Abstract
Two overlapping genomic clones containing the murine granulocyte-macrophage colony stimulating factor (GM-CSF) gene have been isolated. On the basis of transfection experiments, we have established that a 9-kb BamHI fragment from one of these recombinants encodes biologically active GM-CSF. As deduced from nucleotide sequence analysis, the GM-CSF gene comprises four exons encompassing 2.5 kb of genomic DNA. Primer extension analysis of GM-CSF mRNA identifies a transcriptional initiation site 35 bp upstream of a single translational initiation codon in-frame with the GM-CSF coding sequences and 28 bp downstream of a TATA promoter consensus sequence. Pre-GM-CSF molecules encoded by mRNAs originating from this promoter would include a hydrophobic leader sequence typical for a secreted protein. Intriguingly, sequences present at the 5' end of a GM-CSF cDNA clone previously isolated in our laboratory are not contained within either of the genomic clones and must therefore be transcribed from a promoter located at least 10 kb 5' of the main body of the gene. mRNAs transcribed from this alternative upstream promoter possess an additional initiating codon and potentially encode a pre-GM-CSF polypeptide with an atypical NH2-terminal leader peptide. Comparison of the nucleotide sequence of the GM-CSF gene with that of other haemopoietic growth factor genes has revealed a common decanucleotide (5'-GPuGPuTTPyCAPy-3') within their respective 5'-flanking regions which may be involved in their co-ordinate regulation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bazill G. W., Haynes M., Garland J., Dexter T. M. Characterization and partial purification of a haemopoietic cell growth factor in WEHI-3 cell conditioned medium. Biochem J. 1983 Mar 15;210(3):747–759. doi: 10.1042/bj2100747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess A. W., Camakaris J., Metcalf D. Purification and properties of colony-stimulating factor from mouse lung-conditioned medium. J Biol Chem. 1977 Mar 25;252(6):1998–2003. [PubMed] [Google Scholar]
- Burgess A. W., Metcalf D., Russell S. H., Nicola N. A. Granulocyte/macrophage-, megakaryocyte-, eosinophil- and erythroid-colony-stimulating factors produced by mouse spleen cells. Biochem J. 1980 Feb 1;185(2):301–314. doi: 10.1042/bj1850301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danna K. J., Sompayrac L. M. Efficient infection of monkey cells with SV40 DNA. II. Use of low-molecular-weight DEAE-dextran for large-scale experiments. J Virol Methods. 1982 Dec;5(5-6):335–341. doi: 10.1016/0166-0934(82)90025-8. [DOI] [PubMed] [Google Scholar]
- Dexter T. M., Garland J., Scott D., Scolnick E., Metcalf D. Growth of factor-dependent hemopoietic precursor cell lines. J Exp Med. 1980 Oct 1;152(4):1036–1047. doi: 10.1084/jem.152.4.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Early P., Rogers J., Davis M., Calame K., Bond M., Wall R., Hood L. Two mRNAs can be produced from a single immunoglobulin mu gene by alternative RNA processing pathways. Cell. 1980 Jun;20(2):313–319. doi: 10.1016/0092-8674(80)90617-0. [DOI] [PubMed] [Google Scholar]
- Fujita T., Takaoka C., Matsui H., Taniguchi T. Structure of the human interleukin 2 gene. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7437–7441. doi: 10.1073/pnas.80.24.7437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuse A., Fujita T., Yasumitsu H., Kashima N., Hasegawa K., Taniguchi T. Organization and structure of the mouse interleukin-2 gene. Nucleic Acids Res. 1984 Dec 21;12(24):9323–9331. doi: 10.1093/nar/12.24.9323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Gluzman Y. SV40-transformed simian cells support the replication of early SV40 mutants. Cell. 1981 Jan;23(1):175–182. doi: 10.1016/0092-8674(81)90282-8. [DOI] [PubMed] [Google Scholar]
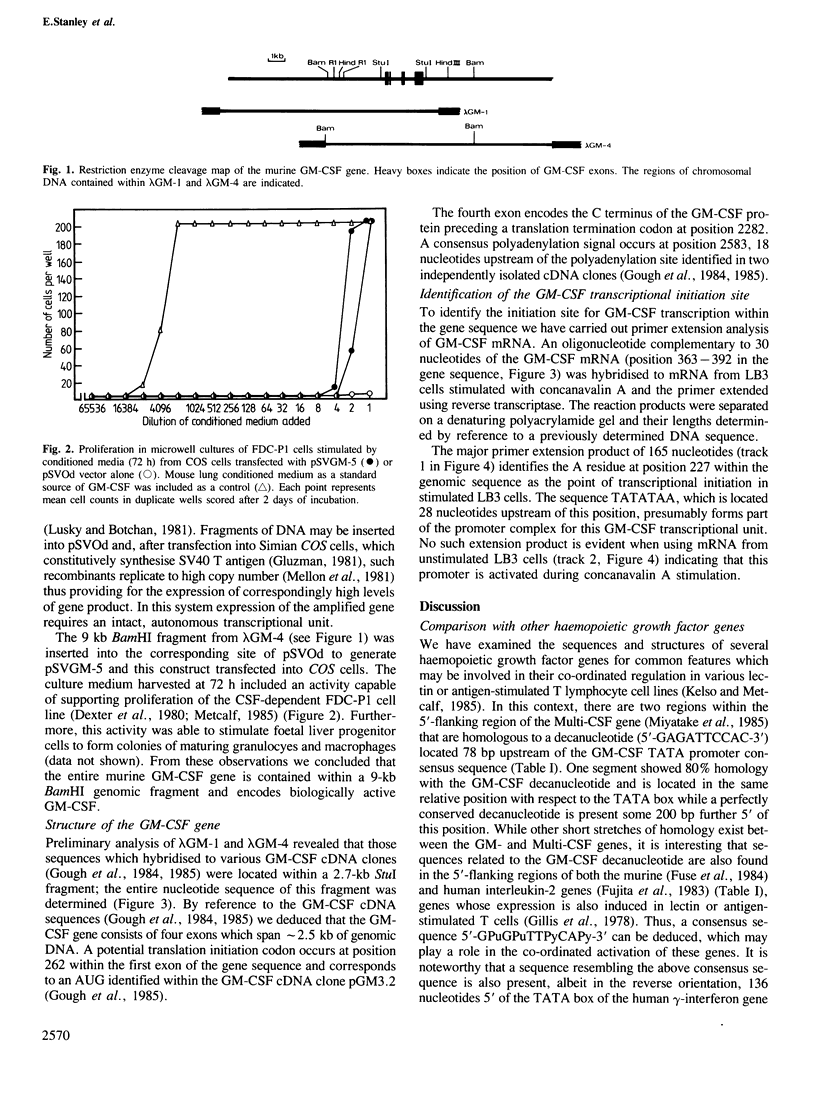
- Gough N. M., Gough J., Metcalf D., Kelso A., Grail D., Nicola N. A., Burgess A. W., Dunn A. R. Molecular cloning of cDNA encoding a murine haematopoietic growth regulator, granulocyte-macrophage colony stimulating factor. 1984 Jun 28-Jul 4Nature. 309(5971):763–767. doi: 10.1038/309763a0. [DOI] [PubMed] [Google Scholar]
- Gough N. M., Metcalf D., Gough J., Grail D., Dunn A. R. Structure and expression of the mRNA for murine granulocyte-macrophage colony stimulating factor. EMBO J. 1985 Mar;4(3):645–653. doi: 10.1002/j.1460-2075.1985.tb03678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihle J. N., Keller J., Henderson L., Klein F., Palaszynski E. Procedures for the purification of interleukin 3 to homogeneity. J Immunol. 1982 Dec;129(6):2431–2436. [PubMed] [Google Scholar]
- Jonas V., Lin C. R., Kawashima E., Semon D., Swanson L. W., Mermod J. J., Evans R. M., Rosenfeld M. G. Alternative RNA processing events in human calcitonin/calcitonin gene-related peptide gene expression. Proc Natl Acad Sci U S A. 1985 Apr;82(7):1994–1998. doi: 10.1073/pnas.82.7.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelso A., MacDonald H. R., Smith K. A., Cerottini J. C., Brunner K. T. Interleukin 2 enhancement of lymphokine secretion by T lymphocytes: analysis of established clones and primary limiting dilution microcultures. J Immunol. 1984 Jun;132(6):2932–2938. [PubMed] [Google Scholar]
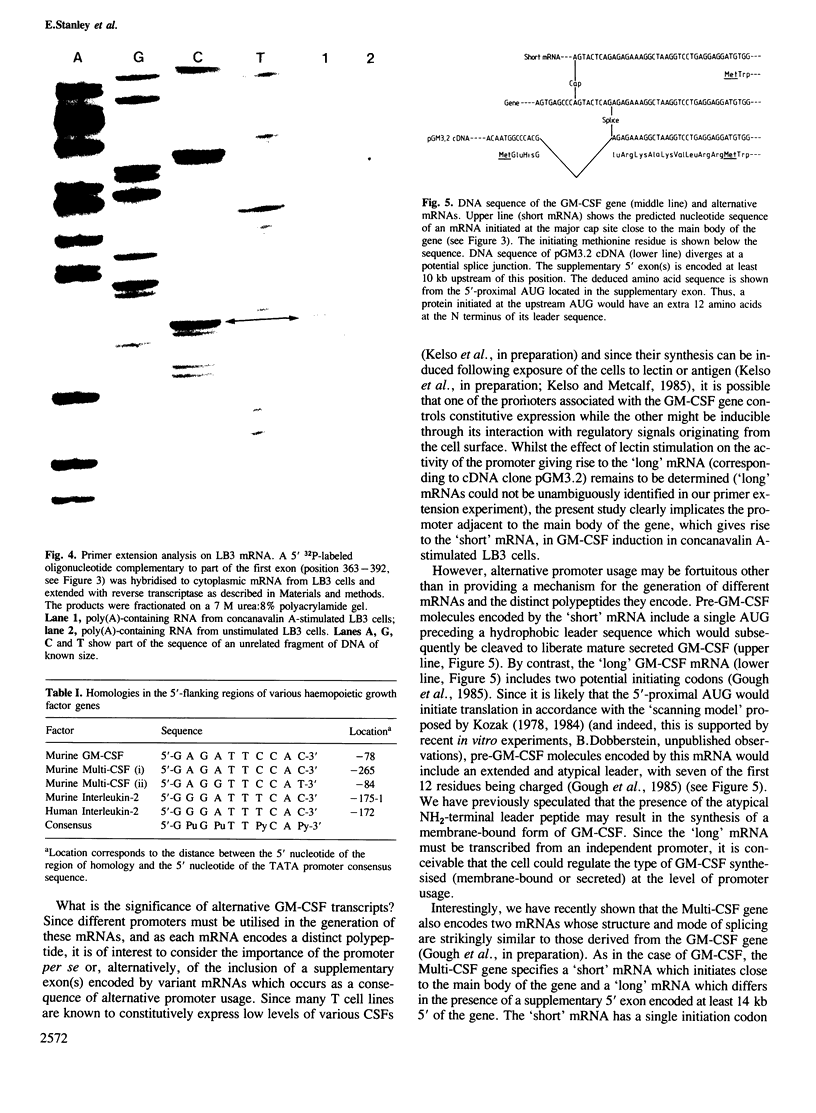
- Kelso A., Metcalf D. Clonal heterogeneity in colony stimulating factor production by murine T lymphocytes. J Cell Physiol. 1985 Apr;123(1):101–110. doi: 10.1002/jcp.1041230115. [DOI] [PubMed] [Google Scholar]
- King C. R., Piatigorsky J. Alternative RNA splicing of the murine alpha A-crystallin gene: protein-coding information within an intron. Cell. 1983 Mar;32(3):707–712. doi: 10.1016/0092-8674(83)90056-9. [DOI] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. How do eucaryotic ribosomes select initiation regions in messenger RNA? Cell. 1978 Dec;15(4):1109–1123. doi: 10.1016/0092-8674(78)90039-9. [DOI] [PubMed] [Google Scholar]
- Lusky M., Botchan M. Inhibition of SV40 replication in simian cells by specific pBR322 DNA sequences. Nature. 1981 Sep 3;293(5827):79–81. doi: 10.1038/293079a0. [DOI] [PubMed] [Google Scholar]
- Maki R., Roeder W., Traunecker A., Sidman C., Wabl M., Raschke W., Tonegawa S. The role of DNA rearrangement and alternative RNA processing in the expression of immunoglobulin delta genes. Cell. 1981 May;24(2):353–365. doi: 10.1016/0092-8674(81)90325-1. [DOI] [PubMed] [Google Scholar]
- McNeil T. A. Release of bone marrow colony stimulating activity during immunological reactions in vitro. Nat New Biol. 1973 Aug 8;244(136):175–176. [PubMed] [Google Scholar]
- Mellon P., Parker V., Gluzman Y., Maniatis T. Identification of DNA sequences required for transcription of the human alpha 1-globin gene in a new SV40 host-vector system. Cell. 1981 Dec;27(2 Pt 1):279–288. doi: 10.1016/0092-8674(81)90411-6. [DOI] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Miyatake S., Yokota T., Lee F., Arai K. Structure of the chromosomal gene for murine interleukin 3. Proc Natl Acad Sci U S A. 1985 Jan;82(2):316–320. doi: 10.1073/pnas.82.2.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabeshima Y., Fujii-Kuriyama Y., Muramatsu M., Ogata K. Alternative transcription and two modes of splicing results in two myosin light chains from one gene. Nature. 1984 Mar 22;308(5957):333–338. doi: 10.1038/308333a0. [DOI] [PubMed] [Google Scholar]
- Nawa H., Kotani H., Nakanishi S. Tissue-specific generation of two preprotachykinin mRNAs from one gene by alternative RNA splicing. Nature. 1984 Dec 20;312(5996):729–734. doi: 10.1038/312729a0. [DOI] [PubMed] [Google Scholar]
- Nicola N. A., Metcalf D., Matsumoto M., Johnson G. R. Purification of a factor inducing differentiation in murine myelomonocytic leukemia cells. Identification as granulocyte colony-stimulating factor. J Biol Chem. 1983 Jul 25;258(14):9017–9023. [PubMed] [Google Scholar]
- Parker J. W., Metcalf D. Production of colony-stimulating factor in mixed leucocyte cultures. Immunology. 1974 May;26(5):1039–1049. [PMC free article] [PubMed] [Google Scholar]
- Rozek C. E., Davidson N. Drosophila has one myosin heavy-chain gene with three developmentally regulated transcripts. Cell. 1983 Jan;32(1):23–34. doi: 10.1016/0092-8674(83)90493-2. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley E. R., Heard P. M. Factors regulating macrophage production and growth. Purification and some properties of the colony stimulating factor from medium conditioned by mouse L cells. J Biol Chem. 1977 Jun 25;252(12):4305–4312. [PubMed] [Google Scholar]
- Yip Y. K., Pang R. H., Urban C., Vilcek J. Partial purification and characterization of human gamma (immune) interferon. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1601–1605. doi: 10.1073/pnas.78.3.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Hagenbüchle O., Schibler U. A single mouse alpha-amylase gene specifies two different tissue-specific mRNAs. Cell. 1981 Feb;23(2):451–458. doi: 10.1016/0092-8674(81)90140-9. [DOI] [PubMed] [Google Scholar]