Abstract
The genus Fritillaria (Liliaceae) comprises of ~140 species of bulbous perennials, which are distributed in the temperate zone of the Northern Hemisphere. Fritillaria species have attracted much attention because of their commercial value, partly as ornamental plants but principally as a source of material for use in traditional medicine. The use of Fritillaria extracts is well established in some countries in Eastern Europe (e.g., Turkey), and Asia (e.g., China, Japan). In traditional Chinese medicine, the medicinal Fritillaria species is called Bulbus Fritillariae Cirrhosae (BFC), which has been used as a traditional medicine for thousands of years. However, to the best of our knowledge, there are no reports on resource investigation of plants of BFC in the last ten years. In this study, we chose 32 traditional producing regions in Qinghai-Tibet Plateau to perform an investigation on resource availability of BFC. In five sites we did not find any plants of BFC. Results show that the average number of the plants of BFC per quadrat in 26 sites was less than 7, and the average resource density was <22 mg/m2. Habitat types and plant morphology of BFC plants were recorded. Our investigation shows that the area for artificial cultivation of BFC is larger than 400 hm2 and productivity was higher than 180 t. In addition, the total alkaloid contents of samples from cultivated bases and plantations are higher than that from wild fields. This study suggests that the wild populations of BFC are still at the risk of depletion. Artificial cultivation of BFC might be an important way to resolve the current contradiction between resource protection and resource utilization. In addition, identifying the closest European relatives of the Fritillaria species used in traditional medicine may resolve this contradiction.
Keywords: Bulbus Fritillariae Cirrhosae, plant resource, total alkaloids, traditional medicine, plant morphology, artificial cultivation, Qinghai-Tibet Plateau
Introduction
The genus Fritillaria (Liliaceae) comprises of ~140 species of bulbous perennials (Day et al., 2014), which are distributed in the temperate zone of the Northern Hemisphere and currently divided into eight subgenera (Rix et al., 2001). Fritillaria species have attracted much attention because of their commercial value, partly as ornamental plants but principally as a source of material for use in traditional medicine (Day et al., 2014). The use of Fritillaria extracts is well established in some countries in Eastern Europe (e.g., Turkey; Atta ur et al., 1994; Day et al., 2014).
Locals of Çatak in Turkey use some Fritillaria species in treatment of several diseases, for example wound healing (Mukemre et al., 2015). The medicinal use of Fritillaria species is also well established in China, the Himalayas (India, Nepal, and Pakistan), Japan, Korea, and Southeast Asia (Day et al., 2014). In traditional Chinese medicine, the medicinal Fritillaria species is called Bulbus Fritillariae Cirrhosae (BFC), also known by the Chinese name “Chuan Bei Mu,” which has been used as a traditional medicine for thousands of years.
BFC was recorded in the traditional Chinese medicine book Shen Nong Ben Cao Jing (The Divine Farmer's Materia Medica Classic), which is one of the earliest classic medical book written in the Eastern Han Dynasty (25–220; Wang, 2012). In the Chinese Pharmacopoeia (2010 Edition), the botanical origins of BFC include six species of the Fritillaria genus: Fritillaria cirrhosa D. Don, F. unibracteata Hsiao et K.C. Hsia, F. przewalskii Maxim, F. delavayi Franch, F. taipaiensis P. Y. Li and F. unibracteata Hsiao et K. C. Hsia var. wabuensis (S. Y. Tang et S. C. Yue) Z. D. Liu, S. Wang et S. C. Chen (Pharmacopoeia, 2010).
BFC is used as a folk medicine because of its remarkable antitussive, expectorant and antiasthmatic activities (Pharmacopoeia, 2010). Previous studies indicated that chemical constituents of BFC mainly include steroidal alkaloids, saponins, terpenoids, and glycosides (Hao et al., 2013). It is believed that alkaloids are the major biological active constituents (Li et al., 2013; Wang et al., 2014a). Recent pharmacological studies found that alkaloids from BFC exhibit remarkable antitussive, expectorant, anti-inflammatory effects (Wang et al., 2011, 2012), antiasthmatic effect (Yan, 2005), hypotensive property (Kang et al., 2004), anti-bacterial activity (Li et al., 2005), and antitumor effect (Wang D. D. et al., 2014; Wang et al., 2014b, 2015).
Currently, there are 400 manufacturers producing over 200 kinds of preparations containing BFC, because of its obvious effects (Li et al., 2012). The production of medicinal preparations containing F. cirrhosa is an industry with an estimated value of US $400 million per year (Day et al., 2014). At present, BFC is mainly harvested from the wild fields. Production of BFC has fallen short of consumption (Wang et al., 2014a). The price of BFC was beyond $260 per kilogram in 2014, almost nine-folds of that in 2004. The growing price of BFC resulted in over-exploitation. Nowadays, these used species in Fritillaria genus have been classified as precious, rare and threaten species by Endangered Species Scientific Commission of China in 2012. Some other countries (e.g., Burma, Turkey) are involved in supplying the increasing demand (Day et al., 2014).
However, to the best of our knowledge, there are still no studies on wild resource of plants of the traditional medicine BFC. The present study focused on investigation of the current resource status of BFC in traditional BFC-producing regions in Sichuan, Chongqing, and Yunnan provinces in Qinghai-Tibet Plateau.
Materials and methods
Sampling sites
The literature survey was performed to obtain information about distribution of the plants of BFC, which would be useful for choosing the sampling sites. According to Flora of China, Flora of Sichuan Province, and Flora of Yunnan Province and previous studies (Luo and Chen, 1996; Liu et al., 2008), 32 sites in Sichuan, Chongqing, and Yunnan provinces, which are the traditional producing regions of BFC, were chosen to perform a survey on plant resource of BFC during 2013–2014 (Table 1). In every site, there was one experienced and local herbalist who helped us to carry out resource investigation.
Table 1.
GPS data and species in different sites, and the content of total alkaloids of the samples from different sites.
| County | The field site | Latitude | Longitude | Altitude (m) | Species | Content of total alkaloids (%) | |
|---|---|---|---|---|---|---|---|
| Sichuan | Kangding | Xingduqiao Town, Waze Village, Enwei Plateau Medicinal Plant Fostering Base | N30.0637 | E101.5827 | 3530 | F. cirrhosa | 0.300 ± 0.026 |
| Daofu | Weita Village | N31.3417 | E101.1594 | 3599 | F. cirrhosa | 0.043 ± 0.002 | |
| Yinen Village | N31.4261 | E101.1043 | 3781 | F. cirrhosa | 0.023 ± 0.002 | ||
| Jiazong Village | N31.0910 | E101.2119 | 4243 | F. cirrhosa | 0.023 ± 0.002 | ||
| Litang | Qudeng Village | N30.1313 | E100.0629 | 4134 | F. cirrhosa | 0.025 ± 0.004 | |
| Yajiang | Milong Village, Beihou Cun | N29.9444 | E101.0323 | 2569 | F. cirrhosa | 0.124 ± 0.010 | |
| Milong Village, Mashizi Cun | N29.9752 | E101.1848 | 3909 | F. unibracteata | 0.175 ± 0.017 | ||
| Milong Village, Lasang Mountain | N29.9515 | E101.2083 | 4503 | F. delavayi | 0.006 ± 0.001 | ||
| Songpan | Shanba Village, Duoji mountain | N33.0342 | E103.7133 | 3502 | F. unibracteata | 0.054 ± 0.014 | |
| Sedi Village, Geliping | N32.9613 | E103.3471 | 3841 | F. unibracteata | 0.072 ± 0.008 | ||
| Shuijing Village, New Lotus Chuanbeimu Cultivation Base | N32.9574 | E103.7113 | 3321 | F. wabuensis | 0.209 ± 0.006 | ||
| Hongyuan | Hongxi Village, Fumin Chuanbeimu Cultivation Base | N32.8806 | E102.6102 | 3510 | F. unibracteata | 0.072 ± 0.008 | |
| Hongxi Village, Luosang Chuanbeimu Cultivation Base | N32.7782 | E102.5625 | 3530 | F. unibracteata | 0.034 ± 0.003 | ||
| Hongxi Village, Caimugou | N32.7420 | E102.5745 | 3578 | F. unibracteata | 0.034 ± 0.002 | ||
| Aba | Jiaerduo Village, Nuoerdang Cun | N33.2103 | E101.4662 | 3723 | F. unibracteata | 0.036 ± 0.005 | |
| Jiaerduo Village, Fenshui Ling | N33.4153 | E101.4985 | 3693 | F. unibracteata | 0.030 ± 0.006 | ||
| Rangtang | Gangmuda Village, Jiudaoguai | N32.3070 | E101.0647 | 3898 | F. unibracteata | 0.027 ± 0.002 | |
| Gangmuda Village, Gage Mountain | N32.3067 | E101.0649 | 3915 | F. unibracteata | 0.025 ± 0.004 | ||
| Luhuo | Renda Village, Guobalong | N31.2178 | E100.8387 | 3119 | - | - | |
| Renda Village, Ribudike | N31.2161 | E100.7911 | 3826 | - | - | ||
| Mao | Songpinggou Village, New Lotus Chuanbeimu Cultivation Base | N32.1988 | E103.4953 | 3729 | F. unibracteata; F. wabuensis | 0.249 ± 0.003 | |
| Xiangcheng | Shuiwa Village | N29.1451 | E100.0675 | 4644 | - | - | |
| Ranwu Village | N28.3141 | E99.7467 | 3887 | - | - | ||
| Yunnan | Shangri-la | Kari Mountain | N27.8149 | E99.7053 | 3352 | - | - |
| Lijiang | Hutiaoxia Village | N26.9904 | E100.0665 | 3857 | F. cirrhosa | 0.140 ± 0.012 | |
| Dadong Village, Haizi Cun | N27.0595 | E100.2768 | 3088 | F. cirrhosa | 0.058 ± 0.017 | ||
| Dadong Village, Lianggushui Mountain | N27.0260 | E100.2930 | 3131 | F. cirrhosa | 0.068 ± 0.014 | ||
| Chongqing | Wuxi | Lanying Village, Xian Cun, Zhonghoujiao | N31.4043 | E109.8737 | 1658 | F. taipaiensis | 0.148 ± 0.015 |
| Lanying Village, Xian Cun, Balicao | N31.4066 | E109.8831 | 1704 | F. taipaiensis | 0.101 ± 0.013 | ||
| Lanying Village, Xian Cun, Huangcaoping Chuanbeimu Cultivation Base | N31.4165 | E109.9238 | 2074 | F. taipaiensis | 0.303 ± 0.005 | ||
| Chengkou | Mingzhong Village | N31.7381 | E108.8206 | 2786 | F. taipaiensis | 0.179 ± 0.016 | |
| Qinghai | Jiuzhi | Ningyou Village | N33.4154 | E101.4990 | 3696 | F. unibracteata | 0.049 ± 0.017 |
The measurement of content of total alkaloids was conducted in triplicate. Data were reported as mean ± standard error of mean (SEM) (n = 3).
Field investigation
The field investigation was carried out in May, June, or July in 2013 and 2014. Field investigation was designed to assess the distribution, density and abundance of the plants of BFC (Delgado-Lemus et al., 2014). In order to estimate their distribution, the coordinate positions were firstly recorded using a Magellan Triton GPS400 in each site (Delgado-Lemus et al., 2014). The quadrat investigation was performed to assess the density and abundance of the plants of BFC as described previously (Zhang et al., 2015). Series of squares (quadrats) with a set size (4 × 4 m) were placed in each site. The mean density of the plants of BFC per quadrat was the number of the plants of BFC per quadrat. The resource density of the quadrat was calculated as: Resource density = total weight of bulbs/quadrat area (g/m2).
Collection of plant specimens and bulbs of BFC
At each site, 5–15 plants were collected as specimens during field investigation. The specimens were prepared in the medicinal plants Herbarium of West China College of Pharmacy. The specimens were examined and identified by experts, and the voucher specimens with labels were deposited at the medicinal plants Herbarium. At each site, photographic records were taken to capture the field sites, growth habitat, and morphology of plants (Chekole et al., 2015). We also collected the bulbs from these sites. All samples were subjected to the described determination of content of the total alkaloid.
Artificial cultivation survey
In this study, we visited six artificial cultivated bases and two individual cultivated plantations. The specific information about area, production, and market value were gathered by inquiring, personal interviewing, or direct surveying (Zhang et al., 2015).
Measurement of content of total alkaloid
The total alkaloid content was determined according to the previously described acid dye colorimetric method (Wang D. D. et al., 2014; Li et al., 2015). For alkaloid standard, a stock standard solution (0.1 mg/ml) of imperialine (in chloroform) was prepared and the stock solution was diluted to different working concentrations (0.0, 1.0, 2.0, 4.0, 8.0, 10.0 μg/ml).
A certain amount (about 2.0 g) of powdered sample was weighed, immersed in 3 ml of ammonia for 1 h, and then extracted under reflux with 40 ml of chloroform-methanol (3:1) solution for 2 h. The solution was filtered, and diluted to a final volume of 50 ml with chloroform-methanol solution. A certain volume (3–5 ml) of the solution was pipetted and evaporated, and the residue was dissolved in chloroform (10 ml) to get the sample solution. According to the absorption spectra of the standard solutions, 412 nm was chosen as the detection wavelength.
To measure total alkaloid content, 10 ml of each sample or working concentrations of standard solution was transferred into a separate funnel. Five milliliters of distilled water and 2 ml of bromocresol green buffer solution were added into it and shaken for 2 min. After 30 min, the chloroform layer was separated and collected. The absorbance of chloroform fraction was measured at 412 nm using Alpha-1900PC UV–Vis spectrophotometer (Shanghai Lab-Spectrum Instruments Co., Ltd., China). The experimental results were expressed as mean ± standard error of mean (SEM).
Results
Resource distribution and density
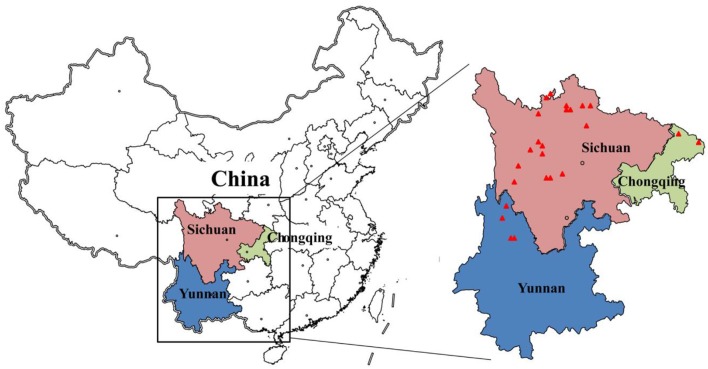
The distribution of wild plants of BFC is shown as Figure 1 and Table 1. To investigate the resource of BFC, we chose 32 traditional producing regions of BFC. In five sites investigated no BFC plants could be found. These sites are Guobalong and Gage Mountain in Renda Village in Luhuo county, Shuiwa Village and Ranwu Village in Xiangcheng county in Sichuan Province, Kari Mountain in Shangri-la in Yunnan province. The sites where plants of F. cirrhosa were found are Kangding, Daofu, Litang, Yajiang counties in Sichuan province and Lijiang county in Yunnan province (Table 1). The sites where plants of F. unibracteata were found are Yajiang, Songpan, Hongyuan, Aba, Rangtang, Litang, Mao counties in Sichuan province and Jiuzhi county in Qinghai province (Table 1). The sites where plants of F. wabuensis were found are Songpan and Mao counties in Sichuan province. The sites where plants of F. taipaiensis were found are Wuxi and Chengkou counties in Chongqing province (Table 1). We only found plants of F. delavayi in Yajiang county in Sichuan province. From the Table 1, the main species of BFC occurring in Sichuan province are F. cirrhosa, F. unibracteata, and F. wabuensis. The main species in Yunnan province is F. cirrhosa. The main species in Chongqing province is F. taipaiensis.
Figure 1.
Location of study areas. The red triangle shows the investigation sites. The traditional producing-regions of Bulbus Fritillariae Cirrhosae (BFC) are mainly distributed in Sichuan, Chongqing, and Yunnan Provinces in Southwest China.
Table 2 shows the mean density of the plants of BFC per quadrat and resource density. The average number of the plants of BFC per quadrat is <7 except for the site in Ningyou Village in Jiuzhi county, Qinghai province. In addition, the average resource density is <22 mg/m2 except for the sites in Lasang Mountain in Milong Village in Yajiang county, Sichuan province and Ningyou Village in Jiuzhi county, Qinghai province. The data reconfirm that the species in the Fritillaria genus are still at risk of depletion. The reason why the average resource density in Lasang Mountain in Yajiang county is higher than other sites may be that the species in this site is F. delavayi which price is much less than the other species. The reason for that in Ningyou Village in Jiuzhi county may be that this site is one of the main producing regions of Chinese Caterpillar Fungus, with a much higher price than FBC.
Table 2.
Density of original plants of Bulbus Fritillariae Cirrhosae and resource density of Bulbus Fritillariae Cirrhosae in the quadrats.
| Province | County | The field site | Density of plants (the number/quadrat) | Resource density (mg/m2) |
|---|---|---|---|---|
| Sichuan | Daofu | Weita Village | 2.23 ± 0.76 | 8.44 ± 0.76 |
| Yinen Village | 1.06 ± 0.82 | 4.13 ± 0.82 | ||
| Jiazong Village | 1.77 ± 0.98 | 7.11 ± 0.98 | ||
| Litang | Qudeng Village | 4.73 ± 0.58 | 17.22 ± 0.58 | |
| Yajiang | Milong Village, Beihou Cun | 0.75 ± 0.67 | 2.95 ± 0.67 | |
| Milong Village, Mashizi Cun | 1.25 ± 0.40 | 5.39 ± 0.40 | ||
| Milong Village, Lasang Mountain | 4.76 ± 0.76 | 38.30 ± 0.76 | ||
| Songpan | Shanba Village, Duoji mountain | 3.23 ± 0.29 | 9.95 ± 0.29 | |
| Sedi Village, Geliping | 3.33 ± 0.77 | 10.56 ± 0.77 | ||
| Hongyuan | Hongxi Village, Caimugou | 4.66 ± 0.32 | 16.48 ± 0.32 | |
| Aba | Jiaerduo Village, Nuoerdang Cun | 5.75 ± 0.52 | 17.43 ± 0.52 | |
| Jiaerduo Village, Fenshui Ling | 6.56 ± 0.53 | 21.73 ± 0.53 | ||
| Rangtang | Gangmuda Village, Jiudaoguai | 2.67 ± 0.82 | 5.69 ± 0.82 | |
| Gangmuda Village, Gage Mountain | 1.75 ± 0.86 | 5.52 ± 0.86 | ||
| Yunnan | Lijiang | Hutiaoxia Village | 3.67 ± 0.46 | 16.52 ± 0.46 |
| Dadong Village, Haizi County | 2.34 ± 0.91 | 8.69 ± 0.91 | ||
| Dadong Village, Lianggushui Mountain | 2.55 ± 0.95 | 11.17 ± 0.95 | ||
| Qinghai | Jiuzhi | Ningyou Village | 21.88 ± 0.49 | 64.40 ± 0.49 |
Data of density of original plants and resource density were reported as mean ± standard error of mean (SEM).
Habitat types
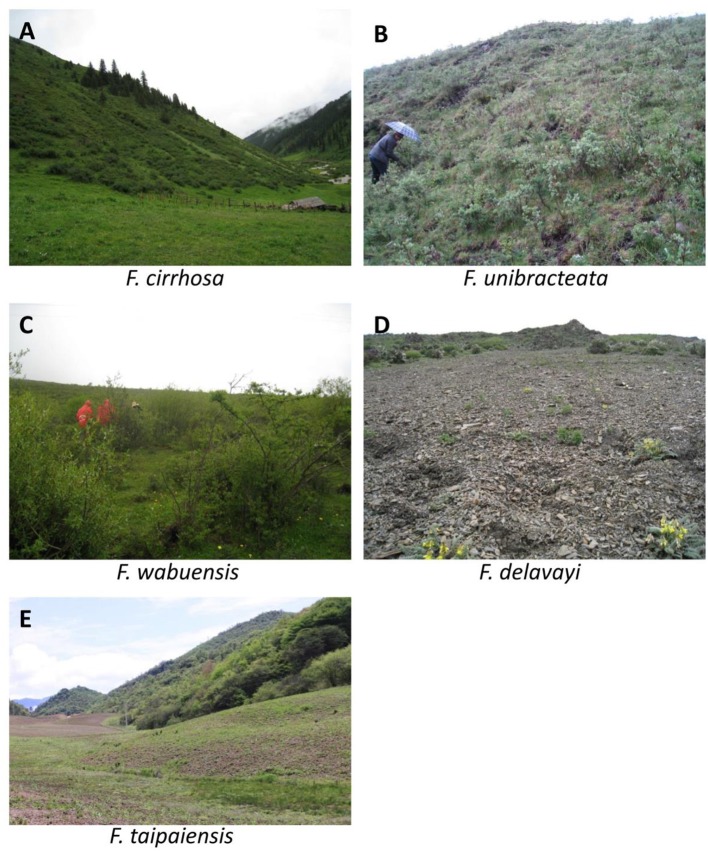
Plants of F. cirrhosa grow under forests, in alpine thickets, or on meadows, and flood lands. They are usually found on shady and moist slopes of valley (Figure 2A). The altitude range of occurrence is from 2,500 to 4,600 m. All altitude data were collected by using Magellan Triton GPS400. The plants of F. unibracteata are usually discovered in moist places of thickets or meadows (Figure 2B). Their altitude range is from 3,200 to 4,700 m. Plants of F. wabuensis grow under forests, or in alpine thickets (Figure 2C) and the altitude range of their occurrence is from 2,500 to 3,500 m. The plants of F. delavayi are usually discovered in sandy and gravelly places or on flood lands (Figure 2D). Their altitude range is often above 4,000 m. Plants of F. taipaiensis grow under forests, in hill thickets, or on grassy slopes (Figure 2E). Their altitude range is from 1,500 to 3,200 m. The habitat types of plants of F. cirrhosa and F. unibracteata are almost the same. The altitudes of plants of F. taipaiensis and F. wabuensis are lower than that of F. cirrhosa and F. unibracteata, and altitude of plants of F. taipaiensis is the lowest. F. taipaiensis and F. wabuensis are thus most suitable for artificial cultivation. The plants of F. delavayi grow in relatively harsh conditions with higher altitude, lower temperature and lower humidity.
Figure 2.
Habitat types of plants of Bulbus Fritillariae Cirrhosae (BFC). (A) Habitat type of plants of F. cirrhosa; (B) Habitat type of plants of F. unibracteata; (C) Habitat type of plants of F. wabuensis; (D) Habitat type of plants of F. delavayi; (E) Habitat type of plants of F. taipaiensis.
Plant morphology
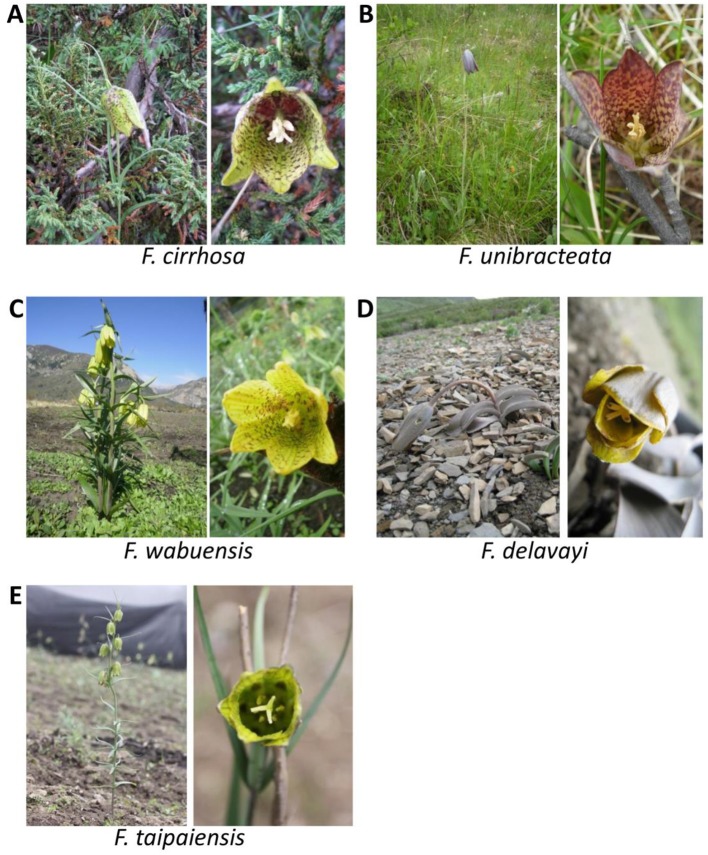
F. cirrhosa (Cheng and Helen, 2000): As shown in Figure 3A, height range of stem is 15–65 cm. Seven to eleven leaves are opposite or sometimes 3- or 4-whorled and alternate. Shape of leaf blade is linear to linear-lanceolate (4–12 cm × 3–15 mm), which apex is often curved or cirrose. The inflorescence is (1-3)-flowered with 3 bracts, whose apex is curved or cirrose. Flowers are bell-shaped, usually nodding. The pedicel is much shorter than tepals. Tepals are yellow or yellowish green, slightly or heavily spotted or tessellated with purple, usually oblong-elliptic (3–5 × 1.2–1.8 cm). Nectaries are elliptic to ovate (3–5 × 2–3 mm), projecting abaxially. Length of stamens is 2–3 cm, filaments sometimes are slightly papillose. The style is 3-lobed with 3–5 mm lobes. Capsule is narrowly winged, and wings are 1–1.5 mm wide.
Figure 3.
Plants of Bulbus Fritillariae Cirrhosae (BFC). (A) Plants of F. cirrhosa; (B) Plants of F. unibracteata; (C) Plants of F. wabuensis; (D) Plants of F. delavayi; (E) Plants of F. taipaiensis.
F. unibracteata (Cheng and Helen, 2000): As shown in Figure 3B, height range of stem is 15–50 cm. There are five to seven leaves, and the basal 2 leaves are usually opposite, others are opposite or alternate. The shape of leaf blade is linear to linear-lanceolate (3.6–5.5 cm × 3–5 mm), apex is not curved or cirrose. Inflorescence is (1-3)-flowered with 1 bract whose apex is acuminate. Flowers are bell-shaped, usually nodding. Pedicel is rather long. Tepals are blackish purple, tessellated with yellowish brown or sometimes with a colored, V-shaped stripe near apex. Nectaries are inconspicuous or strongly projecting abaxially. Length of stamens is 1.2–1.5 cm, filaments sometimes are papillose. The style is scarcely or shortly lobed with 0.5–2 mm lobes. The capsule is narrowly winged, and wings are about 1.1 mm wide.
F. wabuensis (Cheng and Helen, 2000): As shown in Figure 3C, height range of stem is 50–115 cm. There are 12–28 leaves, and the basal 2 leaves are usually opposite, others are whorled or alternate. Shape of leaf blade is linear-lanceolate (6.5–16.5 cm × 6–25 mm), the apex is not curved or cirrose. The inflorescence is (1-7)-flowered with 1-4 bracts whose apex is acuminate. Flowers are bell-shaped, usually nodding. Tepals are yellowish green, spotted with or without blackish purple. Nectaries are elliptic to ovate (5–8 × 2–3 mm), projecting abaxially. The style is 3-lobed with 3–5 mm lobes. The capsule is narrowly winged, and wings are 1–2 mm wide.
F. delavayi (Cheng and Helen, 2000): As shown in Figure 3D, height range of stem is 10–35 cm. Five to seven leaves are closely arranged in middle or distal part of the stem, alternate or subopposite. The shape of the leaf blade is ovate or ovate-elliptic (2–7 × 1–3 cm), apex often is obtuse or rounded. The inflorescence is 1-flowered without bract. Flowers are bell-shaped, usually nodding. The pedicel is long. Tepals are yellowish, spotted or tessellated with reddish brown, narrowly elliptic or oblong-elliptic (3.2–4.5 × 1.2–1.8 cm). Nectaries are inconspicuous. Length of stamens is 1.6–2.2 cm, filaments are glabrous. Anthers are basifixed. The style is 3-lobed with 0.5–5 mm lobes. The capsule is narrowly winged.
F. taipaiensis (Cheng and Helen, 2000): As shown in Figure 3E, height range of stem is 20–100 cm. Five to twenty leaves are usually opposite or sometimes middle and distal ones also whorled and alternate. The shape of the leaf blade is linear to linear-lanceolate (5–13 cm × 3–12 mm), apex often is not curved. The inflorescence is (1-3)-flowered with 3 bracts whose apex is often curved. Flowers are bell-shaped, usually nodding. The pedicel is 2–4 cm. Tepals are yellowish green, densely spotted purple, narrowly oblong or obovate-oblong (2.5–5 × 0.6–1.8 cm). Nectaries are slightly projecting abaxially. Length of stamens is about 3/5 as long as repals, filaments are slightly papillose distally. The style is 3-lobed with 2–4 mm lobes. The capsule is winged, and wings are 0.5–2.0 mm wide.
Artificial cultivation
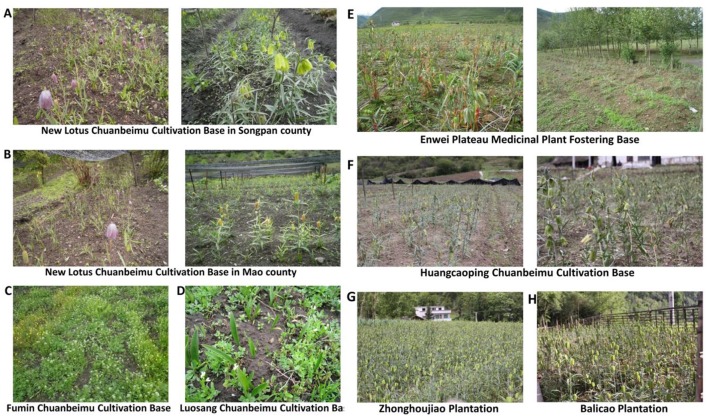
Because production of BFC obtained from the wild field has fallen short of consumption and the price is growing higher in recent decades, the artificial cultivation of BFC has been gradually developed by the traditional Chinese medicinal cultivation companies (Wang et al., 2014a). In this work, we investigated six artificial cultivated bases and two individual cultivated plantations (Figure 4). As shown in Table 3, the cultivated bases or plantations are located in Kangding, Mao, Songpan, and Hongyuan counties in Sichuan province, and Wuxi county in Chongqing province. The species in cultivation include F. cirrhosa, F. unibracteata, F. wabuensis, and F. taipaiensis, which grow in relatively easier habitats compared to other species. We also found that lots of farmers in Wuxi county cultivate F. taipaiensis in gardens or surrounding the home yard. The income of selling bulbs of F. taipaiensis is one of the important economic sources for the region. Enwei Plateau Medicinal Plant Fostering Base and New Lotus Chuanbeimu Cultivation Base are the two largest bases among the investigated bases covering an area of about 400 hm2 and their productivity is up to 180 t. However, the production of BFC from artificial cultivation is far short of what is needed (Liu et al., 2008). So, at present wild populations is still the main source of BFC in the market, which may further aggravate BFC wild resource depletion.
Figure 4.
The artificial cultivated bases and individual cultivated plantations. (A) New Lotus Chuanbeimu Cultivation Base in Songpan county; (B) New Lotus Chuanbeimu Cultivation Base in Mao county; (C) Fumin Chuanbeimu Cultivation Base; (D) Luosang Chuanbeimu Cultivation Base; (E) Enwei Plateau Medicinal Plant Fostering Base; (F) Huangcaoping Chuanbeimu Cultivation Base; (G) Zhonghoujiao Plantation; (H) Balicao Plantation.
Table 3.
The details of the artificial cultivated bases and home cultivated plantations.
| Name of bases or plantation | Species | Area (m2) | Production (t) | Income (dollar) |
|---|---|---|---|---|
| Enwei Plateau Medicinal Plant Fostering Base | F. cirrhosa | 180 × 104 | 20 | 12 × 106 |
| New Lotus Chuanbeimu Cultivation Base in Shuijing Village | F. wabuensis and F. unibracteata | 10 × 104 | 8 | 4.0 × 106 |
| Fumin Chuanbeimu Cultivation Base | F. unibracteata | 20 × 104 | 14 | 8.1 × 106 |
| Luosang Chuanbeimu Cultivation Base | F. unibracteata | 0.12 × 104 | 0.09 | 0.04 × 106 |
| New Lotus Chuanbeimu Cultivation Base in Songpinggou Village | F. wabuensis and F. unibracteata | 200 × 104 | 160 | 80.6 × 106 |
| Huangcaoping Chuanbeimu Cultivation Base | F. taipaiensis | 1.8 × 104 | 1.64 | 0.72 × 106 |
| Xian Cun, Zhonghoujiao plantation in Lanying Village | F. taipaiensis | 0.06 × 104 | 0.07 | 0.024 × 106 |
| Xian Cun, Balicao plantation in Lanying Village | F. taipaiensis | 0.08 × 104 | 0.075 | 0.032 × 106 |
Total alkaloid content determination
The calibration curve was drawn with six standard solutions at concentrations ranging from 0 to 10.43 μg/ml. The calibration curve showed a good linearity with the correlation coefficient being r = 0.9984. The regression equation was Y = 17.5668X + 0.1477, where Y and X correspond to the concentration and absorbance, respectively. The data about the content of total alkaloid in different samples are listed in Figure 5 and Table 1. The results show that samples from Mingzhong Village in Chengkou county, Huangcaoping Chuanbeimu Cultivation Base in Wuxi county, Chongqing province and from Enwei Plateau Medicinal Plant Fostering Base in Kangding county, New Lotus Chuanbeimu Cultivation Base in Songpan and Mao counties, Sichuan province have the higher content of total alkaloids. These samples are from artificial cultivation bases or individual plantation, which suggests that the content of total alkaloid of bulbs from artificial cultivation is higher than that from wild fields. The reason is unknown so far.
Figure 5.
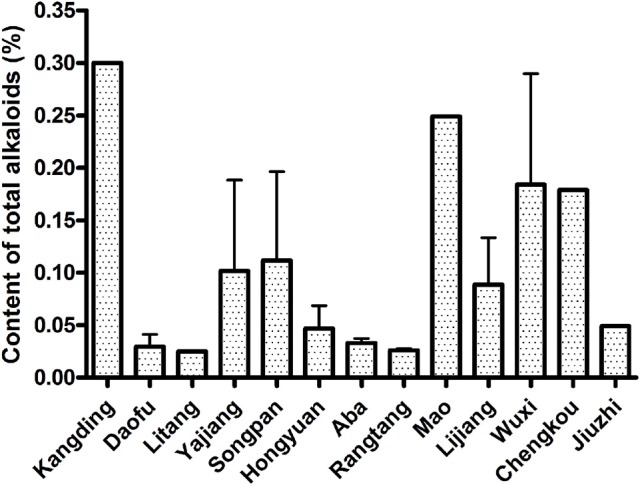
The content of total alkaloids in different traditional producing regions in Qinghai-Tibet Plateau.
Discussion and conclusion
In this investigation, we chose 32 traditional producing regions in Qinghai-Tibet Plateau. There were five traditional producing regions where we did not find the original plants of BFC. In the other investigation sites, the average density of plants is lower than 7, except the site in Ningyou Village in Jiuzhi county, Qinghai province. At the same time, the average resource density is less than 22 mg/m2, except for sites in Lasang Mountain in Milong Village in Yajiang county and Ningyou Village in Jiuzhi county. These results indicate that the wild resource of BFC is still at the risk of depletion. The total alkaloid contents in the BFC from Kangding, Mao, Wuxi, and Chengkou are higher than that from other traditional producing regions (Figure 5). In these regions, there are some artificial cultivation bases or individual plantations, where content of total alkaloids of bulbs is higher compared to wild fields. The total alkaloid contents in the BFC from Daofu, Litang, Aba, and Rangtang are much lower than that from other traditional producing regions (Figure 5). The reason is unknown so far.
In order to ensure sustainable utilization of BFC wild resource, the plants of BFC in the 1980s were classified as third level of protection medicinal plants in regulations on the protection of wild medicinal herb resources established by the Chinese government (Liu et al., 2008). Later in 2012, the plants of BFC were classified as precious, rare and threatened species by Endangered Species Scientific Commission of China. Strategies for resource conservation could include regulation, additional cultivation, harvesting after sexual maturity, investigation of micro-propagation, and natural fostering. For the government, establishment of protective regulation is actually one of the most effective strategies for the conservation of wild plants of BFC. At present, the local authorities are trying to perform programs for the propagation and recovering of populations in collaboration with some traditional Chinese medicinal companies and some research groups. Traditional medicinal plants cultivation companies could increase the area of cultivation of the plants of BFC. In addition, cultivation specialist of plant of BFC could conduct training amongst farmers to cultivate the plants of BFC. Medicinal farmers could harvest the BFC after sexual maturity of the plants, which can would help to sustain populations. Researchers could focus on micro-propagation methods like plant tissue and cell culture. Recently, plant tissue culture methods of F. cirrhosa (Li, 2012), F. unibracteata (Li and Wu, 2011), and F. przewalskii (Wang, 2008) was established. In recent years, some researchers proposed natural fostering as a cultivation type of traditional Chinese medicinal plants. They selected F. cirrhosa to carry out natural fostering and found natural fostering not only increased local BFC production but also conserved local biological diversity (Li et al., 2012). These approaches could have positive effects to resolve the current contradiction between resource protection and resource utilization. In Europe, there are lots of Fritillaria species. Identifying the European closest relatives of the Fritillaria species used in traditional medicine could point to additional species that might be analyzed for their potential medicinal value, which may in turn reduce pressure on those species that are currently being collected intensively (Day et al., 2014).
In conclusion, we performed resource investigation in 32 traditional producing regions and found that the wild resource of BFC is at the risk of depletion. In addition, habitat types, plant morphology, and artificial cultivation were investigated and discussed. Lastly, the results of determination of total alkaloid content of samples from different sites display that total alkaloid content of bulbs from cultivation is higher than that of bulbs form wild fields. Artificially cultivated plants of BFC could be an important way to resolve the current contradiction between resource protection and resource utilization. In addition, identifying the European closest relatives of the Fritillaria species used in traditional medicine may contribute to resolve this contradiction.
Author contributions
DW and XC were involved in the initial design of the study, carried out field investigation, and artificial cultivation survey, collected plant specimens and bulbs of BFC and measured the total alkaloid contents of samples. AA, XY, and DW performed data analysis and drafted the manuscript. SW participated in experiments' design and helped to draft the manuscript. All authors read and approved the final manuscript.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors acknowledge the support by the Polish KNOW (Leading National Research Centre) Scientific Consortium “Healthy Animal—Safe Food,” decision of Ministry of Science and Higher Education No. 05-1/KNOW2/2015, and by the Austrian Science Fund (FWF): P25971-B23.
Glossary
Abbreviations
- BFC
Bulbus Fritillariae Cirrhosae.
References
- Atta ur R., Farooq A., Choudhary M. I., Gilani A. H., Shaheen F., Ali R. A., et al. (1994). A new anticholinergic steroidal alkaloid from Fritillaria imperialis of Turkish origin. Planta Med. 60, 377–379. 10.1055/s-2006-959507 [DOI] [PubMed] [Google Scholar]
- Chekole G., Asfaw Z., Kelbessa E. (2015). Ethnobotanical study of medicinal plants in the environs of Tara-gedam and Amba remnant forests of Libo Kemkem District, northwest Ethiopia. J. Ethnobiol. Ethnomed. 11:4. 10.1186/1746-4269-11-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X., Helen V. M. (2000). Fritillaria linnaeus. Flora China 24, 127–133. Available online at: http://www.efloras.org/florataxon.aspx?flora_id=2&taxon_id=113029 [Google Scholar]
- Day P. D., Berger M., Hill L., Fay M. F., Leitch A. R., Leitch I. J., et al. (2014). Evolutionary relationships in the medicinally important genus Fritillaria, L. (Liliaceae). Mol. Phylogenet. Evol. 80, 11–19. 10.1016/j.ympev.2014.07.024 [DOI] [PubMed] [Google Scholar]
- Delgado-Lemus A., Casas A., Tellez O. (2014). Distribution, abundance and traditional management of Agave potatorum in the Tehuacan Valley, Mexico: bases for sustainable use of non-timber forest products. J. Ethnobiol. Ethnomed. 10:63. 10.1186/1746-4269-10-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao D. C., Gu X. J., Xiao P. G., Peng Y. (2013). Phytochemical and biological research of Fritillaria medicine resources. Chin. J. Nat. Med. 11, 330–344. 10.3724/SP.J.1009.2013.00330 [DOI] [PubMed] [Google Scholar]
- Kang D. G., Sohn E. J., Lee Y. M., Lee A. S., Han J. H., Kim T. Y., et al. (2004). Effects of bulbus Fritillaria water extract on blood pressure and renal functions in the L-NAME-induced hypertensive rats. J. Ethnopharmacol. 91, 51–56. 10.1016/j.jep.2003.11.015 [DOI] [PubMed] [Google Scholar]
- Li L., Long W., Wan X., Ding Q., Zhang F., Wan D. (2015). Studies on quantitative determination of total alkaloids and berberine in five origins of crude medicine “Sankezhen.” J. Chromatogr. Sci. 53, 307–311. 10.1093/chromsci/bmu060 [DOI] [PubMed] [Google Scholar]
- Li M., Wu N. (2011). Study on dynamic accumulation of verticine in plant tissue culture of F. unibracteata at different stages. Life Sci. Instrum. 9, 45–47. 10.3969/j.issn.1671-7929.2011.04.022 [DOI] [Google Scholar]
- Li Q. (2012). Biochemical and Physiological Research of Cultured Fritillaria Cirrhosa, D. Don during Bulb Organogenesis and Development. Sichuan University. [Google Scholar]
- Li S., Liu J., Gong X., Yang X., Zhu Y., Cheng Z. (2013). Characterizing the major morphological traits and chemical compositions in the bulbs of widely cultivated Fritillaria species in China. Biochem. Syst. Ecol. 46, 130–136. 10.1016/j.bse.2012.09.014 [DOI] [Google Scholar]
- Li X., Song J., Wei J., Hu Z., Xie C., Luo G. (2012). Natural fostering in Fritillaria cirrhosa: integrating herbal medicine production with biodiversity conservation. Acta Pharm. Sin. B 2, 77–82. 10.1016/j.apsb.2011.12.006 [DOI] [Google Scholar]
- Li Y., Xu C., Zhang Q., Liu J. Y., Tan R. X. (2005). In vitro anti-Helicobacter pylori action of 30 Chinese herbal medicines used to treat ulcer diseases. J. Ethnopharmacol. 98, 329–333. 10.1016/j.jep.2005.01.020 [DOI] [PubMed] [Google Scholar]
- Liu H., Chen S.-L., Yao H., Li X.-W., Zhang Y. (2008). [Research progress on resources in Bulbus Fritillariae Cirrhosae]. Zhongguo Zhong Yao Za Zhi 33, 1645–1648. [PubMed] [Google Scholar]
- Luo Y., Chen X. (1996). A revision of Fritillaria, L. (Liliaceae) in the Hengduan Moutains and adjacent regions, China (1)-A study of Fritillaria cirrhosa, D. Don and its related species. Acta Phytotax. Sin. 34, 304–312. [Google Scholar]
- Mukemre M., Behcet L., Cakilcioglu U. (2015). Ethnobotanical study on medicinal plants in villages of Catak (Van-Turkey). J. Ethnopharmacol. 166, 361–374. 10.1016/j.jep.2015.03.040 [DOI] [PubMed] [Google Scholar]
- Pharmacopoeia N. C. O. C. (2010). Pharmacopoeia of Peoples Republic of China. Beijing: China Medical Science Press. [Google Scholar]
- Rix M., Frank E., Webster G., Group A. G. S. F. (2001). Fritillaria: A Revised Classification: Together with an Updated List of Species. London: Fritillaria Group of the Alpine Garden Society. [Google Scholar]
- Wang D. (2012). Studies on the Key Techniques of Artificial Cultivation for Fritillaria species, Chemical Constituents from the Fritillariae Cirrhosae Bulbus and their Pharmacology Activity. Chengdu: Sichuan University. [Google Scholar]
- Wang D., Jiang Y., Wu K., Wang S., Wang Y. (2015). Evaluation of antitumor property of extracts and steroidal alkaloids from the cultivated Bulbus Fritillariae ussuriensis and preliminary investigation of its mechanism of action. BMC Complement. Altern. Med. 15:29. 10.1186/s12906-015-0551-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Wang S., Chen X., Xu X., Zhu J., Nie L., et al. (2012). Antitussive, expectorant and anti-inflammatory activities of four alkaloids isolated from Bulbus of Fritillaria wabuensis. J. Ethnopharmacol. 139, 189–193. 10.1016/j.jep.2011.10.036 [DOI] [PubMed] [Google Scholar]
- Wang D., Wang S., Du Q., Wang N., Liu S., Wang X., et al. (2014a). Optimization of extraction and enrichment of steroidal alkaloids from bulbs of cultivated Fritillaria cirrhosa. Biomed Res. Int. 2014:258402. 10.1155/2014/258402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Wang S., Feng Y., Zhang L., Li Z., Ma J., et al. (2014b). Antitumor effects of Bulbus Fritillariae cirrhosae on Lewis lung carcinoma cells in vitro and in vivo. Ind. Crops Prod. 54, 92–101. 10.1016/j.indcrop.2013.12.054 [DOI] [Google Scholar]
- Wang D., Zhu J., Wang S., Wang X., Ou Y., Wei D., et al. (2011). Antitussive, expectorant and anti-inflammatory alkaloids from Bulbus Fritillariae Cirrhosae. Fitoterapia 82, 1290–1294. 10.1016/j.fitote.2011.09.006 [DOI] [PubMed] [Google Scholar]
- Wang D. D., Feng Y., Li Z., Zhang L., Wang S., Zhang C. Y., et al. (2014). In vitro and in vivo antitumor activity of Bulbus Fritillariae Cirrhosae and preliminary investigation of its mechanism. Nutr. Cancer 66, 441–452. 10.1080/01635581.2013.878737 [DOI] [PubMed] [Google Scholar]
- Wang H. (2008). Study on Technology of Plant Tissue Culture of F. przewalskii and Its Effect on Alkaloids Accumulation. Gansu Agricultural University. [Google Scholar]
- Yan X. (2005). Effects of Ethanol Extract of Three Kinds of Bulb Fritillariae Cirrhosae on Guinea Pigs with Allergic Asthma. Sichuan University. [Google Scholar]
- Zhang S., Wang R., Zeng W., Zhu W., Zhang X., Wu C., et al. (2015). Resource investigation of traditional medicinal plant Panax japonicus (T.Nees) C.A. Mey and its varieties in China. J. Ethnopharmacol. 166, 79–85. 10.1016/j.jep.2015.02.051 [DOI] [PubMed] [Google Scholar]