, et al. Congenital hyperinsulinism in Chinese patients: 5-yr treatment outcome of 95 clinical cases with genetic analysis of 55 cases. Pediatr Diabetes. 2016;17(3):227–34. doi: 10.1111/pedi.12254. [DOI] [PubMed] [Google Scholar]
- 4.Li M, Li C, Allen A, Stanley CA, Smith TJ. The structure and allosteric regulation of glutamate dehydrogenase. Neurochem Int. 2011;59(4):445–55. doi: 10.1016/j.neuint.2010.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith TJ, Stanley CA. Untangling the glutamate dehydrogenase allosteric nightmare. Trends Biochem Sci. 2008;33(11):557–64. doi: 10.1016/j.tibs.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 6.Li C, Li M, Chen P, Narayan S, Matschinsky FM, Bennett MJ, Stanley CA, Smith TJ. Green tea polyphenols control dysregulated glutamate dehydrogenase in transgenic mice by hijacking the ADP activation site. J Biol Chem. 2011;286(39):34164–74. doi: 10.1074/jbc.M111.268599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.MacMullen C, Fang J, Hsu BY, Kelly A, de Lonlay-Debeney P, Saudubray JM, Ganguly A, Smith TJ, Stanley CA Hyperinsulinism/hyperammonemia Contributing I. Hyperinsulinism/hyperammonemia syndrome in children with regulatory mutations in the inhibitory guanosine triphosphate-binding domain of glutamate dehydrogenase. J Clin Endocrinol Metab. 2001;86(4):1782–7. doi: 10.1210/jcem.86.4.7414. [DOI] [PubMed] [Google Scholar]
- 8.Stanley CA, Fang J, Kutyna K, Hsu BY, Ming JE, Glaser B, Poncz M. Molecular basis and characterization of the hyperinsulinism/hyperammonemia syndrome: predominance of mutations in exons 11 and 12 of the glutamate dehydrogenase gene. HI/HA Contributing Investigators. Diabetes. 2000;49(4):667–73. doi: 10.2337/diabetes.49.4.667. [DOI] [PubMed] [Google Scholar]
- 9.Fang J, Hsu BY, MacMullen CM, Poncz M, Smith TJ, Stanley CA. Expression, purification and characterization of human glutamate dehydrogenase (GDH) allosteric regulatory mutations. Biochem J. 2002;363(Pt 1):81–7. doi: 10.1042/0264-6021:3630081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li C, Chen P, Palladino A, Narayan S, Russell LK, Sayed S, Xiong G, Chen J, Stokes D, Butt YM, et al. Mechanism of hyperinsulinism in short-chain 3-hydroxyacyl-CoA dehydrogenase deficiency involves activation of glutamate dehydrogenase. J Biol Chem. 2010;285(41):31806–18. doi: 10.1074/jbc.M110.123638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsu BY, Kelly A, Thornton PS, Greenberg CR, Dilling LA, Stanley CA. Protein-sensitive and fasting hypoglycemia in children with the hyperinsulinism/hyperammonemia syndrome. J Pediatr. 2001;138(3):383–9. doi: 10.1067/mpd.2001.111818. [DOI] [PubMed] [Google Scholar]
- 12.De Lonlay P, Benelli C, Fouque F, Ganguly A, Aral B, Dionisi-Vici C, Touati G, Heinrichs C, Rabier D, Kamoun P, et al. Hyperinsulinism and hyperammonemia syndrome: report of twelve unrelated patients. Pediatr Res. 2001;50(3):353–7. doi: 10.1203/00006450-200109000-00010. [DOI] [PubMed] [Google Scholar]
- 13.Ferrara C, Patel P, Becker S, Stanley CA, Kelly A. Biomarkers of Insulin for the Diagnosis of Hyperinsulinemic Hypoglycemia in Infants and Children. J Pediatr. 2016;168:212–9. doi: 10.1016/j.jpeds.2015.09.045. [DOI] [PubMed] [Google Scholar]
- 14.Kibbey RG, Choi CS, Lee HY, Cabrera O, Pongratz RL, Zhao X, Birkenfeld AL, Li C, Berggren PO, Stanley C, et al. Mitochondrial GTP insensitivity contributes to hypoglycemia in hyperinsulinemia hyperammonemia by inhibiting glucagon release. Diabetes. 2014;63(12):4218–29. doi: 10.2337/db14-0783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Treberg JR, Clow KA, Greene KA, Brosnan ME, Brosnan JT. Systemic activation of glutamate dehydrogenase increases renal ammoniagenesis: implications for the hyperinsulinism/hyperammonemia syndrome. Am J Physiol Endocrinol Metab. 2010;298(6):E1219–25. doi: 10.1152/ajpendo.00028.2010. [DOI] [PubMed] [Google Scholar]