Abstract
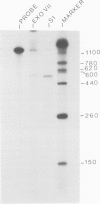
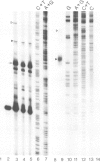
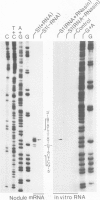
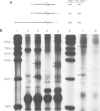
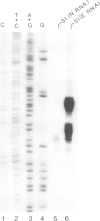
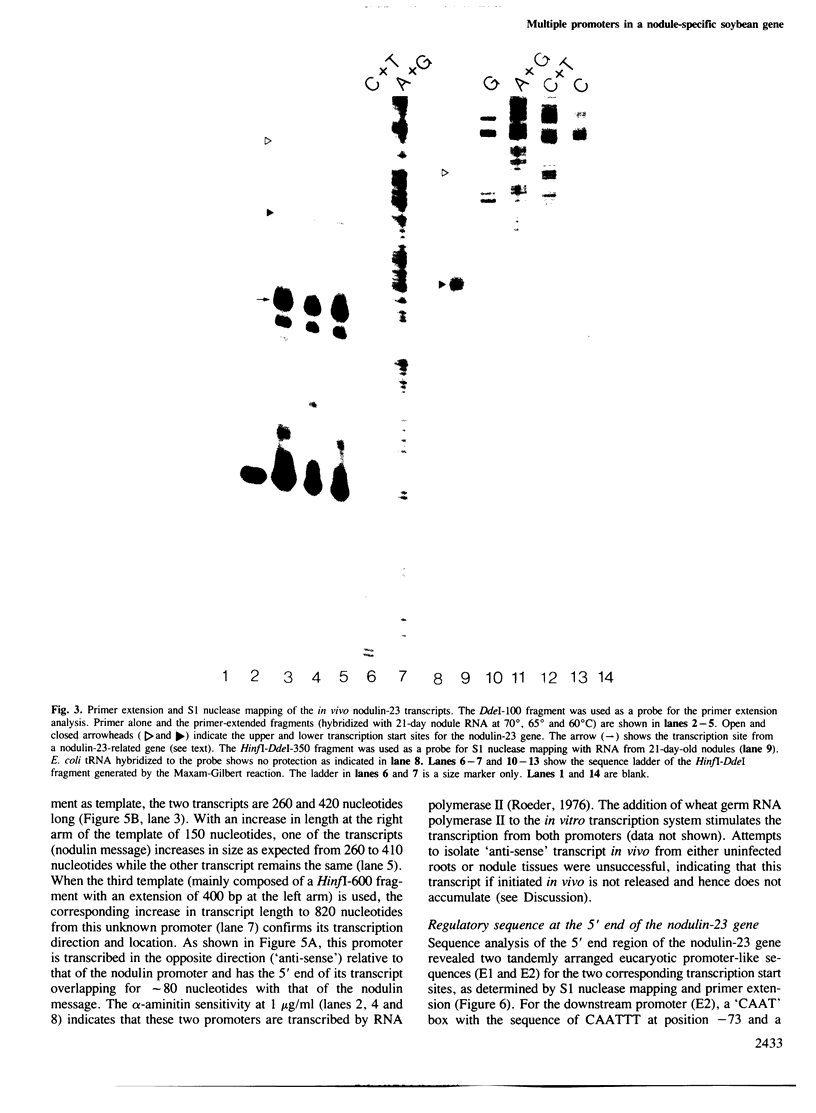
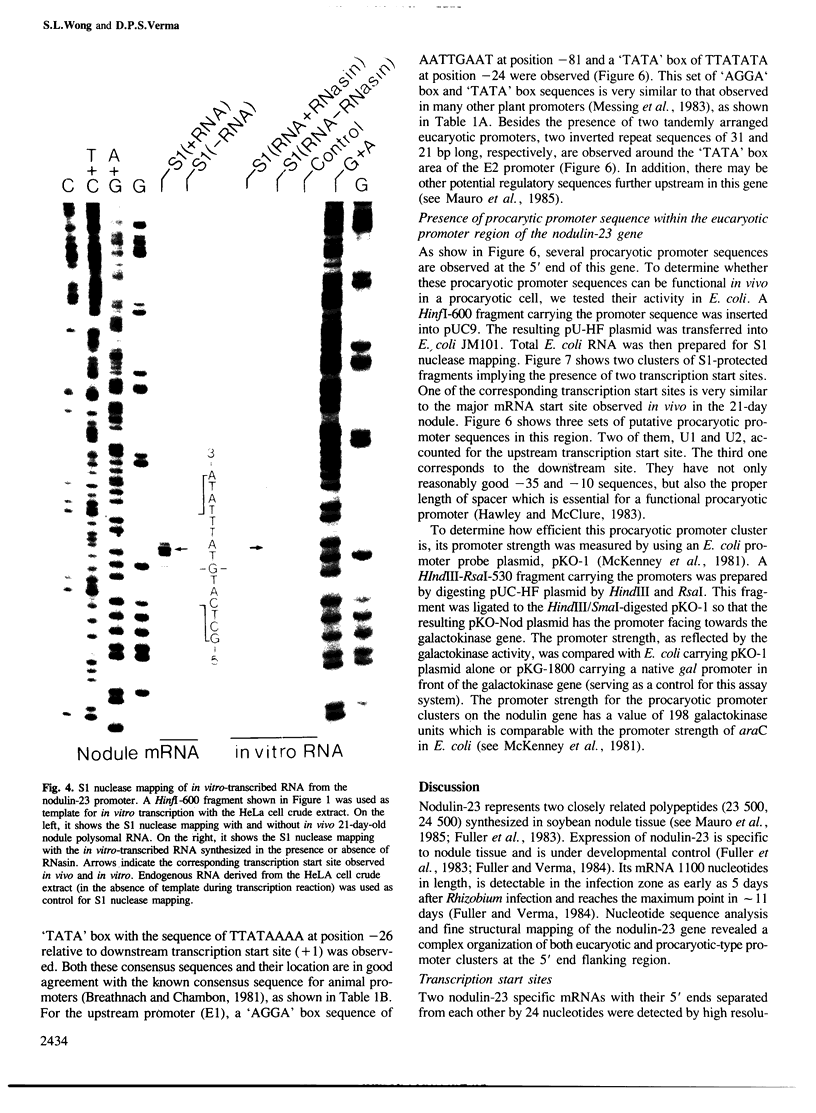
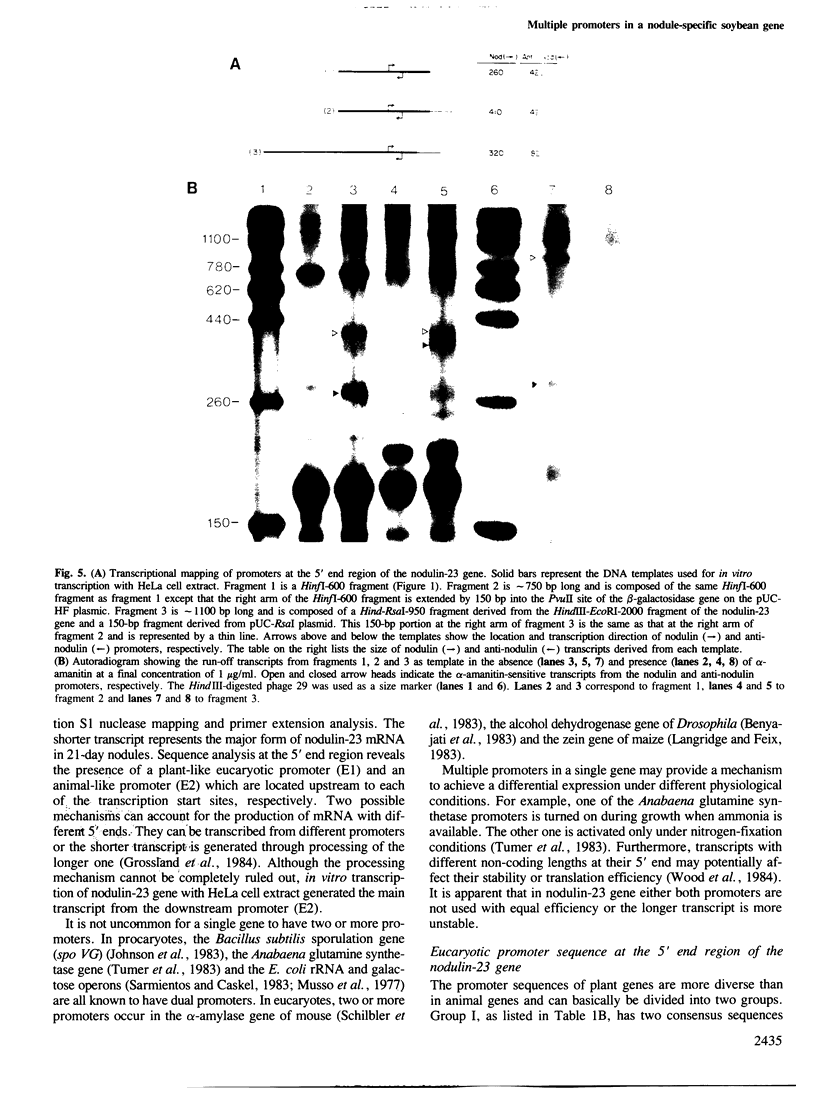
Soybean nodulin-23 gene, induced in nodules formed due to symbiosis with Rhizobium, was found to contain multiple sequences capable of acting as eucaryotic and procaryotic promoters. The transcription start sites of this gene were localized by S1 nuclease mapping, primer extension with nodule mRNA and in vitro run-off transcription analysis. A major transcription start site was observed by S1 mapping; however, the primer extension revealed a second start site. Sequence analysis showed the presence of tandemly arranged eucaryotic promoter sequences, each corresponding to one of the observed transcription start sites. In vitro transcription analysis with HeLa cell extract demonstrated the presence of another α-amanitin-sensitive transcript (`anti-sense') with its direction opposite to that of the nodulin mRNA. The 5' ends for 80 nucleotides of nodulin and `anti-sense' messages are complementary to each other. In addition, three tandemly arranged, functional procaryotic promoters (assayed in Escherichia coli) are found to be located within the eucaryotic promoter regions of this gene. One of the procaryotic transcription start sites is similar to that of the major in vivo transcript in nodules. The potential significance for the presence of several promoter-like sequences in this gene is discussed.
Keywords: nodulin-23 gene, in vitro transcription, Glycine max, symbiotic nitrogen fixation, eucaryotic and procaryotic promoters
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benyajati C., Spoerel N., Haymerle H., Ashburner M. The messenger RNA for alcohol dehydrogenase in Drosophila melanogaster differs in its 5' end in different developmental stages. Cell. 1983 May;33(1):125–133. doi: 10.1016/0092-8674(83)90341-0. [DOI] [PubMed] [Google Scholar]
- Bergmann H., Preddie E., Verma D. P. Nodulin-35: a subunit of specific uricase (uricase II) induced and localized in the uninfected cells of soybean nodules. EMBO J. 1983;2(12):2333–2339. doi: 10.1002/j.1460-2075.1983.tb01743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Bisseling T., Been C., Klugkist J., Kammen A., Nadler K. Nodule-specific host proteins in effective and ineffective root nodules of Pisum sativum. EMBO J. 1983;2(6):961–966. doi: 10.1002/j.1460-2075.1983.tb01528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Crossland L. D., Rodermel S. R., Bogorad L. Single gene for the large subunit of ribulosebisphosphate carboxylase in maize yields two differentially regulated mRNAs. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4060–4064. doi: 10.1073/pnas.81.13.4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depicker A., Stachel S., Dhaese P., Zambryski P., Goodman H. M. Nopaline synthase: transcript mapping and DNA sequence. J Mol Appl Genet. 1982;1(6):561–573. [PubMed] [Google Scholar]
- Donahue T. F., Farabaugh P. J., Fink G. R. The nucleotide sequence of the HIS4 region of yeast. Gene. 1982 Apr;18(1):47–59. doi: 10.1016/0378-1119(82)90055-5. [DOI] [PubMed] [Google Scholar]
- Fuller F., Künstner P. W., Nguyen T., Verma D. P. Soybean nodulin genes: Analysis of cDNA clones reveals several major tissue-specific sequences in nitrogen-fixing root nodules. Proc Natl Acad Sci U S A. 1983 May;80(9):2594–2598. doi: 10.1073/pnas.80.9.2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garger S. J., Griffith O. M., Grill L. K. Rapid purification of plasmid DNA by a single centrifugation in a two-step cesium chloride-ethidium bromide gradient. Biochem Biophys Res Commun. 1983 Dec 28;117(3):835–842. doi: 10.1016/0006-291x(83)91672-8. [DOI] [PubMed] [Google Scholar]
- Gilman M. Z., Chamberlin M. J. Developmental and genetic regulation of Bacillus subtilis genes transcribed by sigma 28-RNA polymerase. Cell. 1983 Nov;35(1):285–293. doi: 10.1016/0092-8674(83)90231-3. [DOI] [PubMed] [Google Scholar]
- Hawley D. K., McClure W. R. Compilation and analysis of Escherichia coli promoter DNA sequences. Nucleic Acids Res. 1983 Apr 25;11(8):2237–2255. doi: 10.1093/nar/11.8.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera-Estrella L., Block M. D., Messens E., Hernalsteens J. P., Montagu M. V., Schell J. Chimeric genes as dominant selectable markers in plant cells. EMBO J. 1983;2(6):987–995. doi: 10.1002/j.1460-2075.1983.tb01532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izant J. G., Weintraub H. Inhibition of thymidine kinase gene expression by anti-sense RNA: a molecular approach to genetic analysis. Cell. 1984 Apr;36(4):1007–1015. doi: 10.1016/0092-8674(84)90050-3. [DOI] [PubMed] [Google Scholar]
- Johnson W. C., Moran C. P., Jr, Losick R. Two RNA polymerase sigma factors from Bacillus subtilis discriminate between overlapping promoters for a developmentally regulated gene. Nature. 1983 Apr 28;302(5911):800–804. doi: 10.1038/302800a0. [DOI] [PubMed] [Google Scholar]
- Lang-Unnasch N., Ausubel F. M. Nodule-specific polypeptides from effective alfalfa root nodules and from ineffective nodules lacking nitrogenase. Plant Physiol. 1985 Apr;77(4):833–839. doi: 10.1104/pp.77.4.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langridge P., Feix G. A zein gene of maize is transcribed from two widely separated promoter regions. Cell. 1983 Oct;34(3):1015–1022. doi: 10.1016/0092-8674(83)90559-7. [DOI] [PubMed] [Google Scholar]
- Legocki R. P., Verma D. P. Identification of "nodule-specific" host proteins (nodoulins) involved in the development of rhizobium-legume symbiosis. Cell. 1980 May;20(1):153–163. doi: 10.1016/0092-8674(80)90243-3. [DOI] [PubMed] [Google Scholar]
- Luse D. S., Haynes J. R., VanLeeuwen D., Schon E. A., Cleary M. L., Shapiro S. G., Lingrel J. B., Roeder R. G. Transcription of the beta-like globin genes and pseudogenes of the goat in a cell-free system. Nucleic Acids Res. 1981 Sep 11;9(17):4339–4354. doi: 10.1093/nar/9.17.4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lycett G. W., Croy R. R., Shirsat A. H., Boulter D. The complete nucleotide sequence of a legumin gene from pea (Pisum sativum L.). Nucleic Acids Res. 1984 Jun 11;12(11):4493–4506. doi: 10.1093/nar/12.11.4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Efstratiadis A. Fractionation of low molecular weight DNA or RNA in polyacrylamide gels containing 98% formamide or 7 M urea. Methods Enzymol. 1980;65(1):299–305. doi: 10.1016/s0076-6879(80)65040-x. [DOI] [PubMed] [Google Scholar]
- Mauro V. P., Nguyen T., Katinakis P., Verma D. P. Primary structure of the soybean nodulin-23 gene and potential regulatory elements in the 5'-flanking regions of nodulin and leghemoglobin genes. Nucleic Acids Res. 1985 Jan 11;13(1):239–249. doi: 10.1093/nar/13.1.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Mizuno T., Chou M. Y., Inouye M. A unique mechanism regulating gene expression: translational inhibition by a complementary RNA transcript (micRNA). Proc Natl Acad Sci U S A. 1984 Apr;81(7):1966–1970. doi: 10.1073/pnas.81.7.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musso R. E., Di Lauro R., Adhya S., de Crombrugghe B. Dual control for transcription of the galactose operon by cyclic AMP and its receptor protein at two interspersed promoters. Cell. 1977 Nov;12(3):847–854. doi: 10.1016/0092-8674(77)90283-5. [DOI] [PubMed] [Google Scholar]
- Rose M., Botstein D. Structure and function of the yeast URA3 gene. Differentially regulated expression of hybrid beta-galactosidase from overlapping coding sequences in yeast. J Mol Biol. 1983 Nov 15;170(4):883–904. doi: 10.1016/s0022-2836(83)80193-4. [DOI] [PubMed] [Google Scholar]
- Sarmientos P., Cashel M. Carbon starvation and growth rate-dependent regulation of the Escherichia coli ribosomal RNA promoters: differential control of dual promoters. Proc Natl Acad Sci U S A. 1983 Nov;80(22):7010–7013. doi: 10.1073/pnas.80.22.7010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schibler U., Hagenbüchle O., Wellauer P. K., Pittet A. C. Two promoters of different strengths control the transcription of the mouse alpha-amylase gene Amy-1a in the parotid gland and the liver. Cell. 1983 Jun;33(2):501–508. doi: 10.1016/0092-8674(83)90431-2. [DOI] [PubMed] [Google Scholar]
- Simons R. W., Kleckner N. Translational control of IS10 transposition. Cell. 1983 Sep;34(2):683–691. doi: 10.1016/0092-8674(83)90401-4. [DOI] [PubMed] [Google Scholar]
- Tomizawa J., Itoh T., Selzer G., Som T. Inhibition of ColE1 RNA primer formation by a plasmid-specified small RNA. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1421–1425. doi: 10.1073/pnas.78.3.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma D. P., Nash D. T., Schulman H. M. Isolation and in vitro translation of soybean leghaemoglobin mRNA. Nature. 1974 Sep 6;251(5470):74–77. doi: 10.1038/251074a0. [DOI] [PubMed] [Google Scholar]
- Weaver R. F., Weissmann C. Mapping of RNA by a modification of the Berk-Sharp procedure: the 5' termini of 15 S beta-globin mRNA precursor and mature 10 s beta-globin mRNA have identical map coordinates. Nucleic Acids Res. 1979 Nov 10;7(5):1175–1193. doi: 10.1093/nar/7.5.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood C. R., Boss M. A., Patel T. P., Emtage J. S. The influence of messenger RNA secondary structure on expression of an immunoglobulin heavy chain in Escherichia coli. Nucleic Acids Res. 1984 May 11;12(9):3937–3950. doi: 10.1093/nar/12.9.3937. [DOI] [PMC free article] [PubMed] [Google Scholar]