Abstract
Most regions of the adult mammalian central nervous system (CNS) do not support axonal growth and regeneration. Laminin, expressed by cultured astrocytes and known to promote neurite outgrowth of cultured neurons, is normally present in brain basement membranes, and only transiently induced in adult brain astrocytes by injury. Here I provide three lines of evidence which suggest that the continued expression of laminin by astrocytes may be a prerequisite for axonal growth and regeneration in adult CNS. Firstly, laminin is continuously present in astrocytes of adult rat olfactory bulb apparently in close association with the olfactory nerve axons. Secondly, laminin is continuously expressed by astrocytes in adult frog brain, and sectioning of the optic tract further increases laminin immunoreactivity in astrocytes of the optic tectum during the period of axonal regeneration. Lastly, laminin appears normally in astrocytes of the frog and goldfish optic nerves which regenerate, but not in astrocytes of the rat or chick optic nerves which do not regenerate. The selective association of laminin with axons that undergo growth and regeneration in vivo is consistent with the possibility that astrocytic laminin provides these central nervous systems with their regenerative potential.
Full text
PDF




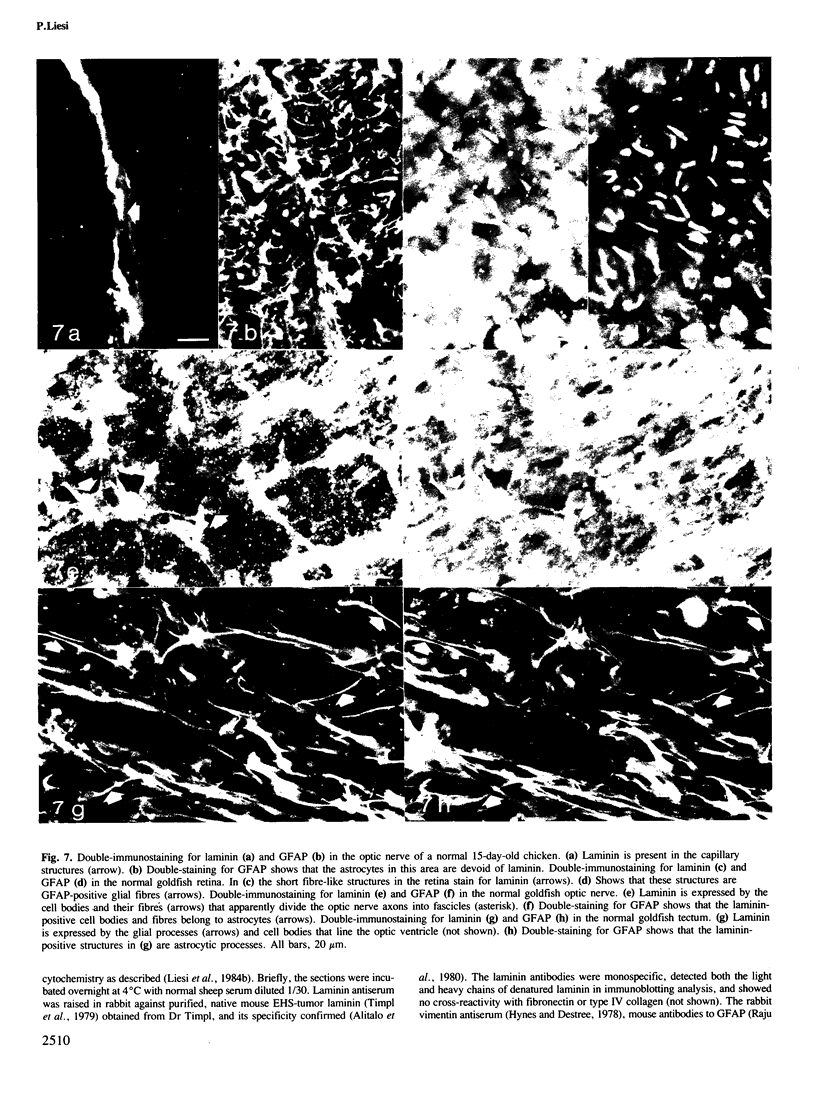

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alitalo K., Kurkinen M., Vaheri A., Virtanen I., Rohde H., Timpl R. Basal lamina glycoproteins are produced by neuroblastoma cells. Nature. 1980 Oct 2;287(5781):465–466. doi: 10.1038/287465a0. [DOI] [PubMed] [Google Scholar]
- Altman J. Autoradiographic and histological studies of postnatal neurogenesis. IV. Cell proliferation and migration in the anterior forebrain, with special reference to persisting neurogenesis in the olfactory bulb. J Comp Neurol. 1969 Dec;137(4):433–457. doi: 10.1002/cne.901370404. [DOI] [PubMed] [Google Scholar]
- Baron-Van Evercooren A., Kleinman H. K., Ohno S., Marangos P., Schwartz J. P., Dubois-Dalcq M. E. Nerve growth factor, laminin, and fibronectin promote neurite growth in human fetal sensory ganglia cultures. J Neurosci Res. 1982;8(2-3):179–193. doi: 10.1002/jnr.490080208. [DOI] [PubMed] [Google Scholar]
- Benfey M., Aguayo A. J. Extensive elongation of axons from rat brain into peripheral nerve grafts. Nature. 1982 Mar 11;296(5853):150–152. doi: 10.1038/296150a0. [DOI] [PubMed] [Google Scholar]
- Bernstein J. J., Getz R., Jefferson M., Kelemen M. Astrocytes secrete basal lamina after hemisection of rat spinal cord. Brain Res. 1985 Feb 18;327(1-2):135–141. doi: 10.1016/0006-8993(85)91507-0. [DOI] [PubMed] [Google Scholar]
- Cornbrooks C. J., Carey D. J., McDonald J. A., Timpl R., Bunge R. P. In vivo and in vitro observations on laminin production by Schwann cells. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3850–3854. doi: 10.1073/pnas.80.12.3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotman C. W., Nieto-Sampedro M. Cell biology of synaptic plasticity. Science. 1984 Sep 21;225(4668):1287–1294. doi: 10.1126/science.6382610. [DOI] [PubMed] [Google Scholar]
- Cotman C. W., Nieto-Sampedro M., Harris E. W. Synapse replacement in the nervous system of adult vertebrates. Physiol Rev. 1981 Jul;61(3):684–784. doi: 10.1152/physrev.1981.61.3.684. [DOI] [PubMed] [Google Scholar]
- Doucette J. R. The glial cells in the nerve fiber layer of the rat olfactory bulb. Anat Rec. 1984 Oct;210(2):385–391. doi: 10.1002/ar.1092100214. [DOI] [PubMed] [Google Scholar]
- Edgar D., Timpl R., Thoenen H. The heparin-binding domain of laminin is responsible for its effects on neurite outgrowth and neuronal survival. EMBO J. 1984 Jul;3(7):1463–1468. doi: 10.1002/j.1460-2075.1984.tb01997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillery R. W. Experiments to determine whether retinogeniculate axons can form translaminar collateral sprouts in the dorsal lateral geniculate nucleus of the cat. J Comp Neurol. 1972 Nov;146(3):407–420. doi: 10.1002/cne.901460306. [DOI] [PubMed] [Google Scholar]
- Hedman K., Vaheri A., Wartiovaara J. External fibronectin of cultured human fibroblasts is predominantly a matrix protein. J Cell Biol. 1978 Mar;76(3):748–760. doi: 10.1083/jcb.76.3.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O., Destree A. T. 10 nm filaments in normal and transformed cells. Cell. 1978 Jan;13(1):151–163. doi: 10.1016/0092-8674(78)90146-0. [DOI] [PubMed] [Google Scholar]
- Kalil K., Reh T. Regrowth of severed axons in the neonatal central nervous system: establishment of normal connections. Science. 1979 Sep 14;205(4411):1158–1161. doi: 10.1126/science.472734. [DOI] [PubMed] [Google Scholar]
- Kiernan J. A. Hypotheses concerned with axonal regeneration in the mammalian nervous system. Biol Rev Camb Philos Soc. 1979 May;54(2):155–197. doi: 10.1111/j.1469-185x.1979.tb00871.x. [DOI] [PubMed] [Google Scholar]
- Liesi P., Dahl D., Vaheri A. Laminin is produced by early rat astrocytes in primary culture. J Cell Biol. 1983 Mar;96(3):920–924. doi: 10.1083/jcb.96.3.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesi P., Dahl D., Vaheri A. Neurons cultured from developing rat brain attach and spread preferentially to laminin. J Neurosci Res. 1984;11(3):241–251. doi: 10.1002/jnr.490110304. [DOI] [PubMed] [Google Scholar]
- Liesi P. Do neurons in the vertebrate CNS migrate on laminin? EMBO J. 1985 May;4(5):1163–1170. doi: 10.1002/j.1460-2075.1985.tb03755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesi P., Kaakkola S., Dahl D., Vaheri A. Laminin is induced in astrocytes of adult brain by injury. EMBO J. 1984 Mar;3(3):683–686. doi: 10.1002/j.1460-2075.1984.tb01867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund R. D., Lund J. S. Synaptic adjustment after deafferentation of the superior colliculus of the rat. Science. 1971 Feb 26;171(3973):804–807. doi: 10.1126/science.171.3973.804. [DOI] [PubMed] [Google Scholar]
- Manthorpe M., Engvall E., Ruoslahti E., Longo F. M., Davis G. E., Varon S. Laminin promotes neuritic regeneration from cultured peripheral and central neurons. J Cell Biol. 1983 Dec;97(6):1882–1890. doi: 10.1083/jcb.97.6.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis F. L. A brain protein unique to the olfactory bulb. Proc Natl Acad Sci U S A. 1972 May;69(5):1221–1224. doi: 10.1073/pnas.69.5.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maturana H. R., Lettvin J. Y., McCulloch W. S., Pitts W. H. Evidence That Cut Optic Nerve Fibers in a Frog Regenerate to Their Proper Places in the Tectum. Science. 1959 Dec 18;130(3390):1709–1710. doi: 10.1126/science.130.3390.1709. [DOI] [PubMed] [Google Scholar]
- Müller H. W., Gebicke-Härter P. J., Hangen D. H., Shooter E. M. A specific 37,000-dalton protein that accumulates in regenerating but not in nonregenerating mammalian nerves. Science. 1985 Apr 26;228(4698):499–501. doi: 10.1126/science.3983637. [DOI] [PubMed] [Google Scholar]
- Ostberg A., Norden J. Ultrastructural study of degeneration and regeneration in the amphibian tectum. Brain Res. 1979 Jun 8;168(3):441–455. doi: 10.1016/0006-8993(79)90301-9. [DOI] [PubMed] [Google Scholar]
- Raisman G. Specialized neuroglial arrangement may explain the capacity of vomeronasal axons to reinnervate central neurons. Neuroscience. 1985 Jan;14(1):237–254. doi: 10.1016/0306-4522(85)90176-9. [DOI] [PubMed] [Google Scholar]
- Raju T., Bignami A., Dahl D. In vivo and in vitro differentiation of neurons and astrocytes in the rat embryo. Immunofluorescence study with neurofilament and glial filament antisera. Dev Biol. 1981 Jul 30;85(2):344–357. doi: 10.1016/0012-1606(81)90266-9. [DOI] [PubMed] [Google Scholar]
- Rogers S. L., Letourneau P. C., Palm S. L., McCarthy J., Furcht L. T. Neurite extension by peripheral and central nervous system neurons in response to substratum-bound fibronectin and laminin. Dev Biol. 1983 Jul;98(1):212–220. doi: 10.1016/0012-1606(83)90350-0. [DOI] [PubMed] [Google Scholar]
- Schnitzer J., Franke W. W., Schachner M. Immunocytochemical demonstration of vimentin in astrocytes and ependymal cells of developing and adult mouse nervous system. J Cell Biol. 1981 Aug;90(2):435–447. doi: 10.1083/jcb.90.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skene J. H. Growth-associated proteins and the curious dichotomies of nerve regeneration. Cell. 1984 Jul;37(3):697–700. doi: 10.1016/0092-8674(84)90404-5. [DOI] [PubMed] [Google Scholar]
- Taub E., Harger M., Grier H. C., Hodos W. Some anatomical observations following chronic dorsal rhizotomy in monkeys. Neuroscience. 1980;5(2):389–401. doi: 10.1016/0306-4522(80)90114-1. [DOI] [PubMed] [Google Scholar]
- Timpl R., Rohde H., Robey P. G., Rennard S. I., Foidart J. M., Martin G. R. Laminin--a glycoprotein from basement membranes. J Biol Chem. 1979 Oct 10;254(19):9933–9937. [PubMed] [Google Scholar]










