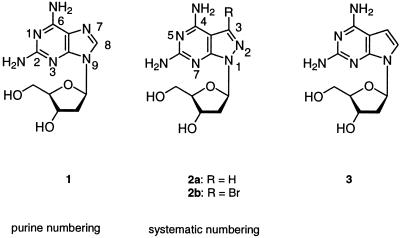
Abstract
Oligonucleotides incorporating 8-aza-7-deazapurin-2,6-diamine (pyrazolo[3,4-d]pyrimidin-4,6-diamine) nucleoside 2a or its 7-bromo derivative 2b show enhanced duplex stability compared to those containing dA. While incorporation of 2a opposite dT increases the Tm value only slightly, the 7-bromo compound 2b forms a very stable base pair which is as strong as the dG-dC pair. Compound 2b shows a similar base discrimination in duplex DNA as dA. The base-modified nucleosides 2a,b have a significantly more stable N-glycosylic bond than the rather labile purin-2,6-diamine 2′-deoxyribonucleoside 1. Base protection with acyl groups, with which we had difficulties in the case of purine nucleoside 1, was effective with pyrazolo[3,4-d]-pyrimidine nucleosides 2a,b. Oligonucleotides containing 2a,b were obtained by solid phase synthesis employing phosphoramidite chemistry. Compound 2b harmonizes the stability of DNA duplexes. Their stability is no longer dependent on the base pair composition while they still maintain their sequence specificity. Thus, they have the potential to reduce the number of mispairs when hybridized in solution or immobilized on arrays.
INTRODUCTION
The stability of natural DNA depends on the base pair composition. The guanine-cytosine pair is more stable than the adenine-thymine pair, which is attributed to the third hydrogen bond. Oligonucleotide duplexes forming other tridentate base pairs are expected to show a similar stability to dG-dC. However, it has been reported that the additional amino group of 2-amino-2′-deoxyadenosine (1) stabilizes the dA-dT pair only very little, resulting in a Tm increase of only 1–2°C per modified residue (1–7). Moreover, stabilization does not correspond to an increasing number of purin-2,6-diamine 2′-deoxyribonucleoside (1) residues (8). A stronger stabilization as reported for DNA is found for duplex RNA and for DNA–RNA hybrids (7,9). High base pair stability is observed when 2-aminoadenine is introduced into peptide nucleic acids (PNA) (10) or hexitol nucleic acids (11).
The unusual behavior of oligonucleotide duplexes containing tridentate 1-dT base pairs has prompted us to search for more stable tridentate base pairs with dA-dT base recognition. Finally, the ‘dA-dT’ base pair should exhibit the same stability as a dG-dC pair. This problem was approached by replacing the purine moiety of compound 1 by other heterocyclic bases. 7-Deazapurines (pyrrolo[2,3-d]pyrimidines) (purine numbering is used throughout Results and Discussion) and 8-aza-7-deazapurines (pyrazolo[3,4-d]pyrimidines) have already been used in the case of adenine and guanine nucleosides to alter duplex stability (12–15). These heterocycles mimic the shapes of the purines and can substitute for purine bases in Watson–Crick base pairs. In the case of analogs of 2′-deoxyadenosine it has been observed that the 7-deaza-2′-deoxyadenosine-dT base pair is less stable than a dA-dT pair while the 8-aza-7-deaza-2′-deoxyadenine-dT pair shows the same stability. Furthermore, it was found that the 7-halogen substituents increase base pair stability significantly (13,14,16). However, it was not possible to increase the stability of the bidentate dA-dT pair to the level of a tridentate dG-dC pair when a modified base was substituted for a regular one. This manuscript reports on oligonucleotides containing the pyrazolo[3,4-d]pyrimidine nucleosides 2a,b (Scheme 1). They represent structural analogs of 1 and are incorporated into oligonucleotides by solid phase synthesis employing phosphoramidite chemistry.
Scheme 1.
MATERIALS AND METHODS
General
Chemicals were purchased from Sigma-Aldrich Chemie GmbH (Deisenhofen, Germany). Solvents were of laboratory grade. Elemental analyses were performed by Mikroanalytisches Laboratorium Beller (Göttingen, Germany). NMR spectra were measured on Avance DPX 250 or AMX 500 spectrometers (Bruker, Karlsruhe, Germany) operating at proton resonance frequencies of 250.13 and 500.14 MHz (125.13 MHz for the 13C nuclei). Chemical shifts are in p.p.m. relative to TMS as internal standard. J values are given in Hz. UV spectra were recorded on a U 3200 spectrometer (Hitachi, Japan). Thin layer chromatography (TLC) was performed on 0.2 mm thick silica gel 60 F254 aluminum sheets (Merck, Germany) and column flash chromatography (FC) on silica gel 60 (Merck, Germany) at 0.4 bar (4 × 104 Pa).
Oligonucleotides
Synthesis and purification. The oligonucleotide syntheses were performed in an ABI 392-08 DNA synthesizer (Applied Biosystems, Weiterstadt, Germany) at the 1 µmol scale using the phosphoramidites 7b and 11a,b and those of 1 and the canonical 2′-deoxyribonucleosides (Applied Biosystems, Weiterstadt, Germany) following the synthesis protocol for 3′-cyanoethyl phosphoramidites (user manual for the 392 DNA synthesizer; Applied Biosystems, Weiterstadt, Germany). The phosphoramidite of nucleoside 1 was a commercial product from Glen Research (USA). After cleavage from the solid support the oligonucleotides were deprotected with 25% aqueous NH3 (10–12 h at 60°C). Composition analysis of the oligomers was performed by tandem hydrolysis with snake venom phosphodiesterase followed by alkaline phosphatase as described (17).
Quantification of the constituents was made on the basis of peak areas, which were divided by the extinction coefficients of the nucleoside (ɛ260 values: dA, 15 400; dC, 7300; dG, 11 400; dT, 8800). Snake venom phophodiesterase (EC 3.1.15.1, Crotallus durissus) and alkaline phosphatase (EC 3.1.3.1, Escherichia coli) were supplied by Roche Diagnostics GmbH (Penzberg, Germany). The MALDI-TOF mass spectra were provided by Dr T.Wenzel (Bruker, Germany). MALDI-TOF data for the modified oligonucleotides are shown in Table 1. Oligonucleotide purification was performed by reverse phase HPLC (RP-18) with a Merck-Hitachi HPLC: 250 × 4 mm RP-18 column with gradients of 0.1 M (Et3NH)OAc (pH 7.0)/MeCN 95:5 (A) and MeCN (B) (gradient I, 50 min 0–50% B in A, flow rate 1.0 ml/min; gradient II, 20 min 0–25% B in A, flow rate 0.7 ml/min plus 30 min 25–40% B in A, flow rate 1.0 ml/min. The concentrated oligonucleotide solutions were treated with 2.5% CHCl2COOH/CH2Cl2 for 5 min at r.t. to remove the 4,4′-dimethoxytrityl residues. The detritylated oligomers were purified by reverse phase HPLC with the gradient III, 20 min 0–25% B in A, flow rate 1.0 ml/min). The oligomers were desalted on a short column (RP-18 silica gel) using H2O for elution of the salt, while the oligomers were eluted with MeOH/H2O (3:2). The oligomers were lyophilized on a Speed-Vac evaporator to yield colorless solids which were frozen at –24°C.
Table 1. Molecular masses (M+) of oligonucleotides determined by MALDI-TOF mass spectroscopy.
| Oligomer |
M+ (calculated) |
M+ (found) |
| 5′-d(TAGGTCAATACT) (12) | 3644.4 | 3645 |
| 5′-d(AGTATTGACCTA) (13) | 3644.4 | 3645 |
| 5′-d(TAGGTC2a2aTACT) (16) | 3674.4 | 3677 |
| 5′-d(AGT2aTTG2aCCTA) (17) | 3674.4 | 3675 |
| 5′-d(TAGGTC2b2bTACT) (21) | 3832.5 | 3830 |
| 5′-d(AGT2bTTG2bCCTA) (18) | 3832.5 | 3832 |
Determination of Tm values and thermodynamic data. Absorbance versus temperature profiles were measured on a Cary 1/1E UV/VIS spectrophotometer (Varian, Australia) equipped with a Cary thermoelectrical controller. The temperature was measured in the reference cell with a Pt-100 resistor. The thermodynamic data ΔH° and ΔS° were obtained by curve shape analysis using the program Meltwin 3.0 (18); the ΔG° values were calculated for 310°C. The CD spectra were recorded in 1.0 cm cuvettes with a Jasco-600 spectropolarimeter (Jasco, Japan) connected to a thermostatically controlled water bath (RCS-6; Lauda, Germany).
3-Bromo-1-(2-deoxy-β-d-erythro-pentofuranosyl)-1H-pyrazolo[3,4-d]-pyrimidin-4,6-diamine (2b)
A solution of 6-amino-3-bromo-1-[2-deoxy-β-d-erythro-pentofuranosyl]-4-isopropoxy-1H-pyrazolo[3,4-d]pyrimidine (4b) (19) (1.0 g, 2.6 mmol) in aqueous 25% NH3 (80 ml) was heated at 70°C for 100 h in an autoclave. The solvent was reduced to a small volume while the residue precipitated. Crystallization of a small sample was performed in H2O. The product was obtained as colorless needles (646 mg, 72%). Melting point 155°C. TLC (CH2Cl2/MeOH 9:1), Rf 0.2; UV (MeOH), λmax 261, 278 nm (ɛ 8700, 9000). 1H NMR [(D6)DMSO], δ 2.16, 2.68 [2m, H2-C(2′)], 3.38, 3.48 [2m, H2-C(5′)], 3.77 [m, H-C(4′)], 4.36 [m, H-C(3′)], 4.72 [t, J = 4.9, HO-C(5′)], 5.19 [d, J = 4.1, HO-C(3′)], 6.32 [‘t’, J = 6.5, H-C(1′)], 6.39 (s, NH2), 6.77 (br, NH2). Calculated for C10H13BrN6O3 (345.2), C 34.80, H 3.80, N 24.35; found, C 34.97, H 3.97, N 24.21.
1-(2-Deoxy-β-d-erythro-pentofuranosyl)-N4,N6-(diphenoxyacetyl)-1H-pyrazolo[3,4-d]-pyrimidin-4,6-diamine (5a)
Compound 2a (20) (1.5 g, 5.6 mmol) was dried by co-evaporation with pyridine. The residue was dissolved in pyridine (25 ml) and trimethylsilyl chloride (3.3 ml, 26 mmol) was added at r.t. with stirring. Stirring was continued for 15 min and a solution of 2,4,5-trichlorophenyl phenoxyacetate (5.4 g, 16.4 mmol) (21) in pyridine (15 ml) was added in one portion. The reaction mixture was stirred for 16 h at 40°C. It was cooled (ice bath) and H2O (4.2 ml) was added. After 5 min the solution was diluted with aqueous 25% NH3 (6 ml) and the mixture was concentrated to dryness. The residue was absorbed on silica gel and subjected to FC (CH2Cl2/MeOH 98:2, CH2Cl2/MeOH 9:1) yielding a colorless foam (900 mg, 30%). TLC (CH2Cl2/MeOH 9:1), Rf 0.4; UV (MeOH), λmax 266 nm (ɛ 9100). 1H NMR [(D6)DMSO], δ 2.26, 2.82 [2m, H2-C(2′)], 3.34, 3.48 [2m, H2-C(5′)], 3.82 [m, H-C(4′)], 4.44 [m, H-C(3′)], 4.73 [t, J = 5.5, HO-C(5′)], 5.14 (s, OCH2), 5.17 [d, J = 4.3, HO-C(3′)], 6.60 [‘t’, J = 6.3, H-C(1′)], 6.92–7.32 (m, arom. H), 8.41 [s, H-C(3)], 10.77 (s, NH), 11.30 (s, NH). Calculated for C26H26N6O7 (534.5), C 58.42, H 4.90, N 15.72; found, C 58.68, H 4.78, N 15.20.
1-[2-Deoxy-5-O-(4,4′-dimethoxytrityl)-β-d-erythro-pento-furanosyl]-N4,N6-(diphenoxyacetyl)-1H-pyrazolo[3,4-d]-pyrimidin-4,6-diamine (6a)
Compound 5a (0.8 g, 1.5 mmol) was co-evaporated twice with pyridine. The residue was dissolved in pyridine (2 ml) and 4,4′-dimethoxytrityl chloride (0.6 g, 1.77 mmol) was added under stirring at r.t. The stirring was continued for 4 h, then the solution was diluted with MeOH (5 ml) and CH2Cl2 (25 ml). After washing with 5% aqueous sodium bicarbonate (3 × 20 ml) the organic layers were combined, dried (Na2SO4) and concentrated to dryness. The residue was purified by FC (CH2Cl2/acetone 95:5, CH2Cl2/acetone 9:1) yielding a colorless foam (310 mg, 25%). TLC (CH2Cl2/acetone 9:1), Rf 0.3; UV (MeOH), λmax 266 nm (ɛ 8900). 1H NMR [(D6)DMSO], 2.32, 2.81 [2m, H2-C(2′)], 2.99 [2m, H2-C(5′)], 3.67 (2s, OCH3), 3.95 [m, H-C(4′)], 4.53 [m, H-C(3′)], 5.15 (s, OCH2), 5.33 [d, J = 4.7, HO-C(3′)], 6.62 [‘t’, J = 6.3, H-C(1′)], 6.69–7.31 (m, arom. H), 8.36 [s, H-C(3)], 10.83 (s, NH), 11.56 (s, NH). Calculated for C47H44N6O9 (836.9), C 67.45, H 5.30, N 10.04; found, C 66.95, H 5.41, N 10.15.
1-[2-Deoxy-5-O-(4,4′-dimethoxytrityl)-β-d-erythro-pentofuranosyl]-N4,N6-(diphenoxyacetyl)-1H-pyrazolo[3,4-d]-pyrimidin-4,6-diamine 3′-[(2-cyanoethyl) N,N-diisopropylphosphoramidite] (7a)
To a solution of compound 6a (0.15 g, 0.18 mmol) in THF (0.5 ml) (i-Pr)2EtN (0.12 ml, 0.71 mmol) and 2-cyanoethyl diisopropylphosphoramido chloridite (64 mg, 0.27 mmol) were added and stirred for 30 min at r.t. (argon atmosphere). The mixture was diluted with CH2Cl2 (20 ml) and 5% aqueous NaHCO3 and extracted with CH2Cl2 (3 × 15 ml). The combined organic layers were dried (Na2SO4) and evaporated to give an oil. This was dissolved in CH2Cl2 and submitted to FC (eluant CH2Cl2/EtOAc 85:15) yielding a colorless foam (80 mg, 43%). TLC (CH2Cl2/EtOAc 85:15), Rf 0.8. 31P NMR (CDCl3), 149.2, 149.4.
1-(2-Deoxy-β-d-erythro-pentofuranosyl)-N4,N6-dibenzoyl-1H-pyrazolo[3,4-d]-pyrimidin-4,6-diamine (5b)
Compound 2a (20) (1.0 g, 3.8 mmol) was co-evaporated twice with toluene and dissolved in pyridine (40 ml). Trimethylsilyl chloride (4.0 g, 36.8 mmol) was added with stirring at r.t. The solution was cooled to 0°C and PhCOCl (6.6 ml, 57 mmol) was added drop-wise over a period of 30 min. After stirring overnight at r.t. the solution was diluted with EtOAc (200 ml), washed with saturated aqueous sodium bicarbonate (200 ml) and ice-cold H2O (200 ml). The aqueous layer was extracted with EtOAc (2 × 400 ml). The combined organic layers were evaporated to dryness and the residue dissolved in THF/MeOH/H2O (5:4:1, 250 ml). The dark orange solution was cooled to 0°C and 2 N NaOH (25 ml) was added while stirring was continued for 40 min. The residue was purified by FC (eluant CH2Cl2/MeOH 98:2, CH2Cl2/MeOH 95:5) yielding an amorphous solid (1.1 g, 61%). TLC (CH2Cl2/MeOH 95:5), Rf 0.3; UV (MeOH), λmax 245, 274 nm (ɛ 16 400, 15 200). 1H NMR [(D6)DMSO], 2.13, 2.67 [2m, H2-C(2′)], 3.38, 3.52 [2m, H2-C(5′)], 3.84 [m, H-C(4′)], 4.46 [m, H-C(3′)], 4.72 [t, J = 5.7, HO-C(5′)], 5.29 [d, J = 4.4, HO-C(3′)], 6.66 [‘t’, J = 6.5, H-C(1′)], 7.51–8.11 (m, arom. H), 8.40 (s, H-C3), 10.95 (s, NH), 11.49 (s, NH). Calculated for C24H22N6O5 (474.5), C 60.75, H 4.67, N 17.71; found, C 61.03, H 4.70, N 17.58.
1-[2-Deoxy-5-O-(4,4′-dimethoxytrityl)-β-d-erythro-pento-furanosyl]-N4,N6-dibenzoyl-1H-pyrazolo[3,4-d]-pyrimidin-4,6-diamine (6b)
As described for 6a with 5b (500 mg, 1.05 mmol) and 4,4′-dimethoxytriphenylmethyl chloride (460 mg, 1.35 mmol) in pyridine (3 ml). The residue was purified by FC (CH2Cl2/acetone 95:5, CH2Cl2/acetone 9:1) yielding a colorless foam (400 mg, 49%). TLC (CH2Cl2/acetone 9:1), Rf 0.4; UV (MeOH), λmax 244, 275 nm (ɛ 16 400, 15 200).1H NMR [(D6)DMSO], 2.35, 2.67 [2m, H2-C(2′)], 3.07, 3.10 [2m, H2-C(5′)], 3.68, 3.69 (2s, OCH3), 3.97 [m, H-C(4′)], 4.56 [m, H-C(3′)], 5.35 [d, J = 4.8, HO-C(3′)], 6.72–8.11 (m, arom. H), 8.36 [s, H-C(3)], 11.01 (s, NH), 11.54 (s, NH).
1-[2-Deoxy-5-O-(4,4′-dimethoxytrityl)-β-d-erythro-pento-furanosyl]-N4,N6-dibenzoyl-1H-pyrazolo[3,4-d]-pyrimidin-4,6-diamine 3′-[(2-cyanoethyl)-N,N-diisopropylphos-phoramidite] (7b)
As described for 7a with 6b (110 mg, 0.14 mmol), (i-Pr)2EtN (75 µl, 0.43 mmol) and 2-cyanoethyl diisopropylphosphoramido chloridite (38 µl, 0.17 mmol) in THF (3 ml) at 30°C. The oily residue was submitted to FC (CH2Cl2/EtOAc 85:15) yielding a colorless foam (92 mg, 67%). TLC (CH2Cl2/EtOAc 85:15), Rf 0.8. 31P NMR (CDCl3), 149.82, 149.63.
1-(2-Deoxy-β-d-erythro-pentofuranosyl)-N4,N6-bis-[(di-n-butylamino)methylidene]-1H-pyrazolo[3,4-d]-pyrimidin-4,6-diamine (8a)
A solution of 2a (300 mg, 1.13 mmol) in MeOH (5 ml) and N,N-di-n-butylformamide dimethyl acetal (22) (790 µl, 3.39 mmol) was added and stirred for 2 h at 40°C. The reaction mixture was evaporated to dryness and the residue adsorbed on silica gel. FC (CH2Cl2/MeOH 98:2→ CH2Cl2/MeOH 95:5) afforded two main products. From the faster migrating zone a colorless foam of 8a (330 mg, 53%) was isolated. TLC (CH2Cl2/MeOH 95:5), Rf 0.4; UV (MeOH), λmax 235, 274, 322 nm (ɛ 22 800, 13 800, 25 700). 1H NMR (CDCl3), 0.87–0.95 (m, CH2CH3), 1.26–1.38 (m, CH2CH3), 1.57–1.60 (m, CH2CH2), 2.42–2.92 [m, H2-C(2′)], 3.26–3.72 [2m, H2-C(5′), NCH2], 3.88 [m, H-C(4′)], 4.39 [m, H-C(3′)], 4.78 [t, J = 5.8, HO-C(5′)], 5.21 [d, J = 4.8, HO-C(3′)], 6.55 [‘t’, J = 6.9, H-C(1′)], 7.93 [s, H-C(3)], 8.66 (s, N=CH), 8.88 (s, N=CH). Calculated for C28H49N8O3 (544.7), C 61.74, H 8.88, N 20.57; found, C 61.71, H 8.91, N 20.57.
1-(2-Deoxy-β-d-erythro-pentofuranosyl)-N6-[(di-n-butyl-amino)methylidene]-1H-pyrazolo[3,4-d]-pyrimidin-4,6-diamine (9a)
The slower migrating zone furnished compound 9a (80 mg, 17%) as a colorless foam. TLC (CH2Cl2/MeOH 95:5), Rf 0.3; UV (MeOH), λmax 235, 275, 320 nm (ɛ 23 000, 14 900, 22 100). 1H NMR [(D6)DMSO], 0.88–0.95 (m, CH2CH3), 1.27–1.36 (m, CH2CH3), 1.53–1.62 (m, CH2CH2), 2.21 [m, H-C(2′)], 2.91 [m, H-C(2′)], 3.38–3.59 [2m, H2-C(5′), NCH2], 3.79 [m, H-C(4′)], 4.40 [m, H-C(3′)], 4.78 [t, J = 5.8, HO-C(5′)], 5.19 [d, J = 4.4, HO-C(3′)], 6.36 (s, NH2), 6.40 [‘t’, J = 6.5, H-C(1′)], 7.77 [s, H-C(3)], 8.69 (s, N=CH). Calculated for C19H31N7O3 (405.5), C 56.28, H 7.71, N 24.18; found, C 55.98, H 7.52, N 24.05.
3-Bromo-1-(2-deoxy-β-d-erythro-pentofuranosyl)-N4,N6-bis-[(di-n-butylamino)methylidene]-1H-pyrazolo[3,4-d]-pyrimidin-4,6-diamine (8b)
As described for 8a, with 2b (350 mg, 1 mmol) and N,N-di-n-butylformamide dimethyl acetal (720 µl, 3.1 mmol) in MeOH (7 ml) for 2 h at 40°C. After FC (CH2Cl2/MeOH 98:2, CH2Cl2/MeOH 95:5) two main products were isolated. The faster migrating zone gave 8b as a foam (310 mg, 50%). TLC(CH2Cl2/MeOH 95:5), Rf 0.3; UV (MeOH), λmax 235, 275, 320 nm (ɛ 22 500, 14 500, 22 100). 1H NMR [(D6)DMSO], 0.94–0.99 (m, CH2CH3), 1.32–1.44 (m, CH2CH3), 1.59–1.73 (m, CH2CH2), 2.21 [m, H-C(2′)], 2.91 [m, H-C(2′)], 3.32–3.78 [2m, H2-C(5′), NCH2], 3.89 [m, H-C(4′)], 4.40 [m, H-C(3′)], 4.78 [t, J = 5.8, HO-C(5′)], 5.19 [d, J = 4.4, HO-C(3′)], 6.81 [‘t’, J = 6.5, H-C(1′)], 8.69 (s, N=CH), 8.94 (s, N=CH). Calculated for C28H47BrN8O3 (623.6), C 53.93, H 7.60, N 17.97; found, C 54.01, H 7.52, N 18.05.
3-Bromo-1-(2-deoxy-β-d-erythro-pentofuranosyl)-N6-[(di-n-butylamino)methylidene]-1H-pyrazolo[3,4-d]-pyrimidin-4,6-diamine (9b)
The slower migrating zone furnished 9b as a colorless foam (75 mg, 16%). TLC (CH2Cl2/MeOH 95:5), Rf 0.3; UV (MeOH), λmax 236, 276, 320 nm (ɛ 21 600, 14 000, 21 900). 1H NMR [(D6)DMSO], 0.90–0.96 (m, CH2CH3), 1.30–1.39 (m, CH2CH3), 1.59–1.73 (m, CH2CH2), 2.23 [m, H-C(2′)], 2.89 [m, H-C(2′)], 3.35–3.78 [2m, H2-C(5′), NCH2], 3.84 [m, H-C(4′)], 4.43 [m, H-C(3′)], 4.80 [t, J = 5.7, HO-C(5′)], 5.23 [d, J = 4.6, HO-C(3′)], 6.40 (s, NH2), 6.81 [‘t’, J = 6.4, H-C(1′)], 8.65 (s, N=CH). Calculated for C19H30BrN7O3 (484.4), C 47.11, H 6.24, N 20.24; found, C 47.23, H 6.52, N 20.35.
1-[2-Deoxy-5-O-(4,4′-dimethoxytrityl)-β-d-erythro-pentofuranosyl]-N4-[(di-n-butylamino)methylidene]-N6-formyl-1H-pyrazolo[3,4-d]-pyrimidin-4,6-diamine (10a)
As described for 6a with 8a (80 mg, 0.15 mmol), 4,4′-dimethoxytriphenylmethyl chloride (60 mg, 0.18 mmol) in pyridine (0.5 ml). The residue was purified by FC (CH2Cl2/acetone 95:5, CH2Cl2/acetone 9:1) yielding a colorless foam (85 mg, 77%). TLC (CH2Cl2/acetone 9:1), Rf 0.3; UV (MeOH), λmax 235, 276, 320 nm (ɛ 22 800, 20 000, 24 800). 1H NMR (CDCl3), 0.98–1.05 (m, CH2CH3), 1.14–1.48 (m, CH2CH3), 1.65–1.78 (m, CH2CH2), 2.42–2.92 [m, H2-C(2′)], 3.35–3.70 [2m, H2-C(5′), NCH2], 3.71 (s, OCH3), 4.06 [m, H-C(4′)], 4.39 [m, H-C(3′)], 5.21 [d, J = 4.8, HO-C(3′)], 6.55 [‘t’, J = 6.6, H-C(1′)], 6.76–7.96 (m, arom. H), 7.94 (d, J = 10.5, NH), 7.96 [s, H-C(3)], 8.81 (s, N=CH), 9.57 (d, J = 10.5, CHO). Calculated for C41H49N7O6 (735.9), C 66.92, H 6.71, N 13.32; found, C 66.85, H 6.56, N 13.40.
3-Bromo-1-[2-deoxy-5-O-(4,4′-dimethoxytrityl)-β-d-erythro-pentofuranosyl]-N4-[(di-n-butylamino)methyl-idene]-N6-formyl-1H-pyrazolo[3,4-d]-pyrimidin-4,6-diamine (10b)
As described for 6a with 8b (310 mg, 0.5 mmol), 4,4′-dimethoxytriphenylmethyl chloride (202 mg, 0.6 mmol) in pyridine (2 ml). The residue was purified twice by FC (CH2Cl2/acetone 95:5, CH2Cl2/acetone 9:1) yielding a colorless foam (250 mg, 61%). TLC (CH2Cl2/acetone 9:1), Rf 0.3; UV (MeOH), λmax 235, 276, 320 nm (ɛ 22 900, 21 100, 25 300). 1H NMR [(D6)DMSO], 0.90–0.96 (m, CH2CH3), 1.28–1.37 (m, CH2CH3), 1.61–1.68 (m, CH2CH2), 2.21 [m, H-C(2′)], 2.91 [m, H-C(2′)], 3.03 [2m, H2-C(5′)], 3.35–3.52 (m, NCH2), 3.69 (s, OCH3), 3.90 [m, H-C(4′)], 4.48 [m, H-C(3′)], 5.32 [d, J = 4.7, HO-C(3′)], 6.46 [‘t’, J = 6.2, H-C(1′)], 6.73–7.31 (m, arom. H), 8.99 (s, N=CH), 9.56 (d, J = 9.89, NH), 10.77 (d, J = 9.88, CHO). Calculated for C41H48BrN7O6 (814.8), C 60.44, H 5.94, N 12.03; found, C 60.36, H 5.73, N 11.85.
1-[2-Deoxy-5-O-(4,4′-dimethoxytrityl)-β-d-erythro-pento-furanosyl]-N4-[(di-n-butylamino)methylidene]-N6-formyl-1H-pyrazolo[3,4-d]-pyrimidin-4,6-diamine 3′-[(2-cyano-ethyl)-N,N-diisopropylphosphoramidite] (11a)
As described for 7a with 10a (110 mg, 0.15 mmol), (i-Pr)2EtN (78 µl, 45 mmol) and 2-cyanoethyl diisopropylphosphoramido chloridite (47 µl, 0.2 mmol) in THF (2 ml). The oily residue was submitted to FC (CH2Cl2/EtOAc 85:15) yielding a colorless foam (75 mg, 53%). TLC (CH2Cl2/EtOAc 85:15), Rf 0.8. 31P NMR (CDCl3), 149.58, 149.52.
3-Bromo-1-[2-deoxy-5-O-(4,4′-dimethoxytrityl)-β-d-erythro-pentofuranosyl]-N4-[(di-n-butylamino)methylidene]-N6-formyl-1H-pyrazolo[3,4-d]-pyrimidin-4,6-diamine 3′-[(2-cyanoethyl)-N,N-diisopropylphosphoramidite] (11b)
As described for 7a with 10b (90 mg, 0.11 mmol), (i-Pr)2EtN (63 µl, 0.36 mmol) and 2-cyanoethyl diisopropylphosphoramido chloridite (31 µl, 0.14 mmol) in THF (1 ml). The oily residue was submitted to FC (CH2Cl2/EtOAc 85:15) yielding a colorless foam (80 mg, 72%). TLC (CH2Cl2/EtOAc 85:15), Rf 0.8. 31P NMR (CDCl3), 149.58, 149.53.
RESULTS AND DISCUSSION
Monomers
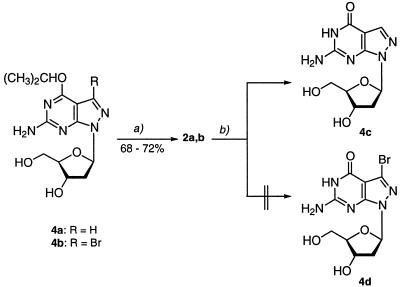
The isopropoxy nucleosides 4a,b, which have been described previously (19), served as precursor molecules for synthesis of the 8-aza-7-deazapurin-2,6-diamine (pyrazolo[3,4-d]pyrimidin- 4,6-diamine) nucleosides 2a,b (Scheme 2). Treatment of the alkoxy derivatives with 25% aqueous NH3 for 4 days at 70°C furnished the diamino compounds 2a,b. Nucleosides 2a,b showed differences in chemical enzymatic stability in comparison to the purin-2,6-diamine nucleoside 1. The half-life of the N-glycosylic bond of compound 2a (τ = 91 min, 0.5 N HCl, 20°C) indicated an ∼15-fold higher glycosylic bond stability than the parent 2-amino-2′-deoxyadenosine (1) (τ = 6 min, 0.5 N HCl, 20°C). The glycosylic bond of the bromo derivative 2b was stable under these conditions; stronger acidic conditions (2 N HCl) were necessary to hydrolyze this molecule (τ = 87 min). A similar stabilization of the glycosylic bond has been reported for other 7-bromo-8-aza-7-deazapurine 2′-deoxyribonucleosides (13,15). Treatment of compound 2a with adenosine deaminase resulted in formation of 8-aza-7-deaza-2′-deoxyguanosine (4c). The reaction was followed by UV spectrophotometry. Deamination of 2a occurred much more slowly than that of purine nucleoside 1. The 7-bromo derivative 2b was stable against deamination even at high enzyme concentrations (23).
Scheme 2. (a) Aqueous 25% NH3, 60°C, 4 days. (b) Adenosine deaminase from calf intestine mucosa (EC 3.5.4.4), Tris–HCl buffer, pH 7.0, 20°C.
The stability of oligonucleotides is influenced by the conformation of the sugar–phosphate backbone and a modified nucleobase can alter the N↔S pseudorotational equilibrium of the sugar moiety significantly. The 1H NMR spectra of nucleosides 1–3 were measured in D2O and 1H,1H NMR coupling constants were determined. Analysis with respect to the sugar ring conformation was then performed using the program PSEUROT (24). According to Table 2 8-aza-7-deazapurin-2,6-diamine nucleosides 2a,b show a more pronounced N conformer population than the corresponding purine nucleoside 1, while the N-type population of the related 7-deazapurine nucleoside 3 is decreased. The conformation around the C(4′)–C(5′) bond indicates that 8-aza-7-deazapurin-2,6-diamine nucleosides 2a,b, as for 8-aza-7-deaza-2′-deoxyguanosines (25), prefer the γt-(–sc) rotamer population, while for the regular purine nucleosides the γ(+)g-(+sc) conformation is predominant (26). This means that in both cases the nucleobase and the CH2OH group undergo a disrotatory motion so that the Coulomb repulsion between N(8) and O(5′) and O(4′) is minimized. These observations indicate that the conformational parameters of nucleosides 2a,b are similar but not the same as those found for purin-2,6-diamine 2′-deoxyribonucleoside 1. These differences can affect base pairing as well as stacking interactions within a DNA duplex.
Table 2. 3JH,H coupling constants of the sugar moieties and conformer populations (N/S) of 2′-deoxyribonucleosides 1–3 at 303 Ka.
| 3JH,H (Hz) | Conformation | ||||||||||||
| |
1′,2′ |
1′,2′′ |
2′,3′ |
2′′,3′ |
3′,4′ |
4′,5′ |
4′,5′′ |
|
N (%) |
S (%) |
γ(+)g |
γt |
γ(–)g |
| 1 | 7.30 | 6.10 | 7.00 | 3.10 | 3.40 | 3.20 | 4.30 | 31 | 69 | 62 | 25 | 13 | |
| 2a | 6.60 | 6.80 | 6.90 | 3.70 | 3.60 | 4.00 | 5.80 | 37 | 63 | 36 | 42 | 22 | |
| 2b | 6.60 | 6.70 | 6.80 | 3.70 | 3.90 | 4.30 | 5.90 | 37 | 63 | 32 | 43 | 25 | |
| 3 | 7.90 | 6.40 | 6.20 | 3.10 | 3.00 | 3.87 | 4.82 | 25 | 75 | 48 | 32 | 20 | |
aSolvent D2O; r.m.s. < 0.4 Hz; ½ΔJmax½ < 0.4 Hz.
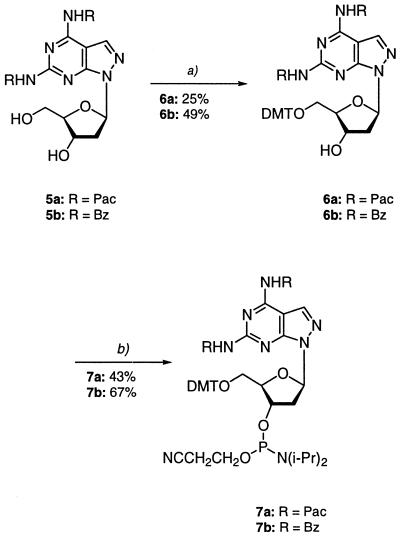
Next, the two amino groups of nucleosides 2a,b were protected. This can be done only with difficulty, as already recognized in the case of purin-2,6-diamine nucleoside 1. In contrast to guanine, the 2-amino group of purin-2,6-diamine is rather basic. As a result, benzoyl protection at that position leads to stable amides, which are difficult to hydrolyze. If a more labile protecting group is chosen for both amino functions, that attached to the 6-amino group readily dissociates. This has already been demonstrated in the case of benzoyl groups (1) and phenoxyacetyl residues (21,27,28). Apart from those difficulties, the acylated derivatives show an increased tendency to depurinate (1). Fortunately, the N-glycosylic bond of nucleosides 2a,b is significantly more stable than that of purine nucleoside 1. Thus, phenoxyacetyl as well as benzoyl groups were successfully introduced into compound 2a when 2,4,5-trichlorophenyl phenoxyacetate (21) or benzoyl chloride were used and the transient protection protocol was employed (29) (2a→5a,b). The yield of the bis-phenoxyacylated derivative 5a was rather low (30%) while the bis-benzoylated nucleoside 5b was isolated in 63% yield (Scheme 3). Both compounds 5a,b were converted into the DMT derivatives 6a,b using standard reaction conditions. Phosphitylation with 2-cyanoethyl diisopropylphosphoramido chloridite afforded the phosphoramidites 7a,b (30). As the DMT derivatives are poorly soluble, a THF-containing solution had to be used for phosphitylation. The yield of the phosphoramidite, which is normally ∼80%, was only 67%.
Scheme 3. (a) Pyridine/(MeO)2TrCl, r.t., 4 h. (b) THF, 2-cyanoethyl diisopropylphosphoramido chloridite, r.t., 30 min.
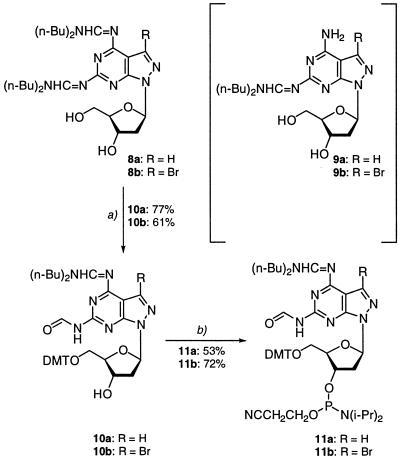
Deprotection of compound 5a (25% aqueous NH3, 40°C, HPLC monitoring) showed that removal of the pac groups is fast and complete deprotection occurs in <1 h. Complete removal of the two benzoyl groups of compound 5b (25% aqueous NH3, 40°C, HPLC monitoring) required 10 h while deprotection of bis-benzoylated purine nucleoside 1 took several days. From this point of view the benzoyl-protected phosphoramidite 7b represents a useful building block for incorporation of compound 2a into oligonucleotides. Nevertheless, the low solubility of the intermediate 6b represents a problem. In spite of this fact, N,N-dialkylaminomethylidene groups were considered for protection. Early attempts to introduce N,N-dimethylaminomethylidene residues into purine nucleoside 1 failed due to the instability of the protecting groups. Our laboratory has used the N,N-di-n-butylaminomethylidene group (22) for protection of the amino function of 8-aza-7-deaza-2′-deoxyisoguanosine and 5-aza-7-deaza-2′-deoxyguanosine (31). This group was now introduced into nucleosides 2a,b. The bis-amidines 8a,b were obtained as the major products (50% yield) while the mono adducts 9a,b were formed as minor components (17%). For the protected nucleosides 8a,b the time required for complete deprotection (concentrated ammonia, 40°C, HPLC monitoring at 260 nm) was determined to be 440 min for 8a and 450 min for 8b. A half-life value for the particular residues was not determined.
Subsequently, the 5′-hydroxyls were protected with 4,4′-dimethoxytrityl chloride. After the work-up followed by silica gel flash chromatography one N,N′-dibutylaminomethylidene residue was hydrolyzed to yield the formyl group (Scheme 4). This group was protecting the 2-amino function, while the 6-amino group carried the dibutylaminomethylidene residue. The structures of compounds 10a,b were established on the basis of NMR spectra (Table 3). Phosphitylation of the DMT derivatives of 10a,b was performed in THF in the presence of 2-cyanoethyl diisopropylphosphoramido chloridite, furnishing the phosphoramidites 11a,b (Scheme 3). These phosphoramidites (11a,b), as well as the corresponding building block 7b with benzoyl protection, were efficiently used in solid phase oligonucleotide synthesis with coupling yields >90%. All compounds were characterized by 1H, 13C and 31P NMR spectra and by elemental analysis (Table 3 and Materials and Methods). The 13C NMR data are particularly useful for monomer assignment as the heterocyclic bases contain only very few protons, making structural characterization from 1H NMR spectra difficult.
Scheme 4. (a) Pyridine/(MeO)2TrCl, r.t., 4 h. (b) THF, 2-cyanoethyl diisopropylphosphoramido chloridite, r.t., 30 min.
Table 3. 13C NMR chemical shifts of pyrazolo[3,4-d]pyrimidine 2′-deoxyribonucleosidesa.
| C(2)b,d | C(4)d | C(5) | C(6)d | C(7) | C=O/=CH | C=O/=CH | C(1′) | C(2′) | C(3′) | C(4′) | C(5′) | |
| |
C(6)c |
C(7a) |
C(3a) |
C(4) |
C(3) |
|
|
|
|
|
|
|
| 2a | 156.9 | 158.3 | 95.5 | 162.7 | 133.3 | – | – | 83.3 | 38.0 | 71.3 | 87.4 | 62.7 |
| 2b | 157.4 | 157.6 | 94.5 | 162.7 | 119.2 | – | – | 83.0 | 37.5 | 70.9 | 87.3 | 62.4 |
| 5a | 152.2 | 154.8 | 100.5 | 156.1 | 136.1 | 168.5 | 169.1 | 83.6 | 37.7 | 71.1 | 87.7 | 62.5 |
| 5b | 153.3 | 155.2 | 102.2 | 156.1 | 134.0 | 165.6 | 166.3 | 83.5 | 37.6 | 70.9 | 87.5 | 62.3 |
| 6a | 152.2 | 154.8 | 100.5 | 155.9 | 135.9 | 168.5 | 169.2 | 83.7 | 38.1 | 70.8 | 85.6 | 64.3 |
| 6b | 153.3 | 155.3 | 102.3 | 156.1 | 132.2 | 165.7 | 166.4 | 83.7 | 38.0 | 70.9 | 85.6 | 64.4 |
| 8af | 157.1 | 157.6 | 106.0 | 158.9 | 134.9 | 164.1 | 166.2 | 86.1 | 41.2 | 73.7 | 89.1 | 64.3 |
| 8b | 156.1 | 156.1 | 103.6 | 157.9 | 121.4 | 162.1 | 164.4 | 83.3 | 37.5 | 70.8 | 87.5 | 62.2 |
| 8bf | 157.1 | 157.7 | 104.7 | 159.2 | 122.9 | 163.4 | 166.7 | 86.2 | 41.0 | 73.8 | 89.0 | 64.2 |
| 9a | 156.6 | 157.3 | 102.4 | 162.3 | 133.7 | 162.9 | – | 83.1 | 37.8 | 71.2 | 87.3 | 62.6 |
| 10af | 155.5 | 155.5 | 107.1 | 156.8 | 134.9 | 161.8 | 163.7 | 84.2 | 38.3 | 73.6 | 86.7 | 64.9 |
| 10b | 156.0 | 156.1 | 103.8 | 157.8 | 121.3 | 162.1 | 164.4 | 83.5 | – e | 70.5 | 85.3 | 64.1 |
aMeasured in (D6)DMSO at 298 K.
bPurine numbering.
cSystematic numbering.
dTentative.
eSuperimposed by (D6)DMSO.
fMeasured in CDCl3.
Oligonucleotides
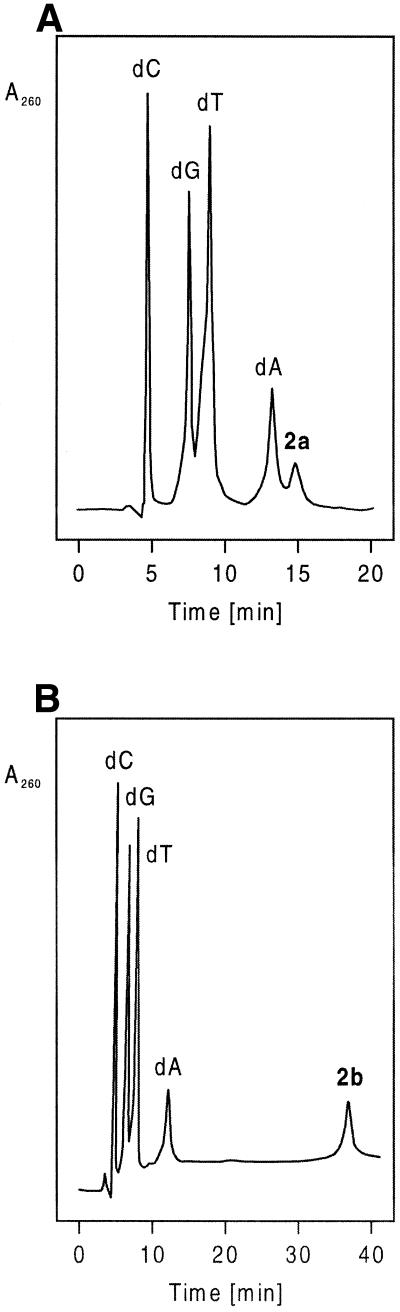
Synthesis and characterization. Automated solid phase synthesis of oligonucleotides 12–26 (Tables 4 and 5) was performed using standard phosphoramidite chemistry. The oligonucleotides were detritylated and purified on oligonucleotide purification cartridges or by reverse phase HPLC (for conditions of purification see Materials and Methods). The homogeneity of the compounds was proven by ion exchange chromatography. The composition of the oligonucleotides was determined by RP-18 HPLC after tandem hydrolysis with snake venom phosphodiesterase and alkaline phosphatase as described (17). 8-Aza-7-deazapurin-2,6-diamine nucleoside 2a migrates slightly slower than dA. The bromo compound 2b is much more hydrophobic, as can be seen from the elution profile (Fig. 1A and B). MALDI-TOF mass spectra were measured for the modified oligonucleotides (Table 1 and Materials and Methods). The correct masses were found in all cases, which underlines that all protecting groups were split off during a 10 h ammonia treatment at 60°C.
Table 4. Tm values and thermodynamic data for oligonucleotide duplexes containing purin-2,6-diamine 2′-deoxyribonucleoside 1 and pyrazolo[3,4-d]pyrimidine analogs 2a,ba.
| |
Tm (°C) |
ΔG0310
(kcal/mol) |
| 5′-d(TAG GTC AAT ACT) (12) | ||
| 3′-d(ATC CAG TTA TGA) (13) | 47 | –10.6 |
| 5′-d(T1G GTC 11T 1CT) (14) | ||
| 3′-d(ATC C1G TT1 TGA) (15) | 50b | – |
| 5′-d(TAG GTC 2a2aT ACT) (16) | ||
| 3′-d(ATC C2aG TT2a TGA) (17) | 51 | –12.3 |
| 5′-d(TAG GTC AAT ACT) (12) | ||
| 3′-d(ATC C1G TT1 TGA) (15) | 49 | –10.9 |
| 5′-d(TAG GTC AAT ACT) (12) | ||
| 3′-d(ATC C2aG TT2a TGA) (17) | 50 | –12.1 |
| 5′-d(TAG GTC AAT ACT) (12) | ||
| 3′-d(ATC C2bG TT2b TGA) (18) | 59 | –13.9 |
| 5′-d(TAG GTC 2b2bT ACT) (21) | ||
| 3′-d(ATC CAG TT A TGA) (13) | 56 | –13.4 |
| 5′-d(TAG GTC 2b2bT ACT) (21) | ||
| 3′-d(ATC C2bG TT 2bTGA) (18) | 67 | –17.0 |
| 5′-d(T2bGGTC2b2bT2bCT) (19) | ||
| 3′-d(A TC CAG T T ATGA) (13) | 64 | –15.8 |
| 5′-d(TAG GTC AAT ACT) (12) | ||
| 3′-(AUC CAG UUA UGA) (20) | 45 | –10.2 |
| 5′-d(T1G GTC 11T 1CT) (14) | ||
| 3′-(AUC CAG UUA UGA) (20) | 48 | –10.2 |
| 5′-d(TAG GTC 2a2aT ACT) (16) | ||
| 3′-(AUC CAG UUA UGA) (20) | 48 | – |
| 5′-d(TAG GTC 2b2bT ACT) (21) | ||
| 3′-(AUC CAG UUA UGA) (20) | 53 | –12.7 |
aMeasured at 260 nm in 0.1 M NaCl, 10 mM MgCl2 and 10 mM Na cacodylate buffer, pH 7.0, with 5 µM + 5 µM single strand concentrations.
bLow cooperativity.
cNot measured.
Table 5. Tm values and thermodynamic data for oligonucleotide duplexes containing dG-dC base pairs, 2b-dT pairs and mismatches.
| |
Tm (°C) |
ΔG0310
(kcal/mol) |
| 5′-d(TAG GTC AAT ACT) (12) | ||
| 3′-d(ATC CAG TTA TGA) (13) | 47a | –10.6 |
| 5′-d(TAG GCC GGC ACT) (22) | ||
| 3′-d(ATC CGG CCG TGA) (23) | 66a | –16.0 |
| 5′-d(TAG GTC 2b2bT ACT) (21) | ||
| 3′-d(ATC C2bG TT2b TGA) (18) | 67a | –17.0 |
| 5′-d(TAG GTC AAT ACT) (12) | ||
| 3′-d(ATC C2bG TT2b TGA) (18) | 59 a | –13.9 |
| 5′-d(TAG GAC AAT ACT) (24) | ||
| 3′-d(ATC C2bG TT2b TGA) (18) | 47a | –10.4 |
| 5′-d(TAG GGC AAT ACT) (25) | ||
| 3′-d(ATC C2bG TT2b TGA) (18) | 51a | –12.3 |
| 5′-d(TAG GCC AAT ACT) (26) | ||
| 3′-d(ATC C2bG TT2b TGA) (18) | 48a | –11.1 |
| 5′-d(TAG GTC AAT ACT) (12) | ||
| 3′-d(ATC CAG TTA TGA) (13) | 50b | –12.0 |
| 5′-d(TAG GAC AAT ACT) (24) | ||
| 3′-d(ATC CAG TTA TGA) (13) | 40b | –8.4 |
| 5′-d(TAG GGC AAT ACT) (25) | ||
| 3′-d(ATC CAG TTA TGA) (13) | 48b | –10.5 |
| 5′-d(TAG GCC AAT ACT) (26) | ||
| 3′-d(ATC CAG TTA TGA) (13) | 38b | –8.0 |
aMeasured at 260 nm in 0.1 M NaCl, 10 mM MgCl2 and 10 mM Na cacodylate, pH 7.0, with 5 µM + 5 µM single strand concentrations.
bMeasured at 260 nm in 1 M NaCl, 100 mM MgCl2 and 60 mM Na cacodylate, pH 7.0, with 5 µM + 5µM single strand concentrations.
Figure 1.
HPLC profiles of the reaction products obtained after enzymatic hydrolysis of oligomers 17 (A) and 18 (B) with snake venom phosphodiesterase and alkaline phosphatase in 0.1 M Tris–HCl buffer (pH 8.0) at 37°C. Column, RP-18 (200 × 10 mm); gradient: 0–20 min 100% B, 20–50 min 0–15% A in B.
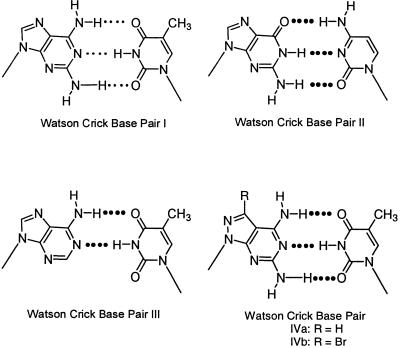
Base pairing and oligonucleotide duplex stability. Because of the presence of three hydrogen bonds the 1-dT base pair (I) is expected to show the same or a similar stability to a dG-dC pair (II) (Scheme 5). Experimental data obtained from DNA melting curves indicate that the thermodynamic stability of duplexes containing a 1-dT base pair is indeed somewhat higher than that of a dA-dT pair (III) but still remains far below that of a dG-dC pair. As the pyrazolo[3,4-d]pyrimidine nucleosides 2a,b can form similar tridentate base pairs as purine compound 1 (motifs IVa,b) it was of interest to investigate the stability of oligonucleotides containing the modified base pairs IVa and IVb.
Scheme 5.
Various studies have been performed to shed some light on the stability differences of a 2,6-diaminopurine-dT pair versus a dG-dC pair (2–6). A rather detailed examination of this matter has been undertaken by Sági et al. (8). The authors compared the thermal stabilities of bidentate base pairs represented by the motifs dA-dU, dI-dC, dA-dT and dI-m5Cd with those of tridentate pairs such as n2Ad-dU, dG-dC, n2Ad-dT and dG-m5Cd. As long as a 2-amino group was absent (bidentate base pairs) the various duplexes all showed very similar stabilities, independently of the particular base structure. On insertion of a 2-amino group, which leads to a tridentate base pair, this similarity disappears. The dG-dC base pair (II) is now much more stable than the n2Ad-dT pair (I). We were interested to compare the base pair stabilities of 8-aza-7-deazapurin-2,6-diamine nucleosides 2a,b with dT. Purine nucleoside 1 was evaluated for comparison. All three compounds were incorporated into the non-self-complementary duplex 5′-d(TAGGTCAATACT) (12), 5′-d(AGTATTGACCTA) (13). This duplex is used as a standard in our laboratory to study the influence of modified nucleosides on the thermal stability and structural behavior of helical formation. The Tm value of 12·13 is 47°C (0.1 M NaCl, 10 mM MgCl2, 10 mM Na cacodylate). The incorporation of six compound 1 residues instead of six dA residues increased the Tm value by only 3°C (see duplex 14·15, Table 4). This corresponds to a 0.5°C Tm increase per residue. Similar findings have been reported from experiments performed in other laboratories (3–8). When only four 8-aza-7-deazapurin-2,6-diamine nucleosides (2a) replaced dA residues an increase in the Tm value from 47 to 51°C was measured, which corresponds to a 1.0°C increase per modified residue (see duplex 12·13 versus 16·17). Thus, the pyrazolo[3,4-d]pyrimidine nucleoside 2a forms a more stable tridentate base pair with dT (motif IVa) than purine nucleoside 1 (motif I).
Earlier our laboratory has shown that 7-substituents of 7-deazapurines as well as of 8-aza-7-deazapurines are well accommodated in the major groove of DNA (12–15). In particular, halogen substituents show favorable properties with regard to duplex stability. This prompted us to replace dA residues by the 7-bromo derivative 2b. According to Table 4 duplex 12·18 carrying only two 2b residues is significantly more stable (Tm = 59°C) than the non-brominated duplex 12·17 (Tm = 50°C). In this case duplex stability was strengthened by 4.5°C per modified base. Likewise, duplex 18·21 was 16°C more stable than duplex 16·17 (Tm = 67°C), which corresponds to a Tm increase of 4°C per modified residue. Also, duplex 12·18, incorporating only two nucleoside 2b residues, was significantly more stable than duplex 14·15, containing six diaminopurine nucleosides (1) (Tm = 59 versus 50°C). A comparison of the stability of duplex 18·21 with the parent hybrid 12·13 shows that replacement of a dA residue by the base-modified nucleoside 2b increases the Tm value by 5°C per residue. Oligonucleotides containing nucleosides 1 or 2a,b were also hybridized with the complementary oligoribonucleotide 20. According to Table 4 DNA–RNA hybrids 14·20 and 16·20 show identical Tm stabilizations for compounds 1 and 2a over that of dA (duplex 12·20). A stronger stabilization was observed when the bromo compound 2b replaced 2a. Nevertheless, stabilization of the DNA–RNA hybrid by 2b was lower than that of DNA–DNA duplexes. The ΔG values shown in the Tables 4 and 5 were calculated at 310 K from the ΔH and ΔS values obtained by curve shape analysis of the melting profiles using the program Meltwin 3.0 (18). The free energies correlate well with the Tm values and correspond to those determined from the concentration dependence of the Tm values within 15%.
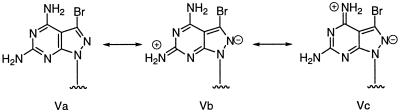
From the data in Table 4 it is apparent that the contribution of compound 2b to DNA–DNA duplex stability is extraordinarily high. Thus, it was of interest to prove whether the modified 2b-dT base pair approaches the stability of the canonical dG-dC pair. For this purpose oligonucleotides 22 and 23 were synthesized and hybridized. Duplex 22·23 is derived from duplex 12·13 by replacing four dA-dT base pairs by four dG-dC pairs. Then the duplex stability of 21·18 containing four 2b-dT base pairs was compared with that of 22·23. From Table 5 it is apparent that both duplexes display very similar Tm values, indicating that the modified dA-dT base pair shows the same stability as a dG-dC pair. An explanation for this observation can be given on the basis of two effects. (i) The more favorable proton donor properties of 8-aza-7-deazapurin-2,6-diamine nucleosides 2a,b compared to those of purin-2,6-diamine nucleoside 1. This is supported by the decreased stability of acyl groups protecting the 2-amino group of 2a,b compared to compound 1. The acylated derivatives of 2a,b are much more easily hydrolyzed than those of nucleoside 1. The mesomeric structures Va–c (Scheme 6) with a positive charge on the amino group and a negative one at the pyrazole N2 underline this observation. (ii) The second effect is caused by the 7-bromo substituent. It reduces the electron density of the heterocycle by the –I effect of the halogen and increases the hydrophobic character of the nucleoside molecule (see Fig. 1A and B). When the bromo nucleoside 2b is present in a DNA duplex water molecules or alkali cations can be expelled from the major groove, resulting in increased stacking interactions of the nucleobases.
Scheme 6.
Next, the base pair discrimination of compound 2b was studied in comparison to dA. Oligonucleotide duplexes containing this nucleoside in one strand and adenine, guanine, thymine and cytosine in the second strand opposite to it were hybridized and the Tm values were determined (Table 5). Almost selective base pairing with dT was observed, as is known for 2′-deoxyadenosine. Discrimination of the modified nucleoside 2b against dG was even more pronounced than for dA. This demonstrates that replacement of dA residues by 2b not only stabilizes the base pair with dT but also shows a selective base recognition for dT. Regarding enzymatic incorporation of 7-bromopyrazolo[3,4-d]pyrimidine 2′-deoxyribonucleoside triphosphates into a growing DNA chain, the Klenow fragment of DNA polymerase has been used successfully (data not shown). Such incorporation is difficult to achieve when duplex-stabilizing nucleoside triphosphates modified in the sugar moiety, such as hexitol nucleotides (32) or sugar locked nucleotides (33), are used.
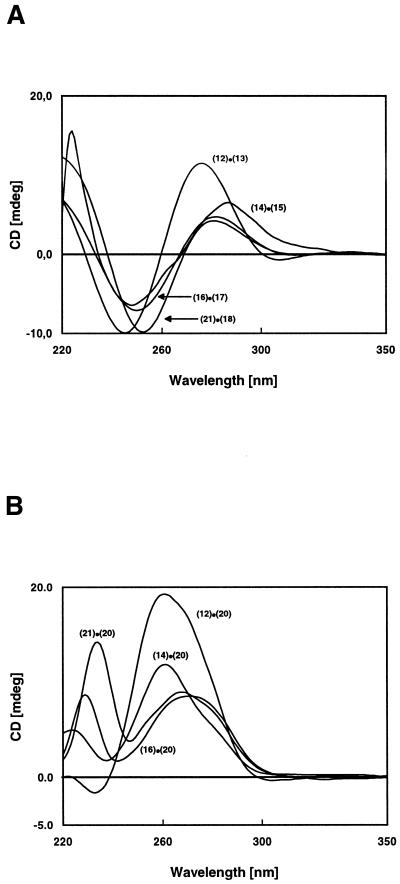
In order to identify the helical structure of the duplexes CD spectra of 12·13, 14·15, 16·17 and 21·18 were measured (Fig. 2A). The CD spectra of all duplexes indicate a B-like DNA structure, with a positive Cotton effect around 270–290 nm and a negative lobe around 250 nm. The CD spectra of the DNA–RNA hybrids (Fig. 2B) adopt the A-DNA form. Nevertheless, purin-2,6-diamine nucleoside 1 as well as the 8-aza-7-deazapurine derivatives 2a,b induce spectral changes which are the result of conformational differences between purine and 8-aza-7-deazapurine nucleosides (25).
Figure 2.
CD spectra of (A) the DNA–DNA duplexes and (B) the DNA–RNA duplexes measured at 20°C in 0.1 M NaCl containing 10 mM MgCl2 and 10 mM Na cacodylate, pH 7.0, at 5 µM + 5 µM single strand concentrations.
CONCLUSIONS
Diaminopurine nucleoside 1 (34), which can form three hydrogen bonds with dT, stabilizes the dA-dT pair only very little, in contrast to the canonical tridentate dG-dC pair. Oligonucleotides incorporating the pyrazolo[3,4-d]pyrimidin-4,6-diamine nucleoside 2a instead of 1 form more stable duplex structures. Nevertheless, the 2a-dT base pair is still considerably less stable than the dG-dC pair. The ‘dA-dT’ base pair reaches the stability of a dG-dC pair when the bromo nucleoside 2b replaces dA. Furthermore, compound 2b is rather stable against acid-catalyzed depurination and resistant to adenosine deaminase compared to the labile purine nucleoside 1. The base pair-stabilizing effect of 2b adjusts the stability of a ‘dA-dT’ pair to that of a dG-dC pair, thereby showing a similar base discrimination and maintaining the sequence specificity of an oligonucleotide. Application of this phenomenon to sequence-specific hybridization in solution or on oligonucleotides immobilized in DNA arrays is currently under investigation.
Acknowledgments
ACKNOWLEDGEMENTS
We thank Dr Junlin He for her valuable contributions, Mr Yang He and Dr H.Rosemeyer for the NMR spectra, Mrs Elisabeth Feiling for the oligonucleotide syntheses and Dr T.Wenzel (Bruker Saxonia, Germany) for the MALDI-TOF mass spectra. Oligonucleotide 20 was a generous gift of Dr L.Beigelman (Ribozyme Pharmaceuticals Inc., Boulder, CO). Financial support by Roche Diagnostics GmbH (Penzberg, Germany) is gratefully acknowledged.
References
- 1.Gaffney B.L., Marky,L.A. and Jones,R.A. (1984) The influence of the purine 2-amino group on DNA conformation and stability II. Tetrahedron, 40, 3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bailly C. and Waring,M.J. (1998) The use of diaminopurine to investigate structural properties of nucleic acids and molecular recognition between ligands and DNA. Nucleic Acids Res., 26, 4309–4314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chollet A. and Kawashima,E. (1988) DNA containing the base analogue 2-aminoadenine: preparation, use as hybridization probes and cleavage by restriction endonucleases. Nucleic Acids Res., 16, 305–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chollet A., Chollet-Damerius,A. and Kawashima,E.H. (1986) Synthesis of oligodeoxyribonucleotides containing the base 2-aminoadenine. Chem. Scripta, 26, 37–40. [Google Scholar]
- 5.Cheong C., Tinoco,I.Jr and Chollet,A. (1988) Thermodynamic studies of base pairing involving 2,6-diaminopurine. Nucleic Acids Res., 16, 5115–5122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lamm G.M., Blencowe,B.J., Sproat,B.S., Iribarren,A.M., Ryder,U. and Lamond,A.I. (1991) Antisense probes containing 2-aminoadenosine allow efficient depletion of U5 snRNP from HeLa splicing extracts. Nucleic Acids Res., 19, 3193–3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoheisel J.D. and Lehrach,H. (1990) Quantitative measurements on the duplex stability of 2,6-diaminopurine and 5-chloro-uracil nucleotides using enzymatically synthesized oligomers. FEBS Lett., 274, 103–106. [DOI] [PubMed] [Google Scholar]
- 8.Sági J., Szakonyi,E., Vorlícková,M. and Kypr,J. (1996) Unusual contribution of 2-aminoadenine to the thermostability of DNA. J. Biomol. Struct. Dyn., 13, 1035–1041. [DOI] [PubMed] [Google Scholar]
- 9.Gryaznov S. and Schultz,R.G. (1994) Stabilization of DNA:DNA and DNA:RNA duplexes by substitution of 2′-deoxyadenosine with 2′-deoxy-2-aminoadenosine. Tetrahedron Lett., 35, 2489–2492. [Google Scholar]
- 10.Haaima G., Hansen,H.F., Christensen,L., Dahl,O. and Nielsen,P.E. (1997) Increased DNA binding and sequence discrimination of PNA oligomers containing 2,6-diamino-purine. Nucleic Acids Res., 25, 4639–4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boudou V., Kerremans,L., De Bouvere,B., Lescrinier,E., Schepers,G., Busson,R., Van Aerschot,A. and Herdewijn,P. (1999) Base pairing of anhydrohexitol nucleosides with 2,6-diaminopurine, 5-methylcytosine and uracil as base moiety. Nucleic Acids Res., 27, 1450–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramzaeva N. and Seela,F. (1996) Duplex stability of 7-deazapurine DNA: oligonucleotides containing 7-bromo- or 7-iodo-7-deazaguanine. Helv. Chim. Acta, 79, 1549–1558. [Google Scholar]
- 13.Seela F. and Zulauf,M. (1999) Synthesis of oligonucleotides containing pyrazolo[3,4-d]-pyrimidines: the influence of 7-substituted 8-aza-7-deazaadenines on the duplex structure and stability. J. Chem. Soc. Perkin Trans., 1, 479–488. [Google Scholar]
- 14.Seela F. and Thomas,H. (1995) Duplex stabilization of DNA: oligonucleotides containing 7-substituted 7-deazaadenines. Helv. Chim. Acta, 78, 94–108. [Google Scholar]
- 15.Seela F. and Becher,G. (1999) Oligonucleotides containing pyrazolo[3,4-d]pyrimidines: the influence of 7-substituted 8-aza-7-deaza-2′-deoxyguanosines on the duplex structure and stability. Helv. Chim. Acta, 82, 1640–1655. [Google Scholar]
- 16.Balow G., Mohan,V., Lesnik,E.A., Johnston,J.F., Monia,B.P. and Acevedo,O.L. (1998) Biophysical and antisense properties of oligodeoxynucleotides containing 7-propynyl-, 7-iodo- and 7-cyano-7-deaza-2-amino-2′-deoxyadenosines. Nucleic Acids Res., 26, 3350–3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seela F. and Lampe,S. (1991) 3-Deazaguanine N7- and N9-(2′-deoxy-β-D-ribofuranosides): building blocks for solid-phase synthesis and incorporation into oligodeoxyribonucleotides. Helv. Chim. Acta, 74, 1790–1800. [Google Scholar]
- 18.McDowell J.A. and Turner,D.H. (1996) Investigation of the structural basis for thermodynamic stabilities of tandem GU mismatches: solution structure of (rGAGGUCUC)2 by two-dimensional NMR and simulated annealing. Biochemistry, 35, 14077–14089. [DOI] [PubMed] [Google Scholar]
- 19.Seela F. and Becher,G. (1998) Synthesis of 7-halogenated 8-aza-7-deaza-2′-deoxyguanosines and related pyrazolo[3,4-d]pyrimidine 2′-deoxyribonucleosides. Synthesis, 207–214. [Google Scholar]
- 20.Seela F. and Driller,H. (1988) 8-Aza-7-deaza-2′,3′-dideoxyguanosine: deoxygenation of its 2′-deoxy-β-D-ribofuranoside. Helv. Chim. Acta, 71, 757–761. [Google Scholar]
- 21.Cano A., Goodman,M.F. and Eritja,R. (1994) Synthesis of oligodeoxyribonucleotides containing 2,6-diaminopurine. Nucl. Nucl., 13, 501–509. [Google Scholar]
- 22.McBride L.J., Kierzek,R., Beaucage,S.L. and Caruthers,M.H. (1986) Amidine protecting groups for oligonucleotide synthesis. J. Am. Chem. Soc., 108, 2040–2048. [Google Scholar]
- 23.Oertel F., Winter,H., Kazimierczuk,Z., Vilpo,J.A., Richter,P. and Seela,F. (1992) Synthesis and properties of methylthiopyrazolo[3,4-d]pyrimidine 2′-deoxy-β-D-ribonucleosides. Liebigs Ann. Chem., 1165–1170. [Google Scholar]
- 24.van Wijk J. and Altona,C. (1993) PSEUROT 6.2—A Program for the Conformational Analysis of the Five-membered Rings. University of Leiden, Leiden, The Netherlands.
- 25.Seela F., Becher,G., Rosemeyer,H., Reuter,H., Kastner,G. and Mikhailopulo,I.A. (1999) The high-anti conformation of 7-halogenated 8-aza-7-deaza-2′-deoxyguanosines: a study of the influence of modified bases on the sugar structure of nucleosides. Helv. Chim. Acta, 82, 105–124. [Google Scholar]
- 26.Rosemeyer H. and Seela,F. (1997) Stereoelectronic effects of modified purine bases on the sugar conformation of nucleosides: pyrrolo[2,3-d]pyrimidines. J. Chem. Soc., Perkin Trans., 2, 2341–2345. [Google Scholar]
- 27.Sproat B.S., Iribarren,A.M., Garcia,R.G. and Beijer,B. (1991) New synthetic routes to synthons suitable for 2′-O-allyloligoribonucleotide assembly. Nucleic Acids Res., 19, 733–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luyten I., Van Aerschot,A., Rozenski,J., Busson,R. and Herdewijn,P. (1997) Protection of 2,6-diaminopurine 2′-deoxyriboside. Nucl. Nucl., 16, 1649–1652. [Google Scholar]
- 29.Ti G.S., Gaffney,B.L. and Jones,R.A. (1982) Transient protection: efficient one-flask syntheses of protected deoxynucleosides. J. Am. Chem. Soc., 104, 1316–1319. [Google Scholar]
- 30.Sinha N.D., Biernat,J., McManus,J. and Köster,H. (1984) Polymer support oligonucleotide synthesis XVIII: use of β-cyanoethyl-N,N-dialkylamino-/N-morpholino phosphoramidite of deoxynucleosides for the synthesis of DNA fragments simplifying deprotection and isolation of the final product. Nucleic Acids Res., 12, 4539–4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seela F., Wei,C., Melenewski,A., He,Y., Kröschel,R. and Feiling,E. P(1999) Parallel-stranded DNA formed by new base pairs related to the isoguanine-cytosine or isocytosine-guanine motifs. Nucl. Nucl., 18, 1543–1548. [Google Scholar]
- 32.Vastmans K., Pochet,S., Peys,A., Kerremans,L., Van Aerschot,A., Hendrix,C., Marlière,P. and Herdewijn,P. (2000) Enzymatic incorporation in DNA of sugar-modified nucleotides. Biochemistry, 39, 12757–12765. [DOI] [PubMed] [Google Scholar]
- 33.Christensen N.K., Petersen,M., Nielsen,P., Jacobsen,J.P., Olsen,C.E. and Wengel,J. (1998) A novel class of oligonucleotide analogues containing 2′-O,3′-C-linked [3.2.0]bicycloarabinonucleoside monomers: synthesis, thermal affinity studies, and molecular modeling. J. Am. Chem. Soc., 120, 5458–5463. [Google Scholar]
- 34.Kirnos M.D., Khudyakov,I.Y., Alexandrushkina,N.I. and Vanyushin,B.F. (1977) 2-Aminoadenine is an adenine substituting for a base in S-2L cyanophage DNA. Nature, 270, 369–370. [DOI] [PubMed] [Google Scholar]