Abstract
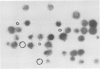
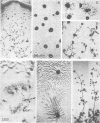
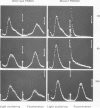
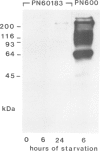
Mutants of the cellular slime mold Polysphondylium pallidum have been selected using a cell sorter and a fluorescentlabeled monoclonal antibody, mAb 293. This antibody blocks cell adhesion when applied as Fab, and recognizes a carbohydrate epitope containing L-fucose. This epitope is expressed on the cell surface and is present on >10 membrane glycoproteins of different apparent mol. wts. Twenty mutants were obtained which did not bind mAb 293 when tested at 2 h of starvation. After longer periods of starvation the epitope became detectable in the mutants. In all these mutants aggregation patterns were atypical. Generally streams of cells that were radially orientated around aggregation centers were missing or were much shorter than in wild-type. Genetic analysis demonstrated that aberrant aggregation was linked to the alteration in carbohydrate epitope expression. One mutant was unstable and gave rise to subclones in which almost no antibody binding was observed, even after 24 h of starvation, and only few aggregation centers with no streams or very short ones were formed. These results indicate that the capability of the cells to aggregate is correlated with the exposure on their surfaces of the carbohydrate epitope recognized by mAb 293, whose function in development remains to be established.
Keywords: fucosylation, contact sites, cell sorting, lectins, cellular slime molds
Full text
PDF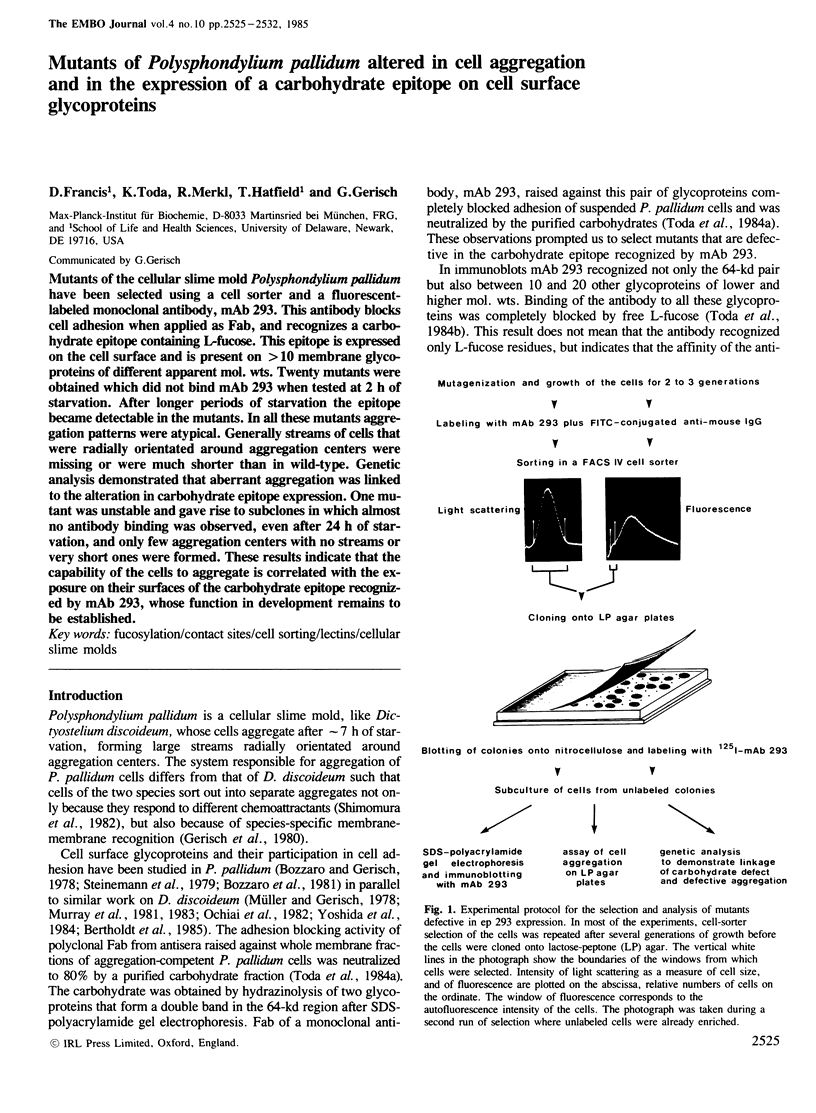
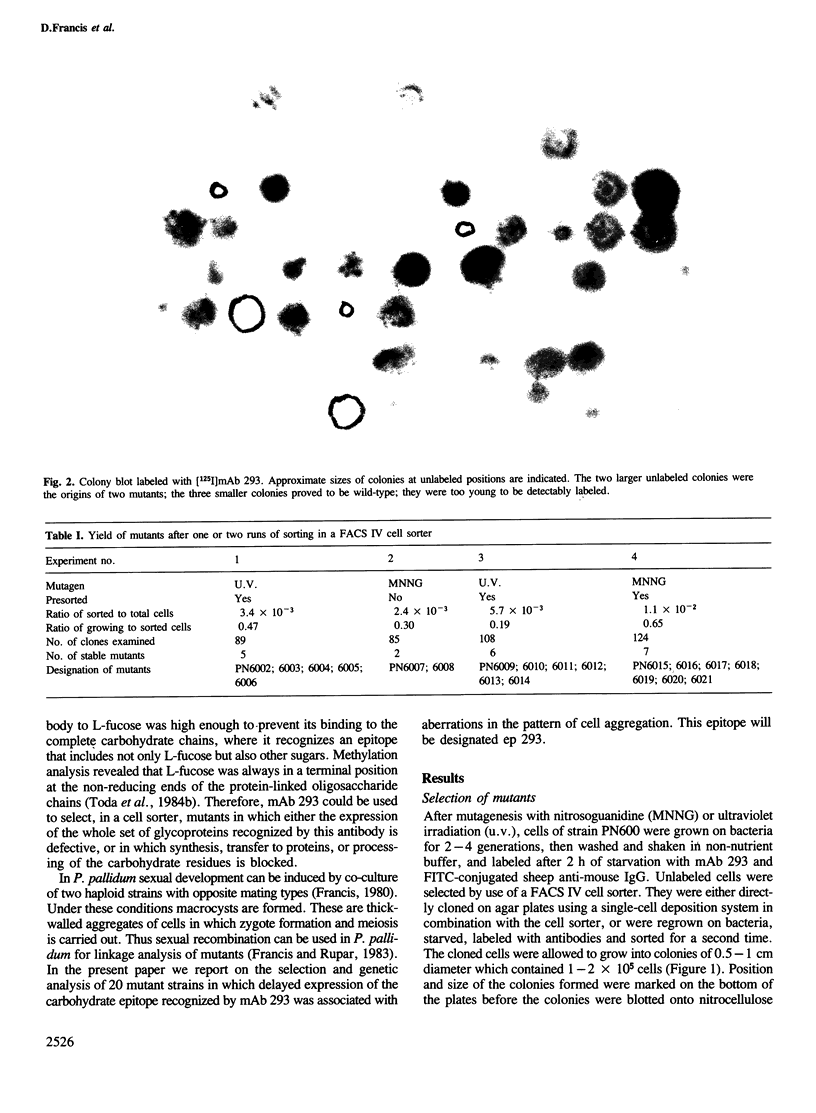
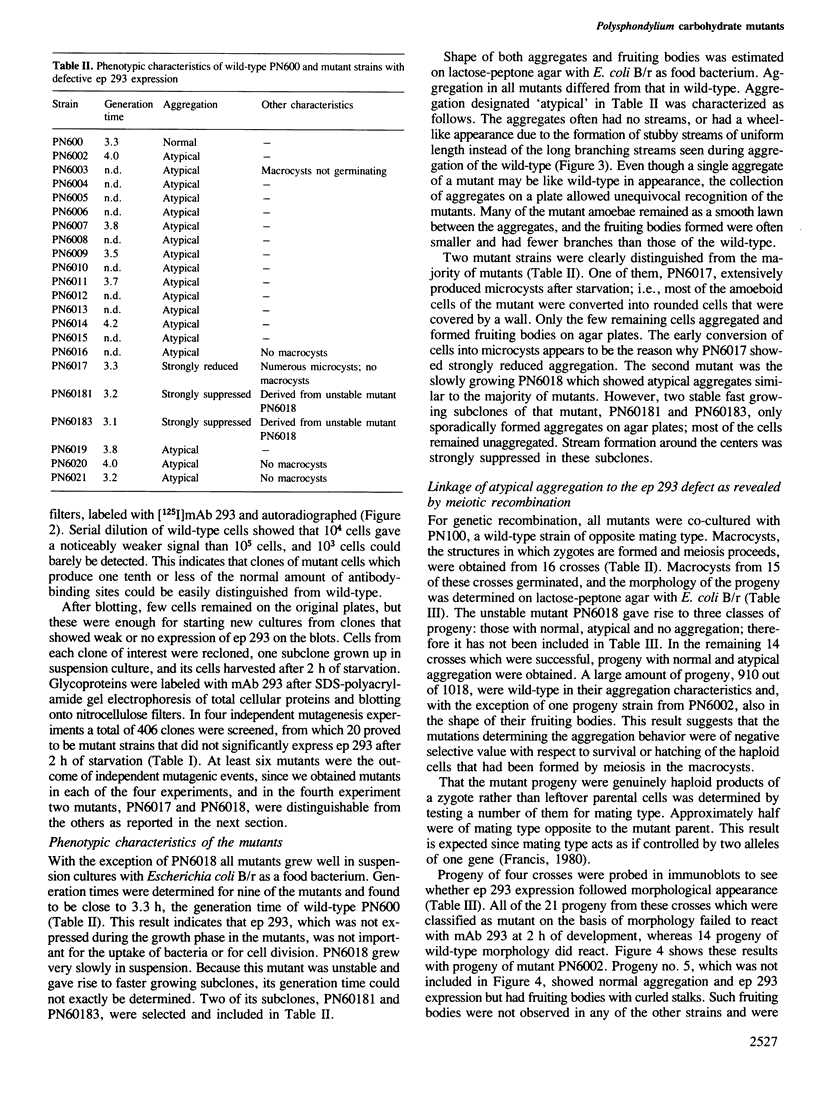
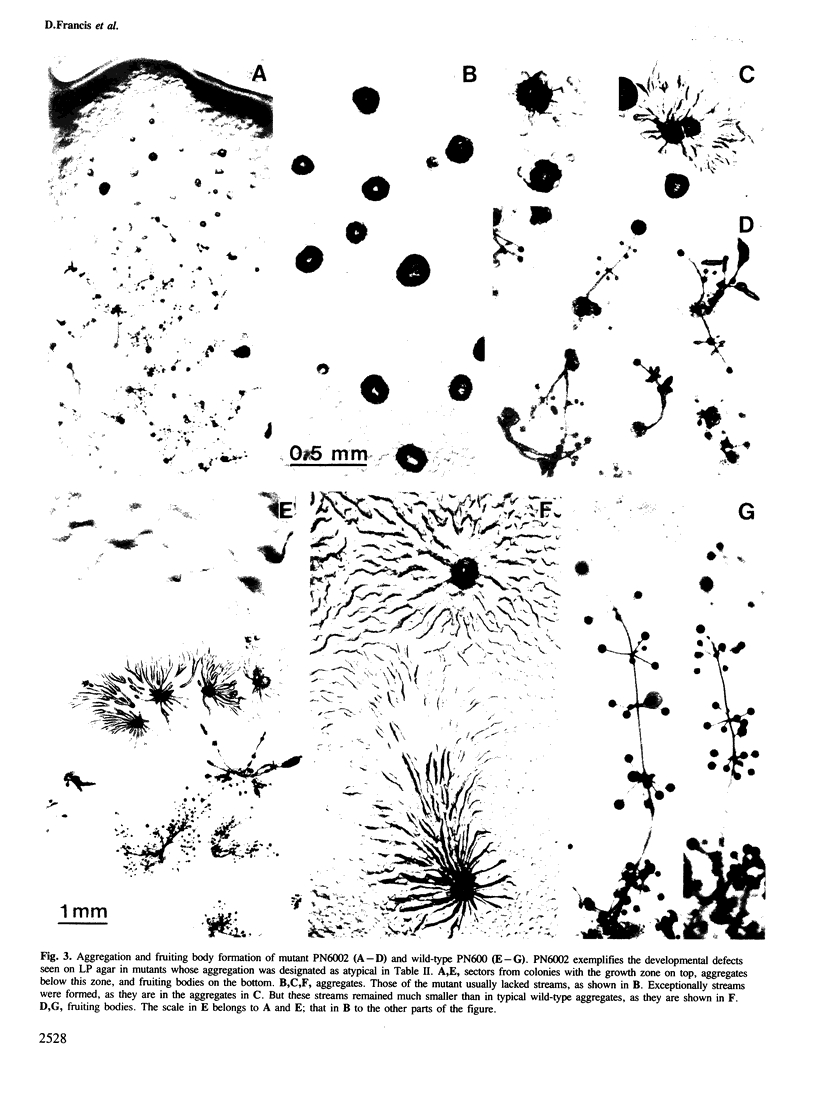

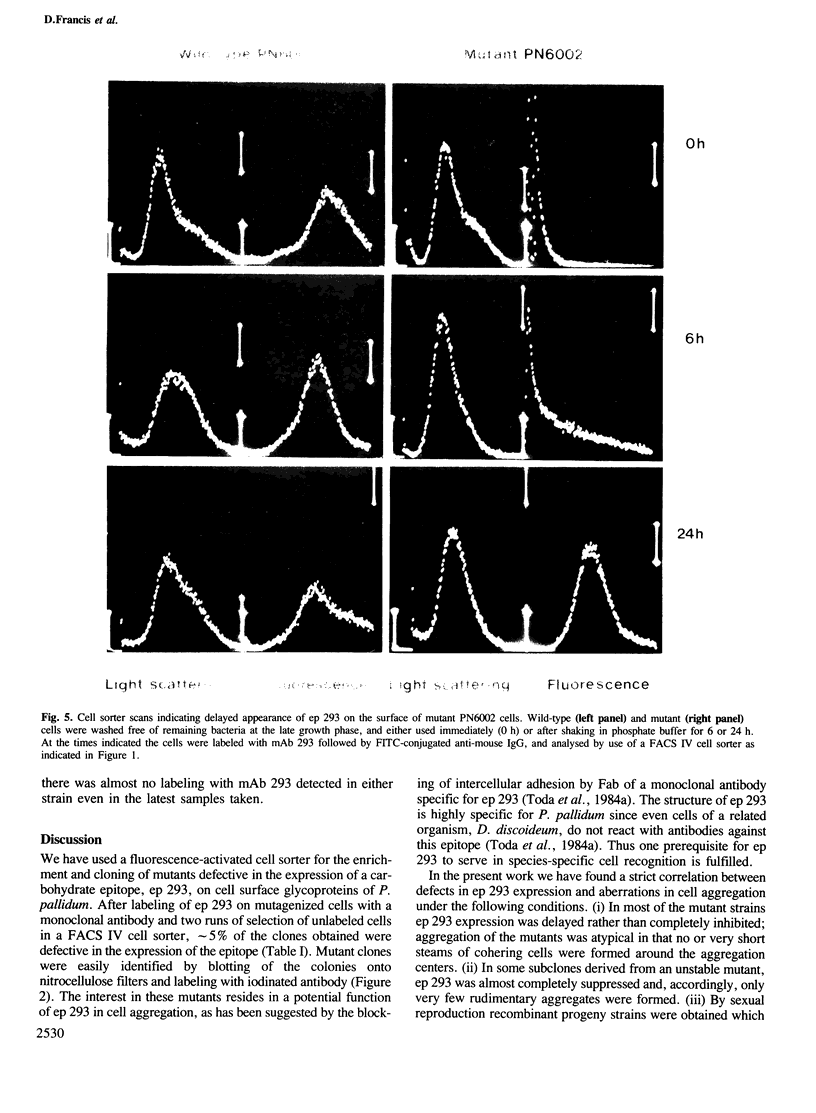


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bertholdt G., Stadler J., Bozzaro S., Fichtner B., Gerisch G. Carbohydrate and other epitopes of the contact site A glycoprotein of Dictyostelium discoideum as characterized by monoclonal antibodies. Cell Differ. 1985 May;16(3):187–202. doi: 10.1016/0045-6039(85)90516-0. [DOI] [PubMed] [Google Scholar]
- Bozzaro S., Gerisch G. Contact sites in aggregating cells of Polysphondylium pallidum. J Mol Biol. 1978 Apr 5;120(2):265–279. doi: 10.1016/0022-2836(78)90067-0. [DOI] [PubMed] [Google Scholar]
- Bozzaro S., Tsugita A., Janku M., Monok G., Opatz K., Gerisch G. Characterization of a purified cell surface glycoprotein as a contact site in Polysphondylium pallidum. Exp Cell Res. 1981 Jul;134(1):181–191. doi: 10.1016/0014-4827(81)90475-4. [DOI] [PubMed] [Google Scholar]
- Francis D. Techniques and Marker Genes for Use in Macrocyst Genetics with POLYSPHONDYLIUM PALLIDUM. Genetics. 1980 Sep;96(1):125–136. doi: 10.1093/genetics/96.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Murray B. A., Niman H. L., Loomis W. F. Monoclonal antibody recognizing gp80, a membrane glycoprotein implicated in intercellular adhesion of Dictyostelium discoideum. Mol Cell Biol. 1983 May;3(5):863–870. doi: 10.1128/mcb.3.5.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray B. A., Yee L. D., Loomis W. F. Immunological analysis of glycoprotein (contact sites A) involved in intercellular adhesion of Dictyostelium discoideum. J Supramol Struct Cell Biochem. 1981;17(3):197–211. doi: 10.1002/jsscb.380170302. [DOI] [PubMed] [Google Scholar]
- Müller K., Gerisch G. A specific glycoprotein as the target site of adhesion blocking Fab in aggregating Dictyostelium cells. Nature. 1978 Aug 3;274(5670):445–449. doi: 10.1038/274445a0. [DOI] [PubMed] [Google Scholar]
- Ochiai H., Stadler J., Westphal M., Wagle G., Merkl R., Gerisch G. Monoclonal antibodies against contact sites A of Dictyostelium discoideum: detection of modifications of the glycoprotein in tunicamycin-treated cells. EMBO J. 1982;1(8):1011–1016. doi: 10.1002/j.1460-2075.1982.tb01286.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimomura O., Suthers H. L., Bonner J. T. Chemical identity of the acrasin of the cellular slime mold Polysphondylium violaceum. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7376–7379. doi: 10.1073/pnas.79.23.7376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler J., Bauer G., Westphal M., Gerisch G. Monoclonal antibody against cytoplasmic lectins of Dictyostelium discoideum: cross-reactivity with a membrane glycoprotein, contact site A, and with E. coli beta-galactosidase and lac repressor. Hoppe Seylers Z Physiol Chem. 1984 Mar;365(3):283–288. doi: 10.1515/bchm2.1984.365.1.283. [DOI] [PubMed] [Google Scholar]
- Steinemann C., Hintermann R., Parish R. W. Identification of a developmentally regulated plasma membrane glycoprotein involved in adhesion of Polysphondylium pallidum cells. FEBS Lett. 1979 Dec 15;108(2):379–384. doi: 10.1016/0014-5793(79)80568-2. [DOI] [PubMed] [Google Scholar]
- Toda K., Bozzaro S., Lottspeich F., Merkl R., Gerisch G. Monoclonal anti-glycoprotein antibody that blocks cell adhesion in Polysphondylium pallidum. Eur J Biochem. 1984 Apr 2;140(1):73–81. doi: 10.1111/j.1432-1033.1984.tb08068.x. [DOI] [PubMed] [Google Scholar]
- Toda K., Tharanathan R. N., Bozzaro S., Gerisch G. Monoclonal antibodies that block cell adhesion in Polysphondylium pallidum: reaction with L-fucose, a terminal sugar in cell-surface glycoproteins. Eur J Biochem. 1984 Sep 17;143(3):477–481. doi: 10.1111/j.1432-1033.1984.tb08395.x. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M., Stadler J., Bertholdt G., Gerisch G. Wheat germ agglutinin binds to the contact site A glycoprotein of Dictyostelium discoideum and inhibits EDTA-stable cell adhesion. EMBO J. 1984 Nov;3(11):2663–2670. doi: 10.1002/j.1460-2075.1984.tb02191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]