Abstract
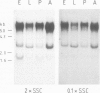
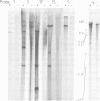
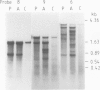
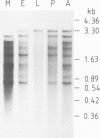
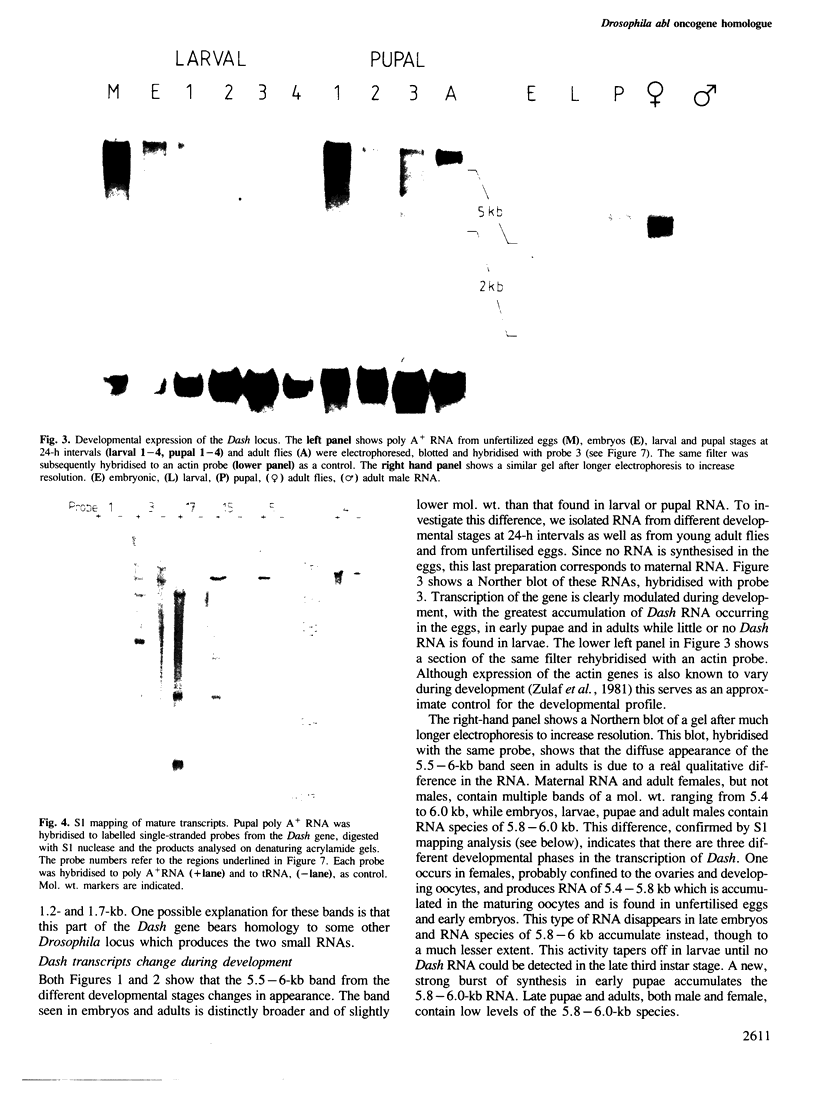
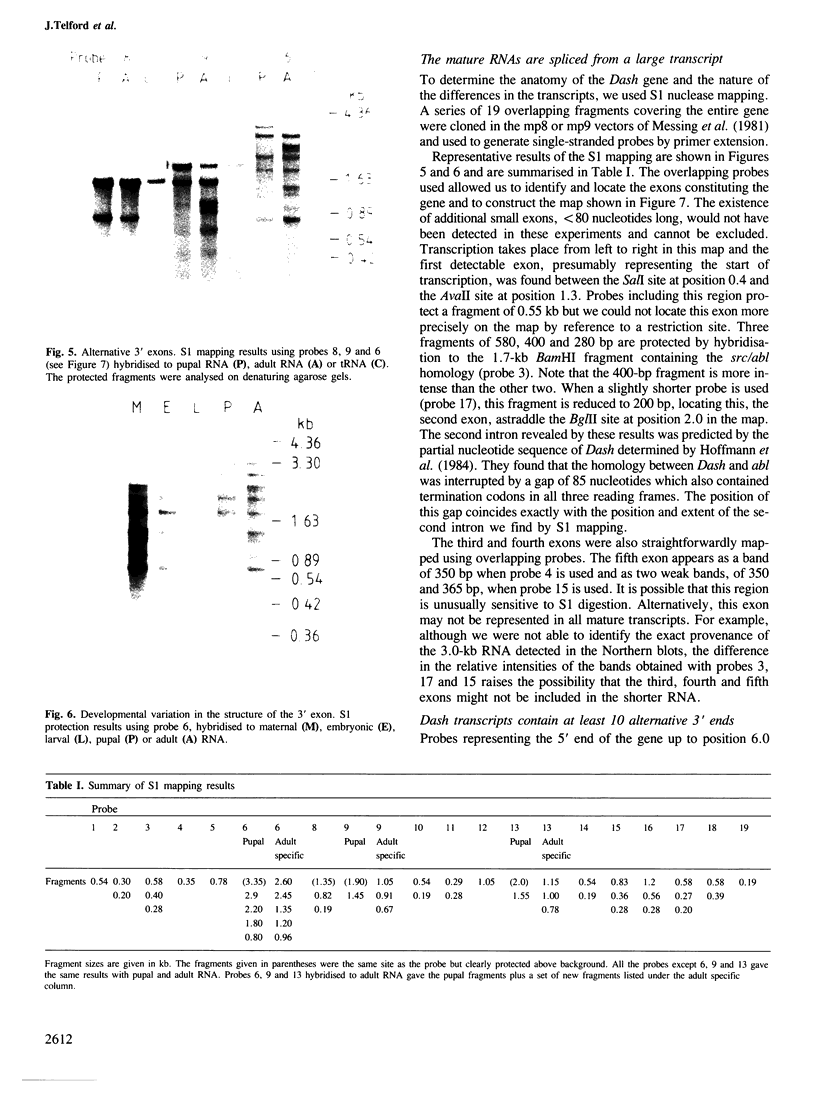
Drosophila sequences homologous to the abl oncogene are located near the 5' end of a gene (Dash). The Dash gene is transcribed to give long RNAs (5-6 kb) and short RNAs (3.0 kb) that lack some of the internal exons of the gene including some of the sequences coding for the protein kinase domain. The gene is composed of at least five short exons and a long 3' exon. The 3' exon is processed in several alternative ways. It contains an intronic sequence which is spliced out in approximately 50% of the transcripts. S1 mapping shows the existence of five different 3' ends, presumed polyadenylation sites, differing by up to 1 kb. Three of these are maternal-specific while the other two are utilised during development. Dash RNA is most abundant in eggs and early embryos, becomes very rare during larval development and returns in a burst of activity in early pupae.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop J. M. Cellular oncogenes and retroviruses. Annu Rev Biochem. 1983;52:301–354. doi: 10.1146/annurev.bi.52.070183.001505. [DOI] [PubMed] [Google Scholar]
- Cheng H. L., Blattner F. R., Fitzmaurice L., Mushinski J. F., Tucker P. W. Structure of genes for membrane and secreted murine IgD heavy chains. Nature. 1982 Apr 1;296(5856):410–415. doi: 10.1038/296410a0. [DOI] [PubMed] [Google Scholar]
- Garcia-Bellido A., Merriam J. R. Clonal parameters of tergite development in Drosophila. Dev Biol. 1971 Oct;26(2):264–276. doi: 10.1016/0012-1606(71)90126-6. [DOI] [PubMed] [Google Scholar]
- Guerra M., Postlethwait J. H., Schneiderman H. A. The development of the imaginal abdomen of Drosophila melanogaster. Dev Biol. 1973 Jun;32(2):361–372. doi: 10.1016/0012-1606(73)90247-9. [DOI] [PubMed] [Google Scholar]
- Heisterkamp N., Groffen J., Stephenson J. R. The human v-abl cellular homologue. J Mol Appl Genet. 1983;2(1):57–68. [PubMed] [Google Scholar]
- Heldin C. H., Westermark B. Growth factors: mechanism of action and relation to oncogenes. Cell. 1984 May;37(1):9–20. doi: 10.1016/0092-8674(84)90296-4. [DOI] [PubMed] [Google Scholar]
- Hoffman-Falk H., Einat P., Shilo B. Z., Hoffmann F. M. Drosophila melanogaster DNA clones homologous to vertebrate oncogenes: evidence for a common ancestor to the src and abl cellular genes. Cell. 1983 Feb;32(2):589–598. doi: 10.1016/0092-8674(83)90478-6. [DOI] [PubMed] [Google Scholar]
- Hoffmann F. M., Fresco L. D., Hoffman-Falk H., Shilo B. Z. Nucleotide sequences of the Drosophila src and abl homologs: conservation and variability in the src family oncogenes. Cell. 1983 Dec;35(2 Pt 1):393–401. doi: 10.1016/0092-8674(83)90172-1. [DOI] [PubMed] [Google Scholar]
- Lev Z., Leibovitz N., Segev O., Shilo B. Z. Expression of the src and abl cellular oncogenes during development of Drosophila melanogaster. Mol Cell Biol. 1984 May;4(5):982–984. doi: 10.1128/mcb.4.5.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. D., Curran T., Verma I. M. c-fos protein can induce cellular transformation: a novel mechanism of activation of a cellular oncogene. Cell. 1984 Jan;36(1):51–60. doi: 10.1016/0092-8674(84)90073-4. [DOI] [PubMed] [Google Scholar]
- Müller R., Slamon D. J., Tremblay J. M., Cline M. J., Verma I. M. Differential expression of cellular oncogenes during pre- and postnatal development of the mouse. Nature. 1982 Oct 14;299(5884):640–644. doi: 10.1038/299640a0. [DOI] [PubMed] [Google Scholar]
- Müller R., Verma I. M. Expression of cellular oncogenes. Curr Top Microbiol Immunol. 1984;112:73–115. doi: 10.1007/978-3-642-69677-0_4. [DOI] [PubMed] [Google Scholar]
- Pirrotta V., Bröckl C. Transcription of the Drosophila white locus and some of its mutants. EMBO J. 1984 Mar;3(3):563–568. doi: 10.1002/j.1460-2075.1984.tb01847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirrotta V., Hadfield C., Pretorius G. H. Microdissection and cloning of the white locus and the 3B1-3C2 region of the Drosophila X chromosome. EMBO J. 1983;2(6):927–934. doi: 10.1002/j.1460-2075.1983.tb01523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy E. P., Smith M. J., Srinivasan A. Nucleotide sequence of Abelson murine leukemia virus genome: structural similarity of its transforming gene product to other onc gene products with tyrosine-specific kinase activity. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3623–3627. doi: 10.1073/pnas.80.12.3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J., Early P., Carter C., Calame K., Bond M., Hood L., Wall R. Two mRNAs with different 3' ends encode membrane-bound and secreted forms of immunoglobulin mu chain. Cell. 1980 Jun;20(2):303–312. doi: 10.1016/0092-8674(80)90616-9. [DOI] [PubMed] [Google Scholar]
- Rosenfeld M. G., Amara S. G., Evans R. M. Alternative RNA processing: determining neuronal phenotype. Science. 1984 Sep 21;225(4668):1315–1320. doi: 10.1126/science.6089345. [DOI] [PubMed] [Google Scholar]
- Setzer D. R., McGrogan M., Nunberg J. H., Schimke R. T. Size heterogeneity in the 3' end of dihydrofolate reductase messenger RNAs in mouse cells. Cell. 1980 Nov;22(2 Pt 2):361–370. doi: 10.1016/0092-8674(80)90346-3. [DOI] [PubMed] [Google Scholar]
- Shilo B. Z., Weinberg R. A. DNA sequences homologous to vertebrate oncogenes are conserved in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6789–6792. doi: 10.1073/pnas.78.11.6789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon M. A., Kornberg T. B., Bishop J. M. Three loci related to the src oncogene and tyrosine-specific protein kinase activity in Drosophila. Nature. 1983 Apr 28;302(5911):837–839. doi: 10.1038/302837a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Wang J. Y., Baltimore D. Cellular RNA homologous to the Abelson murine leukemia virus transforming gene: expression and relationship to the viral sequence. Mol Cell Biol. 1983 May;3(5):773–779. doi: 10.1128/mcb.3.5.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. Y., Ledley F., Goff S., Lee R., Groner Y., Baltimore D. The mouse c-abl locus: molecular cloning and characterization. Cell. 1984 Feb;36(2):349–356. doi: 10.1016/0092-8674(84)90228-9. [DOI] [PubMed] [Google Scholar]
- Zulauf E., Sánchez F., Tobin S. L., Rdest U., McCarthy B. J. Developmental expression of a Drosophila actin gene encoding actin I. Nature. 1981 Aug 6;292(5823):556–558. doi: 10.1038/292556a0. [DOI] [PubMed] [Google Scholar]