Abstract
The treatment of acute myeloid leukemia (AML) in older patients is undergoing rapid changes, with a number of important publications in the past five years. Because of this, a group of Canadian leukemia experts has produced an update to the Canadian Consensus Guidelines that were published in 2013, with several new agents recommended, subject to availability. Recent studies have supported the survival benefit of induction chemotherapy for patients under age 80, except those with major co-morbidities or those with adverse risk cytogenetics who are not candidates for allogeneic hematopoietic stem cell transplantation (HSCT). Midostaurin should be added to induction therapy for patients up to age 70 with a FLT3 mutation, and gemtuzumab ozogamicin for de novo AML up to age 70 with favorable or intermediate risk cytogenetics. Daunorubicin 60 mg/m2 is the recommended dose for 3+7 induction therapy. Acute promyelocytic leukemia should be treated with arsenic trioxide plus all-trans retinoic acid, regardless of age, with cytotoxic therapy added upfront only for those with initial white blood count > 10. HSCT may be considered for selected suitable patients up to age 70-75. Haploidentical donor transplants may be considered for older patients. For non-induction candidates, azacitidine is recommended for those with adverse risk cytogenetics, while either a hypomethylating agent (HMA) or low-dose cytarabine can be used for others. HMA may also be used for relapsed/refractory disease after chemotherapy. For patients with secondary AML, CPX-351 is recommended for fit patients age 60-75.
Keywords: Acute myeloid leukemia, elderly patients, chemotherapy, co-morbidities, hypomethylating agents, cytogenetics, hematopoietic stem cell transplantation
Introduction
The treatment of older patients with AML presents several challenges and uncertainties. To address these issues, and to provide treatment guidance in the Canadian context, a panel of Canadian hematologists with expertise in acute leukemia published a set of comprehensive guidelines for the management of AML in patients age 60 years and over [1]. Since this publication in early 2013, a number of new studies have been completed and published. Many of these studies have profound implications for the management of these patients. As a result, based on these new data, many of the recommendations in the original paper no longer apply, or require revision.
We have therefore revisited the questions addressed in the original document, and have produced a series of revised recommendations for the management of these patients, based on more recent studies. These revisions are summarized below, along with the background rationale and justification. Revised treatment algorithms, based on these recommendations, are also included. Those recommendations which have not been specifically revised from the original paper are still felt to apply, and will not be repeated.
Question #1: What are the major criteria to determine who is a suitable candidate for intensive induction chemotherapy?
At the time of the initial publication, the availability of cytogenetics at diagnosis was felt to be important in determining suitability for intensive chemotherapy. Patients with adverse risk cytogenetics were not felt to be candidates for induction therapy, unless they were potential candidates for allogeneic hematopoietic stem cell transplantation (HSCT). In contrast, suitably fit patients with favourable risk cytogenetics were felt to be potential induction therapy candidates, even in the absence of alloHSCT.
Several studies have recently addressed the question of suitability for induction therapy. The demonstration of an overall survival (OS) benefit for secondary AML patients age 60-75 with MDS-like karyotypes who received CPX-351 (discussed in Question 5 below) potentially alters the decision in favor of candidacy for intensive chemotherapy in fit patients with such adverse cytogenetic profiles. In addition, recent studies have suggested that a higher proportion of older patients should be offered intensive approaches. Sorror et al developed a composite index, combining a modified co-morbidity index with age and cytogenetics, and found this to be highly predictive of one year OS [2]. In a subsequent evaluation of 242 patients age 70-79 treated at 6 centers, those who received intensive induction therapy (41% of patients) had a superior OS as compared with those that received non-intensive approaches (2 years OS 26% versus 13%, HR: 0.73) [3]. Early mortality was not increased in the induction cohort, possibly related to recent improvements in supportive care. Only those with the highest composite scores (> 10) did not demonstrate superior outcomes with induction therapy, suggesting that most patients below the age of 80 should be considered for induction approaches. However, as this was a retrospective study, selection bias could not be excluded, and prospective randomized studies are needed to verify this finding. Nevertheless, these data support previous findings by the Swedish Acute Leukemia Registry, which also found a lower early death rate and superior OS in older patients, particularly those age 70-79, who received more intensive treatment approaches [4]. These trends towards intensive treatment in selected, older patients with AML, are consistent with parallel trends in eligibility for alloHSCT; many centres worldwide are now transplanting selected patients into their mid-70s (see Question #3 below).
Geriatric assessment
A recent single center prospective cohort of patients age 60 and older evaluated several baseline measurements of physical performance, in addition to validated cognitive, depression, distress, and self-reported physical function scores, which were compared to the hematopoietic cell transplantation comorbidity index (HCT-CI) [5]. One objective measurement of physical function, the short physical performance battery (SPPB), and the 100-point modified mini-mental status (3MS, evaluating for impaired cognition) were found to be better predictors of mortality with induction chemotherapy, when compared to the HCT-CI. A more recent study found that a locally-derived geriatric assessment tool to determine frailty correlated with overall survival [6]. These studies need confirmation in a larger prospective multicenter cohort to prove applicability, but the use of such objectively administered testing bears consideration when approaching patients in this age group.
Revised recommendations
1. Intensive induction therapy should be considered for all patients below age 80, except for those with high co-morbidity scores, and those with adverse risk cytogenetics who are not potential candidates for HSCT in CR. However, there is no consensus as to what degree of co-morbidity constitutes an absolute contraindication to such therapy, and further studies are needed to clarify this question.
2. In the case of patients with adverse risk cytogenetics, induction therapy should generally be restricted to patients that are potential candidates for HSCT in CR.
3. Although co-morbidity indices are helpful, geriatric assessment tools for physical function and cognition can aid in decision-making regarding suitability for intensive chemotherapy. However, they should not replace clinical judgement. Discussion of these risks with the patient and their family are important to guide decision making.
Question #2: How should we treat older patients who are considered suitable for induction chemotherapy?
At the time of publication, there were no studies directly comparing daunorubicin 60 mg/m2 to 90 mg/m2 in induction. Results from the UK NCRI AML17 trial have since been published [7], comparing these two dose levels in a randomized trial. Although this study primarily enrolled younger patients, 36% were over age 60. This study showed no differences in complete remission (CR) rate, relapse rate or overall survival (OS) between the two groups, in the entire cohort and within each cytogenetic risk group. However, there was a higher incidence of CTAC grade 3-4 toxicity in the 90 mg/m2 arm. Given the latter, we are now recommending 60 mg/m2 as the standard daunorubicin dosing for induction therapy.
For patients with contraindications to anthracyclines (e.g., impaired left ventricular function, or extensive prior anthracycline exposure), several alternative induction strategies were mentioned. A study has now been published reporting on the use of FLAG chemotherapy (fludarabine, cytarabine and G-CSF), primarily in older patients, many of whom had cardiac-based contraindications to anthracyclines [8]. Although this was an uncontrolled, retrospective study, the CR rate and OS were comparable to those seen with 3+7 inductions in this patient population. We are therefore adding the FLAG regimen as an option in patients with cardiac contraindications to anthracycline-based therapy.
Results from the RATIFY study have been published [9]. In this study, patients age 18-60 with newly-diagnosed FLT3 mutated AML were randomly assigned to receive midostaurin or placebo, added to standard induction and consolidation chemotherapy, and then continued as maintenance therapy for up to one year. This study demonstrated a superior CR rate and OS in the midostaurin arm. Although this study did not include patients over age 60, FLT3-ITD mutations are also associated with an inferior outcome post induction chemotherapy in elderly patients [10,11]. A recent phase II study by the German AML Study Group used midostaurin in combination with induction, consolidation and maintenance therapy in adults with FLT3 mutated AML, 34% of whom were age 60-70 [12]. In this study, the relapse-free survival in patients age 60-70 was similar to that of younger patients, and was significantly better than of an age-matched historical control group.
Our initial recommendations included the use of gemtuzumab ozogamicin (GO), if available, based on the French ALFA study in patients age 50-70 years [13]. Since then, a meta-analysis incorporating data from six frontline randomized trials in AML has been published [14]. This analysis confirmed the OS benefit of adding GO to induction therapy. This benefit was most prominently seen in patients with favorable risk cytogenetics, but a significant benefit was also seen in those with intermediate risk cytogenetics. In contrast, there was no benefit in patients with adverse risk karyotypes. Patients with secondary AML either did not benefit, or were not included in these studies.
Acute promyelocytic leukemia (APL)
At the time of publication, all-trans retinoic acid (ATRA) + anthracycline ± cytarabine was considered the standard of care for APL. The subsequent publication of the APL0406 study, demonstrating the superiority of a chemo-free regimen consisting of ATRA plus arsenic trioxide (ATO) over ATRA plus chemotherapy [15,16], has led to the adoption of this chemo-free approach in many centers as the new standard of care for patients with low and intermediate risk disease. This chemo-free approach was subsequently confirmed in the UK NCRI AML 17 study [17] which employed an alternative ATO regimen.
For patients with high-risk APL (defined as presenting WBC > 10 × 109/L), anthracyclines are generally administered with induction therapy, to provide rapid cytoreduction and reduce the high risk of differentiation syndrome, as in the Australian APML4 protocol [18]. In older higher risk patients, some members of the group recommend reducing the dose of anthracycline in patients over age 70, or eliminating it entirely if left ventricular function is impaired, and substituting either cytarabine or hydroxyurea.
The APL0406, UK NCRI AML 17, and APML4 studies focused primarily on younger patients. APL0406 was restricted to patients below age 71, and most patients were under age 60. The AML 17 study included patients up to age 77, but only ~20% were ≥ age 60. The APML4 study had no upper age cutoff, but only 13% were ≥ age 61. While none of these studies identified older patients as particularly problematic, subsequent experience by one group [19] found that older patients experienced more severe complications, and a higher induction mortality, with this regimen. These patients (particularly those with co-morbidities) have more difficulty tolerating the hyperleukocytosis which frequently occurs on this regimen, and the resulting differentiation syndrome. Nevertheless, when the high curability and low relapse rate of APL are considered, it is clear that all patients should be treated.
While the APL0406, UK NCRI AML 17, and APML4 studies did not make this recommendation, Kota et al [19] suggested a reduced dosing schedule (e.g. reducing the ATO dose from 0.15 to 0.1 mg/kg daily, and the ATRA dose from 45 to 25 mg/m2 daily) in older patients; however, there are no prospective studies that address the efficacy and safety of this approach, and most authors still recommend using full doses. However, all patients should be monitored carefully for early signs of differentiation syndrome, and pre-emptive therapy with dexamethasone should be instituted promptly. Hyperleukocytosis arising during chemo-free therapy also needs to be managed aggressively with cytoreduction.
While the advent of ATRA- + ATO- containing regimens has improved the outcomes of patients with APL, early death (ED) remains a major problem, with up to 30% mortality within 30 days of diagnosis reported in some studies [19-24]. Reasons for ED included delays in diagnosing APL, beginning ATRA and transferring to a leukemia center, as well as failure to diagnose and treat coagulopathy in a suitably aggressive manner. Notably, increased age has been shown to correlate strongly with the likelihood of ED [20]. A high index of suspicion for the diagnosis of APL, and the prompt initiation of therapy, are therefore essential for further improving outcomes in APL, particularly in the elderly patient.
Revised recommendations (see Figure 1 for algorithm)
Figure 1.
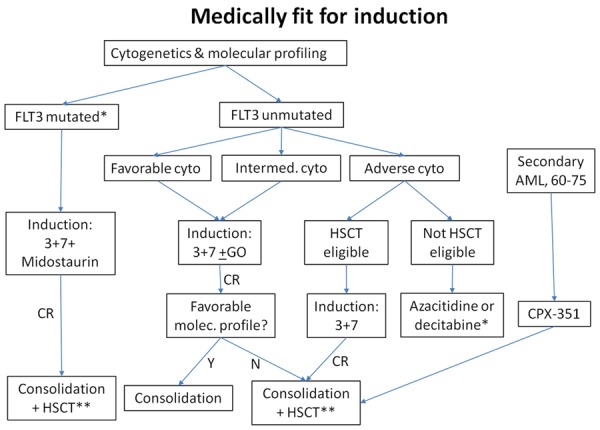
Treatment algorithm for older AML patients who are deemed medically fit for induction therapy. For patients with intermediate-risk cytogenetics (particularly normal karyotype), favorable molecular profile refers to NPM1 mutated or bialleleic CEBPA mutation, in absence of FLT3-ITD. For favorable-risk cytogenetics, favorable molecular profile refers to absence of c-kit mutation. GO = gemtuzumab ozogamicin. HSCT = allogeneic hematopoietic stem cell transplantation. Cyto = cytogenetics. *If not yet received hypomethylating agent. **If HSCT eligible.
1. Older patients with de novo AML and intermediate or favourable risk cytogenetics, who are deemed suitable candidates, should receive induction treatment consisting of an anthracycline or anthracenedione for 3 days plus cytarabine (100-200 mg/m2) for 7 days (3+7). Acceptable anthracyclines/anthracenediones include: Daunorubicin 60 mg/m2 daily × 3 days; Idarubicin 12 mg/m2 daily × 3; Mitoxantrone 12 mg/m2 daily × 3.
2. For patients with contraindications to anthracyclines (e.g., impaired left ventricular function or extensive prior anthracycline exposure), the FLAG regimen (fludarabine, cytarabine and filgrastim) would be a suitable option, in addition to those mentioned in the initial recommendations.
3. For older patients who are candidates for intensive chemotherapy, FLT-ITD and TKD mutation testing results should be provided within one week. For patients up to age 70 with a FLT3-ITD or TKD mutation, midostaurin, if available, should be added to induction and consolidation, and continued as maintenance therapy if not transplanted, in the schedule used in the RATIFY and German AMLSG studies.
4. For non-FLT3 mutated patients up to age 70 with de novo AML and favourable or intermediate risk cytogenetics, gemtuzumab ozogamicin, if available, should be added to induction and consolidation therapy, in the schedule used in the ALFA study.
5. For patients with de novo AML and adverse-risk cytogenetics, induction chemotherapy (either in a standard format or in the context of a clinical trial) should be used for transplant candidates. Non-transplant candidates should be enrolled in a clinical trial or should receive a hypomethylating agent. See Question #5 below for revised recommendations for patients with secondary AML.
6. For patients with APL and low-intermediate risk disease, a chemo-free regimen consisting of ATRA and arsenic trioxide (ATO) should be used. Older patients, in particular, should be closely monitored for treatment-related complications, and further studies are needed to determine the optimal dosing schedules for very elderly and frail patients. High risk patients (defined as baseline WBC > 10 × 109/L) should also receive an anthracycline or, if not eligible, other cytoreductive therapy early during induction.
Question #3: Which older patients should be considered for allogeneic stem cell transplantation (HSCT)?
At the time of publication in 2013 there were insufficient data on HSCT in patients over age 70 years. Recent evidence suggests that HSCT can be performed successfully in selected patients over 70, generally up to age 75 years [25-27].
Comorbidity was considered the most important patient related factor. Since then, other factors such as geriatric assessment (GA), functional status, social support and biomarkers have emerged as good indicators of transplant eligibility [28,29].
While the best results are obtained in patients in CR1, a recent IBMTR study found that selected patients may have a meaningful prolongation of life in CR2, if they have favorable or intermediate risk cytogenetics [30]. This is an important observation, as patients with favorable cytogenetic or mutational profiles are generally not transplanted in CR1.
In the initial review, haploidentical transplants were only recommended in the context of clinical trials. Since then, additional studies of HSCT using haploidentical donors in elderly patients have shown comparable survival to those using matched unrelated donors [31-33], and many centers are now performing such transplants routinely, using the Johns Hopkins protocol with post-transplant cyclophosphamide [33,34]. The advantage of using haploidentical donors is that HSCT can be done more quickly, compared to unrelated donors, and donor availability is wider.
Revised recommendations
1. HSCT may be considered for selected fit candidates up to age 75, depending on the experience of the local transplant center.
2. A suitable haploidentical donor should be considered as an alternative to a matched related or unrelated donor HSCT, based on the experience of the transplant center.
3. To assess fitness and eligibility for allogeneic HSCT, a combination of factors such as chronological age, HCT-CI, Karnofsky Performance Status, Geriatric assessment (GA), and social support should be assessed. As these recommendations have not been studied prospectively, individual transplant centers should establish their own criteria based on local resources for a team evaluation.
4. HSCT for patients in CR2 may be considered, especially for patients with favorable or intermediate risk cytogenetics.
Question #4: How should we treat older patients who are not considered suitable for induction therapy?
At the time of publication in 2013 there was clear evidence supporting the use of azacitidine for patients with AML and 20-30% blasts with dysplasia [35]. However, data for using this agent in those with > 30% blasts were not available. The only randomized study available using hypomethylating agents (HMA) was with decitabine; this study had shown a non-significant trend toward better OS with this agent, compared to low-dose cytarabine (LDAC) or supportive care [36].
Since then, the results of the AZA-AML-001 study have been published. This Phase III trial studied the use of azacitidine in AML patients with more than 30% BM blasts. 488 patients, age > 65 years with de novo or secondary AML, not eligible for HSCT, and with intermediate or adverse-risk cytogenetics, were randomized 1:1 to receive azacitidine 75 mg/m2 for 7 consecutive days every 28 days, or to a pre-determined conventional care regimen (CCR), including intensive chemotherapy, LDAC, or best supportive care only [37]. The azacitidine group showed a trend toward increased median OS at 10.4 months, compared to 6.5 months in the CCR group.
Of the 170 patients with poor-risk cytogenetics, there was a statistically significant improved median OS in the azacitidine arm (6.4 months) compared to the CCR group (3.2 months). In patients with intermediate-risk cytogenetics, median OS did not differ significantly between those who received azacitidine (13.0 months) or CCR (10.1 months) [37,38]. In intermediate-risk patients pre-selected to receive LDAC, OS was also similar between those receiving LDAC or azacitidine.
Several subsequent subgroup analyses of the AZA-AML-001 data have been published only in abstract form. An analysis of the subset of 158 AZA-AML-001 patients, classified locally at participating sites as AML with morphologic dysplastic changes (AML-MDC) at the time of study enrolment, showed that those treated with azacitidine had a statistically significant increase in median OS of 12.7 months, compared to 6.3 months for those treated with CCR [39]. While this analysis has been interpreted speculatively to suggest that AML-MDC patients with intermediate-risk cytogenetics should receive azacitidine rather LDAC, the post hoc nature of these analyses, and the absence of a pre-defined multivariate analysis incorporating cytogenetic risk groups or prior MDS, suggest caution in this regard.
Several additional recent reports have clarified further the role of azacitidine or other hypomethylating agents in the management of AML in the elderly. First, the Austrian Azacitidine Registry (AAR) has confirmed in a ‘real-life’ setting the efficacy of azacitidine in a large cohort of elderly patients (57.9% ≥ age 75 years) with AML with 20-30% blasts. Outcomes in the AAR setting are indistinguishable from those of the AZA-AML-001 study [40]. Second, a retrospective multicenter analysis of over 500 patients who received a hypomethylating agent for relapsed/refractory AML has clarified the role of these agents in this setting [41]. Overall, the CR rate was 11.7%, with an additional 6% and 8% achieving CRi and hematologic improvement (HI), respectively, with no differences seen between azacitidine and decitabine. Notably, the OS of the entire cohort was 11.6 months, while those achieving CR had a median OS of 25.6 months. Thus, while the likelihood of achieving a CR in this setting is low, such responders appear to derive benefit.
Revised recommendations (see Figure 2 for algorithm)
Figure 2.
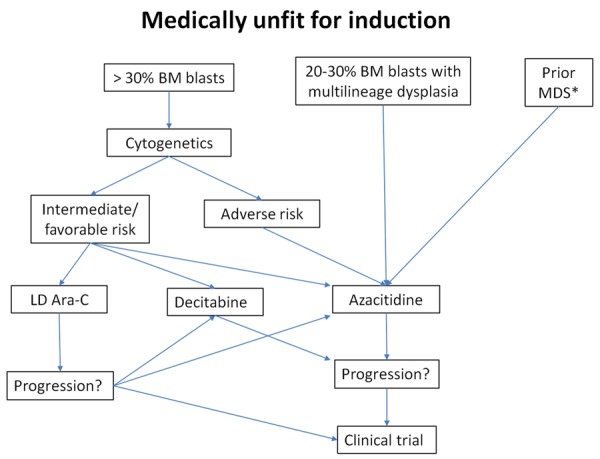
Treatment algorithm for older AML patients who are deemed not medically fit for induction therapy. BM = bone marrow. LD Ara-C = low-dose cytarabine, MDS = myelodysplastic syndrome. *If no prior exposure to hypomethylating agents.
1. For patients who are not considered candidates for intensive chemotherapy, cytogenetic results are important in determining the optimal therapy to use, and should be provided within one week.
2. For patients with 20 to 30% bone marrow blasts with myelodysplasia-related changes, azacitidine should be offered as standard treatment.
3. For all other patients, consider cytogenetic risk group: a. Adverse-risk cytogenetics: Azacitidine should be offered as the preferred frontline treatment. Some authors also recommend this agent for patients with prior MDS not previously exposed to hypomethylating agents, or those with dysplastic morphology; b. Intermediate- and favorable-risk cytogenetics: LDAC, azacitidine and decitabine are all reasonable treatment options.
4. For patients with relapsed or refractory disease following LDAC or other chemotherapies, HMA therapy (either azacitidine or decitabine) are reasonable treatment options if a clinical trial is not available. However, for non-induction candidates progressing on HMAs, there is no recognized effective therapy, LDAC is not recommended, and clinical trial enrolment is suggested.
5. LDAC, azacitidine and decitabine should be used continuously until disease progression, to obtain the optimal clinical response.
Question #5: How should we treat older patients with secondary or therapy-related AML?
As outlined in the initial recommendations, patients with AML arising from an antecedent hematologic disorder (sAML), or those with therapy-related AML (tAML), should be managed similarly to those with de novo AML. However, recent data using CPX-351 suggests a change in the approach to these patients.
CPX-351 is a nanoparticle lipid formulation containing a fixed 5:1 molar ratio of cytarabine and daunorubicin. An initial Phase II study in patients age 60-75 found no significant difference in survival compared to standard 3+7 induction, but a subgroup analysis showed a superior CR rate and OS in patients with secondary AML arising from a prior myelodysplastic syndrome (MDS) [42]. A follow-up study was therefore performed, in which AML patients age 60-75 with either prior MDS, chronic myelomonocytic leukemia (CMML), therapy-related AML or MDS-like cytogenetic abnormalities, were randomized to receive either CPX-351 or 3+7 induction therapy. This study, described to date only in abstract form, showed that the CPX-351 arm had a higher CR rate (48% vs. 33%) and superior OS (P=0.021), without an increase in early mortality [43]. Patients receiving CPX-351 also had a superior outcome following subsequent allogeneic hematopoietic stem cell transplant (HSCT) [44], and had a particularly high CR rate in the presence of a FLT3 mutation. As subgroup analyses have not yet been published, it is unclear whether those with de novo AML and MDS-like cytogenetic abnormalities derived comparable benefit compared to those with secondary AML.
Revised recommendations
1. For AML arising from prior MDS or CMML, or therapy related AML, CPX-351, if available, should be used as induction and post-remission therapy in patients age 60-75 who are eligible for intensive therapy.
2. If CPX-351 is not available, standard induction chemotherapy should be used for HSCT candidates. For non-HSCT candidates otherwise medically fit for intensive therapy, and with intermediate/favorable risk cytogenetics, induction chemotherapy or hypomethylating agents are both reasonable options.
3. For patients not medically fit for intensive therapy, or with adverse risk cytogenetics and not a candidate for alloHSCT, enrolment in a clinical trial, or a hypomethylating agent (if not previously utilized), should be considered (See Question #4 for details).
Conclusion
As noted above, a number of new studies published in the past several years have altered substantially the approach to evaluating and treating older patients with AML. The changes to the recommended treatment algorithms are summarized in Figure 1 (induction candidates) and Figure 2 (non-induction candidates). However, these studies are also changing the fundamental decision regarding candidacy for intensive therapy.
Some of the newer agents have not yet been approved by various regulatory bodies; however, given the strength of the early data, it is felt that such approval is likely in the near future. Therefore, we have incorporated agents which are in the approval process into the revised algorithms.
There are several promising investigational agents which are currently being evaluated in clinical trials in AML, either alone or in combination with existing drugs. Given that outcomes in older patients are still suboptimal, with most patients ultimately dying of progressive disease, the authors continue to strongly encourage older patients to be enrolled in such studies, for all except the terminal stages of their disease. Although the results of these clinical trials are uncertain, it is likely that at least some of these studies will lead to new standards of care, which will necessitate further revisions to the guidelines in the coming years.
Disclosure of conflict of interest
JM Brandwein: Advisory Board & Honoraria - Celgene, Lundbeck, Pfizer, Novartis, Paladin. N Zhu: Advisory Board & Honoraria - Celgene, Novartis. R Kumar: Advisory Board & Honoraria - Celgene. B Leber: Advisory Board and Honoraria - Celgene, Novartis, BMS, Pfizer, Paladin, Amgen, Alexion, Lundbeck, Abbvie, Astellas. M Sabloff: Lundbeck, Amgen. Research funding - Sanofi, Roche. I Sandhu: Advisory Board & Honoraria - Celgene, Novartis. J Kassis: No disclosures. HJ Olney: Advisory Board & Honoraria - Celgene, BMS, Novartis, Paladin, Pfizer. M Elemary: Advisory Board & Honoraria - Celgene, Novartis, Roche, Lundbeck. AC Schuh: Advisory Board & Honoraria - Celgene, Lundbeck, Pfizer.
References
- 1.Brandwein JM, Ashkenas J, Geddes MN, Kew AK, Leber B, Nevill T, Sabloff M, Sandhu I, Schuh A, Storring J. Treatment of older patients with acute myeloid leukemia (AML): a Canadian consensus. Am J Blood Res. 2013;3:141–164. [PMC free article] [PubMed] [Google Scholar]
- 2.Sorror ML, Storer BE, Elsawy M, Fathi AT, Brunner A, Gerds AT, Sekeres MA, Mukherjee S, Medeiros BC, Shami P, Peña E, Wardyn S, Whitten J, Frenkel H, McCune J, Lee SJ, Estey EH. Impact of comorbidities at diagnosis of acute myeloid leukemia on one-year mortality. Blood. 2015;126:532. [Google Scholar]
- 3.Sorror ML, Storer BE, Elsawy M, Fathi AT, Brunner A, Gerds AT, Sekeres MA, Mukherjee S, Medeiros BC, Wang ES, Vachhani P, Shami PJ, Peña E, Wardyn S, Whitten J, Moore R, Becker PS, McCune J, Lee SJ, Sandmaier BM, Appelbaum FR, Estey EH. Intensive versus non-intensive induction therapy for patients (Pts) with newly diagnosed acute myeloid leukemia (AML) using two different novel prognostic models. Blood. 2016;128:216. [Google Scholar]
- 4.Juliusson G, Antunovic P, Derolf A, Lehmann S, Möllgård L, Stockelberg D, Tidefelt U, Wahlin A, Höglund M. Age and acute myeloid leukemia: real world data on decision to treat and outcomes from the Swedish acute leukemia registry. Blood. 2009;113:4179–4187. doi: 10.1182/blood-2008-07-172007. [DOI] [PubMed] [Google Scholar]
- 5.Klepin HD, Geiger AM, Tooze JA, Kritchevsky SB, Williamson JD, Pardee TS, Ellis LR, Powell BL. Geriatric assessment predicts survival for older adults receiving induction chemotherapy for acute myelogenous leukemia. Blood. 2013;121:4287–4294. doi: 10.1182/blood-2012-12-471680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aydin S, Audisio E, D’Ardia S, Allione B, Nicolino B, Busca A, Dellacasa C, Iovino G, Evangelista A, Ciccone G, Vitolo U. Assessment of patients fitness at diagnosis in elderly acute myeloid leukemia. Blood. 2016;128:4012a. [Google Scholar]
- 7.Burnett AK, Russell NH, Hills RK, Kell J, Cavenagh J, Kjeldsen L, McMullin MF, Cahalin P, Dennis M, Friis L, Thomas IF, Milligan D, Clark RE UK NCRI AML Study Group. A randomized comparison of daunorubicin 90 mg/m2 vs 60 mg/m2 in AML induction: results from the UK NCRI AML17 trial in 1206 patients. Blood. 2015;125:3878–8385. doi: 10.1182/blood-2015-01-623447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saini L, Brandwein J, Turner R, Larratt L, Hamilton M, Peters A, Wu C, Zhu N, Patterson JM, Bolster L, Mant M, Ritchie B, Liew E, Ghosh S, Sandhu I. The FLAG chemotherapy regimen is an alternative to anthracycline based therapy for the treatment of acute myeloid leukemia for patients with pre-existing cardiac disease. Eur J Haematol. 2016;97:471–478. doi: 10.1111/ejh.12757. [DOI] [PubMed] [Google Scholar]
- 9.Stone RM, Mandrekar SJ, Sanford BL, Laumann K, Geyer S, Bloomfield CD, Thiede C, Prior TW, Döhner K, Marcucci G, Lo-Coco F, Klisovic RB, Wei A, Sierra J, Sanz MA, Brandwein JM, de Witte T, Niederwieser D, Appelbaum FR, Medeiros BC, Tallman MS, Krauter J, Schlenk RF, Ganser A, Serve H, Ehninger G, Amadori S, Larson RA, Döhner H. Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N Engl J Med. 2017 doi: 10.1056/NEJMoa1614359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whitman SP, Maharry K, Radmacher MD, Becker H, Mrózek K, Margeson D, Holland KB, Wu YZ, Schwind S, Metzeler KH, Wen J, Baer MR, Powell BL, Carter TH, Kolitz JE, Wetzler M, Moore JO, Stone RM, Carroll AJ, Larson RA, Caligiuri MA, Marcucci G, Bloomfield CD. FLT3 internal tandem duplication associates with adverse outcome and gene- and microRNAexpression signatures in patients 60 years of age or older with primary cytogenetically normal acute myeloid leukemia: a cancer and leukemia group B study. Blood. 2010;116:3622–3626. doi: 10.1182/blood-2010-05-283648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lazenby M, Gilkes AF, Marrin C, Hills RK, Burnett AK. The prognostic relevance of flt 3 and npm1 mutations on older patients treated intensively or non-intensively: a study of 11312 patients in the UK NCRI AML 16 trial. Leukemia. 2014;28:1953–1959. doi: 10.1038/leu.2014.90. [DOI] [PubMed] [Google Scholar]
- 12.Schlenk R, Döhner K, Salih H, Kündgen A, Fiedler W, Salwender HJ, Westermann J, Götze KS, Horst HA, Wulf G, Lübbert M, Kraemer D, Kindler T, Ringhoffer M, Brossart P, Held G, Greil R, Südhoff T, Münnich A, Weber D, Gaidzik VI, Teleanu MV, Paschka P, Theis F, Heuser M, Thol F, Benner A, Ganser A, Döhner H. Midostaurin in combination with intensive induction and as single agent maintenance therapy after consolidation therapy with allogeneic hematopoietic stem cell transplantation or highdose cytarabine ( NCT01477606) Blood. 2015;126:322a. [Google Scholar]
- 13.Castaigne S, Pautas C, Terre C, Raffoux E, Bordessoule D, Bastie JN, Legrand O, Thomas X, Turlure P, Reman O, De Revel T, Gastaud L, de Gunsberg N, Contentin N, Henry E, Marolleau JP, Aljijakli A, Rousselot P, Fenaux F, Preudhomme C, Chevret S, Dombret H. Effect of gemtuzumab ozogamicin on survival of adult patients with de-novo acute myeloid leukaemia (ALFA-0701): a randomised, open-label, phase 3 study. Lancet. 2012;379:1508–1516. doi: 10.1016/S0140-6736(12)60485-1. [DOI] [PubMed] [Google Scholar]
- 14.Hills RK, Castaigne S, Appelbaum FR, Delaunay J, Petersdorf S, Othus M, Estey EH, Dombret H, Chevret S, Ifrah N, Cahn JY, Récher C, Chilton L, Moorman AV, Burnett AK. Addition of gemtuzumab ozogamicin to induction chemotherapy in adult patients with acute myeloid leukaemia: a meta-analysis of individual patient data from randomised controlled trials. Lancet Oncol. 2014;15:986–996. doi: 10.1016/S1470-2045(14)70281-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lo-Coco F, Avvisati G, Vignetti M, Thiede C, Orlando SM, Iacobelli S, Ferrara F, Fazi P, Cicconi L, Di Bona E, Specchia G, Sica S, Divona M, Levis A, Fiedler W. Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. N Engl J Med. 2013;369:111–121. doi: 10.1056/NEJMoa1300874. [DOI] [PubMed] [Google Scholar]
- 16.Platzbecker U, Avvisati G, Ehninger G, Cicconi L, Thiede C, Ferrara F, Orlando SM, Vignetti M, Specchia G, Sborgia M, Lo-Coco F. Improved outcome with ATRA-Arsenic trioxide compared to ATRA-chemotherapy in non-high risk acute promyelocytic leukemia-updated results of the Italian-German APL0406 trial on the extended final series. J. Clin. Oncol. 2017;35:605–612. doi: 10.1200/JCO.2016.67.1982. [DOI] [PubMed] [Google Scholar]
- 17.Burnett AK, Russell NH, Hills RK, Bowen D, Kell J, Knapper S, Morgan YG, Lok J, Grech A, Jones G, Khwaja A, Friis L, McMullin MF, Hunter A, Clark RE, Grimwade D UK National Cancer Research Institute Acute Myeloid Leukaemia Working Group. Arsenic trioxide and all-trans retinoic acid treatment for acute promyelocytic leukaemia in all risk groups (AML17): results of a randomized, controlled, phase 3 trial. Lancet Oncol. 2015;16:1295–1305. doi: 10.1016/S1470-2045(15)00193-X. [DOI] [PubMed] [Google Scholar]
- 18.Iland HJ, Bradstock K, Supple SG, Catalano A, Collins M, Hertzberg M, Browett P, Grigg A, Firkin F, Hugman A, Reynolds J, Di Iulio J, Tiley C, Taylor K, Filshie R, Seldon M, Taper J, Szer J, Moore J, Bashford J, Seymour JF. All-trans-retinoic acid, idarubicin, and IV arsenic trioxide as initial therapy in acute promyelocytic leukemia (APML4) Blood. 2012;120:1570–1580. doi: 10.1182/blood-2012-02-410746. [DOI] [PubMed] [Google Scholar]
- 19.Kota V, Karkhanis P, Sharma R, Bolds S, Sitchenko K, Shrestha A, Jonnalagadda V, Gaddh M, Bernal-Mizrachi L, Heffner LT, Winton EF, McLemore ML, Langston A, Galipeau J, Bodó I, Al-Kadhimi Z, Bashey A, Stuart RK, Vidito SI, Pati A, Gerber JM, Grunwald MR, Bradley KT, Tongol J, El-Geneidy M, Khoury HJ, Arellano M, Jillella A. A prospective multi-center trial shows reduction of early deaths (ED) and improved survival in elderly acute promyelocytic leukemia (APL) patients (> 60 years). Results of using a simplified treatment algorithm and expert support in georgia, South Carolina and neighboring states. Blood. 2016;128:1622a. [Google Scholar]
- 20.Lehmann S, Ravn A, Carlsson L, Antunovic P, Deneberg S, Möllgård L, Derolf AR, Stockelberg D, Tidefelt U, Wahlin A, Wennström L, Höglund M, Juliusson G. Continuing high early death rate in acute promyelocytic leukemia: a population-based report from the Swedish adult acute leukemia registry. Leukemia. 2011;25:1128–1134. [Google Scholar]
- 21.Park JH, Qiao B, Panageas KS, Schymura MJ, Jurcic JG, Rosenblat TL, Altman JK, Douer D, Rowe JM, Tallman MS. Early death rate in acute promyelocytic leukemia remains high despite all-trans retinoic acid. Blood. 2011;118:1248–1254. doi: 10.1182/blood-2011-04-346437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McClellan JS, Kohrt HE, Coutre S, Gotlib JR, Majeti R, Alizadeh AA, Medeiros BC. Treatment advances have not improved the early death rate in acute promyelocytic leukemia. Haematologica. 2012;97:133–136. doi: 10.3324/haematol.2011.046490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Altman JK, Rademaker A, Cull E, Weitner BB, Ofran Y, Rosenblat TL, Haidau A, Park JH, Ram SL, Orsini JM, Sandhu S, Catchatourian R, Trifilio SM, Adel NG, Frankfurt O, Stein EM, Mallios G, Deblasio T, Jurcic JG, Nimer S, Peterson LC, Kwaan HC, Rowe JM, Douer D, Tallman MS. Administration of ATRA to newly diagnosed patients with acute promyelocytic leukemia is delayed contributing to early hemorrhagic death. Leuk Res. 2013;37:1004–1009. doi: 10.1016/j.leukres.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 24.de la Serna J, Montesinos P, Vellenga E, Rayón C, Parody R, León A, Esteve J, Bergua JM, Milone G, Debén G, Rivas C, González M, Tormo M, Díaz-Mediavilla J, González JD, Negri S, Amutio E, Brunet S, Lowenberg B, Sanz MA. Causes and prognostic factors of remission induction failure in patients with acute promyelocytic leukemia treated with all-trans retinoic acid and idarubicin. Blood. 2008;111:3395–3402. doi: 10.1182/blood-2007-07-100669. [DOI] [PubMed] [Google Scholar]
- 25.Heidenreich S, Ziagkos D, de Wreede LC, van Biezen A, Finke J, Platzbecker U, Niederwieser D, Einsele H, Bethge W, Schleuning M, Beelen DW, Tischer J, Nagler A, Glass B, Maertens J, Yáñez L, Beguin Y, Sill H, Scheid C, Stelljes M, Ganser A, Zachée P, Selleslag D, de Witte T, Robin M, Kröger N. Allogeneic stem cell transplantation for patients age ≥ 70 years with myelodysplastic syndrome: a retrospective study of the MDS Subcommittee of the chronic malignancies working party of the EBMT. Biol Blood Marrow Transplant. 2017;23:44–52. doi: 10.1016/j.bbmt.2016.09.027. [DOI] [PubMed] [Google Scholar]
- 26.Aoki J, Kanamori H, Tanaka M, Yamasaki S, Fukuda T, Ogawa H, Iwato K, Ohashi K, Okumura H, Onizuka M, Maesako Y, Teshima T, Kobayashi N, Morishima Y, Hirokawa M, Atsuta Y, Yano S, Takami A. Impact of age on outcomes of allogeneic hematopoietic stem cell transplantation with reduced intensity conditioning in elderly patients with acute myeloid leukemia. Am J Hematol. 2016;91:302–307. doi: 10.1002/ajh.24270. [DOI] [PubMed] [Google Scholar]
- 27.Pohlen M, Groth C, Sauer T, Gorlich D, Mesters R, Schliemann C, Lenz G, Müller-Tidow C, Büchner T, Berdel WE, Stelljes M. Outcome of allogeneic stem cell transplantation for AML and myelodysplastic syndrome in elderly patients (60 years) Bone Marrow Transplant. 2016;51:1441–1448. doi: 10.1038/bmt.2016.156. [DOI] [PubMed] [Google Scholar]
- 28.Rosko A, Artz A. Aging: treating the older patient. Biol Blood Marrow Transplant. 2017;23:193–200. doi: 10.1016/j.bbmt.2016.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Artz AS, Logan B, Zhu X, Akpek G, Bufarull RM, Gupta V, Lazarus HM, Litzow M, Loren A, Majhail NS, Maziarz RT, McCarthy P, Popat U, Saber W, Spellman S, Ringden O, Wickrema A, Pasquini MC, Cooke KR from the Center for International Blood and Marrow Transplantation Research. The prognostic value of serum C-reactive protein, ferritin, and albumin prior to allogeneic transplantation for acute myeloid leukemia and myelodysplastic syndromes. Haematologica. 2016;101:1426–1433. doi: 10.3324/haematol.2016.145847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Michelis FV, Gupta V, Zhang MJ, Wang HL, Aljurf M, Bacher U, Beitinjaneh A, Chen YB, DeFilipp Z, Gale RP, Kebriaei P, Kharfan-Dabaja M, Lazarus HM, Nishihori T, Olsson RF, Oran B, Rashidi A, Rizzieri DA, Tallman MS, de Lima M, Khoury HJ, Sandmaier BM, Weisdorf D, Saber W Acute Leukemia Working Committee of the Center for International Blood and Marrow Transplant Research, aresearch collaboration between the National Marrow Donor Program/Be the Match Registry and the Medical College of Wisconsin. Cytogenetic risk determines outcomes after allogeneic transplantation in older patients with acute myeloid leukemia in their second complete remission: a center for international blood and marrow transplant research cohort analysis. Cancer. 2017;123:2035–2042. doi: 10.1002/cncr.30567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blaise D, Furst S, Crocchiolo R, El-Cheikh J, Granata A, Harbi S, Bouabdallah R, Devillier R, Bramanti S, Lemarie C, Picard C, Chabannon C, Weiller PJ, Faucher C, Mohty B, Vey N, Castagna L. Haploidentical T cell-replete transplantation with post-transplantation cyclophosphamide for patients in or above the sixth decade of age compared with allogeneic hematopoietic stem cell transplantation from an human leukocyte antigen-matched related or unrelated donor. Biol Blood Marrow Transplant. 2016;22:119–124. doi: 10.1016/j.bbmt.2015.08.029. [DOI] [PubMed] [Google Scholar]
- 32.Kasamon YL, Bolanos-Meade J, Prince GT, Tsai HL, McCurdy SR, Kanakry JA, Rosner GL, Brodsky RA, Perica K, Smith BD, Gladstone DE, Swinnen LJ, Showel MM, Matsui WH, Huff CA, Borrello I, Pratz KW, McDevitt MA, Gojo I, Dezern AE, Shanbhag S, Levis MJ, Luznik L, Ambinder RF, Fuchs EJ, Jones RJ. Outcomes of nonmyeloablative HLA-haploidentical blood or marrow transplantation with high-dose posttransplantation cyclophosphamide in older adults. J. Clin. Oncol. 2015;33:3152–3161. doi: 10.1200/JCO.2014.60.4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bashey A, Zhang X, Jackson K, Brown S, Ridgeway M, Solh M, Morris LE, Holland HK, Solomon SR. Comparison of outcomes of hematopoietic cell transplants from T-replete haploidentical donors using post-transplantation cyclophosphamide with 10 of 10 HLA-A, -B, -C, -DRB1, and -DQB1 allele-matched unrelated donors and HLA-identical sibling donors: a multivariable analysis including disease risk index. Biol Blood Marrow Transplant. 2016;22:125–33. doi: 10.1016/j.bbmt.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 34.Rashidi A, Slade M, DiPersio JF, Westervelt P, Vij R, Romee R. Post-transplant high-dose cyclophosphamide after HLA-matched vs haploidentical hematopoietic cell transplantation for AML. Bone Marrow Transplant. 2016;51:1561–1564. doi: 10.1038/bmt.2016.217. [DOI] [PubMed] [Google Scholar]
- 35.Fenaux P, Mufti GJ, Hellstrom-Lindberg E, Santini V, Gattermann N, Germing U, Sanz G, List AF, Gore S, Seymour JF, Dombret H, Backstrom J, Zimmerman L, McKenzie D, Beach CL, Silverman LR. Azacitidine prolongs overall survival compared with conventional care regimens in elderly patients with low bone marrow blast count acute myeloid leukemia. J. Clin. Oncol. 2010;28:562–569. doi: 10.1200/JCO.2009.23.8329. [DOI] [PubMed] [Google Scholar]
- 36.Kantarjian HM, Thomas XG, Dmoszynska A, Wierzbowska A, Mazur G, Mayer J, Gau JP, Chou WC, Buckstein R, Cermak J, Kuo CY, Oriol A, Ravandi F, Faderl S, Delaunay J, Lysak D, Minden M, Arthur C. Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J. Clin. Oncol. 2012;30:2670–2677. doi: 10.1200/JCO.2011.38.9429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Drombret H, Seymour JF, Butrym A, Wierzbowska A, Selleslag D, Jang JH, Kumar R, Cavenagh J, Schuh AC, Candoni A, Récher C, Sandhu I, del Castillo TB, Al-Ali HK, Martinelli G, Falantes J, Noppeney R, Stone RM, Minden MD, McIntyre H, Songer S, Lucy LM, Beach CL, Döhner H. International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with > 30% blasts. Blood. 2015;126:291–299. doi: 10.1182/blood-2015-01-621664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dohner H, Seymour JF, Butrym A, Wierzbowska A, Selleslag D, Jang JH, Kumar R, Cavenagh J, Kumar R, Schuh AC, Candoni A, Récher C, Sandhu I, del Castillo TB, Al-Ali HK, Martinelli G, Falantes J, Stone RM, Minden MD, McIntyre H, Songer S, Lucy LM, Beach CL, Döhner H. Overall survival in older patients with newly diagnosed acute myeloid leukemia (AML) with > 30% bone marrow blasts treated with azacitidine by cytogenetic risk status: results of the AZA-AML-001 study. ASH abstracts 2014. Blood 124: 621a. [Google Scholar]
- 39.Seymour JF, Döhner H, Butrym A, Wierzbowska A, Selleslag D, Jang JH, Cavenagh JD, Kumar R, Schuh AC, Candoni A, Récher C, Sandhu I, del Castillo TB, Al-Ali HK, Martinelli G, Falantes J, Stone RM, Minden MD, McIntyre H, Songer S, Lucy LM, Beach CL, Dombret H. Azacitidine (AZA) versus conventional care regimens (CCR) in older patients with newly diagnosed acute myeloid leukemia (> 30% bone marrow blasts) with morphologic dysplastic changes: a subgroup analysis of the AZA-AML-001 trial. Blood. 2014;124:10. [Google Scholar]
- 40.Pleyer L, Döhner H, Dombret H, Seymour JF, Schuh AC, Beach CL, Swern AS, Burgstaller S, Stauder R, Girschikofsky M, Sill H, Schlick K, Thaler J, Halter B, Spandl SM, Zebisch A, Pichler A, Pfeilstöcker M, Autzinger EM, Lang A. Azacitidine for front-line therapy of patients with AML: reproducible efficacy established by direct comparison of international phase 3 trial data with registry data from the Austrian azacitidine registry of the AGMT study group. Int J Mol Sci. 2017;18:1–18. doi: 10.3390/ijms18020415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stahl M, Podoltsev NA, DeVeaux M, Perreault S, Itzykson R, Ritchie EK, Sekeres MA, Fathi AT, Komrokji RS, Bhatt VR, Al-Kali A, Cluzeau T, Santini V, Brunner A, Roboz GJ, Fenaux P, Litzow M, Vey N, Verma V, Germing U, Fernández PM, Zelterman D, Kim TK, Prebet T, Gore SD, Zeidan AM. The use of hypomethylating agents (HMAs) in patients with relapsed and refractory acute myeloid leukemia (RR-AML): clinical outcomes and their predictors in a large international patient cohort. Blood. 2016;128:1063. [Google Scholar]
- 42.Lancet JE, Cortes JE, Hogge DE, Tallman MS, Kovacsovics TJ, Damon LE, Komrokji R, Solomon SR, Kolitz JE, Cooper M, Yeager AM, Louie AC, Feldman EJ. Phase 2 trial of CPX-351, a fixed 5:1 molar ratio of cytarabine/daunorubicin, vs cytarabine/daunorubicin in older adults with untreated AML. Blood. 2014;123:3239–3246. doi: 10.1182/blood-2013-12-540971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lancet JE, Uy GL, Cortes JE, Newell LF, Lin TL, Ritchie EK, Stuart RK, Strickland SA, Hogge D, Solomon SR, Stone RM, Bixby DL, Kolitz JE, Schiller GJ, Wieduwilt MJ, Ryan DH, Hoering A, Chiarella M, Louie AC, Medeiros BC. Final results of a phase III randomized trial of CPX-351 versus 7+3 in older patients with newly diagnosed high risk (secondary) AML. J. Clin. Oncol. 2016;34(Suppl):7000a. [Google Scholar]
- 44.Lancet JE, Hoering A, Uy GL, Cortes JE, Newell LF, Lin TL, Ritchie EK, Stuart RK, Strickland SA, Hogge D, Solomon SR, Stone RM, Bixby DL, Kolitz JE, Schiller GJ, Wieduwilt MJ, Ryan DH, Chiarella MT, Louie AC, Medeiros BC. Survival following allogeneic hematopoietic cell transplantation in older high-risk acute myeloid leukemia patients initially treated with CPX-351 liposome injection versus standard cytarabine and daunorubicin: subgroup analysis of a large phase III trial. Blood. 2016;128:906a. [Google Scholar]


