Abstract
Background
We have expanded the use of tandem mass spectrometry combined with liquid chromatography (HPLC-MS/MS) for multiplex newborn screening of seven lysosomal enzymes in dried blood spots (DBS). The new assays are for enzymes responsible for the mucopolysaccharidoses MPS-I, -II, -IIIB, -IVA, -VI, and −VII) and type 2 neuronal ceroid lipofuscinosis (LINCL).
Methods
New substrates were prepared and characterized for tripeptidyl peptidase 1 (TPP1), α-N-acetylglucosaminidase (NAGLU), and lysosomal β-glucuronidase (GUSB). These assays were combined with previously developed assays to provide a multiplex HPLC-MS/MS assay of seven lysosomal storage diseases (LSDs). Multiple reaction monitoring (MRM) of ion dissociations for enzyme products and deuterium-labeled internal standards was used to quantify the enzyme activities.
Results
Deidentified DBS samples from 62 non-affected newborns were analyzed to simultaneously determine (run time 2 min per DBS) the activities of TPP1, NAGLU, and GUSB, along with those for α-iduronidase (IDUA) iduronate-2-sulfatase (I2S), N-acetylgalactosamine-6-sulfatase (GALNS), and N-acetylgalactosamine-4-sulfatase (ARSB). The activities measured in the 7-plex format showed analytical ranges of 102-909 that clearly separated healthy infants from affected children.
Conclusions
The new multiplex assay provides a robust comprehensive newborn screening assay for the mucopolysaccharidoses, which is the most rapid method reported to date. The method is shown to be expandable to include additional LSDs including neuronal ceroid lipofuscinosis. The assay is also useful for biochemical diagnosis and prognosis studies.
Lysosomal storage disorders (LSDs) are genetic diseases caused by deficiency of activity of enzymes degrading substrates including glycolipids, glycosaminoglycans, and proteins (1). With the development of therapies for several LSDs (for example, 2-6), detection of enzyme activity in dried blood spots (DBS) from newborns is of recent interest because of the potential benefits of early treatment. We have previously reported several enzyme assays of lysosomal enzymes that were developed in a multiplex format using tandem mass spectrometry (MS/MS) (7). Here we report results expanding the diagnostic portfolio to a 7-plex format including new assays for lysosomal enzymes tripeptidyl peptidase 1 (TPP1), α-N-acetylglucosaminidase (NAGLU), and lysosomal β-glucuronidase (GUSB). These are combined with previously reported assays for mucopolysacharidoses (α-iduronidase (IDUA) for MPS-I, iduronate-2-sulfatase (I2S) for MPS-II, N-acetylgalactosamine-6-sulfatase (GALNS) for MPS-IVA, and N-acetylgalactosamine-4-sulfatase (ARSB) for MPS-VI) (8).
TPP1 deficiency results in classic late infantile (type 2) neuronal ceroid lipofuscinosis (LINCL, also called Jansky-Bielschowsky disease) (9). LINCL is an autosomal recessive disorder causing neurodegenerative disease with seizures, mental regression, visual loss, and shortened life expectancy (10). Mucopolysaccharidoses are a group of diseases resulting from deficiency of enzymes degrading glycosaminoglycans (1). MPS-IIIB (also called Sanfilippo B) (11,12) is caused by deficient NAGLU. MPS-VII (Sly syndrome) (13) results from deficient GUSB. Recent advances in enzyme replacement therapies (2-6) provide a strong motivation for the development of analytical methods allowing for the detection of newborns at risk of developing one of the treatable LSD's.
Tandem mass spectrometry (MS/MS) with electrospray ionization (ESI) has been shown to a robust and comprehensive platform for newborn screening of several inborn errors of metabolism in which biomarkers or enzymatic activities are measured (7, 14, 15). We have previously reported multiplex MS/MS assays that targeted a portfolio of several LSDs and utilized flow injection for sample delivery to the mass spectrometer (16-19). A limitation of the flow injection approach is that sulfatase substrates undergo non-enzymatic cleavage to desulfated products in the heated ESI source of the mass spectrometer that interfere with the activity measurements. In parallel efforts, liquid chromatography in combination with MS/MS (HPLC-MS/MS) has been evaluated as an alternative method of enzyme activity analysis in DBS (20-25). HPLC leads to separation of substrates from products prior to MS/MS, thus in-source cleavage is of no concern. HPLC requires fast analyte elution to be applicable in newborn screening. Previous HPLC-MS/MS methods for LSDs require only ∼2 min inject-to-inject times (20-25) and are thus appropriate for newborn screening.
Methods
Experimental details of the reported assays and synthetic procedures are given as supplementary text, schemes and tables. DBS were obtained from the Washington newborn screening laboratory with Institutional Review Board approval.
Results
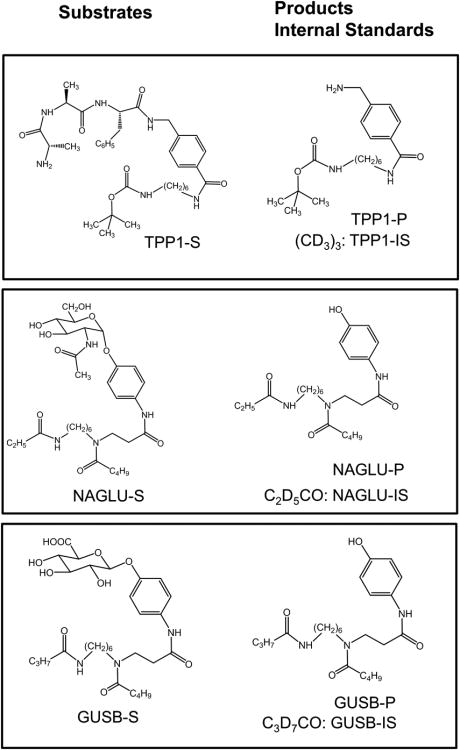
The reported method utilizes substrates and internal standards for IDUA, I2S, GALNS, and ARSB that have been reported previously (8,26). The previously reported substrate (S) and internal standard (IS) for TPP1 (27) have been modified by replacing the C3 linker with a longer C6 aliphatic chain (Figure 1) with the goal of increasing the product and IS hydrophobicity (to increase retention to the LC column). Details of the synthetic procedures and compound characterization are described in the Supplement. Action of the TPP1 enzyme results in benzylamide bond cleavage releasing the product (TPP1-P). The Ala-Ala-Phe tripeptide motif was selected because it is a preferred natural target of TPP1 (28) and has been shown to give high activity in in vitro assays (27). The TPP1 enzyme activity towards the new substrate was fully characterized by incubations in DBS. The activity (μmol hour1 L blood-1) showed a linear increase with the substrate concentration up to 800 μM (Supplemental Figure 1) and incubation time over a 24 hour period (Supplemental Figure 2). The TPP1 activity varied between pH 3.5-6 but did not show a prominent pH-dependent trend within this range (Supplemental Figure 3). The internal standard (TPP-IS) is chemically identical to TPP1-P but is distinguished by a 9 Da mass difference due to the presence of the d9-tert-butyl group.
Figure 1.
Structures of TPP1, NAGLU, and GUSB substrates, products, and internal standards.
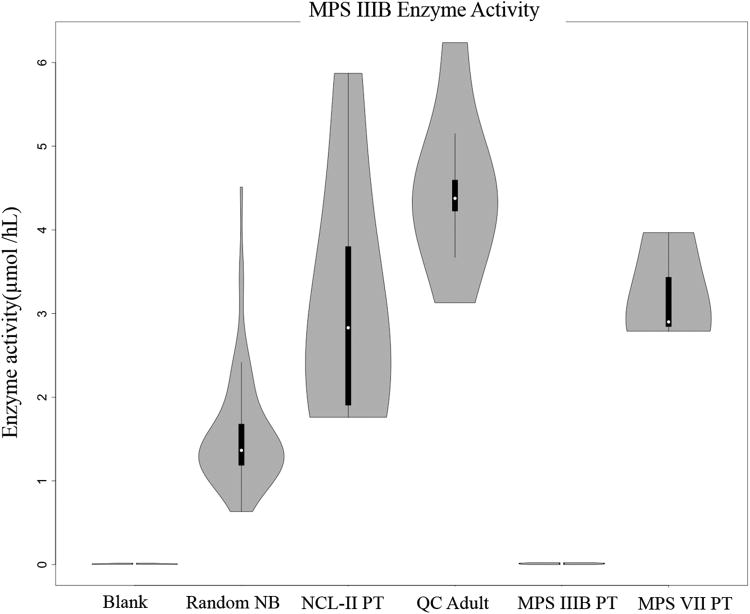
The previously reported NAGLU substrate (NAGLU-S) and internal standard (NAGLU-IS) (29) were redesigned to be suitable for multiplex analysis of MPS IIIB. The new compounds (Figure 1) were synthesized and characterized as described in the Supplement. Action of NAGLU results in hydrolysis of the glycosidic bond in NAGLU-S forming the aglycone which is the enzyme product (NAGLU-P). The new NAGLU aglycon scaffold consists of an acylated aminophenol with a six-carbon linker between a tertiary C5 and secondary C3 amide group. The tertiary amide is presumably involved in facile protonation in electrospray (30), whereas the C5 and C3 amides provide a mass encoding combination for MS/MS and tune the product hydrophobicity for fast HPLC elution. The NAGLU-IS is a d5-derivative of NAGLU-P with the label in the C3-amide group that is retained in the reporter fragment ion in MS/MS.
The GUSB substrate is analogous to that for NAGLU in the composition of the aglycone but differs from it by being a β-glucoside and having a C4 secondary amide (Figure 1). Action of the GUSB enzyme hydrolyzes the glycosidic bond producing the aglycone as GUSB-P. GUSB-IS is a d7-derivative that has the isotope label in the C4 amide group.
The NAGLU and GUSB activities towards the new substrates were fully characterized as a function of the incubation time, substrate concentration, and pH, as described in the Supplement (Figures S4-S9). The NAGLU and GUSB had optimum activities at pH 5.0 (Figure S4) and 4.1 (Figure S9), respectively. With regard to the previously determined optimum pH for the other enzyme substrates (22,26), the multiplex assay pH was adjusted to pH 5.0 to assure that all seven enzymes had activity within a sufficient analytical range, with a particular regard to the least active GALNS (8). The sufatases are inhibited by inorganic phosphate and sulfate that has to be sequestered from the assay medium (22). This is achieved by precipitating sulfate and phosphate with added cerium(III)sulfate at 5 mM concentration during the incubation (22).
The assays were performed in 96-well microtiter plates in 50 mM ammonium acetate buffer (pH 5.0) with an assay cocktail consisting of substrates at the following concentrations: TPP1 (0.2 mM), I2S (1.0 mM), NAGLU (0.5 mM), GALNS (1.0 mM), ARSB (1.0 mM), and GUSB (0.5 mM). The assay cocktail further contained internal standards (7.5-15 μM) and 0.1 mM NAG-thiazoline to inhibit endogenous hexosaminidase A that would otherwise catalyze hydrolysis of the GALNS and ARSP products (8). Also, small amounts of the β-anomer present as an impurity in NAGLU-S would be converted to NAGLU-P by action of hexosaminidase, leading to an anomalous NAGLU-P signal in DBS deficient in NAGLU. A DBS punch (3 mm diameter) was placed in the well with 30 μL of the assay cocktail. The 96-well plate was sealed with adhesive film and incubated in a shaker at 37 °C for 16 hours. The incubation was quenched by adding 0.1 mL of 50:50 methanol:ethyl acetate, and the products and internal standards were extracted into ethyl acetate. The solvent was removed by a stream of oil-free N2, the samples were reconstituted in the HPLC elution solvent, 55:45:0.1 water:acetonitrile:formic acid, and injected via an autosampler on the HPLC column for elution. HPLC separations were performed with isocratic solvent elution on a XSelect charge surface hybrid (CSH™) column (Waters, 130 Å, 3.5 μm, 2.1 mm × 50 mm, 1/pkg [cat. #186005255]) with an XSelect CSH C18 Sentry Guard Cartridge, 130 Å, 3.5 μm, 2.1 mm × 10 mm. To prevent carryover, the injection needle was washed in between injections with a weak (H2O/acetonitrile 90:10 with 0.1% formic acid) and strong (100% acetonitrile with 0.1% formic acid) solvent.
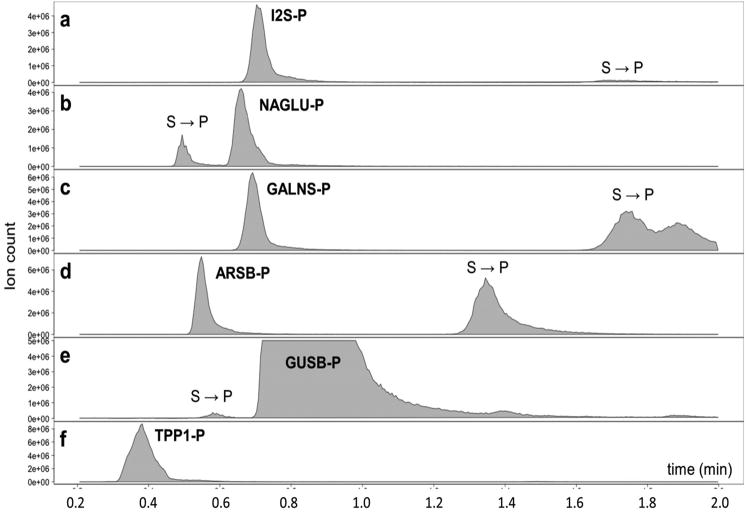
MS/MS measurements were performed in a multiple reaction monitoring (MRM) format on a Waters Xevo TQMS tandem quadrupole mass spectrometer equipped with an Acquity UPLC separation system (an HPLC system is adequate). Experimental conditions including cone voltages and laboratory collision energies were optimized for each parent ion-fragment ion transition. The transition m/z for products and internal standards show no overlaps (Supplemental Table S1), ensuring independent determination of enzyme activities in the multiplex format. The HPLC traces for all product MRM transitions are shown in Figure 2, the analogous traces for the IS are presented in Supplemental Figure S10. The HPLC-MRM traces show two types of signals. One type corresponds to MRM transitions associated with elution of the enzymatic products produced by DBS incubation at their specific retention times. The product retention times coincide with those of the internal standards that are distinguished by different m/z because of deuteration (Table S1). The other signals correspond to elution of residual substrates that underwent partial non-enzymatic dissociation in the hot ESI source of the mass spectrometer, forming gas-phase ions identical with those from products of enzyme incubation. Since the assays are generally run at low substrate conversion, the substrates are major components of the mixture injected on the column. Figure 2 shows that the peaks of the enzymatic products are well separated from those of the substrates, so that the background caused by non-enzymatic cleavage in the ion source does not interfere with the measurements of enzyme activity. The I2S-S, GALNS-S, and ARSB-S elute after the corresponding products because of favorable interactions with the cationic centers in the HPLC matrix, so that tailing of the substrate peaks does not affect the product signal. The NAGLU-S and GUSB-S elute before their products but are baseline separated under the HPLC conditions. The TPP1-S does not dissociate in the ion source, and the HPLC trace shows only the peak of TPP1-P. Under the optimized HPLC conditions, elution of all 18 components was finished within 2 minutes, allowing for fast repetitive injection of multiple samples.
Figure 2.
HPLC-MRM traces for enzyme products on the XSelect CSH C18 column. For the ion transitions see Supplementary Table S1 (there are no isobaric overlaps for all P and IS). S → P indicates product ions formed by decomposition of the substrates in the ion source.
We have also examined HPLC separations on reverse-phase C-18 monolith columns (EMD Chromolith, Fast Gradient, RP18e, 50-2 mm, sorbent lot/column No. U11509/101). When using a well-conditioned column, we were able to obtain satisfactory separation of I2S, NAGLU, GALNS, and ARSB products and internal standards. However, new columns from the supplier required very long (36 h) conditioning to achieve satisfactory separation of the substrates and products or internal standards. The use of Chromolith columns was therefore less preferred.
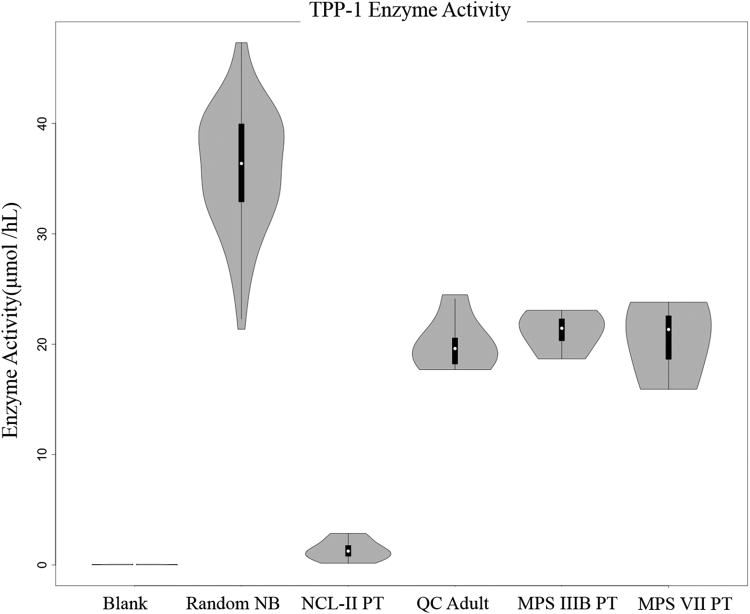
The activities measured for DBS from 62 unaffected infants are visualized in violin plots (Figure 3-5) and summarized in Table 1 (individual values given in Suppl. Table S2). TPP1 shows the highest activity with a 21.3-47.3 μmol h-1 L blood-1 range and 35.9 μmol h-1 L blood-1 mean. GALNS shows the lowest mean activity at 0.67 μmol h-1 L blood-1 followed by NAGLU (1.56), ARSB (4.37), I2S (16.1), and GUSB (28.5) all in μmol h-1 L blood-1 units. The activities measured for an adult volunteer and pooled blood (quality control HIGH) DBS samples were within or close to the ranges of newborn DBS activities (Table 1). In contrast, DBS from patients previously diagnosed with MPS IIIB, MPS VII, and LINCL showed low activities of the relevant enzymes. In particular, the mean NAGLU activity in DBS from four MPS IIIB patients (0.01 μmol h-1 L blood-1) was at 0.64% of the normal mean whereas the activities of the other five enzymes were within the normal ranges. The mean GUSB activity in DBS from three MPS VII patients (0.08 μmol h-1 L blood-1) was at 0.28% of the normal sample mean with the other enzyme activities within the normal ranges. The mean TPP1 activity in DBS from seven LINCL patients (1.33 |imol h-1 L blood-1) was at 3.7% of the of the normal sample mean which is 6.4 standard deviations from the mean.
Figure 3.
TPP1 activity distributions in DBS from the random newborn cohort (NB), quality controls (QC), and affected patients (PT) showing the medians (°), interquantile-range (iqr, q3-q1) (|) and range of (q1-1.5iqr: q3+1.5iqr) except when it is beyond the boundary of the data (|).
Figure 5.
GUSB activity distributions in DBS from the random newborn cohort (NB), quality controls (QC), and affected patients (PT) showing the medians (°), interquantile-range (iqr, q3-q1) (|) and range of (q1-1.5iqr: q3+1.5iqr) except when it is beyond the boundary of the data (|).
Table 1.
Summary of Enzyme Activities in DBS.
| Sample | Mean Activity (μmol h-1 L blood-1)a | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| GALNS | NAGLU | ARSB | I2S | GUSB | TPP1 | |
| Blank (n = 6) | 0.0033 | 0.01 | 0.05 | 0.06 | 0.06 | 0.03 |
| CDC Qlowb (n = 2) | 0.17 | 0.23 | 0.46 | 0.98 | 13.0 | 5.0 |
| CDC Qhighb (n = 2) | 3.0 | 5.8 | 5.1 | 14.6 | 44.4 | 22.7 |
| Adults (n = 4) | 1.1 | 4.1 | 1.2 | 17.0 | 19.5 | 19.0 |
| Newborns (n = 62) | 0.67 | 1.6 | 4.4 | 16.1 | 28.5 | 35.9 |
| MPS IIIB patients (n = 4) | 0.83 | 0.01 | 1.7 | 19.2 | 17.7 | 21.2 |
| MPS VII patients (n = 3) | 1.0 | 3.2 | 1.8 | 26.1 | 0.08 | 20.4 |
| LINCL patients (n = 7) | 1.2 | 3.1 | 1.8 | 17.1 | 27.0 | 1.3 |
For standard deviations in these measurements see supplementary Table S2.
From (39).
All assays showed low blank activities from incubations that used the assay cocktail and a filter paper punch but no blood with an average activity of <0.06 μmol h-1 L-1 from six measurements (Table 1). The analytical range in the activity measurements, defined as the ratio [assay response for the quality control HIGH]]/[blank activity] (8), was 909, 580, 102, 243, 740, and 757 for GALNS, NAGLU, ARSB, I2S, GUSB, and TPP1, respectively.
In our previous study of TPP1 assays (27) we noted a decreased TPP1 activity in samples that were stored at room temperature for long periods. Enzyme stability in DBS is an important issue that has been addressed previously for five lysosomal enzymes (31). We studied the activities of TPP1, NAGLU, and GUSB in DBS that were obtained by a fresh blood prick and then stored in ziplock bags at -20 °C, 4 °C, and ambient temperature for up to 34 days. The TPP1 activity showed a 25-30% decrease over 34 days at all storage temperatures (Supplementary Figure S11). The NAGLU and GUSB activities showed ∼15-20% decreases (Supplementary Figures S12 and S13). However, the activity data measurements performed on different days showed some scatter that made extrapolation to longer times uncertain. The data indicate that DBS storage at -20 °C for a few months is unlikely to degrade TPP1, NAGLU, and GUSB to affect enzyme activity measurements for healthy individuals. DBS aging and degradation may affect measurements for samples with activities close to the level cutoffs by increasing the number of false positives.
IDUA for analysis of MPS-I is typically done as part of a 6-plex LSD newborn screening assay (15, 32). We found that the IDUA substrate could be added to the above assay cocktail so that the full set of mucopolysaccharidoses could be analyzed in a single multiplex run. The detailed experimental protocol, HPLC-MS/MS traces, and a typical data set are shown in Suppl. Material. The analytical range for IDUA was > 100.
Discussion
The results from the multiplex LC-MS/MS assay show a very good separation of enzyme activities in DBS from the normal cohort and affected patients. The Figure 3 data for TPP1 indicate that the activities in the DBS from the LINCL patients are significantly lower than the lowest normals and also lower than the activities from patients affected by the other LSD. Similar results are shown in Figure 4 where the DBS from the MPS IIIB affected patients showed virtually zero NAGLU activities. The results for GUSB (Figure 5) are also conclusive in showing a large separation of the activities in DBS from the MPS VII affected patients from the rest of the set. An added benefit of multiplexing the assay is that a finding of simultaneously low activities for 2 or more lysosomal enzymes can be an indication that the integrity of the DBS sample has been compromised. Also, multiplexing methods are amenable to statistical methods in which co-variances are used to determine screen positive scores rather than absolute cutoff values (14).
Figure 4.
NAGLU activity distributions in DBS from the random newborn cohort (NB), quality controls (QC), and affected patients (PT) showing the medians (°), interquantile-range (iqr, q3-q1) (|) and range of (q1-1.5iqr: q3+1.5iqr) except when it is beyond the boundary of the data (|).
The analytical ranges of the 7 assays are more than 1- to 2-orders of magnitude higher than those of fluorometric assays with 4-methylumbelliferone substrates (8). For example, the analytical range of the HPLC-MS/MS assay for GALNS of 909 is 360-fold higher than the value of ∼2-3 reported for the fluorimetric assay using DBS and the 4-methylumbelliferone substrate (33,34). High analytical ranges of the MS/MS assays are predicted to yield a lower number of false positives compared to fluorometric assays when applied to newborn screening of LSDs as has been observed from large scale pilot studies and live newborn screening reports (7). Also, it is not possible with the fluorometric assays to accurately measure relatively low amounts of residual lysosomal enzymes for post-newborn screening diagnosis and prognosis studies, whereas MS/MS assays provide clear stratification of these samples (35-37). The relatively low analytical ranges for the fluorometric assays are due to the fact that the substrate itself is fluorescent and thus significantly contributes to the blank (8).
As an alternative to direct measurement of lysosomal enzymes in DBS, MS/MS analysis of accumulated glycosaminoglycan (measured as disaccharide degradation products) has been suggested as a first-tier newborn screening analysis of the mucopolysaccharidoses (38). It is thus useful to compare this method to the method reported in the current study. Both methods cover the mucopolysaccharidoses comprehensively. The glycosaminoglycan method requires 2 DBS punches, ∼1 hr of sample preparation time, an overnight incubation with glycosaminoglycan hydrolyzing enzymes, and HPLC-MS/MS with an inject-to-inject time of 5 min (38) resulting in a total time of 42 hr for 300 samples using a single HPLC-MS/MS instrument. The HPLC-MS/MS enzymatic assay described here requires a single DBS punch, ∼2 hr of sample preparation time, an overnight incubation and HPLC-MS/MS with an inject-to-inject time of 2 min resulting in a shorter total time of 28 hr for 300 samples. A recent pilot study of 2,862 DBS using the glycosaminoglycan method applied to MPS-I, -II, and −III gave a false positive rate of 0.9% (38). In marked contrast, the pilot study of the enzymatic analysis method described here (being carried out in the WA newborn screening laboratory, M.H. Gelb and C. R. Scott, unpublished) on the first ∼30,000 DBS gave a false positive rate of 0.02% for the same 3 LSDs (45-fold lower than the glycosaminoglycan method). The screen positive rate increases to 0.045% if all 7 mucopolysaccharidoses are considered. These results suggest that the enzymatic activity assay should be carried out for first-tier newborn screening with glycosaminoglycan analysis used as a possible second-tier assay; this is the method currently being used for newborn screening of MPS-I in the KY state newborn screening lab.
Studies are underway to test these new HPLC-MS/MS enzymatic assays for diagnostic and prognostic purposes using leukocytes as the enzyme source rather than DBS. It is hoped that the large analytical range will lead to a separation of affected individuals from those with pseudodeficiencies as well as to estimate the severity of the disease, for example early versus late onset forms.
Supplementary Material
Acknowledgments
Research Funding: BioMarin Corp. M. H. Gelb, F. Turecek, C. R. Scott: National Institute of Diabetes and Digestive and Kidney Diseases, NIH (R01 DK067859).
Role of Sponsor: The funding organizations played a direct role in the design of study, review and interpretation of data, and preparation and approval of manuscript.
Nonstandard abbreviations
- DBS
dried blood spots
- LSD
lysosomal storage disorders
- TPP1
tripeptidyl peptidase 1
- NAGLU
α-N-acetylglucosaminidase
- GUSB
lysosomal β-glucuronidase
- I2S
iduronate-2-sulfatase
- GALNS
N-acetylgalactosamine-6-sulfatase
- ARSB
N-acetylgalactosamine-4-sulfatase
- MPS-II, IIIB, IVA, VI, VII
mucopolysaccharidoses II, IIIB, IVA, VI, VII
- LINCL
infantile neuronal ceroid lipofuscinosis
- MSMS
tandem mass spectrometry
- ESI
electrospray ionization
- MRM
multiple reaction monitoring
- HPLC
high-performance liquid chromatography
Footnotes
Author Contributions: All authors confirmed they have contributed to the intellectual content of this paper and have met the following 3 requirements: (a) significant contributions to the conception and design, acquisition of data, or analysis and interpretation of data; (b) drafting or revising the article for intellectual content; and (c) final approval of the published article.
Authors' Disclosures or Potential Conflicts of Interest: Upon manuscript\ submission, all authors completed the author disclosure form. Disclosures and/or potential conflicts of interest:
Employment or Leadership: F. Turecek, University of Washington.
Consultant or Advisory Role: M. H. Gelb, Perkin Elmer.
Stock Ownership: None declared
Honoraria: None declared.
Expert Testimony: None declared.
Patents: M. H. Gelb, F. Turecek PCT/US2016/042143 filed 7/13/2016.
References
- 1.Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic and Molecular Basis of Inherited Disease. 8th. New York: Mc-Graw-Hill; 2001. [Google Scholar]
- 2.Ohashi T. Enzyme replacement therapy for lysosomal storage diseases. Pediatr Endocrinol Rev. 2012;10(Suppl 1):26–34. [PubMed] [Google Scholar]
- 3.Lund TC. Hematopoietic stem cell transplant for lysosomal storage diseases. Pediatr Endocrinol Rev. 2013;11(Suppl 1):91–8. [PubMed] [Google Scholar]
- 4.Weinreb NJ. Oral small molecule therapy for lysosomal storage diseases. Pediatr Endocrinol Rev. 2013;11(Suppl 1):77–90. [PubMed] [Google Scholar]
- 5.Biffi A, Montini E, Lorioli L, Cesani M, Fumagalli F, Plati T, et al. Lentiviral hematopoietic stem cell gene therapy benefits metachromatic leukodystrophy. Science. 2013;341(6148):864. doi: 10.1126/science.1233158. [DOI] [PubMed] [Google Scholar]
- 6.Hollak CEM, Wijburg FA. Treatment of lysosomal storage disorders: successes and challenges. J Inherit Metab Dis. 2014;37:587–98. doi: 10.1007/s10545-014-9718-3. [DOI] [PubMed] [Google Scholar]
- 7.Gelb MH, Scott CR, Tureček F. Newborn screening for lysosomal storage diseases. Clin Chem. 2015;61:335–346. doi: 10.1373/clinchem.2014.225771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar AB, Masi S, Ghomashchi F, Chennamaneni N, Ito M, Scott CR, et al. Tandem mass spectrometry has a larger analytical range than fluorescence assays of lysosomal enzymes: application to newborn screening of mucopolysaccharidoses types II, IVA and VI. Clin Chem. 2015;61:1363–1371. doi: 10.1373/clinchem.2015.242560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hofmann S, Peltonen L. The neuronal ceroid lipofuscinoses. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic and Molecular Basis of Inherited Disease. 8th. New york: McGraw-Hill; 2001. pp. 3877–94. [Google Scholar]
- 10.Jalanko A, Braulke T. Neuronal ceroid lipofuscinoses. Biochim Biophys Acta. 2009;1793:697–709. doi: 10.1016/j.bbamcr.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 11.Weber B, Blanch L, Clements PR, Scott HS, Hopwood JJ. Cloning and expression of the gene involved in Sanfilippo B syndrome (mucopolysaccharidosis III B) Hum Mol Genet. 1996:771–7. doi: 10.1093/hmg/5.6.771. [DOI] [PubMed] [Google Scholar]
- 12.Zhao HG, Li HH, Bach G, Schmidtchen A, Neufeld EF. The molecular basis of Sanfilippo syndrome type B. Proc Natl Acad Sci USA. 1996;93:6101–5. doi: 10.1073/pnas.93.12.6101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller RD, Hoffmann JW, Powell PP, Kyle JW, Shipley JM, Bachinsky DR, Sly WS. Cloning and characterization of the human beta-glucuronidase gene. Genomics. 1990;7:280–3. doi: 10.1016/0888-7543(90)90552-6. [DOI] [PubMed] [Google Scholar]
- 14.Marquardt G, et al. Enhanced interpretation of newborn screening results without analyte cutoff values. Genet Med. 2012;14:648–55. doi: 10.1038/gim.2012.2. [DOI] [PubMed] [Google Scholar]
- 15.Tortorelli S, Turgeon CT, Gavrilov DK, Oglesbee D, Raymond KM, Rinaldo P, Matern D. Simultaneous testing for 6 lysosomal storage disorders and X-adrenoleukodystrophy in dried blood spots by tandem mass spectrometry. Clin Chem. 2016;62:1248–54. doi: 10.1373/clinchem.2016.256255. [DOI] [PubMed] [Google Scholar]
- 16.Li Y, Scott CR, Chamoles NA, Ghavami A, Pinto BM, Tureček F, Gelb MH. Direct multiplex assay of lysosomal enzymes in dried blood spots for newborn screening. Clin Chem. 2004;50:1785–96. doi: 10.1373/clinchem.2004.035907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scott CR, Elliott S, Buroker N, Thomas LI, Keutzer J, Glass M, et al. Identification of infants at risk for developing Fabry, Pompe or mucopolysaccharidosis I from newborn blood spots by tandem mass spectrometry. J Pediatr. 2013;162:498–503. doi: 10.1016/j.jpeds.2013.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elliott S, Buroker N, Cournoyer JJ, Potier AM, Trometer JD, Elbin C, et al. Pilot study of newborn screening for six lysosomal storage diseases using tandem mass spectrometry. Mol Genet Metab. 2016;118:304–9. doi: 10.1016/j.ymgme.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang XK, Elbin CS, Turecek F, Scott CR, Chuang WL, Keutzer JM, Gelb MH. Multiplex lysosomal enzyme activity assay on dried blood spots using tandem mass spectrometry. Methods Mol Biol. 2010;603:339–50. doi: 10.1007/978-1-60761-459-3_32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.La Marca G, Casetta B, Malvagia S, Guerrini R, Zammarchi E. New strategy for the screening of lysosomal storage disorders: the use of the online traping-an-cleanup liquid chromatography/mass spectrometry. Anal Chem. 2009;81:6113–21. doi: 10.1021/ac900504s. [DOI] [PubMed] [Google Scholar]
- 21.Ko DH, Jun SH, Park HD, Song SH, Park KU, Kim JQ, et al. Multiplex enzyme assay for galactosemia using ultraperformance liquid chromatography-tandem mass spectrometry. Clin Chem. 2010;56:764–71. doi: 10.1373/clinchem.2009.139618. [DOI] [PubMed] [Google Scholar]
- 22.Spáčil Z, Tatipaka H, Barcenas M, Scott CR, Tureček F, Gelb MH. High-throughput assay of nine lysosomal enzymes for newborn screening. Clin Chem. 2013;59:502–11. doi: 10.1373/clinchem.2012.189936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kasper DC, Herman J, De Jesus VR, Mechtler TP, Metz TF, Shushan B. The application of multiplexed, multi-dimensional ultra-high-performance liquid chromatography /tandem mass spectrometry to the high throughput screening of lysosomal storage disorders in newborn dried bloodspots. Rapid Commun Mass Spectrom. 2010;24:986–94. doi: 10.1002/rcm.4496. [DOI] [PubMed] [Google Scholar]
- 24.Spáčil Z, Elliott S, Reeber SL, Gelb MH, Scott CR, Tureček F. Comparative fast liquid chromatography: application to newborn screening of Pompe, Fabry, and Hurler diseases. Anal Chem. 2011;84:4822–8. doi: 10.1021/ac200417u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mashima R, Sakai E, Kosuga M, Okuyama T. Levels of enzyme activities in six lysosomal storage diseases in Japanese neonates determined by liquid chromatography-tandem mass spectrometry. Genet Metab Rep. 2016;9:6–11. doi: 10.1016/j.ymgmr.2016.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chennamaneni N, Kumar A, Barcenas M, Spá čil Z, Scott CR, Tureček F, Gelb MH. Improved reagents for newborn screening of mucopolysaccharidosis-I, II and VI by tandem mass spectrometry. Anal Chem. 2014;87:4508–14. doi: 10.1021/ac5004135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barcenas M, Xue C, Marushchak-Vlaskin T, Scott CR, Gelb MH, Tureček F. Tandem mass spectrometry assays of palmitoyl protein thioesterase 1 and tripeptidyl peptidase activity in dried blood spots for the detection of neuronal ceroid lipofuscinoses in newborns. Anal Chem. 2014;87:7962–8. doi: 10.1021/ac501994b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tian Y, Sohar L, Taylor JW, Lobel P. Determination of the substrate specificity of tripeptidylpeptidase using combinatorial peptide libraries and development of improved fluorogenic substrates. J Biol Chem. 2006;281:6559–72. doi: 10.1074/jbc.M507336200. [DOI] [PubMed] [Google Scholar]
- 29.Wolfe BJ, Ghomashchi F, Kim T, Abam CA, Sadilek M, Jack R, et al. New substrates and enzyme assays for the detection of mucopolysaccharidosis III (Sanfilippo syndrome) types A, B, C and D by tandem mass spectrometry. Bioconjug Chem. 2012;28:557–64. doi: 10.1021/bc200609x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spáčil Z, Hui R, Gelb MH, Tureček F. Protonation sites and dissociation mechanisms of t-butylcarbamates in tandem mass spectrometric assays for newborn screening. J Mass Spectrom. 2011;46:1089–98. doi: 10.1002/jms.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elbin CS, Olivova P, Marashio CA, Cooper SK, Cullen E, Keutzer JM, Zhang XK. The effect of preparation, storage and shipping of dried blood spots on the activity of five lysosomal enzymes. Clin Chim Acta. 2011;412:1207–12. doi: 10.1016/j.cca.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 32.Elliott S, Buroker N, Counoyer JJ, Potier AM, Trometer JD, Elbin C, Schemer MJ, Kantola J, Boyce A, Turecek F, Gelb MH, Scott CR. Pilot study of newborn screening for six lysosomal storage diseases using tandem mass spectrometry. Mol Genet Metab. 2016;118:304–9. doi: 10.1016/j.ymgme.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Camelier MV, Burin MG, De Mari J, Vieira TA, Marasca G, Giugliani R. Practical and reliable enzyme test for the detection of mucopolysaccharidosis IVA (Morquio Syndrome type A) in dried blood samples. Clin Chim Acta. 2011;412:1805–8. doi: 10.1016/j.cca.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 34.Ullal AJ, Millington DS, Bali DS. Development of a fluorimetric microtiter plate based enzyme assay for MPS IVA (Morquio type A) using dried blood spots. Mol Genet Metab Rep. 2014;1:461–4. doi: 10.1016/j.ymgmr.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin N, Huang J, Violante S, Orsini JJ, Caggana M, Hughes EE, Stevens C, DiAntonio L, Liao HC, Hong X, Ghomashchi F, Kumar AB, Zhou H, Kornreich R, Wasserstein, Gelb MH, Yu C. Liquid Chromatography-Tandem Mass Spectrometry Assay of Leukocyte α-Glucosidase Assay But Not the Fluorimetric Assay Is a Useful Tool For Post-Newborn Screening Evaluation of Pompe Disease. Clin Chem. doi: 10.1373/clinchem.2016.259036. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liao HC, Chan MJ, Chiang CC, Yang CF, Niu DM, Huang CK, Gelb MH. Mass Spectrometry but not Fluorimetry Distinguishes Between Affected and Pseudodeficienies in Newborn Screening for Pompe Disease. Clin Chem. doi: 10.1373/clinchem.2016.269027. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liao HC, Spacil Z, Hong X, Ghomashchi F, Escolar ML, Kurtzberg J, Orsini JJ, Turecek F, Scott CR, Gelb MH. High Accuracy Assay of Galactocerebrosidase by Liquid Chromatography-Tandem Mass Spectrometry for Post-Newborn Screening Evaluation of Krabbe Disease. Clin Chem. doi: 10.1373/clinchem.2016.264952. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kubaski F, Mason RW, Nakatomi A, Shintaku H, Xie L, van Vllies NN, Church H, Giugliani R, Kobayashi H, Yamaguchi S, Suzuki Y, Orii T, Fukao T, Montano AM, Tomatsu S. Newborn screening for mucopolysaccharidoses: a pilot study of measurement of glycosaminoglycans by tandem mass spectrometry. J Inher Metab Dis. 2017;40:151–8. doi: 10.1007/s10545-016-9981-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De Jesus VR, Zhou H, Vogt RF. Dried blood spot quality control materials for newborn screening to detect lysosomal storage disorders. Clin Chem. 2013;59:1275–6. doi: 10.1373/clinchem.2013.209940. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.